Evolution and Plasticity of Gene Expression Under Progressive Warming in Drosophila subobscura
Funding: This work was supported by the Fundação para a Ciência e a Tecnologia, I.P. (FCT, Funder ID = 50110000187) under project PTDC/BIA-EVL/28298/2017 and CE3C Unit FCT funding project UIDB/00329/2020 (DOI: 10.54499/UIDB/00329/2020). P.S. and A.S.Q. are funded by national funds (OE), through FCT, in the scope of the framework contract foreseen in the numbers 4, 5 and 6 of the article 23rd, of the Decree-Law 57/2016, of August 29, changed by Law 57/2017, of July 19. M.S. is funded by grants PID2021-127107NB-I00 from Ministerio de Ciencia e Innovación (Spain), and 2021 SGR 00526 from Generalitat de Catalunya. M.A.A. was funded through an FCT PhD fellowship (2020.09172.BD).
ABSTRACT
Understanding the molecular mechanisms of thermal adaptation is crucial to predict the impacts of global warming. However, there is still a lack of research on the effects of rising temperatures over time and of studies involving different populations from the same species. The present study focuses on these two aspects, which are of great importance in understanding how organisms cope and adapt to ongoing changes in their environment. This study investigates the impact of global warming on the gene expression patterns of Drosophila subobscura populations from two different latitudinal locations after 23 generations of evolution. Our results indicate that evolutionary changes depend on the genetic background of the populations, with different starting points for thermal evolution, and that high-latitude populations show more pronounced evolutionary changes, with some evidence of convergence towards low-latitude populations. We found an interplay between plasticity and selection, with the high-latitude population showing fewer initial plastic genes and lower levels of adaptive plasticity, but a greater magnitude of change in both plastic and selective responses during evolution under warming conditions compared with its low-latitude counterpart. A substantial proportion of the transcriptome was observed to be evolving, despite the lack of observable response at higher-order phenotypic traits. The interplay between plasticity and selection may prove to be an essential component in shaping species’ evolutionary responses to climate change. Furthermore, the value of conducting studies on multiple populations of the same species is emphasised, given the identification of differences between populations with different backgrounds in several contexts.
1 Introduction
Understanding adaptation to global warming is a pressing issue considering the current climatic crisis. Recent data show alarming trends, with continued increases in average surface temperature and thermal amplitude (IPCC 2022). One of the most significant negative impacts of global warming is the disruption of species diversity and distribution patterns (Urban et al. 2016; Antão et al. 2022). For example, declines in insect abundance occur not only in cold-adapted species and habitat specialists, but also in widespread species (Stefanescu et al. 2011; Melero, Stefanescu, and Pino 2016; Wagner et al. 2021). However, recognising the role of climate change in insect declines is one thing, but understanding the processes that mediate its effects on insect populations and the potential for thermal adaptation is quite another. Such knowledge is still lacking.
Plasticity, selection and a combination of both mechanisms can help organisms cope with climate change (Hoffmann and Sgrò 2011; Gibert, Debat, and Ghalambor 2019). Adaptive plasticity refers to the capacity of an organism to adjust its phenotype in response to environmental cues (Bradshaw 1965; Pigliucci 2005; Sultan 2021), whereas selection acts on genetic variation that increases the fitness of an organism in a given environment (Bell 2008). Both types of responses should be analysed in thermal evolutionary studies to better predict the effects of climate change (Hoffmann, Sørensen, and Loeschcke 2003; Franks and Hoffmann 2012; Fox et al. 2019; Kelly 2019). There is evidence that evolution can help organisms adapt to changing temperatures (Hoffmann, Sørensen, and Loeschcke 2003; Hoffmann and Sgrò 2011; Urban et al. 2016), but usually within a specific thermal range. Once thermal shifts fall outside these thermal ranges, lack of genetic variability will limit further adaptation (Kellermann et al. 2012; Hoffmann, Chown, and Clusella-Trullas 2013). In addition, plastic responses may also have limited effectiveness in preventing the effects of climate warming (Gunderson and Stillman 2015). For example, it is possible that new environmental conditions associated with climate warming can result in initial non-adaptive plasticity of populations, as natural selection has not yet shaped the plastic responses to these new conditions (Gibert, Debat, and Ghalambor 2019). However, it is possible that plasticity and selection will interact to shape the adaptive response of populations under new conditions, with initial plastic responses bringing phenotypes closer to the new optimum (Ghalambor et al. 2007; Gibert, Debat, and Ghalambor 2019). In this scenario, plasticity may provide an alternative solution for short-term responses to environmental changes, ‘buying time’ for later evolutionary changes. By buffering against selective pressure, it may even hinder the evolutionary response (Kelly 2019). The debate over the role of plasticity in evolution stems from the observation that many environmentally induced changes appear to be non-adaptive, complicating the understanding of their contribution to adaptation (Ghalambor et al. 2007; Gibert, Debat, and Ghalambor 2019).
In this context, understanding the molecular mechanisms underlying thermal adaptation and plasticity is essential for predicting the evolutionary potential of populations to cope with increasingly warmer environments and, ultimately, for developing strategies to mitigate the impacts of climate change on ecosystems (Urban et al. 2016; Aguirre-Liguori, Ramírez-Barahona, and Gaut 2021). The study of gene expression provides insight into both these mechanisms, revealing how organisms respond to thermal changes (Sørensen, Nielsen, and Loeschcke 2007; Porcelli et al. 2016; Mallard et al. 2018; Manenti, Loeschcke, and Sørensen 2018; Oomen and Hutchings 2022). By examining transcriptome changes in such stressful environmental contexts, researchers can link genotypes to phenotypes, and identify relevant biological and functional processes involved in thermal responses (Lecheta et al. 2020; Oomen and Hutchings 2022). A recent review of studies of transcriptomic responses to temperature change (Logan and Cox 2020) found a pattern of increased genetic variation in performance at both extreme high and low temperatures rather than at intermediate temperatures, suggesting some scope for thermal adaptation to thermal extremes. The authors also highlighted specific biological processes associated with temperature-related responses, including cellular responses to heat and other stress stimuli involving heat shock proteins, underscoring the relevance of transcriptome studies in this context. Moreover, the analysis of transcriptomic changes in evolving populations allows the identification of genes expressed at a given time and their impact on higher-order phenotypic traits under different environmental conditions (Oomen and Hutchings 2022).
Given the importance of understanding the ability of populations to adapt to rapid environmental change, it is relevant to conduct experiments that test the evolutionary potential of populations in environments that reflect ecologically relevant thermal conditions, such as progressive warming and increased thermal amplitude. The study of real-time population changes through Experimental Evolution (Kawecki et al. 2012) has significant potential for addressing thermal adaptation. Indeed, studies of thermal experimental evolution have shown adaptive responses to different imposed thermal regimes (Hoffmann, Sørensen, and Loeschcke 2003; Santos 2007; Castañeda et al. 2019). However, most studies have not included dynamic, progressive changes in thermal conditions across generations, and none have included increased thermal amplitude. The few experimental evolution studies that have addressed evolution under dynamic warming temperatures have not found increased ability to cope with warming environments (Schou et al. 2014; Kinzner et al. 2019; van Heerwaarden and Sgrò 2021).
Experimental Evolution, coupled with sequencing technologies (either genome or transcriptome sequencing)—called Evolve and Reseq (E&R)—allows the characterisation of the molecular mechanisms underlying the evolutionary response of populations (Turner et al. 2011; Schlötterer et al. 2015). This approach is becoming increasingly prevalent, particularly in the context of ectothermic organisms such as Drosophila (Mallard et al. 2018; Manenti, Loeschcke, and Sørensen 2018; Michalak et al. 2019; Mallard, Nolte, and Schlötterer 2020; Sørensen et al. 2020). For instance, Mallard et al. (2018) conducted a thermal E&R study on Drosophila simulans populations that evolved under fluctuating hot conditions that remained constant across generations. This study revealed thermal adaptation with underlying transcriptomic and genomic changes. The authors reported a widespread pattern of down-regulation of glycolysis pathway genes, involving two major genomic targets, Sestrin and SNF4Aγ. Using these populations, Mallard, Nolte, and Schlötterer (2020) focused on the evolution of thermal plasticity of gene expression. After 60 generations of evolution in a hot environment, the authors found that 325 genes had evolved adaptive plasticity, with increased plasticity in 75% of these genes. Michalak et al. (2019) used a rather different experimental design, by artificially selecting populations of Drosophila melanogaster for several heat-related stressors (heat shock and heat knockdown). They found a general highly polymorphic and stress-specific response, highlighting several polymorphisms of interest in the Hsp and Hsc genes. These studies highlight the power of E&R research in providing insight into the molecular mechanisms of thermal plasticity and evolution. However, the molecular response associated with plasticity and evolutionary responses under progressively warmer environments remains unexplored. Additionally, the impact of different genetic backgrounds, or interpopulation variation, on the evolution of thermal plasticity has not been addressed in such E&R studies.
