Deciphering the drivers of negative species–genetic diversity correlation in Alpine amphibians
Abstract
The evolutionary and ecological importance of neutral and adaptive genetic diversity is widely recognized. Nevertheless, genetic diversity is rarely assessed for conservation planning, which often implicitly assumes a positive correlation between species and genetic diversity. Multiple drivers can cause the co-variation between the genetic diversity of one species and the richness of the whole communities, and explicit tests are needed to identify the processes that can determine species–genetic diversity correlations (SGDCs). Here, we tested whether intrapopulation genetic diversity (at neutral loci) and species richness co-vary in the amphibian communities of a southern Alpine region (Trentino, Italy), using the common frog (Rana temporaria) as focal species for the study of genetic diversity. We also analysed ecological similarity, niche overlap and interspecific interactions between the species, to unravel the processes determining SGDC. The neutral genetic diversity of common frogs was negatively related to species richness. The negative SGDC was probably due to an opposite influence of environmental gradients on the two levels of biodiversity, since the focal species and the other amphibians differ in ecological preferences, particularly in terms of thermal optimum. Conversely, we did not find evidence for a role of interspecific interactions in the negative SGDC. Our findings stress that species richness cannot be used as a universal proxy for genetic diversity, and only combining SGDC with analyses on the determinants of biodiversity can allow to identify the processes determining the relationships between genetic and species diversity.
1 INTRODUCTION
Biodiversity embraces three fundamental levels: diversity within species, between species and of ecosystems. The importance of preserving all these three levels of biological diversity has been explicitly stressed by the Aichi Targets, which claim the urgent need to improve the status of biodiversity “by safeguarding ecosystems, species and genetic diversity” (Strategic Goal C; SCBD, 2010).
The role of genetic diversity is widely recognized in evolutionary and ecological theory. Adaptive genetic diversity is required to adapt to a changing environment, determining the evolutionary potential of populations (Allendorf & Luikart, 2012; Booy, Hendriks, Smulders, Van Groenendael, & Vosman, 2000). Neutral genetic diversity provides estimates of genetic drift and inbreeding, which can impact fitness and have detrimental consequences on the viability of populations (Brook, Tonkyn, O'Grady, & Frankham, 2002; Reed & Frankham, 2003; Szulkin, Bierne, & David, 2010). Due to its link with effective population size, neutral genetic diversity can also influence long-term evolutionary potential (Allendorf & Luikart, 2012; Lanfear, Kokko, & Eyre-Walker, 2014); thus, a loss of genetic diversity (either neutral or adaptive) can be associated with increased risk of extinction in natural populations (Frankham et al., 2017; Spielman, Brook, & Frankham, 2004). Moreover, the importance of genetic diversity may be extended to the ecosystem level, due to its influence on ecosystem function and resilience (Hughes, Inouye, Johnson, Underwood, & Vellend, 2008). Nevertheless, in conservation practice, genetic diversity is only considered in certain species-specific conservation programmes, while general strategies for its preservation are largely lacking (Hoban et al., 2013; Laikre et al., 2009; Walpole et al., 2009). For instance, the identification of spatial conservation priorities (e.g., biodiversity hotspots) is generally based on species diversity (Myers, Mittermeier, Mittermeier, Da Fonseca, & Kent, 2000), though its ability to also “capture” genetic diversity patterns has not been properly evaluated.
Although connections between population genetics and community ecology have long been recognized (e.g., Amarasekare, 2000; Antonovics, 2003; Bell, 2001; Hubbell, 2001), only in the last decades have attempts been made to elucidate the relationships between these two levels of biodiversity. Vellend (2003) proposed a general theoretical framework for the correlation between species and genetic diversity (SGDC), and since then, multiple studies have explicitly tested SGDCs in plant and animal communities (reviewed by: Lamy, Laroche, David, Massol, & Jarne, 2017; Vellend, 2003; Vellend & Geber, 2005; Vellend et al., 2014). Despite some work on adaptive genetic diversity (Whitlock, 2014), neutral markers remain the most frequent choice in SGDC studies, particularly for animals (Lamy et al., 2017). From a conservation perspective, SGDCs might be used to predict one level of diversity from the other, in order to simplify spatial prioritization (Kahilainen, Puurtinen, & Kotiaho, 2014). Despite reported SGDCs are often positive (Kahilainen et al., 2014; Vellend et al., 2014), only a fraction of them are actually significant (Lamy et al., 2017). Moreover, recent theoretical and empirical studies have shown that significant negative SGDCs may frequently arise, depending on the selected molecular markers and focal species, as well as the underlying causal processes (Lamy et al., 2017; Laroche, Jarne, Lamy, David, & Massol, 2015).
Even considering only neutral processes, multiple factors can act on genetic and species diversity in both positive and negative ways, thus generating the complex variation in the intensity and sign of observed SGDCs (Lamy et al., 2017; Vellend & Geber, 2005). First, the features of sites (site factors) can simultaneously affect the diversity of communities and the genetic diversity of species. Site factors include the environmental suitability of sites, their area and connectivity. If the focal species is ecologically similar to the other considered species, theory predicts a positive SGDC: for instance, this may be the case when the focal species reaches the largest population size under the same ecological conditions than the other species. Conversely, no or negative SGDCs are predicted if the target species have different or opposite responses to environmental variables (ecological similarity/dissimilarity hypothesis; Lamy et al., 2017; Laroche et al., 2015; Vellend, 2005). Second, interspecific interactions (community factors) can strongly influence the population size of the focal species, thus determining significant SGDC (interspecific interactions hypothesis), with negative correlations expected under strong competition, and positive relationships expected under facilitation (Lamy et al., 2017).
