Parent and offspring genotypes influence gene expression in early life
Abstract
Parents can have profound effects on offspring fitness. Little, however, is known about the mechanisms through which parental genetic variation influences offspring physiology in natural systems. White-throated sparrows (Zonotrichia albicollis, WTSP) exist in two genetic morphs, tan and white, controlled by a large polymorphic supergene. Morphs mate disassortatively, resulting in two pair types: tan male × white female (T × W) pairs, which provide biparental care and white male × tan female (W × T) pairs, which provide female-biased care. To investigate how parental composition impacts offspring, we performed RNA-seq on whole blood of WTSP nestlings sampled from nests of both pair types. Parental pair type had a large effect on nestling gene expression, with 881 genes differentially expressed (DE) and seven correlated gene coexpression modules. The DE genes and modules expressed at higher levels in W × T nests with female-biased parental care function in metabolism and stress-related pathways resulting from the overrepresentation of proteolysis and stress-response genes (e.g., SOD2, NR3C1). These results show that parental genotypes and/or associated behaviours influence nestling physiology, and highlight avenues of further research investigating the ultimate implications for the maintenance of this polymorphism. Nestlings also exhibited morph-specific gene expression, with 92 differentially expressed genes, comprising immunity genes and genes encompassed by the supergene. Remarkably, we identified the same regulatory hub genes in these blood-derived expression networks as were previously identified in adult WTSP brains (EPM2A, BPNT1, TAF5L). These hub genes were located within the supergene, highlighting the importance of this gene complex in structuring regulatory networks across diverse tissues.
1 INTRODUCTION
Parents can have profound impacts on offspring development and fitness. Parental effects can manifest throughout the developmental period, both pre- and postnatally (reviewed in Lupien, McEwen, Gunnar, & Heim, 2009; Meaney, 2001) and can be mediated through parental behaviours, genetics and physiology during early development (Trivers, 1972). Parents play a substantial role in establishing the early life environment of offspring. For example in birds, parental decisions on nest placement, incubation behaviour, and nest defence could strongly impact developmental conditions of the egg. These parental behaviours will impact exposure to sunlight, humidity, temperature, and other environmental impacts of the eggs, which can influence developmental physiology (e.g., Nord & Nilsson, 2011). In addition to parental behaviours, prenatal effects often arise via physiological maternal effects. Developing offspring are susceptible to the maternally created environment (e.g., maternal hormones, immune state, nutrition), which influences offspring physiology (Jacquin, Blottière, Haussy, Perret, & Gasparini, 2012; Mousseau & Fox, 1998; reviewed in Gluckman, Hanson, Cooper, & Thornburg, 2008; Cottrell & Secki, 2009; Wolf & Wade, 2009).
During the post-natal stage, provisioning plays a prominent role in offspring development, with the quality and quantity of food items crucial for offspring development (Griebel, Fairhurst, Marchant, & Clark, 2019; van Oers, Kohn, Hinde, & Naguib, 2015; Royle, Smiseth, & Kölliker, 2012). Similar to the prenatal stage, parental behaviours could also have strong impacts on offspring physiology. In many species, offspring are left alone during parental foraging trips, increasing environmental exposure (Lloyd & Martin, 2004) and predation risk (Lima, 2009). Parental separation can also increase offspring anxiety (Millstein & Holmes, 2007). Siblings must also compete to optimize food intake, body temperature regulation, and preening (Mock & Parker, 1997). Thus, this postnatal environment, largely mediated through parental effects, can be a potential source of early life stress (ELS) in offspring, which may result in life-long fitness effects (reviewed in Monaghan, 2014).
Early life stress has broad effects on organisms, including impaired neural development, neuroendocrine signalling, behaviour, and physiology (McEwen, 2007; Monaghan, 2014). For example, ELS is associated with impaired neuroendocrine function and corresponding impaired hypothalamic-pituitary-adrenal (HPA) development, which leads to an increased stress response sensitivity later in life (e.g., Crespi, Williams, Jessop, & Delehanty, 2012; Heim, Newport, Mletzko, Miller, & Nemeroff, 2008; Spencer, 2017; Spencer, Evans, & Monaghan, 2009). ELS can exacerbate behavioural alterations as organisms develop and mature including symptoms of anxiety and depression in the postnatal environment (Noguera, Kim, & Velando, 2017) and result in impaired behaviour as reproductive adults (e.g., Krause, Honarmand, Wetzel, & Naguib, 2009; reviewed in Bolton, Molet, Ivy, & Baram, 2017). While the organismal effects of ELS are well studied, the genetic underpinnings are relatively underexplored.