Drosophila subobscura is a valuable model organism to study the effects of global warming by experimental evolution, as it is amenable to laboratory rearing, has a short life cycle allowing to study many generations and, most importantly, has been shown to respond to temperature changes and presents adaptive clinal variation for chromosomal inversions frequencies and body size in Europe and in the Americas (Balanyá et al. 2006; Rego et al. 2010; Rezende et al. 2010; Calabria et al. 2012; Simões et al. 2012; Rodríguez-Trelles, Tarrío, and Santos 2013). In addition, a thermal experimental study addressing gene expression evolutionary changes using microarrays showed that this species responds to temperature shifts with changes in gene expression of many genes, particularly those involved in carbohydrate and nucleic acids metabolism and regulation of transcription (Laayouni et al. 2007) .
Our team has been conducting a thermal experimental evolution study to investigate the potential for an evolutionary response to a warming environment in historically differentiated D. subobscura populations, collected from two locations of the European cline, from clearly distinct latitudes. These populations were initially highly differentiated and converged phenotypically after < 30 generations of laboratory evolution, although this was not observed at the karyotypic and genomic levels (Simões et al. 2017; Barreira 2023). After 70 generations in the laboratory, these populations were subjected to thermally dynamic selective regimes that differed in mean temperature and thermal amplitude (Santos et al. 2021). Previous studies of these populations have shown thermal plasticity, but no evidence of thermal adaptation of reproductive performance was observed after 22 generations of evolution under progressive warming conditions (Santos et al. 2023). An important question to explore is whether relevant changes in gene expression are occurring, even if they do not (yet) result in a clear response in fitness-related traits. Unlike higher organisational levels, gene expression is more labile and therefore warrants significant attention to understand the genes and pathways that are changing in response to the new thermal conditions.
- To study the impact of thermal selection on the transcriptome. An overall expectation might be a general polygenic response at the transcriptome level. However, given that no evolutionary response in life-history traits has been detected up to this generation, a broad transcriptomic response may not occur, with only a few key genes showing a stronger signal.
- To analyse the impact of different initial genetic background on the evolutionary response. We expect that the imposition of a new (warming) thermal environment may enhance differentiation between our latitudinal populations at the onset of the thermal experiment, if genetic differences are still present. By analysing transcriptomic variation at baseline and after 23 generations of thermal adaptation, we will address whether differentiation is indeed initially present and whether it is followed by evolutionary convergence or divergence of our latitudinal populations.
- To analyse the interaction between plasticity and selection. An open question is whether there will be a greater number of genes showing plasticity in the warming populations, given the dynamic nature of the warming regime. We expect that, if there is a buffering effect of adaptive plasticity on evolutionary response, populations with higher levels of ancestral adaptive plasticity will show a lower response to selection. We infer ancestral adaptive plasticity in genes with a selective response from concordant direction of selective and ancestral plastic gene expression changes. We further assume that our estimates of ancestral levels of adaptive plasticity in a restricted set of genes can be generalised to the whole transcriptome level. The possibility to compare different populations tested in different thermal environments offers a valuable insight into the relative influence of plasticity and selection on the thermal evolution of the transcriptome.
2 Materials and Methods
2.1 Population Maintenance and Thermal Regimes
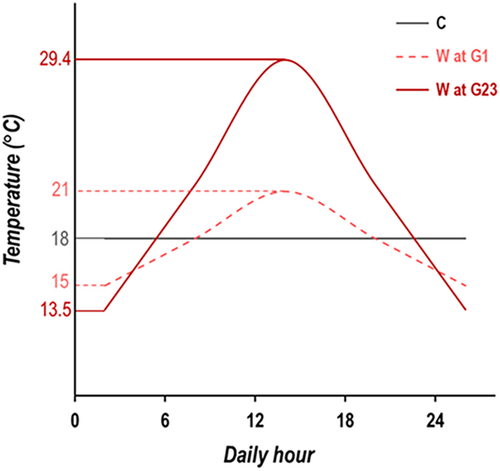
Two laboratory populations of Drosophila subobscura were founded from collections in Adraga (PT, Portugal) and Groningen (NL, the Netherlands) in 2013. These populations were threefold replicated shortly after (originating PT1-3 and NL1-3 populations) and maintained in with census sizes generally ranging between 500 and 1200 individuals (see Table S1), with discrete generations with synchronous 28-day cycle, 12L:12D photoperiod, reproduction at peak fecundity and constant 18°C temperature (Simões et al. 2017). In 2019, after 70 generations of laboratory adaptation, a new thermal regime was defined from each population: the global warming regime (W, specifically WPT and WNL) in which the temperature fluctuated daily (between 15°C and 21°C in the first generation) with increases in both daily mean and daily amplitude per generation (see Figure 1 and details in Santos et al. 2021). The increase in mean temperature in our experimental setup (0.18 per generation) aligns with the expected rate of temperature increase per decade (0.19°C–0.63°C [IPCC 2022]). In our thermal regime, the ratio of the increase in thermal amplitude relative to the mean (0.54/0.18) is also comparable with the IPCC prediction (2/1). The PT and NL populations were maintained in synchrony at a constant temperature of 18°C, serving as controls (C) in this experiment (i.e., assumed to represent the ancestral state of the warming populations; see Figure S1). From this point onwards, the two sets of populations derived from Portugal—PT and WPT—will be referred to the ‘low-latitude’ populations, whereas those derived from the Netherlands—NL and WNL—will be designated as the ‘high-latitude’ populations. This nomenclature is consistent with that employed in other studies (e.g., Santos et al. 2023). Given that only one population per latitude was sampled, and that the populations had already been in the laboratory for several generations when the thermal experiment began, any possible link between the patterns observed and the effects of latitudinal variation in nature must be treated with caution. Upon reaching a peak temperature of 30.2°C (generation 22), it was necessary to halt the increases in the thermal mean and amplitude of the global warming regime because of the significant decline in census size resulting from low viability (Table S1). At that time, the warming populations underwent a bottleneck (census size between 130 and 320 individuals), in contrast with previous generations (average census sizes between 830 and 940). Subsequently, the thermal cycle of the warming populations was reset to that of generation 20. Since that time, populations in the warming regime have been maintained within a temperature cycle with a mean temperature of 21.4°C, a lower extreme of 13.5°C and an upper extreme of 29.4°C (see Figure 1). This was the thermal cycle used for the warming environment in this experiment (see below). Importantly, the census size of our populations increased in the following generation in the warming environment, the assayed generation (see below). Although it was not a prolonged bottleneck in time, its magnitude may have had an impact at the genetic level, reducing the level of genetic variation to some extent and altering patterns of linkage disequilibrium in our populations (Frankham, Ballou, and Briscoe 2010; Schaper et al. 2012).
2.2 Experimental Design
After 23 generations in the warming (W) thermal regime, all populations were maintained for one complete cycle in a common garden environment (constant temperature of 18°C) prior to the assay. This step reduces maternal effects as all mothers of assayed individuals experienced similar environmental conditions. After common garden, the eggs laid by these females were placed in the two test environments, control and warming, to derive the sampled adults. A total of 24 samples were studied, corresponding to two thermal regimes (control vs. warming) × two histories (high latitude vs. low latitude) × three replicate populations (e.g., NL1-3) × two test environments (control vs. warming). Pools of 45 adult females from each population × environment were analysed in this experiment, with individuals being flash frozen (−80°C) on day eight of adult life. The age of the females was chosen as it corresponds to the median age of egg collection for the next generation in the maintenance protocol of both the control and warming regimes. Sex screening was done with CO2 anaesthesia > 48 h before the flash freeze procedure. All sampled females were frozen in the morning of the same day.
