The niches of nuthatches affect their lineage evolution differently across latitude
Abstract
Ecological niche evolution can promote or hinder the differentiation of taxa and determine their distribution. Niche-mediated evolution may differ among climatic regimes, and thus, species that occur across a wide latitudinal range offer a chance to test these heterogeneous evolutionary processes. In this study, we examine (a) how many lineages have evolved across the continent-wide range of the Eurasian nuthatch (Sitta europaea), (b) whether the lineages’ niches are significantly divergent or conserved and (c) how their niche evolution explains their geographic distribution. Phylogenetic reconstruction and ecological niche models (ENMs) showed that the Eurasian nuthatch contained six parapatric lineages that diverged within 2 Myr and did not share identical climatic niches. However, the niche discrepancy between these distinct lineages was relatively conserved compared with the environmental differences between their ranges and thus was unlikely to drive lineage divergence. The ENMs of southern lineages tended to cross-predict with their neighbouring lineages whereas those of northern lineages generally matched with their abutting ranges. The coalescence-based analyses revealed more stable populations for the southern lineages than the northern ones during the last glaciation cycle. In contrast to the overlapping ENMs, the smaller parapatric distribution suggests that the southern lineages might have experienced competitive exclusion to prevent them from becoming sympatric. On the other hand, the northern lineages have expanded their ranges and their current abutting distribution might have resulted from lineages adapting to different climatic conditions in allopatry. This study suggests that niche evolution may affect lineage distribution in different ways across latitude.
1 INTRODUCTION
The role of ecological niche evolution in lineage differentiation has been a long-standing debate (Schluter, 2009; Wiens, 2004; Zink, 2014). One primary controversy stems from the uncertainty about whether niche differentiation is the (a) driving force or (b) consequence of lineage divergence. We argue that beginning the debate with the cause–effect relationship between the two factors helps to examine their seemingly reticulated relationship. In the former situation, niche divergence may contribute to lineage divergence (Schluter, 2009) although niche conservatism may also facilitate divergence between populations when they are separated by unsuitable habitats (i.e., allopatric distribution; Wiens, 2004). When considering the latter situation, genetic drift or natural selection can cause niche differentiation between split lineages. The major effect of niche evolution on the split lineages is to impact their geographic distribution (Zink, 2014). Based on the predominant mode of allopatric speciation in animals, divergent lineages cannot spread to achieve secondary sympatry if they still share similar niches to cause competitive exclusion (Hutchinson, 1959; Weir & Price, 2011). On the contrary, divergent lineages with distinct niches also may not become sympatric if their required niches are not both available in the same regions. Thus, analysing lineages’ niche divergence is critical to distinguish between the two ecological processes determining their nonoverlapping distribution.
Continentally widespread species often survive across different ecological environments and split into multiple allopatric or parapatric lineages (Pyron & Burbrink, 2009). The ecological niches of the divergent lineages may range from highly separated to largely overlapping (Sexton, Montiel, Shay, Stephens, & Slatyer, 2017). In addition, for organisms in boreal, temperate and tropic areas, where historical climate paradigms are different, ecological niche evolution might impact their lineage evolution in different ways (Dong et al., 2017; Lee-Yaw & Irwin, 2015; Song et al., 2016; Weir & Price, 2011). Thus, species with multiple lineages occurring in diverse climatic zones provide an ideal system with reasonable replicates to examine the interaction between ecological niche evolution and lineage differentiation (Pyron & Burbrink, 2009). However, given the difficulties of collecting samples across broad climatic zones, empirical studies for examining these theoretical predictions are rare.
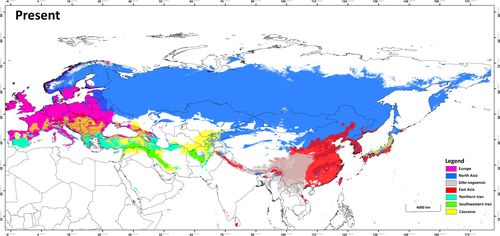
The Eurasian nuthatch (Sitta europaea) is a widespread, resident bird breeding across boreal, temperate and subtropical regions of Europe, North, West and East Asia (Harrap & Quinn, 1995; Figure 1). It is a forest bird, which is a secondary cavity nester that can climb up and down tree trunks, giving it a unique ecological niche in forest ecosystems (Matthysen, 1998). While this bird is a climatic generalist, its lineages occurring in different regions might use different climate niches. However, the number of distinct lineages that have evolved across the wide distribution range of the Eurasian nuthatch remains largely unknown. It is also unclear whether different lineages have experienced distinct phylogeographic histories and adapted to divergent niches.
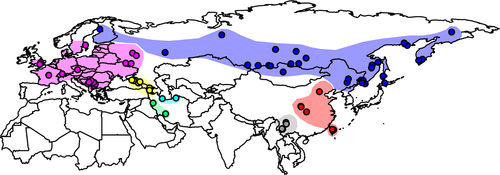
To investigate the interaction between lineage differentiation and niche evolution in the Eurasian nuthatch, we combined multilocus phylogenetic and coalescent analyses with ecological niche modelling (ENM) approaches. First, we estimated the number of independently evolving lineages in this bird and reconstructed the phylogeographic history of each lineage. Second, we compared niche dissimilarity and environmental distinctiveness to assess the level of niche divergence or conservatism between lineages. Finally, we examined the relationship between niche separation and phylogeographic pattern among lineages to infer the role of niche evolution in lineage differentiation.
2 MATERIALS AND METHODS
2.1 Sample and sequence data collection
In this study, the mitochondrial (mtDNA) ND2 gene (Drovetski, Zink, Fadeev, et al., 2004a), two Z-linked introns (ACO-I15, ABCA1; Backström et al., 2006; Kimball et al., 2009) and five autosomal introns (01304, 08352, 12021, 17483, TGFB2-I5; Backström, Fagerberg, & Ellegren, 2008) were sequenced for Eurasian nuthatch samples across most parts of their range (Figure 1 and Supporting Information Table S1). We obtained 230 ND2 sequences, including 47 samples from mainland China and Taiwan (i.e., East Asia) sequenced for this study and 183 samples from North Asia, Europe, Caucasus and Iran sequenced for our previous studies (Hung, Drovetski, & Zink, 2012; Nazarizadeh, Kaboli, Rezaie, Harisini, & Pasquet, 2016; Zink, Drovetski, & Rohwer, 2006) or downloaded from the GenBank (Supporting Information Table S1). For each of the Z-linked and autosomal introns, we sequenced 23–47 samples from East Asia and used 24–138 previously published sequences (Hung et al., 2012) from Caucasus, Europe and North Asia (Supporting Information Table S1). The S. europaea samples from Iran had no nuclear data.