Given the complexity of factors underpinning SGDC, it is important to identify the ongoing processes, integrating into analyses the different potential drivers. Such analyses are rarely performed (Lamy et al., 2017), probably because reconstructing interspecific interactions and understanding the response of multiple species to environmental gradients require extensive data on environmental features, species distribution and ecology.
In this study, we assessed SGDC in amphibian communities, choosing a widespread amphibian, the common frog (Rana temporaria), as focal species for the evaluation of neutral genetic diversity. First, we tested the relationship between diversity at the genetic and community level, considering both species richness and the potential influence of each of the co-occurring amphibians. Second, we decomposed the multivariate relationships between (a) species diversity, (b) neutral genetic diversity and (c) environmental factors, in order to shed light on the processes underlying the recorded SGDC. Finally, we compared the responses of multiple species to environmental gradients and assessed the potential occurrence of interspecific interactions (competition and predation). The integration of these analyses allowed us to assess the support of the ecological similarity/dissimilarity and interspecific interactions hypotheses as explanation for SGDC (see Figure 1).
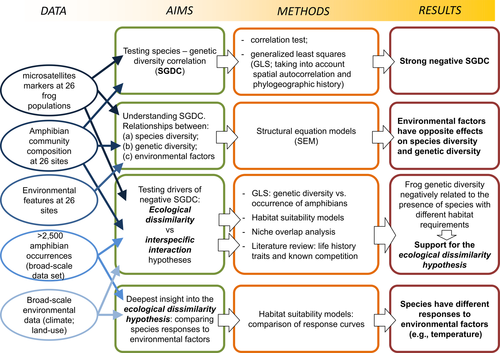
2 MATERIALS AND METHODS
2.1 Ethics statement
All conducted experiments complied with the current laws of Italy. Sampling and monitoring procedures were approved by the Italian Ministry of Environment and the Environmental Unit of the Autonomous Province of Trento (DPN/2D/2003/2267 and 4940- 57/B-09-U265-LS-fd).
2.2 Study system
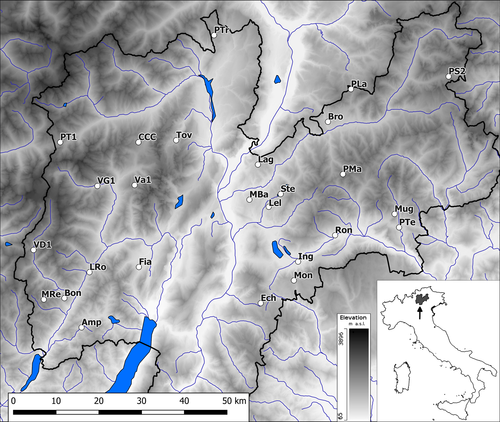
Our study area is Trentino (Autonomous Province of Trento, Italy), a mountainous region of 6,212 km2 in the eastern Alps. The region is characterized by a complex terrain (elevation range: 65–3,764 m a.s.l.; >70% above 1,000 m a.s.l), including part of the Dolomites and Prealps as well as low elevation valleys. The climate varies from the Alpine climate of high elevation areas to the subcontinental moderate climate of the small valleys and the sub-Mediterranean conditions of the southernmost part of the region.
We chose amphibians as a study system because of (a) existing conservation concern, at both global and European scale (Henle et al., 2008; Stuart et al., 2008); (b) availability of accurate distributional data for the study region and (c) a long tradition of community ecology studies for this group of animals (Wells, 2007). Twelve native species inhabit Trentino (Caldonazzi, Pedrini, & Zanghellini, 2002). Following Vellend (2003) and Vellend and Geber (2005), we chose one focal species to assess genetic diversity levels: the common frog (Rana temporaria). This frog is the most widespread amphibian in Europe (Sillero et al., 2014) and is characterized by high adaptability to different ecological conditions. Being often abundant, it is an important component of many ecological communities (Lodé, 1996; Luiselli, Anibaldi, & Capula, 1995) and has been used as a model organism for ecological, evolutionary and genetic studies (e.g., Hitchings & Beebee, 1997; Johansson, Primmer, & Merilä, 2006; Loman, 2004; Shu, Laurila, Suter, & Räsänen, 2016). Common species are widely used in empirical studies reporting SGDCs, due to practical sampling reasons (Laroche et al., 2015), and some studies suggested that SGDCs can be stronger for common species (Gugerli et al., 2008; Taberlet et al., 2012; Vellend, 2005). In Italy, the common frog is regularly present throughout the Alps and in the Northern Apennines; in the study region, it is widespread and abundant, ranging from valley bottoms up to the vegetation limit (approximate elevation range: 200–2,600 m a.s.l; Caldonazzi et al., 2002).
2.3 Richness and composition of amphibian communities
Data on species richness (SR) and composition of amphibian communities for 26 wetland areas (study sites) were derived from the amphibian monitoring programme performed by the regional environmental agency and from accurate monitoring surveys performed by the authors. For each site, an area of approximately 1 km2 was monitored. The selected sites cover the whole study region and different ecological environments (elevation range: 401–2,083 m a.s.l.; see Figure 2 and Supporting Information Table S2). Details on monitoring scheme and methods for each site are reported in Supporting Information Table S1. Overall, each site received at least four (up to twelve) surveys per year, for at least three years. We used the first-order jackknife estimator (Colwell & Coddington, 1994), as implemented in the “vegan” package in R to assess whether surveys provided sufficiently reliable community composition data in each site. The observed species richness was 100% of the estimated species richness for all sites, indicating reliability of presence/absence data.
