Fine-scale geographic patterns of gene flow and reproductive character displacement in Drosophila subquinaria and Drosophila recens
Abstract
When two species are incompletely isolated, strengthening premating isolation barriers in response to the production of low fitness hybrids may complete the speciation process. Here, we use the sister species Drosophila subquinaria and Drosophila recens to study the conditions under which this reinforcement of species boundaries occurs in natural populations. We first extend the region of known sympatry between these species, and then we conduct a fine-scale geographic survey of mate discrimination coupled with estimates of gene flow within and admixture between species. Within D. subquinaria, reinforcement is extremely effective: we find variation in mate discrimination both against D. recens males and against conspecific allopatric males on the scale of a few kilometres and in the face of gene flow both from conspecific populations and introgression from D. recens. In D. recens, we do not find evidence for increased mate discrimination in sympatry, even where D. recens is rare, consistent with substantial gene flow throughout the species’ range. Finally, we find that introgression between species is asymmetric, with more from D. recens into D. subquinaria than vice versa. Within each species, admixture is highest in the geographic region where it is rare relative to the other species, suggesting that when hybrids are produced they are of low fitness. In sum, reinforcement within D. subquinaria is effective at maintaining species boundaries, but even when reinforcing selection is strong it may not always result in a pattern of strong reproductive character displacement due to variation in the frequency of hybridization and gene flow from neighbouring populations.
1 INTRODUCTION
When two species that have been evolving in allopatry come back together in secondary contact, reduced fitness of the hybrid offspring may select for increased prezygotic reproductive barriers (Blair, 1955; Coyne & Orr, 2004; Dobzhansky, 1951). The classic signature of past reinforcement is a pattern of reproductive character displacement (RCD), in which prezygotic isolation is stronger in areas of sympatry relative to allopatry (Butlin, 1987). Empirical, theoretical and comparative studies suggest that the reinforcement of species boundaries is a common outcome of reduced hybrid fitness during secondary contact (Coyne & Orr, 2004; Hopkins, 2013; Noor, 1999; Servedio & Noor, 2003). More recently, it has become apparent that as a consequence of changing mating behaviours between species, reinforcement may also introduce widespread effects on mate choice within species, whereby females from the sympatric populations no longer recognize conspecific males from allopatric populations as suitable mates (termed “cascade” reinforcement; Hoskin & Higgie, 2010; Howard, 1993; Ortiz-Barrientos, Grealy, & Nosil, 2009). In this way, reinforcing selection may play an important role in both completing and initiating speciation events.
The outcome of reinforcing selection can be influenced by many factors, including the strength of intrinsic postzygotic isolation, the relative abundance of each species and the level of gene flow within and between species (Coyne & Orr, 2004; Pfennig & Pfennig, 2012). How these forces interact to promote or inhibit reinforcement is important to understand the process of reinforcement as well as its diversifying effects. Species pairs where one mating direction shows stronger RCD than the reciprocal are often attributed to an asymmetry in postzygotic selection, with stronger hybrid dysfunction leading to higher premating isolation (Cooley, 2007; Yukilevich, 2012). The relative prevalence of each species in the region of sympatry can affect the amount of hybridization that takes place, and it has been suggested that females of species with the smaller population should evolve stronger premating isolation compared to females from the larger population (Noor, 1995; Nosil, 2013; Yukilevich, 2012). Variation in the species prevalence can also result in geographic variation in the strength of RCD (Goldberg & Lande, 2006; Lemmon, Smadja, & Kirkpatrick, 2004; Pfennig & Pfennig, 2012).
