Haemoplasmas in wild rodents: Routes of transmission and infection dynamics
Abstract
The way that some parasites and pathogens persist in the hostile environment of their host for long periods remains to be resolved. Here, longitudinal field surveys were combined with laboratory experiments to investigate the routes of transmission and infection dynamics of such a pathogen—a wild rodent haemotropic bacterium, specifically a Mycoplasma haemomuris-like bacterium. Fleaborne transmission, direct rodent-to-rodent transmission and vertical transmission from fleas or rodents to their offspring were experimentally quantified, and indications were found that the main route of bacterial transmission is direct, although its rate of successful transmission is low (~20%). The bacterium's temporal dynamics was then compared in the field to that observed under a controlled infection experiment in field-infected and laboratory-infected rodents, and indications were found, under all conditions, that the bacterium reached its peak infection level after 25–45 days and then decreased to low bacterial loads, which persist for the rodent's lifetime. These findings suggest that the bacterium relies on persistency with low bacterial loads for long-term coexistence with its rodent host, having both conceptual and applied implications.
1 INTRODUCTION
A host's behavioural, physiological and immunological responses evolve to cope with the selection pressure imposed by parasites/pathogens (Schmid-Hempel, 2011). As a result, most parasites/pathogens living in the host (i.e., endoparasites) do not persist for the host's lifetime. Correspondingly, the way that other parasites and pathogens persist in the hostile environment of their host for long periods remains to be resolved.
Blood constitutes an extreme example of a hostile environment for the parasites/pathogens living therein, as it is unstable, with a high red blood cell turnover rate and a great selective pressure imposed by diverse immune cells and mediators. This hostile nature led scientists to consider blood as a sterile environment that possesses living organisms only during disease (Brooks, Carroll, Butel, & Stephen, 2007; Hall & Lyman, 2006; Motoshima et al., 2012). However, recent evidence points to more permanent blood bacterial residents such as Bartonella and haemoplasma (haemotropic Mycoplasma) species, which may use red blood cells as their primary microhabitat (Cohen, Einav, & Hawlena, 2015; Cohen, Toh, Munro, Dong, & Hawlena, 2015; Gavish et al., 2014; Gutiérrez, Morick, Cohen, Hawlena, & Harrus, 2014; Gutiérrez, Nachum-Biala, & Harrus, 2015). Revealing the mechanisms of these bacteria's transmission and persistence will shed light on the evolutionary strategies underlying host–parasite/pathogen coexistence and will have an applied aspect considering that some of these bacteria are pathogenic to wild animals and humans (Atif, 2015; Breitschwerdt & Kordick, 2000; Eisen & Eisen, 2011; Hoelzle, Zeder, Felder, & Hoelzle, 2014; Liang, Nelson, & Fikrig, 2002; Messick, 2004; Ogden et al., 2015).
Haemoplasmas provide convenient models in which to explore transmission routes and infection dynamics under hostile conditions. They are small pathogens belonging to the class Mollicutes that reside on red blood cells (Hicks et al., 2014; Willi et al., 2007b). This group is remarkably diverse in terms of impact on the mammalian host and prevalence. Their pathogenicity can range, depending on the haemoplasma and mammalian host species, from acutely life-threatening haemolytic anaemia to chronic infection with no apparent clinical manifestation (Henry, 1979; Hoelzle, Adelt, Hoelzle, Heinritzi, & Wittenbrink, 2003; Strait, Hawkins, & Wilson, 2012; Tasker et al., 2009b). Haemoplasmas are also common in wild animals, infecting a range of mammalian hosts at various prevalence levels, ranging from 3% to 97% (Bajer et al., 2014; Boes et al., 2012; Iso et al., 2013; Mascarelli et al., 2015; Millan, Lopez-Roig, Delicado, Serra-Cobo, & Esperon, 2015; Santos et al., 2013; Sashida, Suzuki, Rokuhara, Nagai, & Harasawa, 2014; Sashida et al., 2013; Sharifiyazdi, Nazifi, Aski, & Shayegh, 2014; Volokhov, Hwang, Chizhikov, Danaceau, & Gottdenker, 2017; Willi et al., 2007c). However, to date, experimental studies and long-term surveys have all been conducted with only a few target haemoplasma species of veterinary importance (e.g., Mycoplasma parvum, M. suis, “Ca. M. haemominutum,” “Ca. M. turicensis,” M. haemofelis, M. haematoparvum, M. haemocanis and M. wenyonii) infecting pet and domestic animals (do Nascimento et al., 2014; Hoelzle, 2008; Sasaoka et al., 2015; Tasker et al., 2009b, 2010; Wengi et al., 2008; Willi et al., 2007b). Thus, the transmission and persistence mechanisms of naturally occurring haemoplasmas in wild animals not subjected to antibiotics or vaccinations that do not cause disease in their mammalian host remain to be explored.