2.3 RNA Extraction, Sequencing and Preprocessing of Reads
Female pools were disrupted using the TissueRuptor from Qiagen, and total RNA was extracted using the RNeasy Plus Mini kit (also from Qiagen). Nucleic acid quantification was performed using Nanodrop, and RNA integrity was verified by running the samples through electrophoresis on an agarose gel. Sequencing was carried out with Illumina SBS technology using rRNA removal protocol and strand-specific libraries, and aiming at 50 million reads (15 GB) per sample, with paired-end reads of 150 bp. After sequencing, the quality of the paired-end raw reads was evaluated with FastQC v0.10.1 (Andrews 2010). Reads were pre-processed using fastp version 0.21.1 (Chen et al. 2018) to trim/remove those of poor quality, setting an average quality value of 20 and a minimum read length of 120 base pairs (bp) which corresponds to 80% of read size length. The default value of 5 was used for the n_base_limit parameter. A total of 2,726,550,002 raw reads (pair1 and pair2) were generated (average of 112,783,075 per sample) with a length of 150 bp. After trimming low-quality bases with fastp, 99% of the reads were retained for downstream analyses.
2.4 Mapping of the Reads Against the Reference Genome and Features Assignment
The pre-processed reads from the 24 samples were mapped against the Drosophila subobscura reference genome—assembly UCBerk_Dsub_1.0 (Bracewell et al. 2019) using STAR software version 2.7.9a (Dobin et al. 2013). STAR was run in a 2-pass mapping with default parameters. The assignment of the reads to features (genes) was done with FeatureCounts version 2.0.0 (Liao, Smyth, and Shi 2014) with the following parameters: –p –T 9 –F GFF –t gene –g ID –s 2 –C –a GCF_008121235.1_UCBerk_Dsub_1.0_genomic.gff. The ‘–s’ option was set to 2 as the sequencing protocol used a dUTP second strand marking RNA.
A total of 2,522,844,484 reads (93%) mapped to the reference genome (on average 105,118,520 per sample), with 2,338,445,150 (86%) mapping only once (on average 97,435,215 per sample), hereafter referred to as unique mapped reads (UMR). Mapping results are shown in Tables S2 and S3. A total of 14,306 genes (99% of the genes in the reference genome) were obtained after gene count, and 12,823 (89% of the genes in the reference genome) were used in the overall expression analysis (Figure S2 and Table S4 show additional information per sample).
2.5 Differential Expression Analysis
Read count files were normalised with DESeq2's median of ratios normalisation method using DESeq2 (Love, Huber, and Anders 2014) inside galaxy (Afgan et al. 2018, Galaxy Version 2.11.40.7 + galaxy2). This method enables the estimation of size factors for each sample that are used to normalise samples to account for bias in library sizes and RNA composition across samples (Chatterjee et al. 2018).
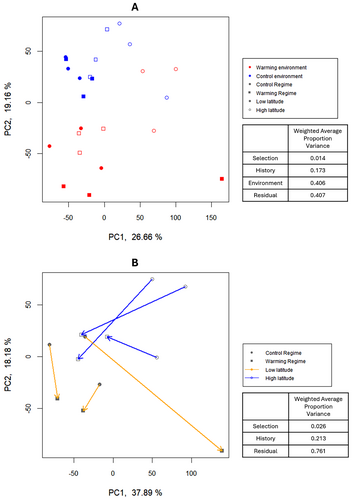
A principal component analysis (PCA) of gene expression variation was computed on the normalised counts for all genes and samples from the different thermal regimes, histories and environments. To further scrutinise evolutionary changes between thermal regimes in the warming environment, additional PCA analysis were performed for samples tested in that environment, using three different datasets: (1) all genes, (2) genes with differential expression between thermal regimes in the high-latitude populations and (3) genes with differential expression between thermal regimes in the low-latitude populations (obtained from models explained below). We also implemented a principal variance component analysis (PVCA) to our data that allow to complement each PCA analysis by estimating the proportion of variation attributed to each effect under study.
Generalised linear mixed effects (GLMMs, Bolker et al. 2009) were applied using the normalised expression counts of each transcript (gene) as raw data to test for the effects of different fixed factors: selection, history and environment allowing to address specific questions (see below). By ‘selection’ here we mean evolutionary differences between thermal regimes, not that they are entirely due to selective forces. Nevertheless, as is common in experimental evolution studies, consistent differences between thermal regimes across replicate populations are tentatively interpreted as related to selective forces. These models incorporated the variation between replicate populations, taking into account the pairing due to their ancestry across regimes and/or environments as a random effect. Specifically, in models including the factor History, such random effect was defined as the ancestral population nested in History (AP:History) to account for the pairing of replicate populations as a function of their ancestry (e.g., PT1 is the ancestral of WPT1, NL1 is the ancestral of WNL1 etc.)—see Table 1, Row 1. To select the most appropriate model and distribution to detect differential expression of normalised read counts, we performed several tests (script available at https://github.com/marta-antunes/WithInteractions) with combinations of different models (with different interaction terms with the random factor tested in a stepwise manner—see Table S5) and distributions (Poisson and negative binomial distributions). For each gene, the best combination was the one with the lowest value of Akaike information criterion (AIC—function AICtab, Burnham and Anderson 2002, Table S1). The combination used for all genes was the best for the majority of them (Figure S3). On this basis, we used the models in Table 1 (without interactions with the random factor) and a negative binomial distribution to perform several analyses.
| Row | Model | Aim |
|---|---|---|
| 1 | Expression ~ History * Environment * Selection + (1|AP:History) | Overall gene expression analysis |
| 2 | Expression ~ Environment + (1|AP) | Analysis of plasticity |
| 3 | Expression ~ Selection + (1|AP) | Analysis of selection |
| 4 | Expression ~ Environment * Selection + (1|AP) | Analysis of the evolution of plasticity |
| 5 | Expression ~ Environment * History + (1|AP) | Analysis of the effects of history on the plasticity of the control populations |
| 6 | Expression ~ History + (1|AP) | Analysis of differences between control populations of distinct history |
| 7 | Expression ~ Selection + (1|Block) | Analysis of the effects of selection in the magnitude of differences between latitudinal populations |
- Note: Expression is the normalised RNA expression values for each gene; History, the fixed factor representing origin of the populations (categories high-latitude and low-latitude); Environment, the fixed factor representing test environment (categories Control and Warming environments); Selection, the fixed factor representing thermal regimes (categories Control and Warming thermal regimes); AP is the random factor that corresponds to the ancestral population nested in the fixed factor History (e.g., PT1 is the ancestral population from which WPT1 and its paired control PT1 derived, nested in PT origin); Block is the random factor that corresponds to the set of same numbered replicate populations (e.g., Block 1 includes NL1, PT1, WNL1 and WPT1).
Different models were applied separately for high- and low-latitude populations to address specific questions (see Rows 2, 3 and 4 of Table 1): (i) the plasticity of the control high-latitude and low-latitude populations, assessed with model (2) comparing the expression in warming and control environments (factor Environment), with a total of six samples per analysis (e.g., three low-latitude replicates tested in the warming environment + three low-latitude replicates tested in the control environment); (ii) the evolutionary changes due to selection in the warming test environment, with the model (3) comparing the gene expression of populations evolving in different thermal regimes (control and warming) and tested in the warming environment; and (iii) the evolution of plasticity, with model (4) with two thermal regimes (control and warming) and two test environments (control and warming), with 12 samples tested per analysis. Additional models were applied to test for the effects of history in the control (ancestral) populations. First, a model was fitted using data from both (high- and low-latitude) populations and the two test environments to directly address the effects of history on the plasticity of the control populations including factors Environment and History as well as their interaction (model (5), Table 1). Furthermore, differences between control populations of distinct history were also assessed at each test environment separately (models with only History as a fixed factor—see model (6), Table 1). Finally, a model (model (7), see Table 1) was applied to directly test for a change in the magnitude of differences between latitudinal populations (e.g., reduction if involving convergence) as a result of thermal selection in the warming environment, by analysing variation in paired differences (absolute values) between replicate populations of distinct latitudes for both control (ancestral) and warming thermal regimes (e.g., NL1–3—PT1–3 vs. WNL1–3—WPT1–3). This was done separately for the genes that showed a differential expression between thermal regimes in low-latitude and high-latitude populations (obtained from model [3]). We also performed the same analyses excluding common genes that showed significant differences between thermal regimes in both low- and high-latitude populations. The model used in these analyses included the fixed factor Selection and the random effect Block (i.e., defined as the set of same numbered replicate populations, e.g., Block 1 with data from NL1-PT1 and WNL1-WPT1 comparisons).