Finally, migration of nonchoosy individuals from allopatry into the contact zone allows for mating mistakes and hybrids to be produced (Coyne & Orr, 2004; Servedio & Noor, 2003). However, even if hybrids have very low fitness, a pattern of RCD may not result if migration is sufficiently high to homogenize allopatric and sympatric populations. Between two species that hybridize, introgression from backcrosses of fertile hybrids can also weaken reinforcing selection, potentially resulting in the collapse of the two species into a hybrid swarm. Theoretical results and experimental evolution studies suggest that classical reinforcing selection is strongest with an intermediate level of gene flow within species and little to no introgression between species (Kirkpatrick, 2000; Matute, 2010a; Nosil, Crespi, & Sandoval, 2003; Servedio & Kirkpatrick, 1997).
While patterns of mate discrimination consistent with cascade reinforcement have been demonstrated in several empirical systems (Higgie & Blows, 2008; Hoskin, Higgie, McDonald, & Moritz, 2005; Jaenike, Dyer, Cornish, & Minhas, 2006; Kozak et al., 2015; Nosil, 2007; Saetre & Saether, 2010), much less is known about the conditions under which cascade reinforcement evolves (Fuller, 2016). Theoretical models by McPeek and Gavrilets (2006) and Pfennig and Ryan (2006) found that interactions with other species could alter female preferences to initiate isolation within species, but their models assumed speciation was nearly complete and no gene flow between sympatric and allopatric populations. Yukilevich and Aoki (2016) used a simulation approach to show that cascade reinforcement only evolved when migration between allopatric and sympatric populations was very low; the only way cascade reinforcement could evolve in the face of gene flow was if substantial ecological divergent selection was also occurring.
Here, we study geographic variation in reinforcement between two closely related species of Drosophila flies, D. subquinaria and D. recens. D. subquinaria and D. recens occur in western and eastern North America, respectively, and their ranges overlap in central Canada. D. subquinaria shows a strong pattern of RCD against D. recens consistent with a history of classical reinforcing selection (Bewick & Dyer, 2014; Jaenike et al., 2006). Specifically, D. subquinaria females from populations where both species are found do not mate with D. recens males, whereas D. subquinaria females from allopatric populations will mate at moderate levels with D. recens males. Furthermore, these “choosy” sympatric females also discriminate against conspecific males from distant populations in a pattern consistent with cascade reinforcement. In contrast, D. recens females do not show a pattern of RCD against mating with D. subquinaria males nor do they show within-species behavioural isolation (Jaenike et al., 2006).
The cost of heterospecific mating is greater for D. subquinaria than for D. recens females, which may contribute to stronger RCD in D. subquinaria. When a D. subquinaria female mates with a D. recens male, most of the hybrid offspring die due to cytoplasmic incompatibility (CI) from a Wolbachia infection that is only present in D. recens (Shoemaker, Katju, & Jaenike, 1999). In contrast, when a D. recens female mates with a D. subquinaria male, there is no CI—the offspring survive and the daughters are fully fertile. Hybrid males are always sterile. Thus, while hybrid fitness is reduced in both crosses, the reduction in hybrid fitness is much higher for D. subquinaria females.
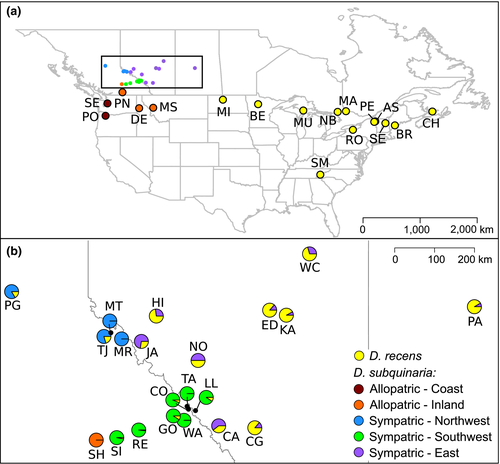
The known region of sympatry between these two species begins at the eastern edge of the Canadian Rocky Mountains in Alberta and extends ~1,200 km to Manitoba (Jaenike et al., 2006). In this region, D. subquinaria is generally at a lower prevalence relative to D. recens (mean 30% D. subquinaria across nine populations; Table 1). No genetic variation for discrimination against D. recens males has been identified within or among sympatric populations of D. subquinaria (Bewick & Dyer, 2014; Jaenike et al., 2006), suggesting reinforcing selection is very effective. In contrast, allopatric populations of D. subquinaria segregate substantial genetic variation for mating with D. recens (Bewick & Dyer, 2014). Within D. recens, mating rates with D. subquinaria are generally low but there is genetic variation for mate discrimination present within both sympatric and allopatric regions (Jaenike et al., 2006).