Much of the genetic work in the context of ELS has focused on gene regulatory impacts, particularly in mammalian biomedical models (reviewed in Alyamani & Murgatroyd, 2018; Silberman, Acosta, & Zorrilla Zubilete, 2016; Szyf, 2009; Szyf, Weaver, & Meaney, 2007). In particular, the quality of parental care can have strong impacts on offspring health resulting from epigenetic modifications (Liu et al., 1997; Meaney, 2001; Weaver et al., 2004). These gene regulation studies primarily use changes in DNA methylation as an indicator of ELS (Kinnally et al., 2011; Lewis & Olive, 2014; Murgatroyd et al., 2009) and recent work has expanded these approaches into nonmammalian organisms (e.g., Gott, 2018; Moghadam et al., 2017; Pértille et al., 2017; Rubenstein et al., 2016; Sheldon, Schrey, Ragsdale, & Griffith, 2018). DNA methylation studies of ELS investigate changes to the structure of DNA, but are often limited in the functional implications of ELS (i.e., transcription and translation). In general, these modifications are thought to alter transcriptional activity of genes in the modified genomic region (Berger, 2007; Lowdon, Jang, & Wang, 2016). Indeed, several studies have also taken candidate gene approaches to investigating gene expression in the context of ELS (Anastasiadi, Esteve-Codina, & Piferrer, 2018; Diaz-Real, Kim, & Velando, 2017; Marco et al., 2014; Reshetnikov, Studenikina, Ryabushkina, Merkulova, & Bondar, 2018). However, very few studies assess genome-wide transcription under ELS (Moghadam et al., 2017), particularly in the context of parental effects (but see: Weaver, Meaney, & Szyf, 2006).
In this study, we examined the white-throated sparrow (Zonotrichia albicollis, WTSP) to assess the role of parental genotype on offspring gene expression. WTSPs exist in two plumage morphs, tan (T) and white (W), that are found in both sexes and in roughly equal frequencies (Lowther, 1961). These morphs are genetically determined by alternative alleles of a supergene, a group of linked genes that are inherited together, show limited recombination, and maintain complex behavioural traits (i.e., WTSP morphs; Schwander, Libbrecht, & Keller, 2014; Taylor & Campagna, 2016). The WTSP supergene resulted from a complex chromosomal rearrangement comprising multiple inversions (hereafter referred to as “inversion” or “inverted”). This inversion contains ~1,100 genes on chromosome two, termed ZAL2m (Romanov et al., 2009; Thomas et al., 2008; Thorneycroft, 1975; Tuttle et al., 2016). W morphs are nearly always heterozygous for the inversion (ZAL2/ZAL2m) and T morphs are always homozygous (ZAL2/ZAL2; Thorneycroft, 1966, 1975).
This unusual polymorphism in WTSPs influences hormonal profiles and the behaviour of both sexes, and thus has the potential to influence pre- and postnatal environments for the offspring of different morphs. W morph males maintain higher levels of testosterone during the prelaying, incubation, and brooding stages and oestradiol during the laying and brooding stages (Horton, Moore, & Maney, 2014). Only oestradiol has been shown to differ between adult female morphs during the breeding season and is higher in W morph females during the prelaying and laying stages (Horton et al., 2014). These genetic and hormonal differences also translate into striking behavioural differences. W morphs of both sexes, for example, are highly territorial and sing frequently whereas T morphs are far less territorial and aggressive (Horton & Holberton, 2010; Horton et al., 2014; Kopachena & Falls, 1993; Lowther, 1962; Tuttle, 2003). More importantly from the perspective of offspring, males of each morph also differ in paternal investment (Horton et al., 2014; Knapton & Falls, 1983). W morph males are promiscuous and provision nestlings very little. T morph males defend their within-pair paternity through mate guarding and are highly paternal. Females tend to provision at intermediate levels, but T morph females may compensate for unassisted care from W morph males and provision more than W morph females (Knapton & Falls, 1983). A final wrinkle in this complex mating system is that morphs nearly always mate with the opposite morph (98.5%; Tuttle et al., 2016), resulting in two stable pair types: T male × W female (T × W) and W male × T female (W × T; Lowther, 1961; Tuttle, 2003; Tuttle et al., 2016). Because males differ in paternal investment, this results in two distinct parental care strategies. T × W pairs provide biparental care and W × T pairs provide female-biased parental care. In this study we examined gene expression profiles of offspring from both pair-types in order to assess the physiological consequences of variation in parental genotype.
2 MATERIALS AND METHODS
2.1 Field-based sample collection
All nestling whole blood samples in this study came from a breeding population of WTSPs at the Cranberry Lake Biological Station in northern New York, USA (SUNY-ESF, 44.15°N, 74.78°W) and were collected during the 2015 breeding season. We only used samples collected during the first clutch (7 June–15 June 2015), as WTSP males may increase paternal investment in replacement broods (Horton et al., 2014). We collected ~80 µl blood in capillary tubes via brachial venipuncture on days 5–7 post-hatch. Approximately 60 µl blood was preserved in Longmire's lysis buffer (Longmire, Gee, Hardekopf, & Mark, 1992) for genotyping and ~20 µl was immediately placed in RNAlater. Within 6 hr of collection, samples were placed temporarily into liquid nitrogen, before being shipped overnight on dry ice to −80°C storage until RNA extraction. All animal sampling protocols were approved by the Indiana State University Institutional Animal Care and Use Committee (IACUC 562158-1:ET/RG, 562192-1:ET/RG).
2.2 Molecular sexing and genotyping
Nestling DNA was extracted from erythrocytes using the DNA IQ magnetic extraction system (Promega Corp). To determine sex and morph, we used PCR to fluorescently label and amplify a region of the chromo-helicase-DNA-binding gene, and a region of the vasoactive intestinal peptide following Griffiths, Double, Orr, and Dawson (1998) and Michopoulos, Maney, Morehouse, and Thomas (2007). The PCR products were run and analyzed on an ABI PRISM 310 genetic analyzer.