All differential expression analyses were carried out in four steps: (1) selection of the read count files to be used in the analysis; (2) normalisation of the read counts using DESeq2 (Love, Huber, and Anders 2014) within galaxy (Afgan et al. 2018); (3) selection of genes that have data in at least three of the samples (> 11,000 genes in each analysis—see Table S7); and (4) detection of differential expression using glmmTMB R package (Brooks et al. 2017) assuming the negative binomial distribution (scripts generated can be found at https://github.com/marta-antunes/WithInteractions). Considering the need to account for the multiple testing involved in our analysis, p values were corrected with false discovery rate (FDR for alpha = 0.01) calculated as follows: where n is the gene count (Benjamini and Yekutieli 2001, theorem 1.3). Veen diagrams were created using InteractiVenn (Heberle et al. 2015).
2.6 Gene Ontology (GO) Analysis
Given the paucity of information available on the D. subobscura genome in comparison to the D. melanogaster genome, we used Proteinortho (Lechner et al. 2011) within galaxy to detect D. melanogaster orthologs for D. subobscura proteins. The input files used were protein sequence files from D. melanogaster (Release 6 plus ISO1 MT) and D. subobscura download from ncbi. All the parameters were set to default values. Among all these proteins, we selected only those corresponding to our candidate genes—obtained from the GFF file of D. subobscura genome (see Github). Around 8% of the proteins of D. melanogaster did not have correspondence in D. subobscura. The gene symbols for each locus accession were obtained by selecting the ‘Gene’ option in Batch Entrez tool from NCBI (https://www.ncbi.nlm.nih.gov/sites/batchentrez). We then used D. melanogaster gene symbols as input to BiNGO (Maere, Heymans, and Kuiper 2005) within cytoscape 3.9.1 to perform an enrichment analysis. The reference list used consists of the complete annotation of the D. melanogaster genome provided by BiNGO. The parameters used were the default ones with two exceptions: Ontology file that was set to GO_Biological_Process, GO_Molecular_Function or GO_Cellular_Component and organism/annotation was set to D. melanogaster.
2.7 Additional Analyses
To test whether the selected and non-selected genes are equally plastic, we used a Fisher's exact test with a 2 × 2 contingency table for each latitudinal population (i.e., number of selected or not selected genes versus being plastic or not plastic; see Table S8A,B; https://github.com/marta-antunes/WithInteractions).
To assess replicate heterogeneity, we calculated the mean Jaccard index of the three possible replicate combinations (e.g., PT1-PT2, PT2-PT3 and PT1-PT3) for low- and high-latitude populations for the candidate genes of the high latitude that were not candidates in low latitude. The Jaccard index for each combination of two replicate populations expression data was calculated by the formula where X and Y are two vectors with length n (Charikar 2002, code used is available at https://github.com/marta-antunes/WithInteractions). Jaccard index of zero denotes no similarity between the combination and Jaccard index of one indicates maximum similarity.
2.8 Analysis of Adaptive Versus Non-Adaptive Plasticity
The genes used in this analysis were the ones that showed both significant ancestral plasticity (e.g., comparing expression of NL populations in warming and control environments) and effects of selection (e.g., comparing expression of NL vs. WNL populations in the warming environment) in each latitudinal population. By analysing only genes with signs of selection, it was possible to ascertain whether the direction of plasticity was ‘adaptive’ or ‘non-adaptive’. We assume adaptive initial plasticity when the differential expression between environments (i.e., plasticity) in the control populations is in the same direction (up or down-regulation) as the evolution of expression between the warming populations and their controls tested in the warming environment.
3 Results
3.1 Overall Gene Expression Analysis
To obtain a measure of the overall variation in gene expression across different thermal regimes, test environments and histories (latitudinal populations) a PCA was performed on the normalised counts of all samples (PCA; Figure 2A). A combined reading of the two PCA axes highlights clear differences between test environments (i.e., plasticity) as the major source of gene expression variation between samples. This is corroborated by a PVCA that allows to assess the effects of different factors on gene expression variation. In fact, this analysis showed that 40.6% of the variation can be attributed to the test environments, whereas only 17.3% and 1.4% can be attributed to differences between latitudinal populations (history) and thermal regimes, respectively (see Figure 2A).
Applying a generalised linear model, we found more differentially expressed genes between samples from the same populations tested in different environments (effect of plasticity) than between populations with a different History or Thermal regime across environments (3741 genes vs. 1426 and 700 genes respectively, FDR < 0.01—see Tables S9 and S10 and Figure S4). Furthermore, from the 700 differentially expressed genes between thermal regimes (detected across the two test environments), 45% (313) were plastic and 33% (233) showed historical differences (see Figure S4). Below, we focus on the detection of candidate genes for selection—those that were significantly differentially expressed between thermal regimes—in populations tested in the warming environment, which is the new environment to which populations are adapting.
3.2 Evolutionary Changes in the Warming Environment
Given the high percentage of genes showing significant interaction between selection and history in the overall analysis (17%, see Table S9), we conducted a separate analysis of the response to the thermal regimes in the two latitudinal populations. Specifically, for each latitude of origin, we analysed gene expression differences between the warming populations and their respective controls tested in the warming environment.
A total of 221 differentially expressed genes were identified as being common to both low- and high-latitude populations (Figure S5), with a high proportion (64%) showing changes in the same direction (142 genes; Table S11). We found a higher proportion of differentially expressed genes between thermal regimes in high-latitude populations than in low-latitude ones (35% vs. 8%; Figure S5 and details below). These genes were evenly distributed along the genome in both populations (Figure 3).

3.2.1 Low-Latitude Populations
We found 949 (out of 11,948 = 8%) differentially expressed genes between thermal regimes in the low-latitude populations. Of these, 555 were up-regulated (higher expression in the warming than in the control populations) and 394 were down-regulated (see Figure 3A and Table S12A).
Although the up-regulated genes were enriched for Gene Ontology (GO) molecular functions of enzymatic catalytic activities (e.g., hydrolases, peptidases and specific enzymes such as lipases that are involved in the breakdown of triglycerides) and GO biological processes of proteolysis and lipid metabolic processes, the down-regulated genes were mostly enriched for GO molecular functions of binding (e.g., nucleotide binding or purine nucleotide binding) associated with processes of organelle and cellular component organisation (see Table S13).
3.2.2 High-Latitude Populations
In the high-latitude populations, 4195 genes (out of 11,958 = 35%) showed significant differential expression between thermal regimes, which is considerably higher than the number of differentially expressed genes in the low-latitude populations. Of these genes, 1923 were up-regulated and 2272 were down-regulated (see Figure 3B and Table S12B).
The up-regulated genes were enriched for GO structural molecular functions, enzymatic activities of GTPases, GTP binding, RNA polymerase II transcription and Guanyl nucleotide binding. The down-regulated genes were enriched for many GO terms, but some can be highlighted considering their higher statistical significance in the enrichment analysis. These were related to binding functions (like protein binding, binding, adenyl ribonucleotide binding, ATP binding, DNA binding, nucleotide binding) and transcription regulator activity (see Table S13).