| Region/ | Abbrev | Fraction | N lines assayed | N genotyped | ||
|---|---|---|---|---|---|---|
| Location | subquinaria (N) | subq | recens | subq | recens | |
| Allopatric—coastal | ||||||
| Portland, OR | PO | 1 (76) | 5* | 29 | 0 | |
| Seattle, WA | SE | 1 (40) | 5* | 28 | 0 | |
| Allopatric—inland | ||||||
| Deary, ID | DE | 1 (7) | 3* | 2 | 0 | |
| Missoula, MT | MS | 1 (33) | 13* | 30 | 0 | |
| Penticton, BC | PN | 1 (1) | 1 | 0 | ||
| Shuswap, BC | SH | 1 (4) | 4* | 4 | 0 | |
| Sympatric—southwest | ||||||
| Sicamous, BC | SI | 0.97 (107) | 3 | 29 | 0 | |
| Revelstoke, BC | RE | 0.98 (55) | 3 | 1 | 24 | 1 |
| Golden, BC | GO | 0.91 (65) | 3 | 1 | 24 | 4 |
| Wapta, BC | WA | 1 (62) | 3 | 31 | 0 | |
| Confluence, BC | CO | 0.96 (23) | 3 | 15 | 0 | |
| Takakkaw Falls, BC | TA | 1 (17) | 4 | 15 | 0 | |
| Lake Louise, AB | LL | 0.92 (39) | 3 | 33 | 1 | |
| Sympatric—northwest | ||||||
| Prince George, BC | PG | 0.84 (73) | 3 | 1 | 61 | 12 |
| Tete Jaune, BC | TJ | 0.80 (83) | 4 | 2 | 15 | 16 |
| Mt. Robson, BC | MT | 1 (35) | 6 | 34 | 0 | |
| Mt. Robson Route, BC | MR | 1 (20) | 6 | 14 | 0 | |
| Sympatric—east | ||||||
| Canmore, AB | CA | 0.61 (33) | 2* | 8 | 10 | |
| Calgary, AB | CG | 0.13 (48) | 3 | 26 | ||
| Jasper, AB | JA | 0.73 (77) | 31 | 8 | ||
| Hinton, AB | HI | 0.27 (118) | 16* | 2 | 22 | 22 |
| Nordegg, AB | NO | 0.50 (2) | 1 | 1 | ||
| Edmonton, AB | ED | 0.14 (255) | 0 | 11 | ||
| Kawtikh, AB | KA | 0.08 (390) | 7* | 2 | 17 | 26 |
| Winston Churchill, AB | WC | 0.29 (197) | 2 | 7 | ||
| Prince Albert, AB | PA | 0.09 (35) | 3 | 32 | ||
| Allopatric | ||||||
| Minot, ND | MI | 0 (164) | 0 | 8 | ||
| Bemidji, MN | BE | 0 (240) | 0 | 7 | ||
| Munising, MI | MU | 0 (25) | 0 | 7 | ||
| North Bay, ON | NB | 0 (25) | 1 | 0 | 0 | |
| Mattawa, ON | MA | 0 (78) | 0 | 30 | ||
| Rochester, NY | RO | 0 (30) | 0 | 17 | ||
| Peru, NY | PE | 0 (25) | 1 | 0 | 24 | |
| Au Sable Forks, NY | AS | 0 (25) | 0 | 7 | ||
| Bethlehem, NH | NH | 0 (7) | 0 | 7 | ||
| Brunswick Pines, ME | BR | 0 (37) | 0 | 30 | ||
| Charlottetown, PEI | CH | 0 (4) | 0 | 4 | ||
| Smoky Mountains, TN | SM | 0 (74) | 1 | 0 | 36 | |
Note
- Allopatric Drosophila subquinaria populations are indicated as coastal or inland based on the proximity to the Coast Mountains, and sympatric populations are divided into western and eastern based on discrimination against Drosophila recens. For each population, the abbreviation (abbrev), the abundance of D. subquinaria (subq) relative to D. recens (recens) with the total number of flies assayed, the number of isofemale lines assayed for mate discrimination and the number of flies genotyped at the microsatellite loci are shown. Lines used in mate trials marked with an asterisk (*) are data included from Bewick and Dyer (2014). See Supporting Information Table S1 for a full description of each population.