2.3 RNA extraction, library preparation, and sequencing
We sampled a total of 45 nestlings for RNA extraction, but due to issues with RNA quality after extraction (RNA concentration = 0 ng/μl or RIN < 7), only 32 were used for sequencing. These samples represented 23 nestlings from eight T × W pairs and nine nestlings from three W × T pairs. The 23 nestlings from T × W nests included 12 female, 11 male, 12 T morph, and 11 W morph individuals. The nine nestlings from W × T nests included six female, three male, three T morph, and six W morph individuals.
We removed RNAlater and homogenized whole blood tissue samples with Tri-Reagent (Molecular Research Company). Total RNA was purified with a Qiagen RNeasy mini kit, followed by DNase treatment and further purification. We quality assessed RNA with an Agilent Bioanalyzer (RIN > 7). Both library preparation and sequencing were performed at the University of Illinois Roy J. Carver Biotechnology Center. A library was prepared for each RNA sample using the Illumina HT TruSeq stranded RNA sample prep kit. Libraries were distributed into four pools with equimolar concentrations and quantitated via qPCR. Each of the pools was sequenced on an individual lane of an Illumina HiSeq 2500 using the Illumina TruSeq SBS sequencing kit v4 producing 100-nucleotide single-end reads.
2.4 Creation of masked reference genome
The WTSP reference genome was generated from a male T morph individual (Tuttle et al., 2016). Thus, the reference genome does not contain any sequence data from the ZAL2m inversion. To avoid any potential bias in mapping reads derived from W morph individuals onto a T morph genome, we generated a masked reference genome for this study. To do so, we used previously published whole genome sequences from three W morph adults (Tuttle et al., 2016). Reads were adapter trimmed with Trim Galore! v0.3.8 (https://github.com/FelixKrueger/TrimGalore) and aligned to the WTSP reference genome with bwa mem v 0.7.10-r789 using default parameters (Li, 2013). We converted and sorted the resulting SAM alignment to BAM format with samtools view and samtools sort, respectively (samtools v1.2, Li et al., 2009). We then merged all genomic scaffolds corresponding to the ZAL2m inversion, as identified in Tuttle et al. (2016), with samtools merge. We called SNPs within the inversion using samtools mpileup and bcftools call v 1.2 (Li, 2011; Li et al., 2009). We only kept SNPs that were heterozygous in each of the three individuals with SnpSift v 4.3p (Cingolani et al., 2012) and used these SNPs to mask the reference genome with bedtools maskfasta v 2.21.0 (Quinlan & Hall, 2010).
2.5 Quality control, read mapping, differential expression, and gene ontology
We trimmed Illumina sequencing adapters from each of the 32 libraries with Trim Galore! v0.3.8 which uses Cutadapt v1.7.1 (Martin, 2011). Trimmed reads were then mapped to the masked reference genome with star v2.5.3a using default parameters (Dobin et al., 2013). The mapping results were then quantified and assigned gene IDs with htseq-count v0.6.0 (Anders, Pyl, & Huber, 2015) specifying “-s reverse” and “-i gene.” We then removed lowly expressed genes by summing the counts for each gene across all 32 samples, dividing by 32 to obtain the study average, and removing genes with an average read count of <5.
All statistical analyses were performed with r v3.5.0 (R Core Team, 2018). We first identified outlier samples based on visual inspection of sample distance in a dendrogram within weighted gene coexpression network analysis (WGCNA; Horvath, 2011). Two samples, one T female and one T male representing an entire T × W nest, were identified as outliers and removed from all future analyses (Figure S1). Using the remaining 30 samples, we normalized reads accounting for sequencing depth and assessed differential expression with DEseq2 (Love, Huber, & Anders, 2014). We performed variance stabilizing transformation of reads in DEseq2 and performed PCA and hierarchical clustering based on Euclidean distance of gene expression profiles with pcaExplorer v2.6.0 (Marini & Binder, 2016). Differential expression analyses utilized pairwise comparisons between nestling morph and pair type (i.e., parental morphs). We controlled for sex in morph comparisons and sex, morph, and nest ID for pair type comparisons. To include nest ID in the pair type comparison, we followed the “individuals nested within groups” guide in the DEseq2 manual. We did not include nestling age in analyses, as most samples were 6 days old (n = 21), limiting comparisons with nestlings aged Day 5 (n = 3) or Day 7 (n = 6). Network analysis (see below) did not reveal any effect of age on variables of interest (morph, pair type; data not shown). We also tested for an interaction between nestling morph and pair type utilizing a grouping variable as outlined in the DEseq2 manual. DEseq2 determines differential expression with a Wald test followed by Benjamini and Hochberg (1995) FDR correction. Genes were considered differentially expressed (DE) if the FDR corrected p-value was <.10. Details for each model run, including the r code used, are in this project's GitHub repository.
We next tested for gene ontology (GO) enrichment among DE genes with GOrilla (Eden, Lipson, Yogev, & Yakhini, 2007; Eden, Navon, Steinfeld, Lipson, & Yakhini, 2009). For each DEseq2 comparison, we ordered the list of genes based on ascending FDR values, excluding any genes in which DEseq2 did not assign a FDR value. The WTSP genome is not completely annotated, so any loci without a gene symbol were excluded from GO analyses (n = 1,926). GOrilla places greater weight on genes located at the top of the list (i.e., DE genes), while accounting for the contribution of each gene in the given comparison. GO categories were considered significantly enriched if the FDR corrected p-value <.05. GOrilla does not support WTSP annotation; so, all analyses were based on homology to human gene symbols.