Here, longitudinal field surveys and laboratory experiments were combined to investigate the routes of haemoplasma transmission and its infection dynamics in the blood of a wild rodent, Gerbillus andersoni. In the Negev Desert sand dunes (Israel), the haemoplasmas found in all the blood samples of this rodent species belong to a single cluster, which is closely related to (90%–95% similarity in the 16S gene), but distinguishable from, M. haemomuris (Kedem, Cohen, Messika, Einav, & Hawlena, 2014). Below, these will thus be termed M. haemomuris-like bacteria (MHLB). Regarding transmission routes, as lice have rarely been detected on wild G. andersoni rodents (two lice specimens were detected from thousands of rodents sampled over the past 17 years), whereas Synosternus cleopatrae fleas are common on these wild rodents (Hawlena, Abramsky, & Krasnov, 2006) and MHLB were detected in 33% of them (Cohen, Einav, et al., 2015), it seems most likely that MHLB are fleaborne. However, the high natural MHLB prevalence (60 ± 3% SE) in rodents’ blood samples and the evidence for positive associations between MHLB and the fleas’ reproductive success (Messika et al., 2017) led to the prediction that in concert with fleaborne transmission, other transmission routes may operate. Such transmission routes may include transovarial transmission from the parent to the offspring flea (Azad et al., 1992; Morick, Krasnov, Khokhlova, Gottlieb, & Harrus, 2011), transmission via blood regurgitations of the flea (Morick et al., 2013b; Rollend, Fish, & Childs, 2013) and transmission by flea faeces (Woods, Brewer, Hawley, Wisnewski, & Lappin, 2005). Transmission may also operate through direct rodent-to-rodent contact (see indirect evidence for “Ca. M. haemominutum” and “Ca. M. turicensis”; Dean, Helps, Jones, & Tasker, 2008; Lappin, Dingman, Levy, Hawley, & Riley, 2008; Museux et al., 2009; Willi et al., 2007a), through rodent faeces (Willi et al., 2007a; Woods et al., 2005) and through vertical transmission from female to offspring rodents (Almy, Ladd, Sponenberg, Crisman, & Messick, 2006; Fujihara et al., 2011; Harvey & Gaskin, 1977; Sasaoka et al., 2015). Regarding infection dynamics, our longitudinal field data suggest that MHLB have high persistency in G. andersoni; 90% of the MHLB-positive individuals were infected 4 months after the first sampling (Cohen, Einav, et al., 2015). However, due to the observational nature of that study, the possibility that these rodents had cleared the infection and were reinfected towards the second sampling period could not be rejected.
To test which of the above transmission routes are exploited by MHLB and to experimentally quantify any long-term persistency in G. andersoni, a series of transmission experiments were conducted, and the MHLB temporal dynamics in the field was compared to that observed under a controlled infection experiment in field-infected and laboratory-infected rodents.
2 MATERIALS AND METHODS
2.1 General study approach and methods
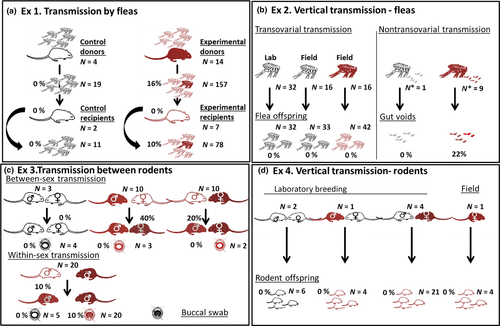
To reveal the transmission routes of MHLB, four laboratory experiments were designed. Experiment 1 tested for fleaborne transmission (Figure 1a), Experiment 2 tested for transovarial and nontransovarial vertical transmission from fleas to offspring (Figure 1b), Experiment 3 tested for direct rodent-to-rodent transmission (Figure 1c), and Experiment 4 tested for vertical transmission from parent rodent to offspring (Figure 1d). To quantify the infection dynamics of MHLB in the laboratory and to confirm that this dynamics fits the field patterns, in Experiment 5, the infection dynamics of the MHLB-positive rodents that were either captured-recaptured in the field, captured in the field and brought to the laboratory, or inoculated under laboratory conditions was followed through time.
All rodents used for the above five experiments were nonreproductive adult G. andersoni. The rodents that were brought from the field were polymerase chain reaction (PCR)-tested for MHLB (see below), and the PCR confirmed them to be Bartonella-negative; then, they were kept isolated and repeatedly cleaned from ectoparasites for at least 2 weeks (see details in Experiment 1 and in Supporting Information, SI 1). The other rodents used in the five experiments were offspring from our laboratory colony that were confirmed to be MHLB- and Bartonella-negative and were free of ectoparasites; thus, none of the rodents received any drug treatment.