3.2.3 Evolutionary Changes Across Latitudinal Populations
To analyse evolutionary differences between low- and high-latitude populations, we performed PCA and PVCA analyses for both latitudinal populations when tested in the warming environment using our full gene dataset (see Figure 2B). The combined reading of the first two PCA axis shows an overall high differentiation between latitudinal populations (21.3% variation, PVCA analysis). We also obtained higher evolutionary changes in the high-latitude populations, that promoted a reduction in historical differences in the warming populations relative to the differences in the controls, except for one outlier population (WPT1). PCA analysis where further performed for the set of candidate genes of each latitudinal population (see Figure S6A,B). The pattern observed in the candidate genes for high-latitude populations was remarkably similar to that observed above for the whole set of genes (see Figure 2B and Figure S6B). On the other hand, candidate genes in low-latitude populations showed lower overall expression changes with no reduction in historical differences during thermal evolution. Comparing these last two analyses, we observed higher expression variation in the candidate genes for the high-latitude populations, which may explain the similarity with the gene expression patterns observed for the whole set of genes. Interestingly, the PVCA analysis confirms what was said above, with a much lower variation (2.3%) associated with evolutionary changes when analysing the low-latitude candidate genes compared with almost three times that variation (6.5%) in the high-latitude candidate genes. Furthermore, the proportion of variation associated with historical differences was slightly lower in the analysis of high-latitude candidate genes (16.2% vs. 19.1%), consistent with the reduction in historical differences reported above (Figure S6A,B).
Using the two sets of candidate genes for thermal selection (in low or high-latitude populations), we also tested for changes in the magnitude of historical differences by comparing the absolute differences between high and low-latitudinal control populations and the corresponding differences in the evolved (warming) populations (see model 7 Table 1 and Table S14). For the high-latitude candidate genes (4195), we observed that 279 of them showed significant variation in the amount of historical differentiation of control versus warming populations. Although this is a rather low proportion (~7%), it is interesting that 176 of them (63.1%) showed evidence of a reduction of differences in the warming populations (i.e., NL-PT > WNL-WPT) confirming the PCA results. On the other hand, the pattern was different for candidate genes in low-latitude populations (949), with 114 genes (12%) showing significant variation in differences between control and warming populations, of which only 30 showed a reduction in differences (26.3%) (see Table S14A,B). When candidate genes common to high- and low-latitude populations are excluded, a similar proportion of genes show significant variation in the amount of historical differentiation between control and warming populations (6% in high-latitude candidates and 12% in low-latitude candidates). The same trend is observed in the proportions reflecting the reduction in differences (see Table S14C,D).
3.3 Evolution of Plasticity
The plasticity of the control populations was analysed as a baseline for studying the evolution of plasticity. This was achieved by comparing the gene expression of each control population (e.g., NL1-3) between the two test environments. A total of 2427 differentially expressed genes were identified between environments (hereafter referred to as ‘plastic genes’) in the control low-latitude populations (PT1-3, Table S15A) and 1567 in the control high-latitude populations (NL1-3, Table S15B). These findings are presented in Table 2. Of these plastic genes, 606 were common to both origins (Figure S7). Interestingly, most of these genes responded in the same direction in both high-latitude and low-latitude populations (593 out of 606 = 98%). When we directly tested how the plastic response varied between control latitudinal populations (the interaction between history and environment, see model 5 Table 1), we found 334 (out of 12,453) genes showing significant differences in plasticity between the two populations (see Table S16A). The number of genes showing overall historical differences (across environments) in the control populations was much higher (3353, see Table S16B). Interestingly, when comparing the control populations in each environment separately (model 6 Table 1), we observed a clearly higher number of genes showing differences between latitudinal populations in the warming environment (3344) than in the control environment (1994)—see Table S17A,B respectively.
| Low latitude | High latitude | |||
|---|---|---|---|---|
| Number of genes | Percentage (%) | Number of genes | Percentage (%) | |
| Plasticity of the control populations | 2427 | 20 | 1567 | 13 |
| Plasticity of the warming populations | 2385 | 20 | 3096 | 26 |
| Evolution of plasticity | 198 | 2 | 325 | 3 |
- Note: Percentage was calculated by dividing the number of differential expressed genes by the total of genes in each analysis (see Table S4).
For the warming populations, we found 2385 plastic genes in the low-latitude populations (WPT1-3, see Table 2 and Table S18A), whereas in the high-latitude populations, we found 3096 plastic genes (WNL1-3, see Table 2 and Table S18B).
Below, for each history, we test whether plasticity has evolved through selection (meaning consistent evolutionary changes in plasticity between thermal regimes across replicate populations) by comparing the plasticity of populations that evolved in the warming regime with that of their respective control regime.
3.3.1 Low-Latitude Populations
The generalised mixed-effects model applied to test the evolution of plasticity (model 4 in Table 1) revealed 198 significant genes (FDR < 0.01) for the interaction between selection and environment, indicating differential evolution of plasticity due to the imposition of the thermal regime (Table 2 and Table S19A).
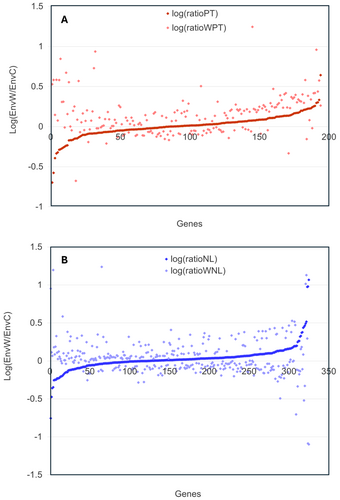
A total of 88 genes out of the 198 analysed (44%) changed their expression in opposite direction between the two environments when comparing the warming versus the control regimes: 54 (61%) genes were down-regulated in PT (lower expression in the warming environment compared with the control environment) and up-regulated in WPT (see left side of Figure 4), whereas 34 (39%) showed the opposite pattern (the few lighter dots below the zero line on the right side of the plot). In general, there is a pattern of up-regulation in WPT with higher expression in the warming environment when compared with expression in the control environment.
3.3.2 High-Latitude Populations
A similar model was applied to the high-latitude populations. We obtained a higher number (325) of significant genes for the interaction between selection and environment (Table 2 and Table S19B) compared with the low-latitude populations.
In the high-latitude populations, we also found that more genes—210 out of 325 (65%)—changed their expression in opposite directions between thermal regimes: Some genes (91) that were down-regulated in NL were up-regulated in WNL (43%, see left side of Figure 4), whereas a higher number of genes that were up-regulated in NL (119) were down-regulated in WNL (57%, right side of the same Figure). Only four genes showed evolution of plasticity in both historical populations (Figure S8).
3.4 Plasticity Versus Evolution
To grasp better the connection between ancestral (control) plasticity and evolution, we examined the overlap of genes associated with control plasticity and evolutionary changes (candidate genes for selection) for both high-latitude and low-latitude populations (Figure 5A,B). This study allows us to understand the extent to which plastic genes are recruited by selection. We found that, in high-latitude populations (Figure 5B), 17% of the genes recruited by selection were plastic (723/(723 + 3472)), which is significantly higher than the 11% (844/(844 + 6861)) of plastic genes that were not recruited by selection. (Fisher's exact test with Table S5B, p value < 2.2e-16). In low-latitude populations (see Figure 5A), 34% of the recruited genes (321/(321 + 628)) were plastic, again significantly higher than the 19% of plastic genes not recruited (2106/(2106 + 8845)) (Fisher's exact test, with Table S8A, p value < 2.2e-16). These results indicate a greater recruitment of plastic genes by selection in low-latitude populations compared with high-latitude populations, even though this impact is significant in both historical populations.
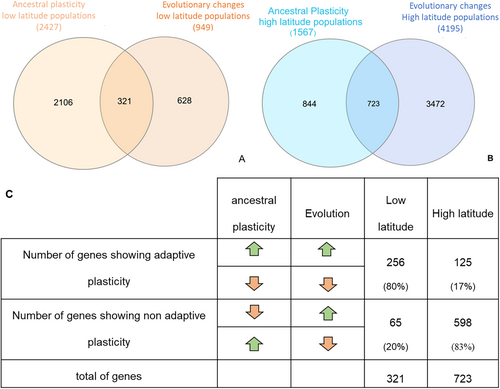
To understand whether candidate genes for selection exhibited ancestral (control) adaptive plasticity, we estimated the number of genes showing expression values with the same sign in both ancestral plasticity and selection (by either consistent up or down-regulation, see Figure 5C). We found that 80% of the selected genes showed ancestral adaptive plasticity in low-latitude populations but only 17% in high-latitude populations (Figure 5C).