Little is known about the patterns of gene flow within each of these species nor how much introgression between species there is due to hybridization. In D. subquinaria, nine autosomal microsatellite markers show strong genetic differentiation between populations to the east and west of the Canadian Rocky Mountains concurrent with the boundary of sympatry with D. recens, whereas the mtDNA shows strong genetic differentiation between populations to the west and east of the Pacific Coast Range (Bewick & Dyer, 2014; Jaenike et al., 2006). In D. recens, the mtDNA shows little population structure across the range, with the exception that flies from the Smoky Mountains (SM) at the southeastern edge of the range tend to be genetically differentiated from flies from the rest of the range. About 3% of D. subquinaria carry a mtDNA haplotype from D. recens (Jaenike et al., 2006; Shoemaker, Dyer, Ahrens, McAbee, & Jaenike, 2004), indicative of occasional hybridization and subsequent introgression from D. recens into D. subquinaria (these lineages have lost Wolbachia).
In this study, we investigate the limits of reinforcing selection and how gene flow may affect patterns of mate discrimination in natural populations of these two species. We previously identified a transition region from sympatry of both species to allopatric D. subquinaria that spans 450 km, from Canmore, Alberta, to Shuswap, British Columbia (Bewick & Dyer, 2014). Here, we collect from populations between these locations along two east–west transects to ask where and how fast the transition from sympatry to allopatry occurs. We find that the species prevalence and mate discrimination patterns change rapidly, on the order of a few kilometres, at the eastern edge of the Rockies, and we identify several populations to the west of the Canadian Rockies where D. recens occurs at low-to-moderate prevalence. We then characterize patterns of gene flow across the geographic ranges of both species and assay patterns of mate discrimination by both species to investigate how migration interacts with reduced hybrid fitness to result in reinforcement.
2 MATERIALS AND METHODS
2.1 Drosophila collections, rearing and mate discrimination assays
We collected flies from natural populations spanning the transition zone from sympatry into allopatry where only Drosophila subquinaria occurs during the summers of 2012–2013 by sweep netting over baits of commercial mushrooms (Table 1; Figure 1; Supporting Information Table S1). Collection sites followed a transect along Canadian Highway 1 in the south and Highway 16 in the north. Wild males were frozen upon collection, and wild females were placed individually in food vials to establish isofemale lines and later frozen. All cultures were maintained at 20°C on a 12 hr light/dark cycle on Instant Drosophila Medium (Carolina Biological, Cary, NC) supplemented with commercial Agaricus bisporus mushroom. To identify flies as Drosophila recens or D. subquinaria, we used multiple molecular markers, as described previously (Bewick & Dyer, 2014).
We used no-choice mating assays to infer patterns of mate discrimination. Lines were maintained in the laboratory for at least ten generations before being used in mating assays, and no-choice mating assays took place as previously described (Bewick & Dyer, 2014). In brief, all flies used in mating experiments were 7–10-day-old virgins, and mating assays took place in 4 ml vials and commenced within an hour of the incubator lights turning on. Flies were placed into vials by air aspiration and we recorded whether the pair mated within 3 hr.