2.4 Genetic diversity data (focal species: common frog)
Genetic diversity in common frog populations was investigated from the 26 study sites using 12 microsatellite markers. In 2009–2012, 1 km2 area was screened in each of the selected sites for common frog spawn during the breeding season. Sampling sites matched the areas of amphibian community monitoring. We collected one fertilized egg from each clutch to avoid full-sibs, as each female lays only one clutch per year (Schmeller & Merilä, 2007; see Marchesini et al., 2017, for more details on sampling). Overall, we collected 700 samples (minimum: 15 samples per site).
Total genomic DNA was extracted using the Qiagen DNeasy 96-Well Plate Kit (QIAGEN Inc., Hilden, Germany), following the manufacturer's protocol. Twenty-one tetranucleotide microsatellite markers originally developed for the common frog (Matsuba & Merilä, 2009) were initially tested on a subset of samples, and the 13 microsatellites that successfully amplified were selected for subsequent genotyping (Supporting Information Table S3a in Supporting Information). The selected loci were amplified in four multiplex PCRs under the conditions described in Supporting Information Table S3b. Contamination throughout the laboratory workflow was checked by means of DNA extraction blanks and PCR negative controls; PCR repeatability was confirmed by re-amplification of samples with known genotypes. PCR products were run on ABI Prism 310 Genetic Analyzer (Applied Biosystems), and two reference samples were included in each run, in order to check for errors due to different electrophoretic conditions. Amplified fragment lengths were scored using genemapper 3.7 software (Applied Biosystems).
Each microsatellite locus was tested for the presence of null alleles, allele dropout and scoring errors using microchecker (Van Oosterhout, Hutchinson, Wills, & Shipley, 2004) and freena (Chapuis & Estoup, 2006). Tests of departure from Hardy–Weinberg equilibrium were performed for each locus in every population with Arlequin 3.5 (Excoffier & Lischer, 2010), using 10,000 steps of dememorization followed by 100,000 Markov chain steps. Genotypic disequilibrium for each pair of loci was checked using genepop 4.1.4 (Rousset, 2008; Markov chain parameters: 10,000 dememorization steps, 100 batches, 10,000 iterations per batch). Significance levels of the tests were adjusted for multiple comparison using false discovery rate (FDR; Benjamini & Hochberg, 1995), as implemented in the p.adjust R function (R Development Core Team, 2016). We chose two standard measures of genetic diversity: allelic richness (AR) and mean expected heterozygosity (He). These measures can capture different processes and/or reflect different properties of the study system (e.g., sample size, mutation rate); thus, they are often jointly considered for assessing SGDC (Lamy et al., 2017; Vellend & Geber, 2005). Allelic richness was estimated using rarefaction (El Mousadik & Petit, 1996) as implemented in fstat 2.9.3.2 (Goudet, 2001), based on minimum sample size of 15 individuals. Mean expected heterozygosity was computed using the unbiased method implemented in genalex 6.5 (Peakall & Smouse, 2006, 2012).
2.5 Species–genetic diversity correlation and relationships between genetic diversity and the occurrence of each amphibian species
To investigate whether genetic diversity (AR and He) in the common frog was correlated with amphibian species richness across the 26 study sites (SGDC), we used the Pearson product-moment correlation. Subsequently, we used generalized least squares (GLS) to assess the robustness of SGDC correlations. GLS are regression models that successfully incorporate spatial structure in the error term (correlation function depending on the geographical distance among sites) and are suitable to analyse spatially explicit data, controlling for potential issues of spatial autocorrelation (Beale, Lennon, Yearsley, Brewer, & Elston, 2010). A previous phylogeographic study (using the mitochondrial COI gene) revealed a complex scenario, with different evolutionary lineages of common frog colonizing the study region after glaciations (Marchesini et al., 2017). Past evolutionary processes can strongly influence present-day genetic diversity (Ficetola, Garner, & De Bernardi, 2007; Petit et al., 2003; Roberts & Hamann, 2015); thus, we repeated SGDC including the number of mitochondrial lineages as covariate, under the assumption that admixture among lineages can increase genetic diversity (see Supporting Information Appendix S1). COI data for the selected populations were derived from Marchesini et al. (2017). Analyses with different proxies of historical factors (frequency of the Alp1 mitochondrial lineage, included as linear or quadratic term; Supporting Information Appendix S1) yielded identical results.
Finally, to evaluate the role of each amphibian species in SGDC, we used GLS to assess the relationships between genetic diversity in the focal species and the occurrence of each amphibian species in the 26 sites.
2.6 Understanding the drivers of SGDC: multivariate relationships between species richness, genetic diversity and ecological factors
In order to shed light on the mechanisms underpinning SGDC, we used structural equation modelling (SEM) to disentangle the multivariate relationships between species richness, genetic diversity and ecological factors (Grace, 2006; Lamy et al., 2017). The model assumed that the dependent variables (species richness and the two measures of genetic diversity, i.e., He and AR) can be determined by the different site factors, also considering the co-variance between the three dependent variables. Eight variables representing the environmental features of sites were considered as potential independent variables: mean annual temperature (proxy for energy availability), annual precipitation, four land cover classes (anthropized areas, i.e., urban + agricultural areas; coniferous forests; broad-leaved and mixed forest; water areas), slope and geological substrate (crystalline vs noncrystalline rocks). All the environmental layers were rescaled at 30 arc-seconds resolution (about 700 x 900 m in the study area); details on variable choice, processing and extraction are provided in Supporting Information Appendix S2. Pearson's correlation and variation inflation factors (VIF) suggested that collinearity was not a major issue for our data (|r| < 0.7 for all pairwise correlations; VIF always < 10; Dormann et al., 2013). We built SEM using “lavaan” package in R 3.4 (Rosseel, 2012), considering all the potential combinations of independent variables, and retained the SEM with lower Akaike's information criterion (AIC). Before performing SEM, environmental variables were scaled (mean = zero, variance = 1), while species richness was square-root-transformed to improve normality. Connectivity is an additional factor potentially determining genetic diversity, and the density of the road network is a major determinant of connectivity for amphibians (Holderegger & Di Giulio, 2010; see also Supporting Information Appendix S2). However, road density was strongly related to the cover of anthropized areas (r = 0.81, p < 0.001), hampering the inclusion of these variables into the same model. All results remained identical if we included road density as independent variable instead of anthropized areas.