In the laboratory, rodents were maintained on sand bedding, in an animal room with an air temperature of 25 ± 1°C and a photoperiod of 12D: 12L, and were provided daily with millet seeds ad libitum and alfalfa as a water source according to Hawlena, Bashary, Abramsky, and Krasnov (2007). Excluding the rodents used for Experiment 3 (which were kept in pairs; see below), all rodents were kept individually in disinfected 20 × 30 cm2 plastic cages with a 1-cm layer of autoclaved sand as substrate.
To infect MHLB-negative rodents for experiments 1, 3 and 5, rodents were subcutaneously inoculated with 150–300 μl blood from MHLB-positive G. andersoni, preserved in 20% DMSO (Sigma-Aldrich, Buchs, Switzerland) at −80°C. MHLB-positive blood contained MHLB loads ranging from 2 × 103 to 4 × 105 copies per inoculum. The bacteria could not be directly inoculated as haemoplasma species are currently uncultivable (Tasker, Helps, Day, Gruffydd-Jones, & Harbour, 2003).
To assess the probability of being infected by MHLB and the MHLB loads in the rodents’ blood collected during experiments 1, 3, 4 and 5 (designated as infection parameters), following Cohen, Einav, et al. (2015), 100–200 μl of blood was collected from the retro-orbital sinus of each individual by capillaries immersed in 0.15% EDTA and was stored in EDTA blood collection tubes at −20°C until further molecular analyses. To assess MHLB infection parameters in fleas collected during experiments 1 and 2, fleas were collected from the rodent body and their DNA was extracted. DNA was extracted from blood samples (experiments 1, 3, 4 and 5) and flea-regurgitated blood (Experiment 2) using a MoBio Bacteremia DNA Isolation Kit, following the manufacturer's instructions (Cohen, Einav, et al., 2015). DNA from fleas (experiments 1 and 2) and buccal swabs (Experiment 3) was extracted using a DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA, USA; Hawlena et al., 2013). DNA from faeces was extracted using the QIAamp DNA Stool Mini Kit (Qiagen). In each extraction session, a negative control was included, in which all of the reagents were added to phosphate-buffered saline (PBS) instead of to the blood, fleas, rodent faeces, buccal swabs or regurgitated blood.
The PCR was performed to test for the presence of MHLB by amplification of the 16S gene using the HM16S-1(F) and HM16S-2(R) primers, following Kedem et al. (2014). Sanger sequencing was performed on 20% of randomly chosen PCR-positive samples, and it confirmed that the tested bands of 762 base pair lengths indeed showed 95% similarity to M. haemomuris. Quantification of the MHLB copy numbers in positive samples was performed by a quantitative PCR (qPCR) (Applied Biosystems 7300, Waltham, Massachusetts, USA), using Qiagen HotStarTaq Master Mix (Precision FAST Blue 2× qPCR Master Mix with ROX) with 10 μM of the 16S rRNA gene (F) GGAGCGGTGGAATGTGTAG and 10 μM of the 16S rRNA gene (R) GGGGTATCTAATCCCATTTGC, 10 μM of probe (TYAAGAACACCAGAGGCGAAGGCG), 25 μM of MgCl2 and 5 μl of DNA in a total volume of 10 μl, following the reaction conditions described in Tasker et al. (2010). To estimate the absolute copy number and validate the repeatability, efficiency and sensitivity of the reactions, in each run, we added a 10-fold serial dilution (i.e., standard curve ranged from 101 to 107 copies per reaction) of previously sequenced plasmids containing the 16S rRNA gene from blood samples that were positive for MHLB. More details are provided in the Supporting Information (SI 2).
The trapping and handling protocol was approved by the Committee for the Ethical Care and Use of Animals in Experiments of Ben-Gurion University of the Negev (# IL-59-09-2015) and by the Israel Nature and Parks Authority (# 41428).
2.2 Experiment 1: Evaluation of transmission by fleas
Eighteen rodent donors were randomly assigned to either experimental (sample size N = 14) or control (N = 4) groups. The 14 experimental donors were composed of four MHLB-positive individuals that were brought from the field to the laboratory and 10 MHLB-negative individuals (six field-captured and four individuals from our breeding colony) that were inoculated by MHLB in the laboratory and became MHLB-positive 10–15 days postinoculation. The four control donors, who were born in the laboratory and were MHLB- and Bartonella-negative, were inoculated with PBS (Figure 1a).
Each rodent was infested by 100 MHLB-negative S. cleopatrae fleas. S. cleopatrae is the most dominant ectoparasite infesting G. andersoni in the Negev Desert sand dunes and has the highest prevalence of MHLB among all G. andersoni ectoparasites (Cohen, Einav, et al., 2015). These fleas were all Bartonella-negative and part of our breeding core, which is maintained as previously described (Krasnov, Khokhlova, Fielden, & Burdelova, 2001a, 2001b; Supporting Information, SI 3). During infestation, the sandy substrate was covered with a wire mesh to prevent the rodents from killing the fleas. This allowed the fleas to feed and reproduce on the rodent, regurgitate blood and lay their eggs in the sand with only minimal disturbances from the rodents (Morick et al., 2013a). Sterilized absorbent paper was added to minimize flea death by rodent urination.