2.2 DNA samples and microsatellite genotyping
To investigate patterns of gene flow within and between species, we sampled from 38 populations that span the geographic ranges of both D. subquinaria and D. recens (Figure 1; Table 1). Many of the DNA samples were initially described in previous studies (Supporting Information Table S1; Bewick & Dyer, 2014; Dyer, Charlesworth, & Jaenike, 2007; Jaenike et al., 2006). When possible, we selected at least 25 wild females of each species from a population for genotyping. For a few populations, we included some wild males, or if isofemale lines had been established but the corresponding wild female sample was lost, we used a single female fly from the line. A total of 830 individuals were used in this study, which included 476 D. subquinaria and 354 D. recens (Table 1).
Our set of 30 microsatellite markers amplified consistently in both D. subquinaria and D. recens (Supporting Information Table S2) and were genotyped using a three-primer fluorescent method (Miles, Isberg, Moran, Hagen, & Glenn, 2008; Schuelke, 2000). Amplified products were run alongside a size standard at the Georgia Genomics Facility, and alleles were scored using genemarker version 2.6 (SoftGenetics, State College, PA). Using a pattern of uniform homozygosity across wild males to assign X-linkage, we identified nine markers as X-linked and 21 as autosomal (Supporting Information Table S2). We converted all X-linked markers in males to haploid status, and for flies taken from laboratory lines, we only included one random allele from each locus. We tested for linkage disequilibrium (LD) and deviations from Hardy–Weinberg equilibrium (HWE) using genepop (Rousset, 2008). For LD and HWE analyses, we only used wild female samples and excluded flies from isofemale lines.
2.3 Patterns of differentiation within and between species
We used arlequin version 3.5 (Excoffier & Lischer, 2010) to calculate estimates of diversity as well as pairwise FST among populations within and between species. We tested for evidence of isolation by distance within each species with a Mantel test using Slatkin's pairwise FST (Slatkin, 1995) and geographic distance (in km), including only populations with at least seven samples. We employed the program structure (Hubisz, Falush, Stephens, & Pritchard, 2009; Pritchard, Stephens, & Donnelly, 2000) to infer genetic clusters. We used an admixture model assuming correlated allele frequencies, with the sampling location as a prior, and for each run included 1,000,000 MCMC steps and a burnin of 100,000 steps. Each run was repeated ten times for each K, with K ranging from 1 to 10. We determined the best K for each data set using the average lnLs and the ΔK method of Evanno, Regnaut, and Goudet (2005), and used clumpp (Jakobsson & Rosenberg, 2007) as implemented in clumpak (Kopelman, Mayzel, Jakobsson, Rosenberg, & Mayrose, 2015) to summarize q-values across runs. We also inferred patterns of population structure with multivariate methods using the program discriminant analysis of principle components (dapc) as implemented in the adegenet r package (Jombart & Ahmed, 2011; Jombart, Devillard, & Balloux, 2010). We used the collecting locations as the a priori populations and retained the number of principle components with the highest overall a-score. For both structure and dapc, we inferred clusters using only samples of D. subquinaria, only samples of D. recens, or all samples of both D. subquinaria and D. recens. For each data set, we used the three sub-data sets (all markers, autosomes only and X-linked only) to ask whether the X-chromosome vs. the autosomes exhibit different patterns of population differentiation or introgression.
We investigated the amount of admixture between species and whether this differs among geographic regions and parts of the genome. We used representative structure runs with K = 2, with samples categorized by species and geographic regions as in Table 1. We used the q score of each individual to estimate the genetic composition from the other species’ cluster and then compared admixture levels between species, regions and parts of the genome using nonparametric Wilcoxon rank sum tests. Unless otherwise noted, statistics were completed with jmp pro version 13 (SAS Institute, Cary, NC).