2.7 Understanding the drivers of SGDC: ecological similarity/dissimilarity vs interspecific interactions hypothesis
We tested the following hypotheses: (1) ecological similarity/dissimilarity and (2) interspecific interactions, for explaining the recorded SGDC pattern.
The ecological similarity/dissimilarity hypothesis predicts positive (negative) SDGC if the different species have the same (opposite) responses to environmental gradients. In order to assess the species’ responses to environmental gradients, we built habitat suitability models (HSMs) for each species using maxent (version 3.3.3; Phillips, Anderson, & Schapire, 2006; Elith et al., 2011). Models were built for the whole Trentino region, considering the eight environmental variables used in SEM analysis. Species distribution data were obtained from a public WebGIS database implementing amphibian distribution records for the whole Trentino region (hereafter: regional data set), including a total of 2,534 individual observations (see Supporting Information Appendix S2); multiple presences on the same grid cell were removed. maxent is based on the maximum entropy approach and estimates environmental suitability for a species based on occurrence data and environmental variables. This method has been found to yield robust predictions, often outperforming alternative approaches (Elith et al., 2006; Hernandez, Graham, Master, & Albert, 2006; Hernandez et al., 2008). Models were built using a 10-fold cross-validation. For each species, data were split in ten sets; we built models using 90% of data (calibration data) and tested predictive performance using the remaining 10% of the data (test data). This procedure was repeated 10 times, each time using a different set of test data (Nogués-Bravo, 2009). All other settings were left as default. Model performance was evaluated using the area under the curve (AUC) (Phillips & Dudík, 2008); models with AUC > 0.75 are considered “fair” predictors of observed data (Elith et al., 2006; Fielding & Bell, 1997; Landis & Koch, 1977); habitat suitability maps were generated using a logistic link function, to yield a suitability value between 0 and 1 (Phillips & Dudík, 2008). To assess whether species respond similarly to ecological gradients, we compared maxent response curves. Furthermore, we performed pairwise correlation tests between the habitat suitability map of the focal species and those of other amphibians. Significance of correlations was tested using the modified t test developed by Dutilleul (Dutilleul, Clifford, Richardson, & Hemon, 1993) to control for potential effects of spatial autocorrelation (R package: SpatialPack; Osorio, Vallejos, & Cuevas, 2014).
The interspecific interactions hypothesis predicts negative (positive) SDGC if predation/competition (facilitation) occurs between focal and nonfocal species. To test this hypothesis, we (a) used niche overlap analysis, (b) compared life-history traits of species and (c) reviewed the literature on interspecific interactions. In amphibian communities, interspecific interactions generally result in competition and predation, while facilitation is rarely reported (Lanza, Andreone, Bologna, Corti, & Razzetti, 2007; Wells, 2007), therefore was not considered in our analyses.
Niche theory predicts that the potential competition between species is related to their degree of niche overlap (Begon, Harper, & Townsend, 1996; Hutchinson, 1957; MacArthur & Levins, 1967): two species with highly similar niches can compete more strongly. We focused on realized Grinnellian niche (i.e., considering noninteractive, nonconsumable scenopoetic variables), which can be measured on the basis of broad-scale environmental features (Soberón & Nakamura, 2009). If the interspecific interactions hypothesis holds, we expect that species with higher niche overlap with the common frog should have a negative relationship with its genetic diversity, while species with lower niche overlap should have no relationships. We used PCA-env (Broennimann et al., 2012) to measure niche overlap between the common frog and all the other amphibians. PCA-env performs a PCA translating the multivariate environmental space available for the species into a two-dimensional space and then uses a kernel density function to compute the density of occurrences in the multivariate space, in order to take into account potential bias caused by unequal sampling effort (Broennimann et al., 2012). Niche overlap was then computed by means of the Schoener's D metric (Warren, Glor, & Turelli, 2008). Schoener's D ranges between 0 (lack of overlap) and 1 (complete overlap) and is particularly suitable to compute overlaps in Grinnellian niches (Rödder & Engler, 2011). We then performed pairwise tests of niche similarity between the common frog and the other amphibians. We considered the same environmental variables and species occurrence data used for HSMs. Niche similarity test evaluates whether the niche occupied by one species is more similar to the niche of the other species than expected by chance, while taking into account background environmental heterogeneity, that is, the differences in available habitat between two species (Broennimann et al., 2012; Warren et al., 2008). Niche similarity is tested by comparing the observed niche overlap (Schoener's D) to the expected distribution of overlaps obtained by randomizing the occurrences of one species across its range of occupancy, while keeping constant the occurrences distribution of the other species. This approach provides more robust estimates of niche differences, compared to species distribution models (Broennimann et al., 2012). The significance of similarity tests was assessed with 1000 replications. Rejection of the null hypothesis indicates that the niches of the considered species are more similar than expected by chance. Niche overlap and similarity analyses were performed using the “ecospat” package (Di Cola et al., 2017) in r 3.1.3 (R Core Team, 2016). The aim of these analysis was not detecting actual competition (for which experimental studies are needed), but to assess the relative competition potential of species. Moreover, niche similarity considered broad-scale bioclimatic variables, mainly related to terrestrial habitats, but interspecific interactions (competition and predation) in amphibians often occur at finer scale during the aquatic phase (breeding activity and larval stage; Wells, 2007). To evaluate actual interspecific interactions at the larval stage, we reviewed the literature to obtain information on the ecology of amphibian tadpoles and aquatic stages and searched the Web of Science (August 6, 2017) using the key words “Rana temporaria” and “interspecific” and “competition.”