Beginning from the first flea infestation, fleas were collected every 72 hr from the donor rodents and were placed on nine MHLB-negative laboratory-born rodents, designated as “recipient” rodents. In each infestation event, all recipients were infested by 50 fleas per rodent; the experimental recipients were infested by fleas removed from experimental donors, and the control recipients were infested by fleas removed from control donors (Figure 1a). Then, 40–60 newly emerged MHLB-negative fleas were placed on the donor rodents.
To estimate the MHLB infection parameters of the transmitted fleas (see general methods), during every infestation event, all the dead fleas were collected, in addition to five live fleas, from the body of each donor and recipient rodent and were stored in 70% ethanol at −20°C until further molecular analyses. It was then tested whether the MHLB organisms remained viable within the flea or were just remnants in the bloodmeal and would thereby be lost after complete digestion. This was done by randomly selecting one flea per rodent in each flea collection event and allowing it to fully digest its bloodmeal for 10 additional days at 25°C and 85% relative humidity before placing it into ethanol at −20°C until further molecular analyses for MHLB detection.
This design resulted in MHLB screening of DNA extracts from a total of 69 dead and 88 live fleas fed on experimental donor rodents, 40 dead and 38 live fleas fed on experimental recipients, and 16 live fleas that were fed on donors and were allowed to digest their bloodmeal for 10 days. We then compared the infection parameters of these fleas, and 30 fleas fed on control donors and recipients (sample size = 19 and 11, respectively).
The experiment lasted 65 days to allow sufficient time for fleas to defecate on recipient rodents and for MHLB multiplication and transmission, as well as to allow an overlap between flea generations, which might be essential for fleaborne transmission.
2.3 Experiment 2: Evaluation of vertical transmission in fleas
In order to examine transovarial MHLB transmission, female fleas from MHLB-positive rodents in the field were randomly collected and allowed to lay eggs for 48 hr of incubation at 95% ± 3 relative humidity and 23 ± 2°C (one flea per rodent; Figure 1b), following Messika et al. (2017). The eggs, developed larva and cocoons were further incubated, and their emerging offspring were collected. The female parents and their offspring were then subjected to DNA extraction and PCR to screen for MHLB. Simultaneously, 32 females from our laboratory colony (see Supporting Information, SI 3) were allowed to lay eggs under the same conditions, and their newly emerged fleas served as controls. This design resulted in 42 offspring of 16 MHLB-positive female fleas and 65 control offspring of 48 MHLB-negative female fleas (16 field-collected females who had 33 offspring plus 32 females from our laboratory colony who had 32 offspring; Figure 1b).
To test for nontransovarial vertical transmission via MHLB-positive regurgitated blood, nine pools of fleas (28 ± 17 fleas per pool) collected from the female flea-positive donors in Experiment 1 were allowed to regurgitate blood into a glass flask for an hour. Then, the voids were subjected to molecular analyses, following Morick et al. (2013b). In parallel, the regurgitated blood of one pool of fleas collected from an MHLB-negative donor served as a control (Figure 1b).
2.4 Experiment 3: Evaluation of direct transmission between rodents
Haemoplasma species may be directly transmitted between individuals from the same mammalian host species via aggression either between sexes during reproduction or between males during male–male competition (within-sex) (Dean et al., 2008; Museux et al., 2009; Willi et al., 2007a). Between-sex transmission was evaluated by pairing (one pair per cage) 20 field-captured couples of MHLB-positive (designated as “donors”) and MHLB-negative (designated as “recipients”) individuals (in half of them, the females in the pair were donors) for 2 weeks to allow reproductive activity. Three MHLB-negative couples served as controls (Figure 1c). In the within-sex transmission experiment, direct transmission was evaluated by placing each of five MHLB-positive males (designated as “donors”) in a cage with one of four different MHLB-negative males (designated as “recipients”) for 3 hr during a period of peak foraging activity (19:30–22:30), resulting in 20 unique pairs (Figure 1c). Individuals used for the within-sex transmission experiment were part of our breeding colony. The infection status of all the individuals that participated in Experiment 3 was assessed for 6 weeks.