Nine samples of Sitta nagaensis, closely related to S. europaea (Pasquet et al., 2014), from Yunnan and southern Sichuan, China (Figure 1), were sequenced for ND2 and the seven nuclear introns (Supporting Information Table S1). We also sequenced one Sitta himalayensis sample and one Sitta yunnanensis sample and used the sequences of two Sitta arctica samples from our previous study (Hung et al., 2012) for ND2 and the seven nuclear introns (Supporting Information Table S1). In addition, we obtained the ND2 sequences of one Sitta himalayensis sample and one Sitta cashmirensis sample from the GenBank (Supporting Information Table S1).
2.2 Sequence data analyses
The phases of sequences containing indels were resolved using the program champuru v1.0 (Flot, 2007). Multi- and single-base indels were treated as single-base polymorphisms for further analyses (Hung et al., 2012). phase 2.1.1 (Stephens, Smith, & Donnelly, 2001) implemented in DnaSP 5.10.01 (Librado & Rozas, 2009) was applied to determine the phases of individuals with multiple heterozygous sites but no indel. The number of interactions, the thinning interval and the number of burn-in iterations of phase analyses were set as 5,000, 10 and 500, respectively, while other variables remained as the default settings. DnaSP 5.10.01 and Arlequin 3.5 (Excoffier & Lischer, 2010) were used to calculate the nucleotide diversity (π) for each gene and each lineage and net nucleotide distance (DA) and genetic differentiation (ΦST) among lineages for each gene. We also examined whether the sequences of each gene have experienced substitution saturation and consequently contained no phylogenetic information using an entropy-based approach (Xia, Xie, Salemi, Chen, & Wang, 2003) implemented in dambe 7.0.28 (Xia, 2018; Xia & Xie, 2001). The substitution saturation tests were performed based on 10,000 simulations with the numbers of operational taxonomic units (NOTU) set as 4, 8, 16 and 32.
The neutrality of mtDNA was tested using the McDonald–Kreitman test (MK test; McDonald & Kreitman, 1991) for pairwise comparisons among three main groups: the S. nagaensis group; the East Asia group; the remaining S. europaea individuals, which formed one big group in the mtDNA gene tree (see Section 3 for details). The neutrality of mtDNA plus nuclear introns and nuclear introns alone was tested using the Hudson–Kreitman–Aguade (HKA) test implemented in the HKA program (http://genfaculty.rutgers.edu/hey/software) with comparison settings set the same as those for the MK tests. We further used the Fu's Fs test (Fu, 1997) and mismatch distribution analysis (Rogers & Harpending, 1992; Slatkin & Hudson, 1991) implemented in DnaSP to examine the neutrality of mtDNA and introns for the same three main groups examined by the MK and HKA tests. The Fu's Fs test and mismatch distribution analysis could also be used to detect a population expansion because it leaves a similar signature in DNA sequences as does natural selection (Ramos-Onsins & Rozas, 2002). The frequency distribution of the mismatch between pairwise sequences of empirical data was compared to the expected distribution under a demographic scenario of a constant population. Model fit was examined by estimating the raggedness statistic (r), measuring the irregularity of the observed distribution. The confidence intervals of the Fs and r values were estimated by performing 10,000 simulations assuming selective neutrality and population equilibrium.
2.3 Phylogenetic reconstruction
We reconstructed maximum-likelihood (ML) phylogeny trees for ND2 sequences using phyml (Guindon & Gascuel, 2003) with 1,000 bootstrap replicates; the trees were rooted with S. yunnanensis. JModelTest 2.1.10 (Darriba, Taboada, Doallo, & Posada, 2012; Guindon & Gascuel, 2003) was used to identify the mutation model (i.e., TrN+G) for the ND2 samples based on Akaike information criterion (AIC) tests. paup 4 (Swofford, 2002) was employed to build the 50% majority consensus tree. Bayesian inferences (BI) analyses for phylogenetic relationships based on ND2 sequences and the above mutation model were reconstructed using MrBayes 3.2 (Ronquist & Huelsenbeck, 2003) with the following conditions: four Markov chain Monte Carlo (MCMC) chains, each of which contained 40 million steps that were sampled every 1,000 steps, with the first 25% of the steps discarded as burn-in. To visualize the relationship among the clusters of ND2 haplotypes, a median-joining network was generated using network 5 (http://www.fluxus-engineering.com; Bandelt, Forster, & Röhl, 1999).
We reconstructed “species trees” to examine the relationships among the lineages identified by the ND2 gene tree (see Section 3 for details) based on the data sets of (a) mtDNA plus introns and (b) introns only using beast 2 (Bouckaert et al., 2014) under the following conditions: MCMC chains, containing 500–750 million steps, were sampled every 2,000–5,000 steps and the first 10% of the steps were discarded as burn-in. The mutation models for each gene were determined using JModelTest. tracer v1.6 (Rambaut, Suchard, Xie, & Drummond, 2014) was used to examine the convergence of each run and assess the ESS values (>200) of likelihood in MCMC analyses. Trees were compiled using TreeAnnotator v2.4.5 (Rambaut & Drummond, 2008) with “maximum sum of clade credibility” and “mean height” options and visualized using figtree v1.4.3 (Rambaut & Drummond, 2009). To further examine whether the multilocus (mtDNA plus introns) data set could recover a significant pattern of lineage delimitation, we also conducted the species tree analysis by setting the analytical units as sampled localities and the running conditions were the same as above.
2.4 Demographic history inference
The Extended Bayesian Skyline Plot (EBSP; Heled & Drummond, 2008) implemented in beast 2 was applied to reconstruct demographic history for each lineage based on the ND2 and seven introns. Strict molecular clocks were used and ND2 with a substitution rate of 0.029 substitutions/site/myr (Lerner, Meyer, James, Hofreiter, & Fleischer, 2011) was set as a reference locus for calibrating the molecular clock. The MCMC sampling procedure was set to 500–750 million steps, stored every 10,000–50,000 steps. tracer was used to examine the convergence of each run and assess the posterior and likelihood ESS values (>200) in MCMC analyses. Skyline plots were generated using the r program plot (EBSP).