3 RESULTS
3.1 Genetic diversity of common frog and amphibian species richness
A total of 700 samples from the 26 selected sites were successfully genotyped at the 13 selected loci. MicroChecker excluded the presence of allelic dropout or scoring errors. FreeNA detected evidence for null alleles at locus BFG072 in most populations. We therefore excluded BFG072 from further analyses. Neither loci nor populations showed systematic deviations from HWE, and only 10 of 312 combinations were significant after adjustment using false discovery rate (FDR). No evidence of genotypic disequilibrium was observed between the selected loci (only 1/766 significant value after FDR correction). Despite all loci were claimed to be tetranucleotides, BFG131 showed an unexpected dinucleotide allelic pattern. After sequencing by means of nonmarked primers, we concluded that the recorded allelic pattern was due to a deletion in the flanking region, and not to mutations in the repeat motif (which proved to be a tetranucleotide microsatellite). Due to this deletion, allele size was not proportional to number of repeats. However, since the computation of genetic variability measures does not rely on mutation models, we retained this locus (see Supporting Information Appendix S3 for a detailed discussion). All the 12 retained loci were polymorphic, with a total number of 177 alleles (average across loci = 14.75).
We detected heterogeneous levels of genetic variability among populations. Allelic richness varied from 4.83 (MBa) to 6.68 (MRe), with an average value of 5.91; expected heterozygosity varied from 0.50 (MBa) to 0.70 (MRe), with an average value of 0.61 (Supporting Information Table S4). AR and He were highly correlated (Pearson's r = 0.854; df = 24, p < 0.001).
Seven amphibian species were recorded in the study sites (reported according to their frequency of occurrence O): common frog (Rana temporaria; focal species for genetic diversity), common toad (Bufo bufo; occurrence O = 0.96), Alpine newt (Ichthyosaura alpestris, O = 0.58), fire salamander (Salamandra salamandra, O = 0.35), pool frog (Pelophylax synkl. esculentus, O = 0.23), yellow-bellied toad (Bombina variegata) and agile frog (Rana dalmatina, both O = 0.08). The richness of amphibian communities varied from 1 to 7 (Supporting Information Table S4). Amphibians present in the region, but undetected in the study sites (5), are rare and spatially localized species (see Supporting Information Appendix S4).
3.2 Species–genetic diversity correlation
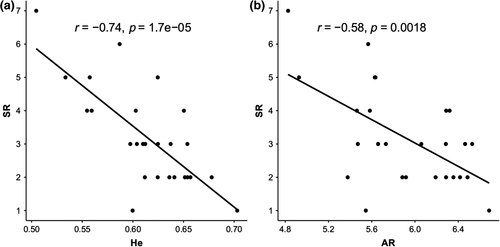
We found a strong and significant negative correlation between species richness of amphibian communities and neutral genetic diversity of common frog populations, for both expected heterozygosity (r = −0.738; df = 24, p < 0.001) and allelic richness (r = −0.583; df = 24, p = 0.002) (Figure 3). All correlations remained strongly significant also taking into account spatial autocorrelation and including the N of mitochondrial lineages as covariate (GLS models; Table 1 ).
| Dependent variable | Independent variable | B | t | p | R 2 |
|---|---|---|---|---|---|
| H e | Community richness | −0.019 | −4.12 | <0.001 | 0.58 |
| N of mitochondrial lineages | −0.007 | 0.726 | 0.475 | ||
| AR | Community richness | −0.189 | −2.99 | 0.007 | 0.35 |
| N of mitochondrial lineages | 0.216 | 0.346 | 0.538 |
3.3 Structural equation modelling
Structural equation models (SEMs) showed that neutral genetic diversity and species richness were determined by the interplay of multiple processes. The SEM with lowest AIC value included three environmental variables: mean annual temperature, water areas and slope (Figure 4). Both measures of genetic diversity were strongly related to environmental variables, being highest in sites with low temperature and in relatively steep areas. Furthermore, heterozygosity was highest in sites characterized by abundance of water areas. The effect of environmental features on community richness was the opposite, as the richest communities were found in sites with warm temperature and low abundance of water areas. When taking into account the effect of environmental features, the relationships between genetic diversity measures and species richness were much weaker, and the relationship between species richness and allelic richness became nonsignificant.

3.4 Relationships between common frog genetic diversity and the occurrence of other amphibians
Relationships between common frog genetic diversity and the occurrence of the six amphibian species were mostly negative, but only some of them were significant. Allelic richness (AR) was particularly low in sites where the agile frog was recorded, while heterozygosity (He) was particularly low in sites with the yellow-bellied toad (Table 2). Other species exhibiting significant negative relationships with common frog genetic diversity were: fire salamander (He, AR), pool frog (AR) and Alpine newt (He; but only in 1/4 GLS models). Results including proxies for historical factors (e.g., N of mitochondrial lineages) yielded similar results (Supporting Information Table S5).
| Species | Dependent variable | B | t 24 | p | R 2 |
|---|---|---|---|---|---|
| Common toad | H e | −0.021 | −0.75 | 0.460 | 0.27 |
| AR | −0.074 | −0.22 | 0.831 | 0.10 | |
| Alpine newt | H e | −0.042 | −2.88 | 0.008 | 0.43 |
| AR | −0.211 | −1.09 | 0.289 | 0.14 | |
| Fire salamander | H e | −0.034 | −2.15 | 0.042 | 0.36 |
| AR | −0.479 | −2.63 | 0.014 | 0.29 | |
| Pool frog | H e | −0.033 | −1.74 | 0.094 | 0.34 |
| AR | −0.526 | −2.41 | 0.024 | 0.27 | |
| Yellow-bellied toad | H e | −0.066 | −4.29 | 0.0004 | 0.57 |
| AR | −0.468 | −1.66 | 0.101 | 0.19 | |
| Agile frog | H e | −0.058 | −2.18 | 0.039 | 0.38 |
| AR | −0.853 | −2.71 | 0.012 | 0.31 |
3.5 Habitat suitability modelling and response to ecological variables
Habitat suitability models showed fair to excellent performance (test AUC ranging from 0.75 to 0.92; see Supporting Information Table S6) for all species. Common toad and common frog yielded the lowest AUC values, probably because they are the species with the broadest geographical range (Phillips et al., 2006).