2.4 Mate discrimination by D. recens females
We conducted mating assays using five isofemale lines of D. recens from the populations in British Columbia where D. recens is at low prevalence to investigate whether these females displayed stronger mate discrimination than D. recens from the main part of the range (Table 1). We paired females with conspecific males from their own line, with conspecific D. recens males from a mixed eastern sympatric stock (created from lines collected in Alberta), and with heterospecific D. subquinaria males from a mixed coastal allopatric stock (created from lines collected in Seattle, WA and Portland, OR). These and the other mixed stocks are described in Bewick and Dyer (2014). Females from two of these lines were also tested for mating with heterospecific D. subquinaria males from the same geographic location as they occurred.
As a control, we assayed patterns of mate discrimination of several lines of D. recens collected from populations east of the Rockies. We used females from eight representative D. recens stocks (Table 1): three isofemale lines from allopatric populations, three isofemale lines from Alberta where both species occur, a mixed stock from sympatric Kawtikh Alberta, and a mixed stock from three sympatric populations in Alberta (the lines in these mixed stocks were different from the isofemale lines tested). Females were paired with conspecific males from the same stock, with D. subquinaria males from a mixed coastal allopatric stock, and with D. subquinaria males from a mixed eastern sympatric stock.
For each line, we tested for a block (i.e., day) effect using a nominal logistic regression with male type and block as fixed effects. After Bonferroni correction, there were no significant block effects, thus we combined the data across blocks in further analyses. Within each line, we tested for variation in mating rate both between species and among lines within each species using a nominal logistic regression with male species (D. recens or D. subquinaria) and male strain within male species as fixed effects in the model. As there was no significant variation in mating with different strains of either D. recens or D. subquinaria males, we combined the data from across strains of each species to investigate regional variation in mating rate against D. subquinaria using a nominal logistic regression with geographic region and line within region as fixed effects in the model.
2.5 Mate discrimination by D. subquinaria females
We tested the patterns of mate discrimination of D. subquinaria females from 41 isofemale lines collected from 11 populations in the western sympatric region (Table 1). Females from each line were paired with three types of males, including males from the same line, conspecific males from a mixed coastal allopatric stock of D. subquinaria and heterospecific males from a mixed eastern sympatric stock of D. recens. We identified a significant block effect for two lines (MT1 and MR11). We combined data from across mating assay days for further analyses; results were similar when these two lines were excluded. First, we asked if there was significant variation in mating rate against each male type using a nominal logistic regression with region (northern or southern transect), population nested within region and line nested within population and region as fixed effects. Second, within each line we compared mating rates with males from the same line vs. D. subquinaria allopatric coastal mixed males. Third, within each line we compared mating rates with males from the same line vs. D. recens sympatric males.
To assess the broad geographic patterns in mate discrimination, we incorporated data from Bewick and Dyer (2014) of mating rates of 55 lines from five allopatric and three eastern sympatric populations against D. recens and coastal allopatric D. subquinaria. Bewick and Dyer (2014) subdivided the allopatric populations into coastal and inland, referring to populations on either side of the Coast Mountains, and we maintain this distinction here. To investigate variation among geographic regions, we calculated the proportion of pairs of each line that mated with each male type; we used a Wilcoxon rank sum test to test for variation among geographic regions followed by pairwise comparisons among geographic regions using the Steel-Dwass method. Finally, we asked whether the prevalence of D. recens in a population is correlated with the level of mate discrimination against D. recens and/or coastal allopatric D. subquinaria males.
2.6 Cline analyses in D. subquinaria
To compare the geographic patterns of gene flow and mate discrimination, we used the HZAR platform in r (Derryberry, Derryberry, Maley, & Brumfield, 2014). For patterns of mate discrimination against conspecific D. subquinaria allopatric males and heterospecific D. recens males, we included mating rates of the 41 lines from this study and the 55 lines from Bewick and Dyer (2014). For each line and male type, the proportion of pairs that mated was used as a data point. To infer clines in gene flow within D. subquinaria and admixture between D. subquinaria and D. recens, we used the mean q score from each population, using representative structure runs of all loci with K = 2. Finally, to estimate the cline in species composition we used the fraction of D. subquinaria within each population (Table 1). As the geographic patterns do not follow a simple east–west gradient, we used six mountain peaks in the Rockies to make a transect that roughly follows the continental divide (Supporting Information Table S3); in Canada, this closely follows the border between British Columbia and Alberta (Figure 1). We then we used the function dist2line in r to calculate the closest distance of each population to that line. To make all distances positive, we set the westernmost population of Portland, Oregon, to 0 km, and then recalculated each distance relative to this; in this way, the continental divide is at 765 km (Supporting Information Table S1).