As direct haemoplasma transmission is expected to occur through biting (Dean et al., 2008; Lappin et al., 2008; Museux et al., 2009; Willi et al., 2007a), the presence of MHLB on the buccal mucosa of the donor and recipient rodents was also examined. To do this, the infection parameters were assessed in 25 swabs collected from the buccal mucosa of 10 experimental donors (two swabs from female donors and three swabs from male donors from the between-sex experiment and four consecutive monthly swabs from each of the five donors in the within-sex experiment). As a control, the MHLB infection parameters in five swabs from recipients, before pairing, in the within-sex experiment and four swabs from control recipients from the between-sex experiment were assessed (Figure 1c). The sampling of the buccal mucosa was carried out by gently swabbing the mucous membranes of the upper and lower jaws using a sterilized cotton swab immersed in PBS. To ensure that the source of these samples was the buccal mucosa, swabs contaminated with blood were excluded. The rest of the swabs were then stored in Eppendorf tubes containing 200 μl of PBS, at −20°C until molecular analyses. Finally, to test for the possibility of direct MHLB transmission through rodent faeces, 21 G. andersoni were inoculated with MHLB, and after 27 days, around peak infection, they were confirmed to be MHLB-positive, and their faeces were collected and subjected to DNA extraction and MHLB PCR. Faeces from two G. andersoni, who were inoculated with MHLB-negative blood, served as positive and negative controls by adding to them 50 μl of either MHLB-positive blood or PBS, respectively.
2.5 Experiment 4: Evaluation of vertical transmission in rodents
We assessed the infection status of 35 offspring belonging to eight litters of (a) an MHLB-positive pregnant female field-captured rodent (one litter), (b) an MHLB-positive female and an MHLB-negative male (four litters of couples from Experiment 3), (c) an MHLB-negative female and an MHLB-positive male (one litter of a couple from Experiment 3) and (d) an MHLB-negative female and male (two litters of control couples from Experiment 3) for 6 weeks after birth (Figure 1d).
2.6 Experiment 5: Comparison of infection dynamics under natural conditions and in field-infected and laboratory-infected rodents under laboratory conditions
The MHLB infection load of three groups of rodents was assessed over time. Group 1 comprised 13 female and six male MHLB-positive rodents, sampled in the field during the spring, the main reproductive season, and recaptured 4 months later in the autumn (Cohen, Einav, et al., 2015). Group 2 comprised two female and five male MHLB-positive rodents, captured during the summer in the same region and brought into the laboratory for further assessment of their infection dynamics. Group 3 comprised four female and six male MHLB-negative rodents inoculated by MHLB-positive preserved blood.
2.7 Statistical analysis
Generalized linear models (GLMs) were applied to our data. In Experiment 1, the effects of rodent role (donor versus recipient), flea sex, flea condition (dead or live), sample origin (field or laboratory) and sample type (rodent blood or fleas) (independent variables) on the probability of being infected by MHLB (binomial distribution) and the MHLB load (Gamma distribution) (dependent variables) were explored. In Experiment 3, a GLM with a binomial distribution was applied to quantify the probability of a recipient rodent becoming infected by MHLB (dependent variable) as a function of the route of direct transmission (between- or within-sex) while the donor identification was treated as a random variable. In Experiment 5, a GLM with a Gamma distribution was applied to explore the effects of the rodent's sex and body mass, and infection load (independent variables) on the day of peak infection and of the MHLB load at peak infection (dependent variables). Statistical analyses were performed in r (version 3.3.3 R Development Core Team, 2017).
3 RESULTS
3.1 Experiment 1: Evaluation of transmission by fleas
The infection loads of the donor rodents were comparable to the loads observed in MHLB-positive rodents in the field (N = 10 and 19, respectively; p = 0.4; Figure 2b). None of the fleas who were allowed to complete digestion or the fleas fed on control rodents were positive for MHLB. In contrast, some of the fleas fed on experimental donors and recipients became MHLB-positive (Figures 1a and 2a). However, the values of both infection parameters were low, that is 0.14 ± 0.03 (N = 235, including dead fleas) and 76 ± 28 (N = 32, including dead fleas), for means ± SE of the probability of being infected by MHLB and of MHLB load (copy numbers in 1 μl of DNA), respectively, and they were not significantly affected by the rodent role, flea sex or flea condition (p > 0.08 for all tests; Figures 1a and 2).
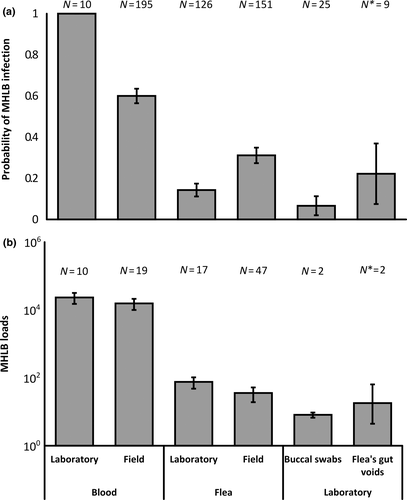
To test whether these infection values fell within the natural infection ranges, the infection values in the 126 live fleas sampled from donor and recipient experimental rodents were compared to those estimated in 151 live fleas removed from MHLB-positive field-captured rodents. The probability of live fleas fed on experimental rodents to become infected by MHLB was 0.13 ± 0.03 and was significantly lower than that of the live field-captured fleas (0.31 ± 0.04; p < 0.001; Figure 2a). However, the MHLB loads in the two groups of fleas were similar (N = 17 and 47, respectively; p = 0.24; Figure 2b). Despite the detection of MHLB in some of the experimental fleas, none of the recipient rodents became positive for MHLB (Figure 1a).