For the common frog, temperature was the variable most important for explaining species distribution (Supporting Information Table S7). Temperature was among the most important variables also for all the other amphibians, but responses to temperature showed opposite patterns among species, probably reflecting different temperature optima (Figures 5; Supporting Information S2). The common frog was associated with the coldest temperatures, with highest suitability in areas with mean annual temperature <8°C. Conversely, for the other amphibians, suitability increased with temperature, peaking in areas characterized by mean temperature above 8–10°C (Figure 5).
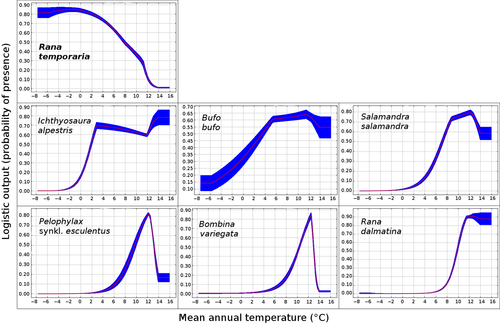
The correlation tests between the habitat suitability map of common frog and those of other amphibian species yielded heterogeneous outcomes (Table 3). The habitat suitability map of common frog was positively related to the maps of alpine newt and common toad, while it was negatively related to the one of all the other amphibians. For a graphical comparison of the habitat suitability maps for the different species, see Supporting Information Figure S1.
| Species | Niche overlap (D) | Niche similarity test (p value) | Correlation of habitat suitability maps | |||
|---|---|---|---|---|---|---|
| CF vs Sp.2 | Sp.2 vs CF | CF vs Sp.2 | p value | df | ||
| Common toad | 0.354 | 0.256 | 0.249 | 0.212 | <0.001 | 3969.1 |
| Alpine newt | 0.533 | 0.114 | 0.113 | 0.224 | <0.001 | 1327.8 |
| Fire salamandera | 0.288 | 0.355 | 0.314 | −0.350 | <0.001 | 4624.9 |
| Pool froga | 0.148 | 0.179 | 0.172 | −0.245 | <0.001 | 2221.6 |
| Yellow-bellied toada | 0.134 | 0.279 | 0.322 | −0.284 | <0.001 | 892.0 |
| Agile froga | 0.108 | 0.285 | 0.261 | −0.188 | <0.001 | 1541.8 |
- a These species exhibited significant relationships with genetic diversity in the common frog (see Table 2).
3.6 Niche overlap analysis (focal vs nonfocal species)
The first two PCA axes generated in PCA-env explained 31.4% and 20.8% of the original environmental variation, respectively (Supporting Information Figure S3b). The most important explanatory variables for axis 1 were mean annual temperature and geological substrate, followed by annual precipitation and slope; the most important explanatory variables for axis 2 were precipitation, anthropized areas, coniferous forests and slope. Common frog showed a broad niche, with species occurrences scattered in an area covering the 50% of the available (background) environment, and it was different from the niches of the other species (Supporting Information Figure S3). Two other species (alpine newt and common toad) showed very broad niches, while pool frog, agile frog and yellow-bellied toad showed narrow niches. Niche overlap between the common frog and the other six amphibians ranged from 0.108 to 0.532 (Table 3; Supporting Information Figure S3). The highest overlap was observed with Alpine newt and common toad, while agile frog, pool frog and yellow-bellied toad showed the lower niche overlaps with the focal species. The species showing highest niche overlap with common frog also show positive and significant correlations of habitat suitability maps, while negative correlations between habitat suitability maps occurred in the species showing the lowest niche overlap with common frog (Table 3).
Similarity tests suggested that the realized niche of the common frog is not more similar to the niches of the other six amphibian species than expected by chance (all p > 0.05; Table 3 and Supporting Information Figure S3), indicating limited niche overlap between the common frog and all the other amphibians in the study area.
3.7 Literature review: interspecific interactions (common frog vs. other amphibians)
According to literature, the aquatic period of the common frog widely overlaps with that of most amphibian species, and some interspecific interactions are known (Supporting Information Table S8). The Alpine newt regularly feeds on common frog eggs (Supporting Information Table S8). The Web of Science search on interspecific competition returned 27 papers. Interspecific competition has been reported in experimental and field studies between common frog and common toad larvae, and between common frog and agile frog males. However, in nearly all cases, the common frog has been described as a superior competitor, both at the larval and adult stage (Supporting Information Table S8).
4 DISCUSSION
4.1 Negative species–genetic diversity correlation in Alpine amphibians
Our data revealed a strong and significant negative correlation between community richness and the neutral genetic diversity of common frog populations. The correlation remained significant also considering the past evolutionary history of populations, a factor which is often neglected in SGDCs studies even though it can heavily shape genetic diversity and may potentially affect SGDC (e.g., Taberlet et al., 2012). The features of our study system mirror those of most empirical SGDC studies carried out so far (Laroche et al., 2015; Vellend, 2003; Vellend & Geber, 2005): (1) Species diversity was measured as species richness at a single taxonomic level; (2) genetic diversity was measured at neutral loci (microsatellites) within one focal species, and (3) choosing a widespread, abundant organism. Moreover, our sampling units (wetlands) can be considered as discrete habitat patches (with regard to the focal organisms), and SGDCs are predicted to be positive and stronger in studies focusing on discrete sampling units rather than in continuous habitats, given the greater potential for strong drift and limited dispersal (Vellend et al., 2014).