2.6 Weighted gene coexpression network analysis (WGCNA)
We used the WGCNA package in r (Langfelder & Horvath, 2008; Zhang & Horvath, 2005) to identify modules of genes with highly correlated expression patterns in our data set. WGCNA identifies modules of coregulated genes blind to the experimental design. These modules are then correlated with external traits, offering a systems-level view into how conditions impact transcriptional networks. Within these networks, we can then perform GO analyses as described above and identify network hubs, which are the most highly connected genes within that network. To create networks, we first exported variance stabilizing transformed (vst) read counts from DEseq2, removed genes with an average vst <5 averaged across all 30 samples, and imported the subsequent list of 8,982 genes into WGCNA. To build the coexpression matrix, we chose a soft thresholding power (β) value of 12, at which the network reaches scale-free topology (Figure S2). We generated a signed network with minimum module size of 30 genes and merged highly correlated modules (dissimilarity threshold = 0.20, which corresponds to R2 = .80). We then correlated the eigengene, which is the first principal component of a module, of these merged modules with external traits (pair type, nestling morph, nestling sex, nest ID). Modules with p < .05 were considered significantly correlated with a given trait. For all morph-specific results, we tested for an enrichment of inversion genes with a chi-squared test using a Fisher's exact test (p < .05).
To visualize the interaction of genes within a module, we generated the intramodular connectivity (IM) score for each gene, which represents the interconnection of module genes. We exported all IM scores for modules of interest and imported into VisAnt v5.51 (Hu et al., 2013) for visualization. To maximize network clarity, we only plotted the top 300 interactions based on IM scores. Thus, we only visualized the most connected genes. To identify hub genes, we visualized the Degree Distribution (DD) for the network and selected the most connected genes above a natural break in the distribution. This resulted in one to nine hub genes per module.
To understand the biological function of modules correlated with traits of interest, we performed a target versus background GO analysis in GOrilla. For each module, we tested the assigned genes for each module against the entire list of 8,982 genes used for the WGCNA analysis. GO categories were significant with a FDR corrected p-value <.05.
3 RESULTS
3.1 Sequencing results
We sequenced each sample to an average depth of 29.4 million reads (range = 16.2–58.5 million reads). The 32 libraries were distributed into four pools in equimolar concentration. One pool contained only four samples, which corresponded to the four samples with lowest RNA concentrations. This pool was sequenced to an average depth of 56.17 million reads per library. The remaining three pools were sequenced to an average depth of 25.62 million reads per library. Samples mapped to our masked genome at an average rate of 91.08% (range = 88.19%–92.87%; Table S1). A total of 8,982 genes had count values ≥5 across all samples, which included 641 located in the W morph inversion. Samples did not segregate by pair type or morph in PCA or hierarchical clustering (Figures S3 and S4).
3.2 Differential expression—morph
A total of 92 genes were differentially expressed between morphs. Sixty-five of these genes (71%) were located in the inversion, representing a significant enrichment (χ2 = 553.73, df = 1, p < .00001; Table S2). The inversion represents only 641 out the 8,892 genes (7%) sampled here. Additionally, expression of 59 of these 92 genes was higher in W morph nestlings, including several innate immune related genes (e.g., IFIT5, IL20RA, EIF2AK2, RSAD2). There was GO enrichment of three categories: “immune response” (p = .019), “mitotic cell cycle process” (p = .029), and “defence response to virus” (p = .049; Table S3).
3.3 Differential expression—pair type
Pair type had the largest effect on gene expression, with 881 genes DE between offspring from the two different pair types (FDR < 0.10, Table S2). Some known stress response genes were more highly expressed in nestlings in W × T nests, including the glucocorticoid receptor (NR3C1), superoxide dismutase (SOD)1 & SOD2, DEP domain-containing mTOR-interacting protein (DEPTOR), and several ubiquitin-mediated proteolysis pathway genes (e.g., UBE2D3, PSMD3, PSMD6). Additionally, immune system related genes were also expressed more highly in W × T nests, including cytokines (e.g., IL2RA, IL7R), suppressor of cytokine signalling 1 (SOCS1), and five putative major histocompatibility complex (MHC) class I loci. However, no GO categories were significantly enriched.
We next tested for a morph-specific response to pair type. Within W × T nests, 40 genes were DE (p < .10) between T and W morph nestlings. Twelve of these genes (30%) are located within the inversion, again reflecting an enrichment of inversion genes among those differentially expressed between morph (χ2 = 34.44, df = 1, p < .00001). Only two genes (THSD7B & CFAP44) were DE between morphs within T × W nests, both of which are uniquely DE between morphs in T × W nests. No GO categories were enriched in either comparison.