3.2 Experiment 2: Evaluation of vertical transmission in fleas
None of the 107 flea offspring were positive for MHLB (Figure 1b). In contrast, in two of the nine flea pools, MHLB-positive regurgitated blood was detected (Figure 1a,b). However, the MHLB load in it was low (Figure 2b).
3.3 Experiment 3: Evaluation of direct transmission between rodents
In all sex combinations of the donor–recipient experiments, some of the recipients became positive, and although the probability of the recipient becoming infected by MHLB was higher for the between-sex combination than for the within-sex combination, the differences were only marginally significant (N = 40 pairs; p = 0.058; Figure 1c). During the within-sex transmission experiment, the two recipient males that became infected were the only males that suffered from injuries, followed by the development of abscesses.
From the 20 buccal swabs that were sampled from the five male donors in the within-sex transmission experiment, two samples, which were sampled 20 days postinoculation, were positive for MHLB and showed low loads (Figure 2b). The other swabs that were sampled were MHLB-negative (Figure 2a). Although the two positive controls of the faeces were MHLB-positive, there were no indications for MHLB in the faeces of the MHLB-positive rodents or of the negative controls; therefore, the possibility of transmission through rodent faeces was not further investigated.
3.4 Experiment 4: Evaluation of vertical transmission in rodents
None of the 35 rodent offspring were positive for MHLB (Figure 1d).
3.5 Experiment 5: Comparison of infection dynamics
Regardless of whether the rodents were naturally or laboratory-infected with MHLB and whether or not they were brought into the laboratory, all individuals were persistently infected (Figure 3). MHLB were detected throughout each rodent's life, reaching 800 days of continuous infection in field-captured rodents that were maintained in the laboratory (Figure 3b). In the field, the MHLB load was significantly higher in the spring than in the autumn (N = 19; p < 0.001; Figure 3a). In the laboratory, all rodents infected with MHLB-positive blood became infected (Figures 2a and 3c). MHLB were first detected in the blood after 10–15 days of infection and peaked by 25–45 days postinoculation. MHLB loads then gradually reduced in all individuals until they stabilized at only a few tens of copy numbers per 1 μl of blood (Figure 3c). MHLB loads at peak bacteraemia and the timing of the peak were not significantly associated with the MHLB load in the inoculum (N = 10, p = 0.42 and p = 0.11, respectively).
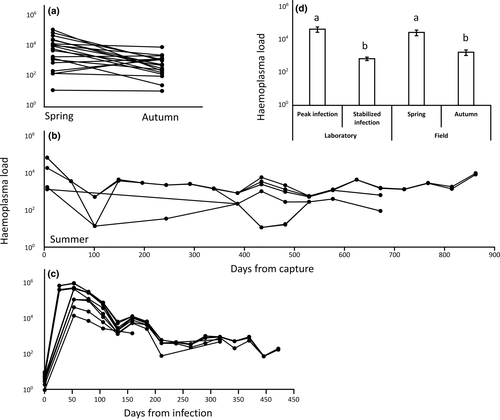
The infection loads and dynamics under controlled infection in the laboratory (Figure 3c) reflected well the infection loads under natural infection in the field (Figures 3a-b). MHLB loads in the spring were similar to the loads observed at peak infection under controlled infection (25–45 days postinfection), and MHLB loads in the autumn were similar to the loads observed during the stabilized period (65–400 days postinfection) under controlled infection (Figure 3d).
4 DISCUSSION
Knowledge of parasite/pathogen transmission routes and their infection dynamics is crucial to our understanding of how parasites/pathogens persist within host populations in nature. Here, the transmission routes and infection dynamics of MHLB in wild rodents were experimentally examined. Both the laboratory manipulations and the infection patterns reported in the field support a major role of direct rodent-to-rodent transmission rates and long persistency. Below the results are discussed in the light of the study goals and of their conceptual and applied implications.
4.1 Transmission routes
Transmission is a fundamental process in disease ecology, determining the evolved virulence levels of the parasite/pathogen, the host response and host–parasite/pathogen dynamics (Antonovics et al., 2017; Ebert, 2013; Sorrell, White, Pedersen, Hails, & Boots, 2009). However, due to the challenges associated with sampling the mammalian hosts, the arthropod vectors and their parasites/pathogens in nature, for many parasites and pathogens, the exact routes of transmission are still unknown. Throughout four experiments in which both field-infected and laboratory-infected rodents were used, evidence suggested that the MHLB are mainly transmitted by rodent-to-rodent contact.
In Experiment 3, in total, 20% of the MHLB-negative recipient rodents became infected by MHLB after being in contact with their donor counterparts for at least 3 hr. Moreover, between-sex transmission was established from donors that were infected by only tens to hundreds of copy numbers per 1 μl of blood, during their stabilized infection period. Considering that gerbils are solitary, the rates of encounters in nature may be lower and may fluctuate seasonally. Seasonality is also supported by the observed infection dynamics in the field wherein MHLB loads during the spring, the main reproductive season, were similar to peak infection in the laboratory but were significantly lower during autumn (Figure 3a,d).