We used the program IMa (Hey & Nielsen, 2007) to estimate divergence times and gene flow between adjacent lineages and their effective population sizes based on the mtDNA plus intron data set. The MCMC analyses contained 5,000,000 steps after a burn-in period of 1,000,000 steps, employing 40 geometric heating chains. The scaled parameter estimates were converted into absolute values based on a generation time of 2 years (Nadachowska-Brzyska, Li, Smeds, Zhang, & Ellegren, 2015) and the geometric mean of the substitution rates of these eight loci, which were calculated by multiplying the sequence length of each locus by 2.9 × 10−8 substitutions/site/year for ND2 (Lerner et al., 2011), 1.62 × 10−9 substitutions/site/year for Z-linked introns or 1.35 × 10−9 substitutions/site/year for autosomal introns (Ellegren, 2007).
2.5 Ecological niche models
We performed ENM analyses to assess ecological niche evolution among S. europaea and S. nagaensis lineages and predicted their historical range shifts based on their georeferenced localities and environmental variables (Arteaga, McCormack, Eguiarte, & Medellín, 2011; Schluter, 2009; Wiens & Graham, 2005). The occurrence points of the lineages were obtained from public databases including the GBIF (http://data.gbif.org/), Ebird (http://ebird.org/ebird/explore), Bold system v4 (http://v4.boldsystems.org) and VerNet (http://vertnet.org), China bird report database (http://www.birdreport.cn/) and the sampling sites of Zink et al. (2006) as well as this study. We discarded occurrence points separated from one another by <0.1° to reduce the effect of spatial autocorrelation. We used two different approaches to assign the occurrence points to each of the seven lineages identified in this study (see Section 3 for details): (a) The occurrence points were split based on the distribution ranges of all subspecies (de Hoyo, Elliot, Sargatal, Christie, & Juana, 2018; Harrap & Quinn, 1995) included in each lineage (Supporting Information Figure S1a). (b) We generated a minimum convex polygon based on the geographic locations of genetic samples for each lineage (Supporting Information Figure S1b) using the Minimum Bounding Geometry function of ArcGIS v10.2 (ESRI, Redlands, CA, USA). We only used the occurrence points covered by each lineage's polygon for the ENM analyses. However, the genetic sample locations of the four smaller lineages (i.e., the Caucasus, northern Iran, southwestern Iran and S. nagaensis lineages) were not enough to generate polygons with reasonable sizes, and thus, we used the occurrence points selected by the first approach for them. The ENMs and relevant analytic results (see below for details) based on the occurrence data sets generated by the two approaches were consistent, suggesting that our analyses were robust to occurrence point assignment. Therefore, we presented the results of the first data set in the main text and those of the second data set (hereafter referred as the polygon data set) in the Supporting Information.
Nineteen bioclimatic variables with a resolution of 2.5-arc min were downloaded from WorldClim (Hijmans, Cameron, Parra, Jones, & Jarvis, 2005; http://www.worldclim.org/) for three time points—the present time, the last glacial maximum (LGM; 21,000 ybp) and the last interglacial period (LIG; 120,000 ybp)—and used as the environmental variables for the ENM analyses. Because of the high levels of autocorrelation between some climatic variables (Peterson, 2011), we only used nine variables with low correlation coefficients (r < 0.7, where r is the Pearson correlation coefficient; Supporting Information Table S2) for further analyses. The ENMs based on three LGM paleoclimate models (i.e., CCSM4, MIROC-ESM and MPI-ESM-P) returned similar results (Supporting Information Figure S2). We averaged the predicted values from the three LGM models using ArcGIS v10.2 for downstream analyses to reduce model-associated uncertainty.
We assessed historical niche shifts in geographic distribution for each lineage across the present time, the LGM and the LIG based on ENMs in geographic (G) space using MaxEnt 3.3.3K (Phillips, Anderson, & Schapire, 2006) with 2,000 iterations and 10 replicates. We evaluated the model performance using AUC (area under the receiver operator curve) based on 30% of the sampled occurrence points used for testing. The distribution map of each lineage was coded as predicted presence/absence using a logistic threshold with equal training sensitivity and specificity for habitat suitability. Levin's B1 (inverse concentration) and B2 (uncertainty) were obtained for each lineage as a measure of niche breadth: the higher values of these indexes indicate the broader niches.
To test niche divergence or conservatism between lineages, we first estimated ENMs in spatial environmental (E) space using the r packages (ade4, adehabitat, dismo, sp, gam, mass, mvtnorm, gbm, dismo and ecospat; Broennimann et al., 2012). The nine climatic (i.e., environmental) variables were transformed into a two-dimensional surface using the first and the second principal component in principal components analysis (PCA). To calibrate the PCA, we used environmental data from both lineage ranges (i.e., background data). Occurrence data for each lineage was assigned a score, which was later projected onto a 100 × 100 PCA grid based on minimum and maximum values of PCA in the data set. We calculated the D values (Rödder & Engler, 2011) to present niche overlap between lineages over the E space. Niche overlap was estimated using a kernel density function that corrected the density of observed occurrence (Broennimann et al., 2012).
The niche equivalency test (Graham, Ron, Santos, Schneider, & Moritz, 2004) and niche similarity test (Peterson, Soberón, & Sánchez-Cordero, 1999), modified by Broennimann et al. (2012), were used to test niche divergence or conservatism. The niche equivalency test examined whether niches of two lineages were equivalent by comparing the empirical D value with 100 simulated ones calculated from all occurrences that were randomly relocated into two groups. The niche similarity test examined whether niches were significantly divergent or conserved when taking into account background environmental differences between two lineages. The similarity test compared the empirical D value with one null distribution of 100 simulated D values between occurrences of one lineage and random points from the range of the other lineage, and another null distribution based on comparisons from the other direction.
2.6 Relationships among genetic distance, ecological niche and geographic distance
Both genetic drift and natural selection could cause niche differentiation between lineages. If it was the former, a positive relationship between niche difference and genetic distance would be expected; if the latter, no particular relationship would be predicted. Given that geographic distance could also impact genetic structure, the former factor should be considered when examining the relationship between genetic and niche divergences (Wang, 2013). To test whether niche evolution is the result of genetic drift, we used the multiple matrix regressions with randomization (MMRR) test to examine whether niche distance (1 − D [niche overlap in the E space]) was correlated with genetic differentiation (ΦST) or distances (DA) while taking into account geographic distance between lineages based on a multiple regression model (Wang, 2013). The ΦST or DA values were (a) based solely on the ND2 or (b) averaged across the ND2 and seven introns. The MMRR test was implemented in r and conducted 10,000 permutations to assess the additive effect of both independent factors (i.e., geographic and niche distances) on genetic divergence.