3.4 WGCNA: morph
Weighted gene coexpression network analysis revealed 26 modules, five of which were correlated with morph (Table 1, Figure 1). The light cyan module (183 genes, R2 = .67, p = 5 × 10−5) and ivory module (72 genes, R2 = −.66, p = 9 × 10−5) contained genes expressed higher and lower, respectively, in W morph nestlings relative to T morph nestlings. These modules are both enriched for genes located within the chromosomal inversion (light cyan module = 70/183 [38%] genes, χ2 = 266.49, df = 1, p < .00001; ivory module = 40/72 [56%], χ2 = 261.60, df = 1, p < .00001; Figure S5). The hubs of each of these modules are also located in the chromosomal inversion (Table 1, Figure S5). Additionally, the sky blue module (58 genes, R2 = .53, p = .003) and dark red module (102 genes, R2 = .47, p = .009; Figure S6) contained genes expressed at higher levels in W morph nestlings and many of these genes overlap with the immune related genes described in the morph DE tests above. The hubs of these networks (e.g., sky blue: EIF2AK2, IFIT5, OASL; dark red: TRAF5; Table 1) reflect a conserved innate immunity network structure in avian blood (Kernbach et al., 2019; Figure S6). Lastly, the salmon module (294 genes, R2 = −.50, p = .005) contained genes expressed at lower levels in W morph nestlings and did not exhibit any enriched GO categories.
| Module | R 2 | p-value | Hub genes | DD of hub gene(s) |
|---|---|---|---|---|
| Dark red | .47 | .009 | TRAF5 | 32 |
| Ivory | −.66 | 9 × 10−5 | GOPC, HDAC2, HINT3, TAF5L, TRMT61B, MARC2 | >29 |
| Light cyan | .67 | 5 × 10−5 | BPNT1, EPM2A, LOC102066536 (GST-like), MAN1A1, MEI4, RNASET2, SLC18B1, TTC32 | >27 |
| Salmon | −.50 | .005 | NSL1 | 39 |
| Sky blue | .53 | .003 | DTX3L, EIF2AK2, IFIT5, LOC102064521 (OASL), LOC102065196 (IFI27L2), PARP9, PARP14, RSAD2, ZNFX1 | >22 |
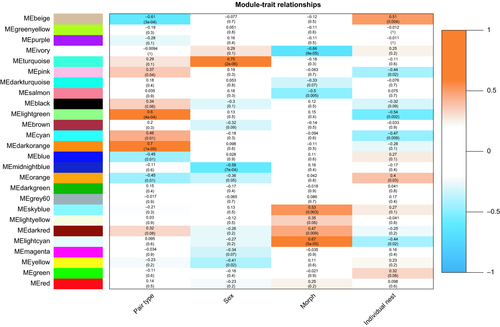
3.5 WGCNA: pair type
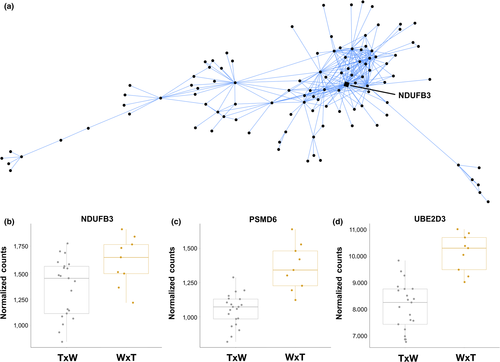
We found seven modules correlated with pair type (Table 2, Figure 1). The blue module represented genes that are expressed at higher levels in nestlings from W × T nests (1,142 genes, R2 = −.45, p = .01). This module contained both the largest number of genes and correspondingly, the strongest functional enrichment. Many of these GO enrichments were related to protein function, resulting from the presence of ribosomal genes. Interestingly, several GO categories for metabolism, catabolism, and proteolysis were also enriched, driven by genes encoding ubiquitin-conjugating enzymes and proteasome subunits (e.g., “proteasomal protein catabolic process,” p = 2.34 × 10−4; “proteasome-mediated ubiquitin-dependent protein catabolic process,” p = 5.32 × 10−4; Table S4). Many of these (e.g., PSMF1, PSMD3, PSMD6, UBE2D2, UBE2D3, UBE3C) were also DE between offspring of the two pair types (Figure 2). Lastly, the blue module contains one hub gene, NDUFB3 (DD = 42; Figure 2), which is involved in the mitochondrial electron transport chain.
| Module | R 2 | p-value | Hub genes | DD of hub gene(s) |
|---|---|---|---|---|
| Beige | −.61 | 3 × 10−4 | DEPTOR | 39 |
| Blue | −.45 | .01 | NDUFB3 | 42 |
| Cyan | .46 | .01 | HELZ | 36 |
| Dark orange | .70 | 1 × 10−5 | NCOA6 | 45 |
| Light green | .60 | 4 × 10−4 | CDK19, CHD4, EPG5 | >28 |
| Orange | −.45 | .01 | ZFX | 31 |
| Pink | .37 | .04 | LOC102060916 (C12orf4) | 19 |
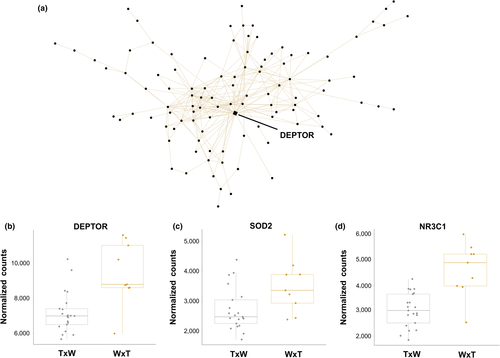
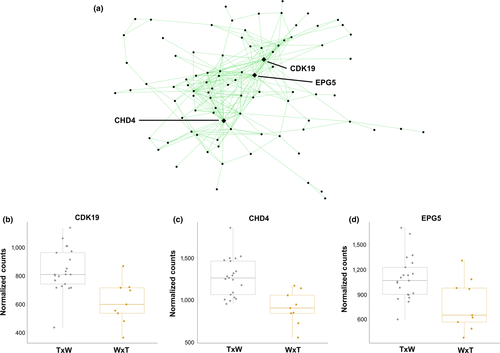
The beige and light green modules represented candidate stress response networks. These modules showed contrasting expression patterns in nestlings from W × T nests (Figure 4). Although not significantly enriched for any GO categories, the beige module comprised 335 genes that shower greater expression in W × T nests than in T × W nests (R2 = −.61, p = 3 × 10−4). DEPTOR, which functions as an inhibitor of the mTOR pathway in response to stress (e.g., Desantis et al., 2015), was the single hub in the beige module (DD = 39, Figure 3). The beige module also contained NR3C1, which is activated in response to increased glucocorticoid secretion. Lastly, the light green module (116 genes, R2 = .60, p = 4 × 10−4) contained genes with low expression in T × W nests relative to W × T nests. There were three hub genes (DD > 28), CDK19, CHD4, and EPG5, each with previously described roles in the stress response (Figure 4).