We used the genetic models within HZAR to fit clines (Derryberry et al., 2014). We ran 15 separate models that varied in the number of cline shape parameters estimated, although all models estimated cline centre (distance from sampling location 1, c) and width (1/maximum slope, w). Models varied in whether they estimated different combinations of the exponential decay curve (tail) parameters δ and τ (neither tail, both tails separately, right or left tail only, or mirrored tails), where δ and τ represent the distance from the cline centre to the tail and the tail slope, respectively. Models also varied in whether they estimated the allele frequencies at the cline ends (pmin and pmax), used the empirical values, or fixed these values at 0 and 1. For each data set, we identified the best-fitting model using sample size corrected Akaike information criterion, extracted the best-fitting maximum likelihood cline and the 2-unit support envelope around that cline and generated summary statistics including the cline width and centre. We considered clines concordant if their two log-likelihood unit support limits (CIs) overlapped. Finally, we estimated the strength of selection (s) acting along each cline, as in Bewick and Dyer (2014). In brief, we used an ecotone model (Endler, 1977; Slatkin, 1973) and estimated s from w = σ/√s, where σ is the standard deviation (SD) of the adult-offspring dispersal distance (as in Gay, Crochet, Bell, & Lenormand, 2008). We calculated selection under dispersal variances of 1, 2, 5 and 10 km (Bewick & Dyer, 2014).
3 RESULTS
3.1 Geographic ranges
We collected from populations from near the ridge of the Canadian Rockies west for several hundred km and found Drosophila recens as far west as Sicamous, BC, in the south, and Prince George, BC, in the north (Table 1; Figure 1). Thus, the region of sympatry of D. recens and Drosophila subquinaria extends more than 300 km farther west than previously realized and is ~1,500 km wide. In this western sympatric region, D. recens occurs at much lower frequency than D. subquinaria, whereas in the eastern sympatric region, D. subquinaria is generally at a lower frequency than D. recens.
3.2 Population differentiation within each species
The microsatellite loci exhibit substantial diversity within each species (Supporting Information Tables S4 and S5). Three loci (3091, 1083, 3047) show a significant deficit of heterozygotes in 14 or more populations; we repeated analyses that assume HWE without these populations and no conclusions were affected. Furthermore, no pair of loci showed LD in more than two populations. Thus, we included all 30 loci in the following analyses.
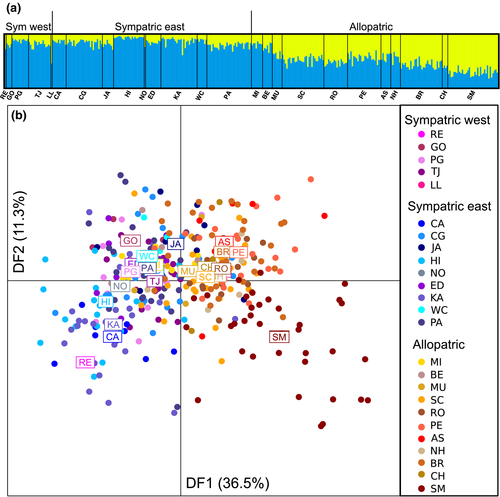
Within D. recens, there is some east–west differentiation, with most of the population structure between the main part of the range and the southernmost SM population (Figure 2). Using structure with all loci, the most likely supported number of clusters is K = 1 based on lnL values and K = 2 based on the Evanno method (Supporting Information Table S6). We note that K = 1 is not a possible result using the Evanno method. With K = 2, the western and eastern sympatric populations are similar in composition to each other and to the allopatric populations to the west of the Great Lakes (Figure 2a and Supporting Information Figure S1). The dapc results are similar, where discriminant function 1 (DF1) differentiates populations on an east–west gradient with the middle being at the west end of the Great Lakes, and with the SM population being the most distinct population (Figure 2b). Pairwise FST values between populations are significant for 20 of 300 comparisons (p < 0.001), and 10 of these included SM populations (Supporting Information Table S7). There is a highly significant pattern of isolation by distance (r = 0.296; p < 0.0001), and this pattern remains when the SM population is removed (r = 0.290; p < 0.0001).