3 RESULTS
3.1 Gene trees
The mtDNA ND2 gene tree revealed six clades in the Eurasian nuthatch: Europe, North Asia, Caucasus, southwestern Iran, northern Iran and East Asia (Figure 2). The Caucasus and southwestern Iran lineages were sisters, which formed a monophyletic group with the northern Iran, North Asia and Europe lineages, but the relationships among these lineages in this group were unresolved (ML bootstrap <70%) or suggested the northern Iran and North Asia as sister lineages (BI Posterior probability [Pp] = 0.93). In addition, this group had a trifurcating relationship between the East Asia lineage and another group containing S. nagaensis and S. cashmirensis. Given the close relationship between S. nagaensis and S. europaea (Figure 2), we included the former in all genetic and ecological analyses and treated it as one lineage in this study (see details below). The ND2 haplotype network also revealed the same seven clusters for the samples (Supporting Information Figure S3).
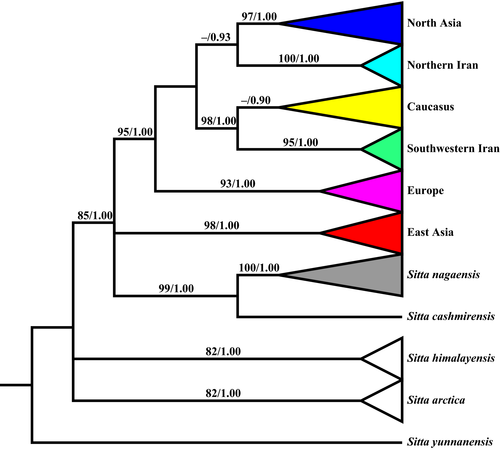
The mtDNA gene tree suggested that S. europaea, S. nagaensis and S. cashmirensis formed a super-species group. Interestingly, we found that S. arctica, a recently named species split from S. europaea (Red'kin & Konovalova, 2006; Zink et al., 2006), was as distinctly related to the super-species group as was S. himalayensis. The results confirmed the distinct status of S. arctica as a species and suggested that S. europaea, S. nagaensis and Sitta cashmriensis were more closely related to each other than any of them were to S. arctica.
3.2 Species trees
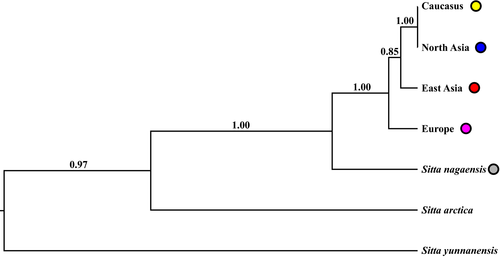
The species trees based on multilocus data also could not completely resolve the relationships among these lineages. The species tree based on the ND2 and seven introns showed partially resolved relationships among the five lineages, Europe, Caucasus, North Asia, East Asia and S. nagaensis (we did not have nuclear samples for the southwestern Iran and northern Iran lineages; Figure 3). The Caucasus and North Asia lineages were the most closely related to each other and had a trifurcating relationship (Pp < 0.9) with the East Asia and Europe lineages. The S. nagaensis lineage was sister to the groups containing all S. europaea samples. The species tree based solely on the seven introns showed a similar pattern with a group containing the North Asia, Caucasus, Europe and East Asia lineages, but their relationships were unresolved (Pps < 0.9; Supporting Information Figure S4). The S. nagaensis lineage was sister to the S. europeae group. The species tree with analytical units set as sampled localities based on the ND2 and seven introns recovered the North Asia, Caucasus, Europe and East Asia lineages and a sister relationship between them and the S. nagaensis lineage (Pps > 0.9), but the relationships among the S. europaea lineages were unresolved (Pps < 0.9, Supporting Information Figure S5).
3.3 Summary statistics of genetic sequences
The East Asia lineage had the highest π values (0.0066 ± 0.0021 [SD]) averaged over mtDNA and introns, followed by that of the North Asia lineage (0.0046 ± 0.0026). The Caucasus lineage had the lowest average π values (0.0015 ± 0.0017) of the five lineages containing both mtDNA and intron sequences (Table 1). The East Asia lineage also had the highest π value (0.0032 ± 0.0002) for mtDNA, followed by that of the Europe lineage (0.0025 ± 0.0002), and the southwestern Iran lineage had the lowest one (0.0006 ± 0.0002) of the all seven lineages.
| Locus | Chr | L | N | Haplotype | π | π NA | π EU | π EA | π CA | π SNA | π NI | π SWI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ND2 | Mt | 1,041 | 230 (116/45/26/15/9/9/10) | 95 | 0.0334 | 0.0016 | 0.0025 | 0.0032 | 0.0018 | 0.0016 | 0.0017 | 0.0006 |
| ACO-I15 | Z | 531 | 183 (100/25/36/10/12/–/–) | 29 | 0.0066 | 0.0020 | 0.0010 | 0.0077 | 0.0007 | 0.0013 | – | – |
| ABCA1 | Z | 365 | 99 (26/7/49/4/13/–/–) | 19 | 0.0077 | 0.0032 | 0 | 0.0078 | 0 | 0.0023 | – | – |
| 01304 | 11 | 685 | 88 (22/8/34/6/18/–/–) | 48 | 0.0069 | 0.0052 | 0.0020 | 0.0071 | 0 | 0.0031 | – | – |
| 08352 | 5 | 378 | 90(22/8/38/6/16/–/–) | 35 | 0.0093 | 0.0087 | 0.0065 | 0.0092 | 0.0048 | 0.0034 | – | – |
| 12021 | 24 | 691 | 140 (50/12/50/10/18/–/–) | 61 | 0.0059 | 0.0057 | 0.0019 | 0.0042 | 0.0005 | 0.0060 | – | – |
| 17483 | 4 | 486 | 114 (40/14/32/10/18/–/–) | 32 | 0.0065 | 0.0026 | 0.0041 | 0.0052 | 0.0030 | 0.0044 | – | – |
| TGFB2-I5 | 3 | 476 | 232 (108/22/52/32/18/–/–) | 89 | 0.0083 | 0.0079 | 0.0026 | 0.0080 | 0.0010 | 0.0084 | – | – |
| Mean | 0.0106 | 0.0046 | 0.0026 | 0.0066 | 0.0015 | 0.0038 | ||||||
| SD | 0.0093 | 0.0027 | 0.0020 | 0.0021 | 0.0017 | 0.0024 |
Note
- Chr indicates the chromosome where the gene is located. L indicates the length of the locus used in the analyses. N shows the number of alleles sampled, and the value within the parentheses indicates the number of alleles in each lineage arranged in the same order as the π values. π indicates the nucleotide diversity of alleles sampled in all lineages. πNA indicates the nucleotide diversity of alleles sampled in the Northern Asia lineage, πEU for the Europe lineage, πEA for the East Asia lineage, πCA for the Caucasus lineage, πSNA for the Sitta nagaensis lineage, πNI for the northern Iran lineage and πSWI for the southwestern Iran lineage. Mean indicates the π value averaged across all loci, and SD indicates its standard deviation.