The exact mechanism of rodent-to-rodent transmission needs to be experimentally confirmed. In the meantime, our study suggests that transmission does not occur through rodent faeces. Furthermore, three pieces of evidence suggest that the transmission is likely to occur by passage of the donor rodent's infected saliva into the recipient's open wounds. First, the detection of MHLB in the buccal swabs of donor rodents during peak infection demonstrated the presence of MHLB on rodent buccal mucous membranes. Second, the two male recipients that became infected suffered from open wounds. Finally, between- and within-sex aggressiveness in G. andersoni is common under laboratory conditions (I. S. Khokhlova and C. Cohen, unpublished data) and was documented during foraging in the field (Ovadia, Abramsky, Kotler, & Pinshow, 2005). Taken together, the above evidence suggests that rodent aggressiveness may enhance MHLB transmission, thereby offering an explanation for the skewed distribution of MHLB towards G. andersoni (Kedem et al., 2014)—the most aggressive species among the three co-occurring rodent species (i.e., G. andersoni, G. pyramidum and G. gerbillus) in the Negev Desert sand dunes (S. Halle, personal communication; (Ovadia, Abramsky, Kotler, & Pinshow, 2005)).
Our study also suggests that other possible transmission routes for haemoplasmas, namely fleaborne and flea or rodent vertical transmission (e.g., Fujihara et al., 2011; Hornok et al., 2015; Taroura et al., 2005), are less likely in our system. No evidence for transovarial vertical transmission between fleas or between rodents was found. Moreover, although about 16% of the fleas became positive for MHLB after feeding on MHLB-positive rodents and allowing for flea defecation, none of the recipient rodents became infected (Figure 1a). It is unlikely that our failure to simulate flea-to-rodent transmission was the result of the low probability of fleas to be infected by MHLB in Experiment 1 (Figure 2a). This is because the mean number of infected fleas per rodent in Experiment 1 was 16 (100 fleas on an average adult rodent multiplied by 0.16, the probability that a flea will become infected in Experiment 1), which exceeded six, the mean number of infected fleas per G. andersoni host in the Negev sand dunes (21 fleas on an average adult rodent and in its burrow multiplied by 0.3, the probability that a flea will become infected in the field; Hawlena et al., 2006), whereas MHLB loads per infected flea were comparable under both conditions (Figure 2b). An alternative explanation for our failure to simulate flea-to-rodent transmission is that fleaborne transmission does not occur in this system. It is likely that the MHLB we detected in the fleas and their regurgitated blood during the laboratory and field sampling were dead bacterial copies detected in the fleas’ bloodmeal and thus were not transmissible to the recipient rodents. Four pieces of evidence support this “dead bacterial copies” explanation, namely (a) the absence of a correlation between the probability of a rodent and its fleas to be infected by MHLB in nature (Messika-Madmon, 2015), (b) the absence of a correlation between flea burden and the probability of a flea being infected by MHLB (Kedem et al., 2014), (c) the similarity between the MHLB loads of dead and live fleas and between those of fleas fed on donor and those fed on recipient rodents in Experiment 1 and (d) the lack of MHLB in fleas that were allowed to complete bloodmeal digestion during Experiment 1. Even if we are wrong, and fleas can transmit MHLB by their gut or their mouth parts without replication (Schorderet-Weber, Noack, Selzer, & Kaminsky, 2017; Shaw, Kenny, Tasker, & Birtles, 2004; Vobis, D'Haese, Mehlhorn, & Mencke, 2003), our results indicate that fleaborne transmission is likely not their main transmission route.
4.2 Infection dynamics
Considering the above evidence for only low transmission rates between rodents and the hostile conditions found in the mammalian host's blood, the question of how haemoplasmas survive in nature is intriguing. The results of Experiment 5 provide us with some clues to this question. First, it seems that very low loads (~1 × 104 MHLB copies in 150–300 μl whole blood subcutaneous inoculum) of MHLB are sufficient for successful infection and that transmission success, infection load and the timing of peak infection are all inoculum dose-independent. Thus, even if transmission opportunities are rare, they are likely to be successful. Second, the laboratory experiment suggests that once infection is established, it is lifelong, maintaining high numbers of infectious rodents in the population. Third, the short infection peak is followed by a low and stable load of asymptomatic MHLB infection in which only a few tens of copy numbers per 1 μl of blood persist. This low and persistent MHLB infection dynamics is similar to several other haemoplasmas of domestic animals (Groebel, Hoelzle, Wittenbrink, Ziegler, & Hoelzle, 2009; Hoelzle et al., 2014; Museux et al., 2009; Tasker et al., 2009b), as well as to other parasites/pathogens (Bartonella spp., Mycobacterium tuberculosis, Plasmodium spp., Brucella spp., Helicobacter pylori, Burkholderia pseudomallei, Coxiella burnetii and Salmonella enterica serovar Typhi; Chomel et al., 2009; Cory, 2015; Merrell & Falkow, 2004; Monack, Mueller, & Falkow, 2004; Okamura, 2016; Rhen, Eriksson, Clements, Bergstrom, & Normark, 2003). Such an infection dynamics may be the result of the parasites/pathogens’ strategy to evade the immune response, compensating for their low loads with their low level of damage to their mammalian host, which reduces their mortality probability (the transmission–virulence trade-off; Anderson & May, 1982; Ewald, 1995; Sorrell et al., 2009). However, it is also possible that this infection dynamics is the result of the mammalian host's immune system's ability to control the parasites/pathogens below harmful levels or even to protect the mammalian host against subsequent infection (Haine, 2008; Miller, White, & Boots, 2005).