3.5 Comparison With Candidate Genes From Other Thermal E&R Studies
We have compiled a list of candidate genes for heat selection from several studies of thermal experimental evolution in Drosophila (Laayouni et al. 2007; Mallard et al. 2018; Michalak et al. 2019; Sørensen et al. 2020). Those genes that have a match in the D. subobscura reference genome are listed in Table 3, along with an indication of a significant effect for the term Selection and direction of change (up or down-regulation) in our warming populations of low and high latitude. It is important to note that this list is not exhaustive, but aims to provide a representative sample of the genes previously identified and highlighted as relevant in the studies mentioned above.
| Up- or Down-regulated | |||||
|---|---|---|---|---|---|
| Gene name in publication | Publication | Gene description in D. subobscura reference genome | Gene ID in the D. subobscura reference genome | Low latitude | High latitude |
| CG11876 | Mallard et al. (2018) | Pyruvate dehydrogenase E1 component subunit beta, mitochondrial | LOC117896292 | Non-sig. | Up |
| CG4183 (Hsp26*) | Laayouni et al. (2007), Michalak et al. (2019) | Heat shock protein 26 | LOC117895522 | Non-sig. | Non-sig. |
| Eno | Mallard et al. (2018) | Enolase | LOC117901927 | Non-sig. | Non-sig. |
| Fbp | Mallard et al. (2018) | Fructose-1,6-bisphosphatase 1 | LOC117902317 | Non-sig. | Non-sig. |
| Gapdh1 | Mallard et al. (2018) | Glyceraldehyde-3-phosphate dehydrogenase | LOC117890520 | Non-sig. | Excluded due to high missing data |
| GD10865* | Sorensen et al. (2020) | Endoplasmic reticulum metallopeptidase 1 | LOC117892204 | Non-sig. | Non-sig. |
| GD11003* | Sorensen et al. (2020) | Probable G-protein coupled receptor Mth-like 14 | LOC117894718 | Up | Non-sig. |
| GD16416* | Sorensen et al. (2020) | Delta-sarcoglycan | LOC117902577 | Non-sig. | Non-sig. |
| GD16815* | Sorensen et al. (2020) | 5-demethoxyubiquinone hydroxylase, mitochondrial | LOC117902507 | Non-sig. | Up |
| GD21769* | Sorensen et al. (2020) | Claspin | LOC117894285 | Non-sig. | Down |
| GD23312* | Sorensen et al. (2020) | DNA-directed RNA polymerases I and III subunit RPAC1 | LOC117902220 | Non-sig. | Up |
| GD23922* | Sorensen et al. (2020) | Zinc finger BED domain-containing protein 4 | LOC117902436 | Non-sig. | Non-sig. |
| GD24459* | Sorensen et al. (2020) | Probable ATP-dependent RNA helicase DHX34 | LOC117889582 | Down | Non-sig. |
| GD25858* | Sorensen et al. (2020) | Ornithine decarboxylase antizyme | LOC117890021 | Up |
Up |
| GD27160* | Sorensen et al. (2020) | Cell cycle control protein 50A | LOC117903544 | Non-sig. | Non-sig. |
| GD27324* | Sorensen et al. (2020) | Vinculin | LOC117903461 | Non-sig. | Down |
| GD27583* | Sorensen et al. (2020) | N-alpha-acetyltransferase 16, NatA auxiliary subunit | LOC117889583 | Non-sig. | Down |
| GD28851* | Sorensen et al. (2020) | T-complex protein 1 subunit epsilon | LOC117890527 | Non-sig. | Down |
| Grp* | Michalak et al. (2019) | Serine/threonine-protein kinase grp | LOC117900415 | Non-sig. | Non-sig. |
| Hsc70-5* (Sorensen et al. 2020) and Hsc70-1* (Michalak et al. 2019) | Michalak et al. (2019) and Sorensen et al. (2020) | hsc70-interacting protein 1-like | LOC117890693 | Non-sig. | Down |
| Hsp22* | Michalak et al. (2019) | Heat shock protein 22 | LOC117895525 | Non-sig. | Non-sig. |
| Hsp68 | Laayouni et al. (2007) | Heat shock protein 68 | LOC117897026 | Non-sig. | Non-sig. |
| ImpL3 | Mallard et al. (2018) | L-lactate dehydrogenase | LOC117895027 | Non-sig. | Up |
| l (1G0334) | Mallard et al. (2018) | Pyruvate dehydrogenase E1 component subunit alpha, mitochondrial | LOC117903707 | Up | Down |
| Pfk | Mallard et al. (2018) | ATP-dependent 6-phosphofructokinase | LOC117891670 | Non-sig | Non-sig |
| Ppn | Michalak et al. (2019) | Papilin | LOC117896779 | Non-sig | Non-sig |
| Qua* | Michalak et al. (2019) | Villin-like protein quail | LOC117901464 | Non-sig. | Non-sig. |
| Sestrin* | Mallard et al. (2018) | Sestrin homologue | LOC117891210 | Down 0.01 < FDR < 0.05 |
Non-sig |
| SNF4Aalpha | Not mentioned in previous publications | 5'-AMP-activated protein kinase catalytic subunit alpha-2 | LOC117896570 | Non-sig. | Down |
| SNF4Agamma* | Mallard et al. (2018) and Michalak et al. (2019) | 5'-AMP-activated protein kinase subunit gamma-1 | LOC117898834 | Non-sig. | Down |
| Tpi | Mallard et al. (2018) | Triosephosphate isomerase | LOC117898999 | Non-sig. | Up |
| Treh | Laayouni et al. (2007) | Trehalase | LOC117891655 | Up | Non-sig. |
| Turandot M | Manenti, Loeschcke, and Sørensen (2018) | Turandot M-like | LOC117896950 | Non-sig. | Non-sig. |
| Turandot X* | Manenti, Loeschcke, and Sørensen (2018) and Sorensen et al. (2020) | Turandot X | LOC117897127 | Non-sig. | Non-sig. |
| Ush* | Michalak et al. (2019) | Zinc finger protein ush | LOC117901953 | Non-sig. | Down |
- Note: Significance for the factor Selection is shown for each gene. Differential expression was considered significant for p values corrected with FDR < 0.01. Significant p values using a looser criterium (FDR < 0.05) are also mentioned in the table. Up-regulation occurs when expression is higher in populations that evolved in the warming environment when compared to their controls. The symbol ‘*’ denotes genes found under selection in genomic analyses, whereas the remainder of the reported candidate genes were obtained from transcriptomic analyses.
Among this repertoire of genes, we found that only 17% (6 out of 35) showed signs of selection in our low-latitude populations, whereas a much higher percentage (44%—15 out of 34) was obtained for the high-latitude populations (Table 3). Also, only two genes show significant signs of selection in both populations. One of them, gene GD25858, coding for ornithine decarboxylase antizyme, shows patterns of up-regulation in both populations. Sestrin and SNF4Agamma genes, which were highlighted by Mallard et al. (2018) as major candidate genes for thermal adaptation in their genome analysis, also show evidence of thermal selection in our study. However, Sestrin only showed a significant sign of selection in the low-latitude populations, whereas SNF4Agamma was only significant in the high-latitude populations (see Table 3). The catalytic subunit α of SNF4A, which was not mentioned by Mallard et al. (2018), was also found to be down-regulated in our high-latitude populations showing the same pattern as the gamma subunit.