The highest DA value averaged over mtDNA and introns (0.0152 ± 0.0044) was found between the S. nagaensis and Caucasus lineages, and the lowest one (0.0068 ± 0.0022) was between the Europe and Caucasus lineages (Supporting Information Table S3). The highest DA value of ND2 (0.0870 ± 0.0175) was found between the S. nagaensis and northern Iran lineages and the lowest one (0.0054 ± 0.0016) was between southwestern Iran and Caucasus lineages (Supporting Information Table S4). The highest ΦST value averaged over mtDNA and introns (0.7235) was found between the Europe and Caucasus lineages, and the lowest one (0.2431) was between the North Asia and East Asia lineages (Supporting Information Table S5). We found the highest ΦST value of ND2 (0.9868) between the S. nagaensis and southwestern Iran lineages and the lowest one (0.8021) between the Caucasus and southwestern Iran lineages (Supporting Information Table S6).
The substitution saturation tests suggested no saturation signal in the mtDNA and intron sequences used in this study. The index values of substitution saturation (Iss) were significantly smaller than critical values assuming symmetrical and asymmetrical tree topologies (Iss.cSym and Iss.cAsym, respectively) for all genes regardless the values of NOTU (Supporting Information Table S7). The results suggested that these sequences have experienced no or little substitution saturation and could be used for phylogenetic reconstruction.
The MK tests could only reject the neutral model for the comparison between the East Asia lineage and the rest of the S. europaea individuals (p = 0.006), but not for the other two comparisons. The HKA tests based on mtDNA plus nuclear introns could only reject the neutral model for the comparison between the S. nagaensis and East Asia lineages (p = 0.025); the HKA tests based only on nuclear introns showed no significant results for any comparison. The Fu's Fs tests showed that four introns in the East Asia lineage, three introns in the group containing all other S. europaea individuals, and three introns in the S. nagaensis lineage had significantly negative Fs values, whereas the mtDNA had no significant Fs values in any of the three groups (Supporting Information Table S8). Given that introns were unlikely impacted by selection (which was supported by the HKA tests), the significantly negative Fs values in some of the introns should be caused by population expansions. The mismatch distributions showed no significant difference from the expected distributions of a constant population and selective neutrality in all genes except for one intron in the three groups (r, p < 0.05; Supporting Information Table S8). Overall, the neutrality test results showed that the mtDNA of S. europaea might be under low or negligible levels of selection, but the signals were not always consistent across tests and could be confounded by population expansions.
3.4 Demographic history
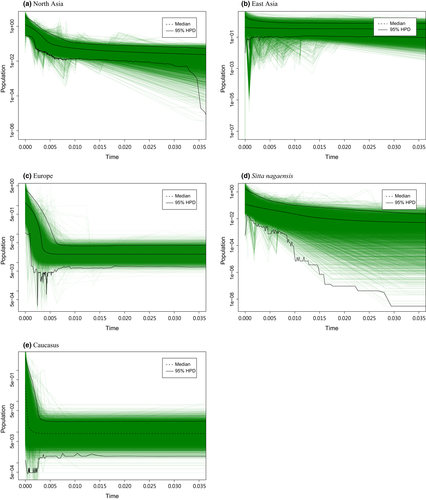
The EBSP results showed that the North Asia and Europe lineages have experienced a recent population expansion (Figure 4). Although the East Asia and S. nagaensis lineages were also found to have experienced a population expansion based on the 95% confidence interval of the number of size-change steps, the changes in population size were not as dramatic as those of the North Asia and Europe lineages (Figure 4). In addition, the variation in the skyline plot curves for the S. nagaensis lineage became large 20 kyr and earlier (Figure 4), which might cause a false rejection of the constant population hypothesis. The Caucasus lineage did not experience a significant population expansion. Overall, the skyline plots showed that the northern (i.e., North Asia and Europe) lineages tended to experience recent population expansions whereas the southern (i.e., Caucasus, East Asia and S. nagaensis) ones had more stable population sizes.
The IMa analyses based on the mtDNA and seven introns showed that the S. nagaensis lineage separated from the East Asia lineage around 1.9 myr (i.e., the peak value of the posterior distribution of estimated divergence times) and the currently neighbouring lineages of S. europaea separated from each other around 0.2–1.6 myr (Supporting Information Figure S6). The IMa estimates showed that levels of gene flow significantly larger than zero were only found from the Europe to Caucasus (0.02 individual/generation) and the North Asia to East Asia (1.16 individual/generation; Supporting Information Figure S7). The geneflow estimates from the East Asia to North Asia, the Europe to North Asia and the North Asia to Europe showed a nonzero peak in their posterior distributions, which, however, contained the value of 0 and thus could not reject the possibility of zero gene flow (Supporting Information Figure S7). The IMa estimated based solely on the seven introns showed results with similar patterns and trends (Supporting Information Figures S8 and S9).
3.5 Ecological niche models
Ecological niche models predicted the geographic niche distribution of the seven lineages (Figure 5 and Supporting Information Figure S10; Figure S11 based on the polygon data set) with AUC values ≥0.9 (except for that of the North Asia lineage = 0.88 based on the first occurrence data set; Supporting Information Table S9; Table S10 based on the polygon data set). ENMs for the lineages did not always match their current ranges, especially for the southern ones. The ENMs for the Caucasus, southwestern Iran, Northern Iran and Europe (in its southern part) lineages somewhat overlapped with one another and so did those of the East Asia and S. nagaensis lineages (Figure 5). In contrast, the northern lineages’ (i.e., North Asia and Europe) ENMs generally matched their parapatric distribution (Figure 5).