However, our results did not match the prediction of positive SGDC, as we found a strong, negative correlation. Past meta-analyses claimed a prevalence of positive SGDCs in empirical studies (Kahilainen et al., 2014; Vellend et al., 2014), but in recent years, numerous examples of negative and nonsignificant SGDCs are emerging (Lamy et al., 2017). Despite the important implications of negative SGDCs, their ecological drivers are rarely investigated analytically (Kahilainen et al., 2014). Given the heterogeneous pattern found in SGDC studies, it is essential to go beyond the mere description of SGDC values and to unravel the underlying processes. In our study, the combination of SGDC analysis with structural equation modelling (SEM) and the assessment of ecological preferences and niche overlaps allowed us to tease apart the role of interspecific interactions and ecological similarity/dissimilarity among species.
4.2 The drivers of negative SGDC: opposite effects of environmental factors on the two levels of diversity
If site characteristics influence species and genetic diversity in a parallel manner, a positive SGDC is expected (Vellend & Geber, 2005). Conversely, in our study, SEM highlighted an opposite influence of site factors on the two levels of diversity (Figure 4). This outcome suggests that the focal species (common frog) shows different ecological responses, compared to the other species of the community (Lamy et al., 2017), although not directly excluding different potential explanations for the negative SGDC (e.g., interspecific interactions).
Interestingly, the three environmental variables most important for common frog distribution (mean annual temperature, water areas and slope; Supporting Information Table S7) were also included in the SEM with best support, indicating that neutral genetic diversity was highest in sites with low temperature and in landscapes with many wetlands and high slope (Figure 4). Neutral genetic diversity reflects demographic processes; thus, the variation in genetic diversity is likely related to differences in demographic features of populations, such as effective population size and connectivity. Population size is often positively related to habitat suitability (Weber, Stevens, Diniz-Filho, & Grelle, 2017; Lunghi et al., 2018), and this may explain why the same variables determine both habitat suitability and genetic diversity. However, total species richness showed opposite response to these variables (Fig. 4), thus determining a negative SGDC.
4.3 The drivers of negative SGDC: interspecific interactions vs. ecological dissimilarity
Most amphibians exhibited a limited niche overlap with the focal species (Table 3), and the ones with the highest overlap, therefore the highest competition potential, exhibited no significant relationships in most of GLS models (Table 2). On the other hand, species with the lowest overlap (i.e., yellow-bellied toad, agile frog and pool frog) exhibited consistent and strong negative relationships with genetic diversity. Snapshot spatial patterns of niche overlap do not provide a direct measure of competition, and species with strong interspecific interactions can even be allopatric, for example, in cases of competitive exclusion. On the other hand, competition can influence genetic diversity if it affects population size, and this requires some overlap in space (Lamy et al., 2017); therefore, low niche overlap helps to identify species pairs for which competition has a limited potential to influence genetic diversity.
Niche overlap allows assessing whether species can interact in space; still, direct measures of competition are needed to assess the actual impact of interspecific interactions. Experimental and field studies did not detect negative interactions between the common frog and species with low niche overlap (Supporting Information Table S8). The common frog is perhaps the most widespread amphibian in Europe (Sillero et al., 2014) and is among the amphibians for which more studies on interspecific interactions exist. The available literature shows that, when competition was observed, common frog tadpoles and adults often are superior competitors (e.g., Gazzola & Van Buskirk, 2015; Vági & Hettyey, 2016), even though competition strength might be stronger at the edge of species distribution. The strongest known interactions between nonfocal and focal species involve alpine newts and common frog tadpoles. Newts are generalist predators, and frog eggs and tadpoles can be food sources for the Alpine newt (e.g., Covaciu-Marcov et al., 2010; Denoël & Demars, 2008). In principle, it is possible that interspecific interactions between newts and common frogs could contribute to the negative SGDC. However, the survival of frog tadpoles shows strong negative density dependence; thus, mortality at early life-history stages is expected to have limited impact on the overall population dynamics and genetic diversity of frogs (Vonesh & De la Cruz, 2002). It is also worth noting that interspecific interactions in amphibians mainly occur at the larval stage, that is, at the micro-habitat scale (e.g., within pond; Wells, 2007), while genetic analyses were performed at a broader scale (1 km2, i.e., wetland, network of ponds). This is the scale at which demographic and microevolutionary processes generally take place in amphibians (Marsh & Trenham, 2001) and is also the scale of most SGDC studies (Lamy et al., 2017).
Since we did not detect effects from potential competition, and effects of actual competition and amphibian predation were generally weak (Supporting Information Table S8), the interspecific interactions hypothesis cannot be considered the main explanation for the recorded negative SGDC. On the other hand, in support of the ecological dissimilarity hypothesis, some of the amphibians exhibiting the strongest negative relationship with genetic diversity of the focal species (salamander, pool frog, agile frog and yellow-bellied toad) showed a very different response to ecological gradients, compared to the common frog (Table 3). This might be viewed as evidence for a key role of ecological differences between focal and nonfocal species in explaining the negative SGDC. Nevertheless, it must be noted that the pool frog and agile frog are rare in the 26 sites (Supporting Information Table S4 and Appendix S4): caution is required for the interpretation of results for the two above-mentioned species. Relationships with rare species are a general issue in SGDC studies, as in ecological communities the majority of species within a higher taxon are rare (Hubbell, 2001).