For each pair type module, the correlation was stronger for the overall effect of pair type than any individual nest, indicating that one nest did not drive the correlation. This trend was reflected in gene expression plots of hub genes and candidate genes described above (Figure S7). We did not observe modules correlated with pair type that were also correlated with nestling morph or sex, suggesting there is no morph or sex-specific response to a given pair type at the network level.
4 DISCUSSION
By assessing genome-wide transcription in nestlings raised by different WTSP pair types we have identified distinct transcriptomic signatures that suggest nestlings raised by W × T pairs exhibited a stronger stress response relative to nestlings raise by T × W pairs. This is reflected both by differential expression of several genes involved in protein degradation as well as networks of coexpressed genes with stress response hubs. Additionally, we identified morph-specific gene expression driven by innate immunity genes and genes located in the chromosome 2 inversion. As adults, the genes within the inversion strongly influence the WTSP neural transcriptome (Balakrishnan et al., 2014; Zinzow-Kramer et al., 2015). Our results here suggest that as nestlings, parental genotypes and associated behaviours, rather than nestling genotype, have the strongest influence on the nestling transcriptome.
4.1 Gene expression differences resulting from pair type
We found 881 genes DE between nestlings raised by the two pair types. Many of these genes function in the proteasome or ubiquitin-mediated proteolysis. Cells naturally use the proteasome for degradation of proteins targeted by the ubiquitination process, but genes involved in proteasome formation (e.g., PSMD6, PSMD11) and ubiquitination (e.g., UBE2B) are upregulated in cells experiencing mild oxidative stress (Aiken, Kaake, Wang, & Huang, 2011; Livneh, Cohen-Kaplan, Cohen-Rosenzweig, Avni, & Ciechanover, 2016; Shang & Taylor, 2011) or organisms experiencing abiotic stress (Dhanasiri, Fernandes, & Kiron, 2013; Tomalty et al., 2015). Thus, increased expression of these genes in nestlings from W × T nests suggests they are responding to oxidative stress. As a result, there may be a cost to having a W morph father and T morph mother at the nestling stage.
To complement our differential expression approach, we also constructed coexpression networks with WGCNA. Using this approach, we identified 26 modules of coregulated genes in this data set (Figure 1), seven of which were significantly correlated with parental pair type. The blue module contains genes that are expressed at higher levels in nestlings in W × T nests. The blue module hub gene was NDUFB3 (Module Membership [MM] = 0.938, DD = 42; Figure 2), which encodes a subunit of the mitochondrial membrane respiratory chain. Interestingly, many of the same proteolysis-related genes highlighted in the differential expression results are also present in this module, resulting in the enrichment of several metabolism and stress-related GO categories (Table S4).
Two modules, light green and beige, contained stress responsive hub genes. The light green module contains genes that are expressed at lower levels in nestlings in W × T nests, with three hub genes: CDK19, CHD4, and EPG5 (Figure 4). The absence of EPG5 expression (via knockout) and reduction in CHD4 expression (via knockdown) has been associated with increased DNA damage (Larsen et al., 2010; Zhao et al., 2013). Similarly, downregulation of CDK19 following knockdown is associated with an increased stress response (Audetat et al., 2017). Suppression of these genes in these nestlings could be indicative of increased cellular damage. The beige module contains genes whose expression is higher in nestlings from W × T nests and contains one hub gene, DEPTOR, which is an inhibitor of mTOR signalling (Figure 3). The exact role of DEPTOR remains unclear, but upregulation probably inhibits the mTORC1 pathway to reduce endoplasmic reticulum stress, promote cell survival, and avoid apoptosis (Catena et al., 2016; Desantis et al., 2015; Peterson et al., 2009). Thus, nestlings raised in W × T nests may be activating DEPTOR to alleviate the effects of endoplasmic reticulum stress.