4 Discussion
We found fundamental differences in transcriptome plasticity and adaptation between historically differentiated Drosophila subobscura populations that have evolved under increasing temperatures. Notably, the impact of selection on the high-latitude populations involved a considerably higher number of genes (4195 genes, representing 35% of the total genes across the transcriptome) compared with those involved in the selective response of the low-latitude populations (949 genes, representing 8% of the total genes across the transcriptome). Furthermore, evolutionary changes in the magnitude of gene expression were also higher in the high-latitude populations, with some evidence of convergence towards low-latitude populations. Conversely, ancestral plasticity was observed to be higher in low-latitude populations than in high-latitude populations (20% vs. 13% of the total number of genes in the respective control populations). Interestingly, after evolution under warming conditions, the high-latitude populations outperformed their low-latitude counterparts, showing increased plasticity. Moreover, the low-latitude populations, which showed a weaker response to selection, were the ones presenting higher levels of ancestral adaptive plasticity. These findings support the hypothesis that the plastic response plays a role in buffering evolutionary changes (Kelly 2019). They also illustrate the influence of historical background on transcriptomic evolution, which will be discussed in more detail below.
4.1 Ample Gene Expression Response to Selection
Given that we did not find an evolutionary response for life-history traits in our populations by generation 22 (Santos et al. 2023), we anticipated that only a relatively low proportion of the transcriptome would exhibit signs of selection. However, this expectation was not met. In fact, we identified hundreds to thousands of genes differentially expressed between thermal selective regimes. It is likely that the bottleneck suffered by our warming populations two generations before this analysis caused depletion of genetic variation. However, our estimates of the total number of variable sites derived from our transcriptomic data do not show evidence of the expected decline in the variability of the warming populations (see Supporting information). This suggests that the bottleneck did not have a substantial impact on the overall genetic variability of our populations. Two factors may have contributed to this: firstly, the bottleneck was not too drastic (the lowest census size was 130), and secondly, census sizes recovered rapidly. Nevertheless, it is possible that the bottleneck may have resulted in the loss of rare alleles and the generation of some linkage disequilibrium patterns (Frankham, Ballou, and Briscoe 2010; Schaper et al. 2012).
It is crucial to acknowledge that changes in gene expression in some of these candidate genes may occur as a correlated response also leading to consistent evolutionary changes in all replicate warming populations, rather than being the direct targets of selection. Pleiotropy, linkage disequilibrium and the side effects of changes in regulatory genes may contribute to the observed variation. Moreover, we cannot exclude effects of consistent drift between replicated warming populations given spurious indications of selective response. The decoupling between life-history traits and gene expression patterns is noteworthy and might be associated with changes in other relevant traits—such as adult survival or thermal tolerance (Rogell et al. 2014; Schou et al. 2014; Manenti, Loeschcke, and Sørensen 2018) not studied directly by Santos et al. (2023). Nevertheless, that study focused on reproductive output, expected to be a most relevant trait, as a surrogate for fitness. Our whole transcriptome approach covers gene expression changes impacting on a wide range of phenotypic functions and traits, including those related to metabolism, physiology and morphology. These might have altogether given higher power to be detected, as correlated responses to the ultimate target of selection, which is fitness (Falconer and Mackay 1996). In any case, the decoupling is likely to reflect a different timing of thermal evolution between the various biological levels. To rephrase, there may be a temporal discrepancy between the evolution of gene expression patterns and fitness-related traits, with a comparatively slower response (and/or capacity to detect it) in the latter relative to the former. Indeed, we have observed the evolution of life-history traits after 39 generations of thermal evolution in our warming populations, particularly in the low-latitude populations (Santos et al. 2023). This slow response serves to reinforce the role of initially adaptive plasticity as a primary mechanism for coping with a warming environment. Furthermore, it also highlights the importance of long-term studies.
We anticipated that the control populations were already adapted to the laboratory environment at the time the warming selective regime was imposed. Our team has followed the dynamics of laboratory adaptation and observed a quick response during the first 20 generations in the laboratory followed by a reduction in the evolutionary rate (Simões et al. 2007, 2019). This suggests that our control populations were already very close to the fitness optimum when the experiment started—after 70 generations of adaptation to the laboratory environment. However, the underlying genes may continue to change frequencies even after the trait optimum has been reached (Franssen et al. 2017; Seabra et al. 2018). According to Franssen et al. (2017), adaptation to the trait optimum involves (1) directional allele frequency changes followed by (2) a plateauing and (3) further allele frequency changes eventually leading to fixation or loss of alleles after the trait optimum was reached. Considering this, it is possible that some changes in gene expression might still occur in the control populations in our study, though of a low magnitude. Having said that, changes in allele frequencies in response to the general laboratory conditions may also occur in the warming populations. However, more pronounced gene expression changes are expected to occur in the warming populations as a result of thermal adaptation. Our stringent criteria to detect candidate genes reinforces this expectation.
A relevant finding is that the numerous candidate genes detected were evenly distributed along the genome. This contrasts with the finding by Laayouni et al. (2007), who found a higher clustering of candidate genes within segregating inversions in D. subobscura populations under thermal selective regimes. Such clustering could be expected because of low recombination within and in the vicinity of inverted regions (Hoffmann and Rieseberg 2010), which could result in the identification of false positives due to hitchhiking (Schlötterer et al. 2015). Our data do not show such a prevalent clustering pattern. Importantly, our populations still presented polymorphisms for inversions by the start of the experiment with only one of the five chromosomes presenting an almost fixed inversion (average expected heterozygosity of 0.271). One simple explanation for the discrepancy between studies is that ours has tested a much higher number of genes than the Laayouni et al. microarray approach.
Common patterns were identified between the two geographical populations, with evidence for a consistent down-regulation of binding functions in both. It was anticipated that down-regulation would occur for non-essential genes (Mallard et al. 2018; Masoomi-Aladizgeh et al. 2022) and potentially involve a reduction in energy production (as described in Mallard et al. 2018). The consistent down-regulation of binding functions that we observed in both populations may be associated with this reduction in non-essential metabolic activities. This is also in agreement with our observations of specific genes showing common selective responses in both geographical populations. Of particular interest are genes coding for trichohyalin, larval serum proteins and fat body protein, which were highly down-regulated in the warming populations from both low- and high-latitude origin (Table S11). According to the Online GEne Essentiality (OGEE) database (Gurumayum et al. 2021), larval serum proteins1, gene CG31551 which codes for trichohyalin (The Uniprot Consortium 2023) and fat body protein 2 in D. melanogaster—are classified as non-essential or conditional (see also empirical studies by Meng et al. 2008; and Kasuya, Kaas, and Kitamoto 2009). The consistent down-regulation of larval serum proteins and fat body protein 2 suggests alterations in the fat body of the flies (Sato and Roberts 1983; Lehmann 1996; Jiang et al. 2005). This is in agreement with previous observations in Drosophila studies of thermal plasticity (Klepsatel et al. 2016) and adaptation (Mallard et al. 2018).
4.2 Selection Is Contingent on Previous History
A greater number of genes were identified as being candidates for selection in the high-latitude populations (4195 vs. 949). Importantly, there was no striking difference in coverage between latitudinal populations (see Figure S2). Also, differences between replicates (particularly in the candidate genes detected only in high-latitude populations) were, if anything, higher in the high-latitude populations (mean Jaccard index = 0.89 for low-latitude population and 0.90 in high-latitude populations). These observations suggest that the differences between low- and high-latitude populations were not due to reduced statistical power (due to increased replicate variability) or other sources of bias that would favour the detection of gene expression differences in the latter.
We also found clear functional differences between low- and high-latitude populations in genes that responded to selection by up-regulation. In fact, we found up-regulation of genes enriched for enzymatic activity (hydrolases such as peptidases and lipases) in low-latitude populations, whereas structural integrity was found to be more highly up-regulated in high-latitude populations. It seems plausible to suggest that these observed differences, along with the differing number of genes responding to selection, may be related to the historical adaptation of high-latitude populations to colder climates in nature. This would entail a subsequent need for greater adjustments in cellular metabolism and regulation to adapt to the warmer temperatures imposed in our experimental evolution regime. Consistent with this expectation, we do in fact observe higher evolutionary changes in the gene expression patterns of high-latitude populations. In particular, we found evidence for a convergence pattern for some of the candidate genes of the high-latitude populations, that evolved in those populations towards the expression patterns of their low-latitude counterparts. Our findings at the functional level also follow that expectation, with the high-latitude populations changing in a broader range of cellular functions than the low-latitude ones. Nevertheless, it is important to note that this potential association with natural environments must be interpreted with caution, given that our populations had already evolved for 70 generations under common, laboratory conditions when the new selective, warming regime was imposed (Santos et al. 2021, 2023). There is a conspicuous absence of studies addressing the interpopulation variation in transcriptome evolution. An exception to this is the geographical survey of D. subobscura European populations by Porcelli et al. (2016), which employed both common garden (i.e., laboratory) and in situ approaches to study gene expression. Their findings indicated that gene expression patterns reflect local adaptation along the European cline of the species, with a trade-off between metabolism and reproduction. The present study demonstrates that interpopulation variation can have a discernible impact on the evolution of gene expression in the context of climate change.
