Linking gene expression patterns with survival studies elucidates adaptive potential in changing environments
Abstract
A major goal in ecology, evolution and conservation biology is understanding how species adapt to changing conditions and using that information to improve conservation actions. Primary to advancing our understanding of adaptation and adaptive potential is determining the causes and consequences of the genetic and phenotypic intraspecific variation that allows for adaptation. However, few studies have been able to link the variation present in molecular process under differing conditions to intraspecific variation in survival. In a From the Cover article in this issue of Molecular Ecology, Harder et al explore the relationship between molecular process and survival to understand the adaptive variation underlying tolerance to low thiamine (vitamin B1, an essential micronutrient) conditions in Atlantic salmon (Salmo salar). Thiamine is required for metabolic functioning, including energy production and nervous and cardiovascular system functioning. By combining controlled breeding and phenotyping with a survival study and transcriptomics, the authors are able to quantify among-family differences in survival under low thiamine conditions. They find wide variation in survival among families, and this survival is linked to differences in gene expression patterns. Their study elucidates the importance of combining different data types to characterize within-population phenotypic variation and understand how that variation may lead to genetic adaptation under stressful conditions.
Thiamine deficiency is an emerging conservation issue worldwide (Gilbert, 2018; Sutherland et al., 2018) and is of particular concern in aquatic ecosystems where it has been linked to reduced survival and reproductive success (Harder et al., 2018). Thiamine deficiency can result from decreased availability of thiamine-containing food in aquatic systems; however, comparatively more cases of thiamine deficiency have been linked to increased consumption of thiaminase, a thiamine-degrading enzyme (Harder et al., 2018). The alewife (Alosa pseudoharengus) is an example of an organism containing high levels of thiaminase. Alewife have invaded multiple lake systems (e.g., Great Lakes and Lake Champlain) and its consumption has been linked to severe thiamine deficiency in alewife predators, including threatened Atlantic salmon (Ketola, Bowser, Wooster, Wedge, & Hurst, 2000). Understanding the potential for populations to adapt to low thiamine conditions not only provides important insights into the process of rapid adaptation but is also vital to our ability to manage for long-term persistence of species in ecosystems where thiamine deficiency is a threat.
In this issue of Molecular Ecology, Harder, Willoughby, Ardren, and Christie (2020) make an excellent contribution to our understanding of the potential for rapid adaptation to thiamine deficiency and the molecular mechanisms underlying this phenomenon. They pair transcriptomics with a survival study to uncover the link between molecular responses to thiamine deficiency and fitness in Atlantic salmon from Lake Champlain (Figure 1). The novel combination of these approaches enables Harder et al. not only to quantify the differences among family survival rates under low thiamine conditions, but also to directly link differences in gene expression patterns with survival.

Historically, studies have used a targeted gene approach to assess intraspecific differences in responses to stress, assaying only those genes known to be related to the stress response of interest. This approach is powerful because it provides testable hypotheses, but it is limited because it requires a priori knowledge of the molecular pathways involved. Such targeted approaches are therefore unable to uncover molecular responses that have not yet been described, of which there are probably many given that we understand only a small fraction of molecular processes. With whole genome transcriptomics, we are no longer limited by our current understanding of the molecular pathways involved in a stress response and can now assay entire transcriptomes. Harder et al. (2020) use both of these approaches, investigating 106 genes known to be involved in thiamine-related biological processes, and pairing this with a whole transcriptome differential expression analysis (Figure 2). They found 17 of the a priori genes were differentially expressed between thiamine treatments (eggs treated or untreated with thiamine), and an additional 3,603 differentially expressed genes were identified via whole transcriptome analysis. This set of genes collectively explained 59% of the variation in response to thiamine treatment. These findings represent a substantial expansion in our understanding of the genes involved in responding to this important environmental stressor.
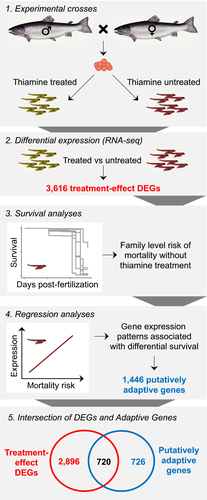
In addition to increasing our understanding of the genes involved in thiamine deficiency, Harder et al. (2020) also explore the possibility that Lake Champlain Atlantic salmon contain the genomic variation needed to adapt to survival under low thiamine conditions. Currently, the re-introduction of Atlantic salmon into Lake Champlain depends on hatchery propagation because rates of natural reproduction are not sufficient for population maintenance (Prévost, Hill, Grant, Ardren, & Fraser, 2020). Eggs are treated with thiamine in the hatchery to alleviate the survival bottleneck caused by severe thiamine deficiency from the consumption of introduced alewife. However, Harder et al. (2020) show there is among-family variation in gene expression patterns that is linked to survival under low thiamine conditions. Of 114 genes where the degree of expression change in response to thiamine treatment was equal across families, 30 of those genes showed variation in the overall amount of expression among families, and in some of these genes this variation was correlated with offspring survival, demonstrating possible adaptive capacity. Additionally, Harder et al. (2020) identified a large number of genes (597) with significant genotype × environment (GxE) interactions. In most of these GxE genes, families with the highest survival under low thiamine conditions exhibited very little difference in expression with or without thiamine treatment, with the difference between treatments increasing with decreasing survival. This demonstrates that the ability to maintain normal homeostatic conditions may lead to increased survival under these stressful conditions. Because Harder et al. (2020) had survival data for each family, they were also uniquely able to directly link survival rates with expression patterns. Interestingly, of the 1,446 genes they identified as linked to survival, about half of them (720) were also associated with the molecular response to low thiamine (Figure 2). This relationship further demonstrates the robustness of their findings and the power in their approach to understand adaptive potential.
The genes found to be significantly linked to both survival and response to low thiamine conditions provide exceptionally fruitful grounds for further exploration of the genetics of adaptation, as well as a means for identifying specific adaptive alleles. An exciting next step would involve pairing expression patterns in genes showing adaptive potential with whole genome sequence data to determine if the causative sequence variants (or expression quantitative trait loci) can be identified. Identifying particular alleles that are associated with expression patterns that confer greater survival would allow the Lake Champlain hatchery to manage their breeding programme to increase the frequency of adaptive alleles, as well as provide the means for quantifying the presence of these alleles in other populations where thiamine deficiency is a concern. This approach would be particularly beneficial in the context of future re-introduction efforts to ensure re-introduced populations are initiated with the genomic variation that will facilitate the greatest potential for persistence. Selectively breeding for adaptive alleles may be preferable over simply ceasing thiamine treatment and allowing adaptation in the hatchery population to occur naturally via strong natural selection, due to the decreased risk of driving the population to extremely low numbers where patterns of genetic drift and inbreeding may predominate.
The methodological approach taken by Harder et al. (2020) is an exciting example of how we can increase our understanding of the process of adaptation by pairing experimental transcriptomics with the measurement of fitness-related traits. Their study not only demonstrates how we can assess the possibility for adaptation to low thiamine conditions, but also is an excellent model for using functional genomics to study additional emerging conservation threats, such as increasing temperature and environmental contaminants. As anthropogenic stressors increase, it is going to be increasingly important to identify and conserve the genomic diversity that serves as the building blocks for population adaptation and persistence.