Higher expression levels for genes in the beige module in these nestlings and the high connectivity of DEPTOR to other coexpressed genes provide further support for a transcriptional stress response in nestlings sampled within W × T nests. The beige module also contains two well-studied stress responsive genes, superoxide dismutase 2 (SOD2) and the glucocorticoid receptor (NR3C1). SOD2 mitigates the effects of exposure to reactive oxygen species by scavenging free radicals (Zelko, Mariani, & Folz, 2002). NR3C1 binds glucocorticoids and has primarily been studied in the context of ELS and methylation of an upstream promoter. NRC3C1 methylation is often associated with downregulation of NR3C1 (e.g., McGowan et al., 2009) and impairment of the HPA axis, but upregulation following methylation has also been observed as part of the stress response (Bockmühl et al., 2015; Turner, Schote, Macedo, Pelascini, & Muller, 2006). The expression pattern observed here directly implicates the HPA axis and suggests these nestlings may be activating SOD2 and NR3C1 to cope with elevated levels of reactive oxygen species and corticosterone, respectively (Finsterwald & Alberini, 2014; Wang, Branicky, Noë, & Hekimi, 2018). However, further work is needed to investigate stress physiology, corticosterone levels, and uncover the epigenetic state of NR3C1 in these nestlings and how this may relate to ELS (Banerjee, Arterbery, Fergus, & Adkins-Regan, 2011; Greggor, Spencer, Clayton, & Thornton, 2017; McCoy et al., 2016; Quirici, Guerrero, Krause, Wingfield, & Vásquez, 2016; Rubenstein et al., 2016).
4.2 How does parental genotype influence offspring gene expression?
In a nonexperimental study, we have limited power to make inferences about the mechanism by which parental genotype impacted offspring gene expression. Given the well-studied reproductive biology of WTSPs, however, two mechanisms seem especially likely: hormone-mediated maternal effects and/or differences in parental provisioning and behaviour. In weighing the evidence for these two nonmutually exclusive possibilities, we conclude that the difference in parental provisioning is the most plausible explanation for the observed gene expression differences. As described above, WTSP morphs differ in hormone levels. Only oestradiol, however, has so far been shown to differ between adult female morphs during the breeding season and is higher in W morph females during the prelaying and laying stages (Horton et al., 2014). No baseline differences in any other hormone measured to date (corticosterone, testosterone, DHEA, DHT) have been described during the breeding season (Horton & Holberton, 2010; Horton et al., 2014; Spinney, Bentley, & Hau, 2006; Swett & Breuner, 2009). Taken together this suggests that hormone deposition into eggs may not differ dramatically between the morphs. By contrast, there is strong evidence of differences in provisioning among morph types (Horton & Holberton, 2010; Horton et al., 2014; Knapton & Falls, 1983; Kopachena & Falls, 1993). Reduced provisioning by W morph males appears to be stable across populations resulting in female-biased parental care in W × T nests (Horton et al., 2014; Knapton & Falls, 1983). Therefore, parental care variation is a likely source of behaviourally mediated maternal or paternal effects (see Crean & Bonduriansky, 2014) that could explain the strong signature of stress exposure in the expression data.
Previous work revealed no difference in clutch size between pair types (Formica, Gonser, Ramsay, & Tuttle, 2004; Knapton, Cartar, & Falls, 1984) and no effect of pair type on nestling mass (Knapton et al., 1984; Tuttle et al., 2017). Also, nestlings did not differ in mass at time of sampling between the T × W and W × T nests used in this study (Smith, Newhouse, Balakrishnan, Tuttle, & Gonser, 2019). Increased provisioning by females to compensate for reduced care by males could explain this observation, and this has been observed previously in a separate WTSP population (Knapton & Falls, 1983). In this scenario reduced brooding and increased maternal separation could also negatively impact nestling physiology and act as a source of ELS (reviewed in Ledón-Rettig, Richards, & Martin, 2013). Somewhat surprisingly, given the gene expression findings described here, a recent study in our study population did not detect differences in reactive oxygen metabolites in plasma of offspring of the two different pair types (Grunst, Grunst, Gonser, & Tuttle, 2019). ROM, however, only provides a limited overview of the stress response and the RNA-seq response we observed could even mitigate long-term consequences of ELS. The results here further highlight the utility of blood RNA-seq as a highly sensitive measure of environmental exposures (Louder, Hauber, & Balakrishnan, 2018).
Our study was limited by the fact that we did not perform a cross-fostering experiment. We aimed to mitigate potential environmental confounds by restricting sampling of nestlings to a short time period of 9 days and sampling nests of both pair types throughout this period. Certainly, the environment may influence gene expression in our samples, but consistent changes among the samples in the two pair types suggest the role of parents is a significant driver of nestling gene expression, rather than temporal or spatial environmental variation. Although the two pair types are equally abundant in our study population, our study had unbalanced sample sizes between the pair types (21 T × W, 9 W × T). The biased sample size resulted from technical difficulties in RNA extraction, as many of these samples contained very little starting tissue. Future studies should prioritize larger tissue samples for RNA based analyses. Lastly, aspects of male behaviour during incubation (e.g., provisioning females) could also influence nestling stress and warrant further targeted behavioural observations of males.