Interestingly, in contrast to most of the other parasites/pathogens, which are characterized by low and persistent infection dynamics, some species of haemoplasmas including MHLB (a) are persistent in all individuals and (b) are horizontally transmitted; additionally, there is (c) no indication that they may convert to disease-causing states (as for Ca. M. haemominutum, Barker et al., 2012; Tasker et al., 2009b; Willi et al., 2007c). Future experiments should thus reveal the mechanisms of persistent haemoplasma infection. In particular, it is important to understand whether (a) the low stable infection levels result from the bacterium's or the mammalian host's actions, (b) the bacterium hides in other tissues in the rodent's body (Novacco, Riond, Meli, Grest, & Hofmann-Lehmann, 2013; Tasker et al., 2009a; Wolf-Jäckel et al., 2012), and (c) the bacterium replicates during the stabilized infection period or remains in a dormant state (Mandell & Beverley, 2017; Monack & Hultgren, 2013; Potgieter, Bester, Kell, & Pretorius, 2015; Vadivelu et al., 2017).
4.3 Ecological and applied implications
The knowledge of MHLB transmission routes and persistence sheds light on the way that parasites/pathogens perceive their mammalian host as an environment. Together with previous evidence, it appears that MHLB spend most of their time in a stable population size (the current study), exhibit narrow rodent ranges (Kedem et al., 2014), have low rates of dispersal (= transmission rate between rodents; the current study) and cause only minimal damage to the rodent (Cohen, Einav, et al., 2015). It is likely that the described pattern reflects a bacterial strategy to persist in a population of solitary mammalian hosts (Weiss, 2002) and to survive in hostile environments such as a mammalian host's blood. Under more favourable conditions for the parasite/pathogen (e.g., a less protected environment or an environment that enables high mammalian host-to-host transmission), it may be more adaptive for it to actively destroy the mammalian host's biomass (Andrews & Rouse, 1982; Esch, Hazen, & Aho, 1977). The knowledge gained in this study can also be applied to control MHLB in natural gerbil communities. In particular, it suggests that (a) the most critical season for MHLB transmission coincides with the breeding season, that (b) all MHLB-positive G. andersoni rodents should be considered as infectious individuals and that (c) ectoparasites do not provide a serious risk factor for bacterial transmission.
ACKNOWLEDGEMENTS
We thank Chen Ben-Zvi, Asa Tirosh, Ricardo Gutiérrez, Nadia Burdelova and Chelsea Hick for valuable help during this study. This study was supported by the Marie Curie Career Integration Grant (CIG) number FP7-293713 and the Israel Science Foundation (ISF) Grant number 1391/15 to H.H. C.C. was sponsored by the Faran Fellowship for excellent Ph.D. students (Ben-Gurion University of the Negev). M.G. was sponsored by the Kreitman School of Advanced Graduate Studies (Ben-Gurion University of the Negev) and the Blaustein Center for Scientific Cooperation (Jacob Blaustein Institutes for Desert Research, Ben-Gurion University of the Negev). This is publication number 972 of the Mitrani Department of Desert Ecology.
DATA ACCESSIBILITY
Raw data can be accessed via the public archive “Figshare.com.” Accession address is https://doi.org/10.6084/m9.figshare.6741518.
AUTHOR CONTRIBUTION
C.C. participated in the design of the study, carried out the field sampling, experiments and molecular analysis, performed the statistical analysis and wrote the manuscript. M.S. participated in the design of the study and carried out experiments and molecular analyses. M.G. participated in the laboratory experiments and assisted in the statistical analyses. I.M. contributed to the laboratory experiments. M.E. participated in the design and analysis of the molecular assays. I.K. participated in experimental design and flea rearing. S.T. participated in the experimental design and consultation regarding the haemoplasma group. H.H. conceived of the study and participated in its design, the design of the molecular assays and the statistical analysis and coordination, and helped to draft the manuscript. All authors read and approved the final manuscript.