4.3 Candidate Genes for Thermal Selection
A survey was conducted on genes highlighted as relevant for heat-selective response in the thermal E&R literature. These genes were then compared with our own populations. Of the 35 selected genes from other studies, 44% and 17% were also highlighted in our high- and low-latitude populations respectively. In contrast, 16 genes (46%) of those 35 were not detected in our populations. This last finding is to be expected given the differences in thermal selective regimes and species used in the literature. Furthermore, of all compiled genes, only two presented a selective response in both high- and low-latitude populations. One of these genes, ornithine decarboxylase antizyme, shows an evolutionary up-regulation in both populations and is involved in positive regulation of protein catabolic process (Hayashi, Murakami, and Matsufuji 1996). This agrees with a plastic response of reduction in protein biosynthesis, growth and proliferation that occurs under heat stress (Klepsatel et al. 2016).
Our findings indicate that genes with a robust selective response in one population may show only a slight (or even absence of) response in another population. Consequently, inferences on targets of thermal selection based on studies in a single population can be misleading, potentially resulting in inaccurate assumptions regarding adaptation to temperature. Standardising experimental protocols by pursuing responses to ecologically relevant conditions (e.g., fluctuating temperatures and progressive warming) could help to alleviate this trend.
4.4 Interplay Between Plasticity and Selection Associated With Historical Origin
Given the dynamic nature of the warming regime, we anticipated a greater number of plastic genes in the warming populations. This expectation was only met for the high-latitude populations, which exhibited a doubling of the number of plastic genes expressed after evolution compared with their controls (from 1567 genes—corresponding to 13%—to 3096%–26%). In contrast, for the low-latitude populations, the number was virtually identical between warming and control populations. An interesting aspect is that the ancestral populations presented different starting points for thermal plasticity, with low-latitude populations having more plastic genes (2427 corresponding to 20% of genes) than the high-latitude ones (1567 genes corresponding to 13%). Interestingly, these differences in thermal plasticity translate into a higher degree of historical differentiation in the warming environment than in the ancestral one. This increased differentiation in the ancestral populations when exposed to the new (warming) environment may have favoured the evolutionary differences between populations with contrasting histories observed in this environment.
During thermal evolution, a greater number of genes changed their degree of plasticity in the high-latitude populations (325 of all genes) than in the low-latitude ones (198). In the low-latitude populations, the plasticity evolved primarily through up-regulation, whereas in the high-latitude populations, it evolved through both up- and down-regulation. It is noteworthy that the up-regulated genes are not identical in the high- and low-latitude populations, with only one gene in common. The differential evolution of plasticity between populations reinforces the relevance of their historical genetic background. These results highlight how thermal responses may vary between different populations and reinforce the importance of conducting studies in several populations to make correct assessments on adaptation to temperature changes. The magnitude of plasticity changes observed in our populations is comparable to that reported in another study addressing thermal plasticity of gene expression in D. simulans (Mallard, Nolte, and Schlötterer 2020). In that study, 2.9% (325/11,200) of genes evolved a change in plasticity relative to the control population, after > 60 generations in the hot environment. Interestingly, we did not require as many generations to obtain a rather similar response (1.6% and 2.6% in low- and high-latitude populations). It remains to be seen whether Mallard, Nolte, and Schlötterer (2020) would have obtained similar results had they conducted the experiment over a shorter time span. Nevertheless, the selective regimes applied differ (milder in the Mallard et al. study), likely leading to different selective pressures and thus different rates of response. Surprisingly, in a thermal experimental evolution study using a fluctuating regime (daily variation between 13°C and 28°C), Manenti, Loeschcke, and Sørensen (2018) did not find indications of evolution of plasticity in the transcriptome of D. simulans populations after 20 generations. These populations also did not show differences in several phenotypic traits across environments (Manenti et al. 2015). Overall, the existence of several sources of differences between studies, namely the species used, the thermal regimes applied and the different time spans of thermal evolution, prevents wider comparisons and generalisations across studies.
It is important to note that the probability of plastic genes being recruited by selection was found to be higher than that of non-plastic genes. This pattern was observed to be more pronounced in low-latitude populations (34% of genes responding to selection were initially plastic in low-latitude populations) than in high-latitude populations (17% in high-latitude populations). Furthermore, we expected that populations with a stronger selective response would show lower ancestral adaptive plasticity. In fact, this pattern was observed in our populations, with the high-latitude populations exhibiting a higher selective response and lower ancestral adaptive plasticity (17%) relative to the low-latitude populations (80%; see Figure 5). It is crucial to note that this analysis only includes the subset of genes that both responded to selection and exhibited plasticity in the control group. We are assuming the levels of adaptive plasticity that we observed can be extrapolated to the entire transcriptome. If this assumption is met, it can be concluded that the observed patterns are consistent with a buffering effect of adaptive plasticity in the evolutionary response (Kelly 2019). Conversely, a lower initial adaptive plasticity implies that a population will be further away from the ‘optimum’ (Ghalambor et al. 2007, 2015; Morris and Rogers 2013; Gibert, Debat, and Ghalambor 2019). This can explain the higher evolutionary response observed in our high-latitude populations. Altogether, these findings demonstrate the influence of the interaction between plasticity and selection (Ghalambor et al. 2007) in the context of adaptation to warming environments (Fox et al. 2019; Gibert, Debat, and Ghalambor 2019; Kelly 2019). Furthermore, it emphasises the necessity of considering interpopulation variation, which enables the assessment of the relevance of different genetic backgrounds and enhances the capacity to identify the relative influence of plasticity and selection in distinct populations.
5 Conclusions
To the best of our knowledge, this is the first study to address the consequences of prolonged evolution under a progressively warming temperature at the gene expression level in populations with different natural histories. Our findings indicate that a significant proportion of the transcriptome is under selection, despite the lack of observable response at a higher level of organisation, such as life-history traits, within the same time span. The effects of the historic background were potentiated in the warming environment and exerted a significant influence on transcriptome evolution. In fact, we observed a markedly higher genome-wide selective response in the higher latitude populations compared with low-latitude populations, with some evidence for evolutionary convergence particularly in candidate genes for thermal selection of high-latitude populations. Moreover, our results suggest that there is an interplay between plasticity and selection. In particular, the high-latitude populations exhibited lower levels of ancestral adaptive plasticity, which may contribute to the overall higher magnitude of changes in gene expression throughout evolution in warming conditions.
The disparate gene expression patterns observed between populations during thermal evolution underscore the importance of conducting studies encompassing multiple populations of the same species.
Author Contributions
M.A.A. experimental work and writing (original draft). M.A.S. and M.M. conceptualisation of the experiment and experimental work. A.S.Q. and M.S. conceptualisation of the experiment and writing (review and editing). P.S. conceptualisation of the experiment, experimental work and writing (review and editing).
Acknowledgements
The authors thank Ana Carromeu-Santos for helping in the maintenance of populations. We thank the Subject Editor (Christian Schlötterer), and three anonymous reviewers for their very helpful comments and suggestions.
Conflicts of Interest
The authors declare no conflicts of interest.
Benefit-Sharing Statement
Benefits Generated: Benefits from this research accrue from the sharing of our data and results on public databases as described above.
Open Research
Data Availability Statement
The sequence data for all samples are available at NCBI BioProject PRJNA1161223 (Antunes et al. 2024b). Customised scripts used are available in Github: https://github.com/marta-antunes/WithInteractions (Antunes et al. 2024a).