Ecological niche models revealed that the southern lineages (i.e., Caucasus, northern Iran, southwestern Iran, East Asia and S. nagaensis) tend to have more stable niche sizes throughout the present time, LGM and LIG than the northern ones (i.e., North Asia and Europe; Supporting Information Figure S10; Figure S11 based on the polygon data set). The results were generally consistent with the population size changes suggested by the EBSP, although we did not have skyline plots for the northern Iran and southwestern Iran lineages. The ENMs suggested that the North Asia lineage experienced range shrinkage towards the south during the LGM (Supporting Information Figures S10 and S11). The North Asia lineage had a LIG niche model more similar to the present one than the LGM one (Supporting Information Figures S10 and S11). The Europe lineage also had a range shrinking towards the south during the LGM (Supporting Information Figures S10 and S11). Compared with the LGM one, the LIG niche distribution of the Europe lineage had a more northern range in Western and Northern Europe, but it was smaller than the present one because the ENM did not include Central and Eastern Europe. The present ENM of the Caucasus lineage was larger than its current known distribution and partially overlapped with those of the northern Iran and Europe lineages (Figure 5). Overall, the ENMs of the Caucasus lineage were similar throughout the present time, LGM and LIG, and so did the southwestern Iran lineage (Supporting Information Figures S10 and S11). The northern Iran lineage had similar niche distributions between the present time and LGM, but its LIG model contained more areas in Southern Europe (Supporting Information Figures S10 and S11). The East Asia lineage had similar ENMs between the present time and LGM, but the northwestern part of the above niche range reduced during the LIG (Supporting Information Figure S10). The S. nagaensis lineage had similar predicted distributions throughout the present time, LGM and LIG, but its LIG distribution did not cover the very northern part of the former two (Supporting Information Figures S10 and S11).
The North Asia lineage had the broadest niche (B1 = 0.41 and B2 = 0.95) followed by the Caucasus lineage (B1 = 0.15 and B2 = 0.90), while the southwestern Iran lineage showed the narrowest niche at the present time (B1 = 0.01 and B2 = 0.73; Supporting Information Table S5). The patterns were generally consistent across the present, LGM and LIG (Supporting Information Table S9). The results based on the polygon data set showed the same pattern (Supporting Information Table S10).
3.6 Niche evolution
The niche equivalency tests rejected the null hypothesis of niche identity for all pairwise comparisons among the seven lineages (p < 0.02), suggesting that they had nonidentical niches (Table 2). In contrast, no comparisons showed significant niche divergence based on the similarity tests (Table 2). Out of 21 pairwise comparisons in the similarity tests, one comparison (i.e., Europe vs. North Asia) showed significant niche conservatism in both directions and 15 comparisons showed significant niche conservatism in one direction (Table 2). The Europe and Caucasus lineages had the highest level of niche overlap (D = 0.226). The lowest level of niche overlap (D = 0) was found in the comparisons between the S. nagaensis lineage and all other lineages except the East Asia and North Asia (Table 2). The analyses based on the polygon data set showed similar results (Supporting Information Table S11).
| Lineages (a) | Lineages (b) | Niche overlap (D) | Similarity test | Equivalency test | |
|---|---|---|---|---|---|
| a → b | b → a | ||||
| EU | SNA | 0 | 1 | 1 | 0.02* |
| EA | 0.065 | 0.02* | 0.93 | 0.02* | |
| NA | 0.145 | 0.02* | 0.04* | 0.02* | |
| NI | 0.033 | 0.02* | 0.08 | 0.02* | |
| SWI | 0.008 | 0.06 | 0.02* | 0.02* | |
| CA | 0.226 | 0.04* | 0.18 | 0.02* | |
| NA | SNA | 0.087 | 0.99 | 0.08 | 0.02* |
| EA | 0.079 | 0.02* | 0.71 | 0.02* | |
| NI | 0.007 | 0.02* | 0.12 | 0.02* | |
| SWI | 0.003 | 0.02* | 0.20 | 0.02* | |
| CA | 0.154 | 0.02* | 0.32 | 0.02* | |
| SNA | EA | 0.094 | 0.04* | 0.63 | 0.02* |
| NI | 0 | 1 | 1 | 0.02* | |
| SWI | 0 | 1 | 1 | 0.02* | |
| CA | 0 | 1 | 1 | 0.02* | |
| EA | NI | 0.049 | 0.14 | 0.02* | 0.02* |
| SWI | 0.034 | 0.93 | 0.02* | 0.02* | |
| CA | 0.116 | 0.55 | 0.02* | 0.02* | |
| NI | SWI | 0.117 | 0.31 | 0.02* | 0.02* |
| CA | 0.149 | 0.02* | 0.34 | 0.02* | |
| SWI | CA | 0.037 | 0.02* | 0.87 | 0.02* |
Note
- The p values for the two tests are shown. The symbol “*” indicates significant niche conservatism in the similarity tests and significant difference in the equivalency tests. EU indicates Europe, NA indicates North Asia, EA indicates East Asia, CA indicates Caucasus, NI indicates northern Iran, SWI indicates southwestern Iran, and SNA indicates Sitta nagaensis.
3.7 MMRR test
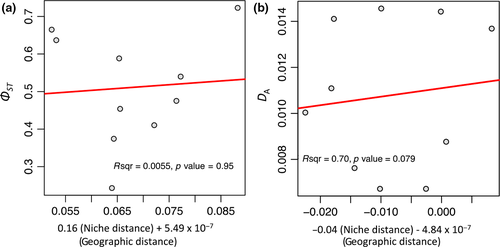
The MMRR test was used to determine whether ecological niche dissimilarity and geographic isolation reflect the observed genetic differences among the S. europaea (and S. nagaensis) lineages. The MMRR results showed that genetic divergence (ΦST, p = 0.95 for ND2 plus introns and 0.15 for ND2) or distance (DA, p = 0.79 for ND2 plus introns and 0.12 for ND2) among the lineages was not significantly associated with ecological niche and geographic distances (Figure 6 and Supporting Information Figure S12). The MMRR tests based on the polygon data set also showed nonsignificant results for all cases (Supporting Information Figure S13).
4 DISCUSSION
Our study reveals six parapatric lineages within S. europaea: the North Asia and Europe lineages are distributed in the northern Palearctic and the other four in the southern Palearctic. The lineages’ niche evolution is relatively conserved when considering the environmental difference between their ranges and thus is unlikely to be the driving force of lineage divergence. Nevertheless, the levels of niche overlap and their impacts on lineages’ distribution differ across latitude. Adaptation to different ecological niches after lineages split might have prevented the northern lineages from becoming sympatric, whereas competition due to similar niches among southern lineages might have led to their abutting ranges.