4.4 Different responses of amphibians to environmental factors
Habitat suitability models highlighted temperature as a major driver of amphibian distribution, with the common frog being more frequently associated with the coldest climates. Within its distributional range, the common frog can be considered a generalist species that exploits wide range of habitats, showing local adaptation and high phenotypic plasticity (Johansson, Veldhoen, Lind, & Helbing, 2013; Muir, Biek, Thomas, & Mable, 2014; Richter-Boix, Teplitsky, Rogell, & Laurila, 2010). Nevertheless, this frog is sensitive to warm temperatures, particularly when associated with low humidity (Lanza, Nistri, & Vanni, 2009). In the study region, the species is widespread but more frequent at high elevations (1,500–2,000 m a.s.l.), while it is less abundant in valley bottoms (Caldonazzi et al., 2002). Conversely, community richness was highest in low-altitude sites with warm temperatures (Figure 4, Supporting Information Tables S2 and S4). At low elevations, specialists of warm microclimates find their ecological optimum, while conditions can be suboptimal for other species (including the common frog). Suboptimal ecological conditions may in turn determine smaller population size and density and consequently a loss of genetic diversity in the focal species in species-rich sites, giving rise to a negative SGDC (see Lamy et al., 2017).
Even though we cannot rule out additional contributing factors, the ecological differences between the common frog and most other amphibians suggest that local environmental features are major drivers of the negative SGDC (ecological dissimilarity hypothesis). In an Alpine region characterized by a wide diversity of climatic regimes and habitats, species sorting by abiotic features preventing the establishment or persistence of certain species plays a major role in community assembly (environmental filtering; Kraft et al., 2015; Weiher, Clarke, & Keddy, 1998). This applies particularly at a large spatial scale, where key climatic gradients such as temperature generally act, while biotic interactions may have a stronger effect at the micro-habitat scale (Soberón & Nakamura, 2009). In systems where both genetic diversity and community richness are shaped by environmental gradients, SGDCs are not necessarily positive (Xu et al., 2016), and the sign and strength of SGDCs depend on the particular ecological requirements of the focal species, compared to the other species in the community (Lamy et al., 2017). Understanding the effects of these gradients on both the species and genetic level of biodiversity can allow predicting the sign of SGDCs; however, caution is needed. These effects may vary depending on the considered functional level, are often species-specific and may be influenced by other processes in complex ways (e.g., Wei & Jiang, 2012). In this study, we measured genetic diversity using neutral markers, as the majority of SGDC studies so far. Nevertheless, patterns may be different for markers under selection. Genomics technologies are providing unprecedented insights into adaptive variation (Li et al., 2017) and will offer the opportunity for investigating the effects of adaptive processes on SGDCs in the near future.
5 CONCLUSION
Theoretical ecology is increasingly recognizing the links between community ecology and population genetics (e.g., see Hendry, 2016; Vellend, 2016), and empirical SGDC studies are needed to verify hypotheses and predictions in natural communities. In conservation practice, SGDCs might be used to infer diversity from one level to the other, for example, using species richness as a surrogate of genetic diversity, since the latter may be more difficult to measure (Kahilainen et al., 2014; Taberlet et al., 2012; Vellend et al., 2014). Similarly, genetic diversity of common species has been proposed to predict species diversity hotspots in taxonomic groups that are difficult to monitor (Kahilainen et al., 2014). In principle, this could be a promising approach for species rich communities, where most species are locally rare (Hubbell, 2001), and/or for elusive animals, such as tropical amphibians (Heyer, Donnelly, McDiarmid, Hayek, & Foster, 1994). However, SGDC patterns can be extremely complex, given the multiple processes that determine them (Lamy et al., 2017). In single-species SGDC studies, the choice of the focal species may determine the sign and strength of the correlation, since different species may differ in ecological preferences and interspecific interactions. The emerging field of community genetics would benefit from multispecies approaches, which are easier to implement with the increasing availability of cost-effective high-throughput sequencing technologies (Lamy et al., 2017).
Our study showed that SGDCs can deviate from a priori expectations even in communities with limited species richness and was performed at the regional scale, that is, the lowest level of conservation planning. Our results thus warn against the indiscriminate use of species richness as unique biodiversity proxy in spatial prioritization (see also Taberlet et al., 2012). We rarely can derive one level of diversity from the other one without a proper knowledge of the context-dependent processes determining genetic and species diversity, and multiple potential factors must be taken into account if we want to understand the links between the different biodiversity levels. Genetic diversity assessment should be explicitly and more extensively implemented in conservation strategies, possibly also including common species, considering their crucial role in ecosystem functioning and stability (Gaston, 2011).
ACKNOWLEDGEMENTS
We thank L. Cornetti, B. Crestanello and M. Girardi for their help during field sampling and lab activities. M. Neteler and D. Rocchini helped for the generation of maps and environmental data. We are grateful to P. Pedrini, M. Menegon, M. Caldonazzi, S. Zanghellini and L. Sottovia for providing amphibian distribution data. This research was partially funded by Autonomous Province of Trento (Italy) as part of the ACE-SAP project (University and Scientific Research Service regulation number 23, June 12, 2008) and by FIRST (FEM International Research School of Trentino).
DATA ACCESSIBILITY
Microsatellite data, environmental data and regional amphibian distribution data set available from the Dryad Digital Repository (https://doi.org/10.5061/dryad.689110r).
AUTHOR CONTRIBUTIONS
A.M. and C.V. conceived the project with contribution from G.F.F. about specific ecological issues. A.M. performed sampling and laboratory work. A.M. and G.F.F. analysed the data with input from C.V. A.M., G.F.F. and C.V. wrote the manuscript. All the authors revised the final version of the manuscript.