4.3 Morph-specific gene expression
We were also interested in morph-specific gene expression and how nestling morph may respond to differences in parental pair type. WTSPs have been studied extensively as adults, but very rarely in other life stages. W morph males and T morph females exhibit earlier reproductive and actuarial senescence, potentially resulting from the high energy expenditure lifestyle of W morph males and biased parental care given by T morph females (Grunst et al., 2018a, 2018b). There also appears to be annual variation in fitness between the morphs as adults. Following cold, wet winters, W morph males exhibit lower recruitment in the breeding grounds, leading to an overproduction of W morph male nestlings, potentially to stabilize morph frequencies in the population (Tuttle et al., 2017). Thus, morph specific differences may arise in early life. We found 92 genes DE between morphs, including 14 innate immune-related genes and genes located within the inversion (65/92 genes, Table S2). WGCNA revealed five modules correlated with morph (Figure 1). These included two innate immunity-related modules with increased expression in W morphs (dark red & sky blue) and two modules enriched with genes located in the inversion (ivory = 40/72, light cyan = 70/183; Figures S5 and S6). The sky blue module contains nine hub genes and the dark red module contains one hub gene, both of which include well-studied antiviral genes (e.g., sky blue: OASL, RSAD2; dark red: TRAF5). These genes also form a coexpression module in avian blood following West Nile virus infection (Kernbach et al., 2019). Adult WTSP morphs differ in their ability to clear infection (Boyd, Kelly, MacDougall-Shackleton, & MacDougall-Shackleton, 2018), so the immune activation here may be indicative of an increased parasite load in W morph nestlings, although further investigation is required. The light cyan module contains genes expressed at higher levels in W morph nestlings and contains eight hub genes, each located in the inversion (Table 1). Three of these, EPM2A, BPNT1, and TAF5L, were also identified as hub genes in brain tissues of adult W morph males (Zinzow-Kramer et al., 2015). These nestlings thus exhibit expression differences in inversion genes prior to any phenotypic or behavioural differences, revealing the importance of the inversion in maintaining morph phenotypes throughout life. Additionally, the conservation of network hub genes in a different tissue and life stage highlights avenues for further investigation into WTSP gene regulation.
Despite broad gene expression differences between the morphs, within pair types morph-specific expression was limited. Nestlings in T × W nests only had two genes DE between morphs. There was a larger effect of morph within W × T nests, where the number of DE genes increased to 40. These genes encompassed a wide range of gene functions without any obvious stress-related candidate genes. Of these 40 genes, 34 are uniquely DE within W × T nests and do not overlap with the overall list of 92 genes DE between morphs using all samples. Interestingly, W morph nestlings in W × T nests expressed glucocorticoid-induced transcript 1 (GLCCI1) at higher levels than T morph nestlings. The function of GLCCI1 remains unclear (Kim, Kim, & Kim, 2016), but expression differences between morphs observed here implicates the role of glucocorticoids in response to pair type. This suggests that nestling morphs may respond differently to the parental pair type though larger sample sizes will be needed to explore this further.
In conclusion, using the WTSP, a system with alternative parental care strategies, we show that nestlings in W × T nests (female-biased parental care) have increased expression of stress-related genes, and parental genotypes may act as a source of ELS in the species. Nestling morph also influences transcription, but parental pair type appears to have the greatest effect on their transcriptome. Combined, this supports the parental effects hypothesis (Schrader, Jarrett, & Kilner, 2018; Wade, 1998), where offspring phenotypes are primarily a result of the nest environment and care received, rather than from offspring genotypes (i.e., T vs. W). Nearly 54% of observed pairs have been W × T (Tuttle et al., 2016). Thus, roughly half of the nestlings in every population will experience female-biased parental care. Our results suggest that these differences in parental pair type have at least short-term consequences on offspring physiology. While we identified impacts at the level of transcription, an integrative approach assessing nestling WTSP physiology and performing cross-fostering experiments will further elucidate the consequences of variation in parental pair type. Importantly, it remains unclear whether female-biased parental care or differences in maternal effects translate into long-term fitness consequences for offspring. There appears to be a cost associated with parental genotype, as less cooperative reproductive strategy (W × T pairs) accelerates senescence (Grunst et al., 2018a, 2018b). We show here that this cost is also translated into nestlings within W × T nests via increased stress-related gene expression. This work sets the stage to further explore morph-specific fitness consequences in nestlings experiencing alternative parental care strategies.
ACKNOWLEDGEMENTS
We would like to thank Lindsay Forrette, Andrea Grunst, and Melissa Grunst for assistance in the field, Sarah Ford for assistance with molecular work, Rachel Wright for WGCNA code, and Cranberry Lake Biological Station. Funding was provided by East Carolina University, Indiana State University, the National Science Foundation (grant No. DUE-0934648), the National Institutes of Health (grant No. 1R01Gm084229 to E.M.T and R.A.G.), and a Sigma Xi Grants in Aid of Research award to DJN. Birds were banded with colour bands and a Fish and Wildlife band (Master Banding Permit 22296 to EMT and permit 24105 to RAG). Dr Alvaro Hernandez and Chris Wright provided guidance and oversight on sequencing carried out at the University of Illinois. All methods were conducted in accordance with legal and ethical standards and were approved by Indiana State University's Institutional Animal Care and Use Committee (protocols 562158-1:ET/RG and 562192-1:ET/RG).
AUTHOR CONTRIBUTIONS
D.J.N. designed and performed research, analyzed the data, and wrote the paper. M.B.S. performed research, contributed samples, and reviewed drafts of the paper. E.M.T. designed and performed research and contributed samples. R.A.G. designed and performed research, contributed samples, and reviewed drafts of the paper. C.N.B. designed and performed research, contributed reagents, and reviewed drafts of the paper.
Open Research
DATA AVAILABILITY STATEMENT
The 32 RNA-seq libraries used in this study are deposited in the NCBI SRA under Bioproject PRJNA546611. Count files and R code are located in this project's GitHub page: https://github.com/danielnewhouse/wtsp