4.1 Phylogeographic history of divergent lineages
The topologies of the ND2 gene tree and species trees are not identical, but they do not conflict. The ND2 gene tree revealed six S. europaea lineages with unresolved relationships except for a supported sister relationship between the Caucasus and southwestern Iran lineages (Figure 2). The species tree based on the ND2 and seven introns supported a sister relationship between the Caucasus and North Asia lineages but showed unresolved relationships among other S. europaea lineages (Figure 3), whereas the species tree based on the seven introns showed no resolved relationship among the S. europaea lineages (Supporting Information Figure S4). Given that (a) the species trees contain no Iran samples and thus it is not possible to reveal the relationship between the southwestern Iran and Caucasus lineages and that (b) the relationship between the Caucasus and Northern Asia lineages is unresolved in the ND2 tree, the topologies of these three trees do not conflict because unresolved phylogenetic relationships are consistent with any hypothesis (Zink & Barrowclough, 2008). The relationships among the lineages are largely unresolved likely because they separated from one another too closely in time (revealed by the IMa analyses) to allow unambiguous lineage sorting in the reconstructed phylogenetic trees (Hung et al., 2012).
The IMa analyses suggest that the S. nagaensis and East Asia (S. europaea) lineages diverged around 1.9 myr, and the S. europaea lineages diverged from one another around 0.2–1.6 myr when Middle and Late Pleistocene glacial–interglacial cycles were the dominant climatic regime. The Pleistocene climate appears to have impacted the lineage divergence of several species, including the Eurasian wren (Troglodytes troglodytes; Drovetski, Zink, Rohwer, et al., 2004b), the common rosefinch (Carpodacus erythrinus; Hung, Drovetski, & Zink, 2013), the horned lark (Eremophila alpestris; Drovetski, Raković, Semenov, Fadeev, & Red'kin, 2014), the Eurasian treecreeper (Certhia familiaris; Pons et al., 2015) and the long-tailed tit (Aegithalos caudatus; Song et al., 2016), in the Eurasian area. Our study suggests that climate fluctuations during the Pleistocene also might have caused the splits among the Eurasian nuthatch lineages.
The coalescent analyses of demographic history (i.e., EBSP) suggest that the northern lineages tend to have higher levels of fluctuations in their effective population sizes than the southern lineages during their evolutionary history. For example, the North Asia and Europe lineages have experienced dramatic population expansions since the LGM, a typical scenario for temperate species (Hewitt, 2000), whereas the southern lineages have had more stable population sizes. The coalescence-based estimates are consistent with the ENMs showing that the northern lineages have tended to expand ranges (or niches) northwards since the LGM compared with the southern lineages, which have not (Supporting Information Figures S10 and S11). In addition, the IMa-estimated gene flow is only detected from the northern lineages to the southern ones (i.e., from Europe to Caucasus and from North Asia to East Asia), suggesting that introgression was likely to occur when the former shifted their distribution southwards and came in contact with the latter during glaciation periods. The Fu's Fs tests also suggest that these lineages have experienced population expansions, which, however, were not substantial enough or occurred too recently to leave detectable signals in all studied genes (Fu, 1997; Ramos-Onsins & Rozas, 2002); the mismatch distribution analyses, which are more conserved in detecting population expansions, showed even fewer genes with significant signals of expansion than the Fu's Fs tests further supporting this argument (Ramos-Onsins & Rozas, 2002).
The distribution ranges of the two northern lineages combined are larger than those of the southern four lineages combined, and thus, the lineage density of the Eurasian nuthatch in the south is higher than the north. The southern climate and environment could be more stable than the northern ones over evolutionary time. Thus, populations in the south (in particular, the Caucasus, northern Iran and southwestern Iran lineages) might have survived long enough to diverge in relatively small areas, whereas the northern lineages might have experienced frequent range contractions and expansions (and associated population extirpations), leaving little time for populations within each lineage to further diverge (Haffer, 1969).
4.2 The causes of the abutting ranges among the lineages
Analysing the causes of the geographic distribution of divergent lineages is critical for understanding how evolution unfolds in nature (Hewitt, 2000). The currently continuous but little- or nonoverlapping (parapatric) distribution of the Eurasian nuthatch lineages (Figure 1) might have been caused by secondary contact between split lineages (Bull, 1991; Haffer, 1969). An interesting question is why the lineages are not sympatric at present (Zink, 2014). The reason could be that (a) the effect of local adaptation is strong enough to prevent invasion between neighbouring lineages, (b) the lineages exclude each other through competition, or (c) the secondary contact occurred too recently to mix different lineages.
For the first scenario, the rejection of niche equivalency tests among all lineages suggests that each lineage might have occupied a niche unique enough to impede their admixture. The ENMs of the northern lineages show little overlap in geographic space whereas those of the southern ones have higher levels of overlap (Figure 5) although the similarity tests suggest that the niches of all lineages are not significantly divergent against their environmental backgrounds. It is also possible that some lineages might have diverging traits other than or only indirectly linked to the climate-related niches predicted by the ENMs (Harrap & Quinn, 1995). The physiological, morphological or behavioural differences resulting from adaptation to different environments via divergent selection (i.e., local adaptation; Williams, 1966) can be strong enough to cause invading lineages to have lower fitness than native lineages and thus inhibit admixture. For example, the North Asia, Europe, East Asia and Caucasus lineages have different belly colorations (Dickinson, 2006; Harrap & Quinn, 1995), suggesting divergent plumage-associated niches (e.g., cryptic coloration), although intra-lineage plumage variations exist in North Asia and Europe. In contrast, the Caucasus, northern Iran and southwestern Iran lineages have similar plumage colorations (Harrap & Quinn, 1995). Thus, the northern lineages are more likely to have higher levels of niche divergence to prevent sympatric distribution than the southern ones. However, it is also possible that the different plumage colorations resulted from genetic drift, rather than divergent selection, when the lineages were isolated in refugia during glacial periods. That is plumage divergence might not always reflect adaptive evolution. We will need evidence showing that the plumage differences among the lineages are adaptive to further prove the role of local adaptation in maintaining their parapatric distribution. In addition, other potentially adaptive characters such as physiological responses to different temperature and humidity regimes can be measured to test for local adaptation. Such evidence requires reciprocal transplant or common garden experiments (Kawecki & Ebert, 2004), which are, however, difficult to conduct in this species and most wild birds.
The second scenario is consistent with the conserved results of niche similarity tests and the cross-predicted ENMs among southern lineages (Figure 5), which imply that they have a potential to invade neighbouring lineages’ ranges because they still share ancestral portions of climate niches (Lee-Yaw & Irwin, 2015; Zink, 2014). ENMs (fundamental niches) larger than the current distribution ranges (realized niches) of the southern lineages suggest that niche filling might be prevented by competition between neighbouring lineages with similar niches (Sexton et al., 2017). Given that Eurasian nuthatches are secondary cavity nesters (Bani Assadi, Kaboli, Etemad, Khanaposhtani, & Tohidifar, 2015; Harrap & Quinn, 1995) and tree holes are limited resources, competition for nest sites may be one of the factors that prevent different lineages from coexisting. Nevertheless, observation of competitive behaviour between lineages in their contact zones is warranted to directly test this hypothesis. The southern lineages might have consistently interacted with each other given their stable historical effective population sizes and ENMs and thus likely have experienced long-term competition leading to their abutting ranges. In contrast, the current ENMs of the northern lineages (i.e., North Asia and Europe) are similar to their real distribution ranges, suggesting that biological competition is less likely the factor contributing to their parapatric distribution although the possibility cannot be completely excluded in this study.
The third scenario is implausible because there should be enough time for most lineages to mingle given the strong dispersal ability of birds, even if the secondary contact is a recent result of post-LGM expansions (Zink, 2014). The current distribution range of the single largest North Asia lineage (about 7,000 km) is larger than the distance between any neighbouring lineages. This means that the neighbouring lineages could have had enough time to become sympatric if they had experienced similar levels of post-LGM range expansions, as the North Asia lineage did. Furthermore, the median natal dispersal distance for the Eurasian nuthatch could range from 1 to 3 km (Matthysen, Adriaensen, & Dhondt, 1995). That is, the Eurasian nuthatch could have dispersed 20,000–60,000 km since the LGM (20,000 ybp), much longer than the current distribution range of the North Asia lineage. However, all lineages currently have parapatric distributions instead.
Overall, the results suggest that the southern and northern lineages might have experienced different scenarios leading to their currently abutting distributions. That is, the distribution of the northern lineages is more likely caused by local adaptation, whereas that of the southern lineages could be mainly caused by competitive exclusion. Whether such an altitudinal pattern is universal to species with continentally wide distribution warrants further examination. It is also worth noting that these hypotheses may not always be mutually exclusive. For example, local adaptation and biological competition might work together, but with varying degrees of contribution, to determine the lineages’ distribution.
4.3 The role of ecological niche evolution in lineage differentiation
Widespread species that occupy broad niches may contain multiple lineages that have either highly overlapping niches or well separate niches, summed up as a broad niche (Sexton et al., 2017). We find both types of niche segregation among the six S. europaea lineages and one S. nagaensis lineage. Interestingly, the niche similarity tests that consider differences in available environmental condition within each lineage's range indicate no significant divergence among their niches. In fact, for all similarity test comparisons, niches are significantly conserved in at least one direction—except those involving the S. nagaensis lineage, for which most (5/6) tests could not reject the null hypotheses (Table 2). The conserved niches indicate that the lineages occupy similar ecological niches in different ecosystems. Now the question becomes how lineages diverged with conserved ecological niches.
It has been assumed that niche conservatism may prevent gene flow between populations because species with conserved niches cannot travel through unsuitable habitats that separate populations (Wiens, 2004). In contrast, niche conservatism might increase gene flow between populations of mobile species because such species can easily move through unsuitable habitats and interbreed with populations occupying similar niches (Wiens, 2004; Wiens & Graham, 2005). Although birds are organisms with relatively high mobility, the Eurasian nuthatch is a nonmigratory forest species that is more likely to diverge due to habitat fragmentation compared to migratory, habitat–generalist birds (Hung, Drovetski, & Zink, 2017). Moreover, historical habitat fragmentation might contribute to their differentiation despite the fact that Eurasian nuthatch lineages currently have no obvious geographic barriers or unsuitable habitats between many of them. That is, habitat changes through evolutionary time might have caused the once continuous distribution of this bird to be separated by intervening habitats (e.g., ice caps or tundra during glacial periods), initiating population divergence, as long as the rate of its niche evolution is slower than the rate of environmental change. If populations are separated long enough, they may diverge into different lineages (or species) with distinct genetic variation.
Since the well-diverged lineages of the Eurasian nuthatch do not have strongly diverged niches, niche separation is less likely to be the driving force of lineage differentiation in this widespread species. Instead, the niche divergence is more likely to be the consequence of lineage split. If the process of the niche evolution has occurred in a neutral manner such as drift, we expect the levels of niche divergence to increase proportionally to the genetic divergence between lineages. However, the MMRR tests reveal no correlation between genetic distance and climate niche divergence when considering geographic distance. The results indicate that the climate niche differences among the Eurasian nuthatch lineages may have not been caused by genetic drift, but selection forces such as local adaptation, biological competition or stabilizing selection (Pyron, Costa, Patten, & Burbrink, 2015; Winkelmann, Genner, Takahashi, & Rüber, 2014) after the lineages split.
ACKNOWLEDGEMENTS
We thank Shou-Hsien Li for providing tissue samples for this study and Noah Last for improving the manuscript. We thank Anna Santure, Mohammad Kaboli and one anonymous reviewer for their helpful comments and suggestions to our manuscript. We are grateful to Chagsaldulam Odonjavkhlan, I-Pin Gao and Hao-Chih Kuo for their help with sequence data collection and analyses and Ya-Jung Lu for his help in obtaining occurrence data. We thank the museums and institutes that loaned tissues or provided sequence data for this study: Institute of Zoology, Chinese Academy of Science; Kunming Institute of Zoology, Chinese Academy of Sciences; Guangdong Institute of Applied Biological Resources; Beijing Normal University; Burke Museum of Natural History; Bell Museum of Natural History; State Darwin Museum; Natural History Museum in Belgrade; Felitsyn's Krasnodar State Historical and Archaeological Museum; Natural Environment Zoological Museum of University of Tehran. This study is supported by the internal fund of Academia Sinica.
AUTHOR CONTRIBUTIONS
C.M.H. designed research. Y.C.C. and M.N. analysed data. F.M.L., X.J.Y., C.T.Y., F.D., L.D., F.S.Z., S.V.D. and Y.L. provided tissue samples or sequence data. C.M.H. led the paper writing with contributions from Y.C.C., M.N., C.C.H., S.V.D., F.M.L. and Y.L. All authors participated in research discussion.
DATA ACCESSIBILITY
The NCBI access numbers of newly sequenced DNA and the ones downloaded from GenBank are provided in Table S1. The input data and scripts for the ENM and MMRR tests are available from the Dryad Digital Repository: https://doi:10.5061/dryad.3472nk1 (link for temporary review: https://datadryad.org/review?doi=doi:10.5061/dryad.3472nk1).