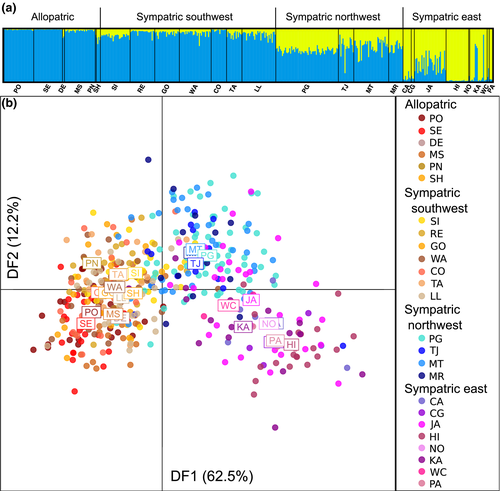
In D. subquinaria, the primary signature of genetic differentiation is also on an east–west gradient, with substantial differentiation between the eastern sympatric populations and the rest of the geographic range (Figure 3). Using structure, the best supported number of clusters is K = 2 using the Evanno method and K = 5 using the lnL values (Supporting Information Table S6). Considering K = 2, geographically distant western allopatric and eastern sympatric populations are the most differentiated (Figure 3a). The sympatric west region in the middle consists of both clusters, with the southern transect more similar to the western allopatric populations. Increasing the K value highlights these differences (Supporting Information Figure S2). The dapc results are similar, where discriminant function 1 differentiates populations on an east–west gradient (Figure 3b). The populations fall into four rough clusters by geographic region, including the allopatric populations, sympatric from the southwest, sympatric from the northwest and sympatric from the east (see Table 1). The exceptions are the Shuswap (SH) and Penticton (PN) populations, which are allopatric based on our collections but are very close both geographically and genetically to Sicamous (SI) and other southwest sympatric populations. Only two of the 95 significant pairwise estimates of FST (p < 0.001) are between populations in same geographic region (Supporting Information Table S8). While there is no significant pattern of genetic isolation by geographic distance when all populations are considered (r2 = 0.148; p = 0.14), patterns of isolation by distance can be obscured by higher level population structure and we do find significant genetic isolation by geographic distance when considering only eastern sympatric (r = 0.986; p = 0.044) or only western sympatric and allopatric populations (r = 0.996; p < 0.0001). Completing the structure and dapc analyses separately for the autosomal and X-linked loci reveals similar patterns within each species (Supporting Information Figures S3–S5, Supporting Information Table S6).
3.3 Admixture between species
Results from both structure and dapc indicate clear genetic differentiation between D. subquinaria and D. recens (Figure 4). Using structure, based on the Evanno method the most likely number of clusters is K = 2 (Figure 4a and Supporting Information Figure S6, Supporting Information Table S6), with clear clustering by species, and based on the lnL values the most likely value of K = 4, with clear differences between species and also additional population differentiation within D. subquinaria. The dapc results are similar: discriminant function 1 differentiates the two species, and discriminant function 2 indicates east–west population structure within D. subquinaria (Figure 4b). Similar patterns were found using only the X-linked loci or autosomal loci (Supporting Information Table S6, Supporting Information Figures S7–S8).
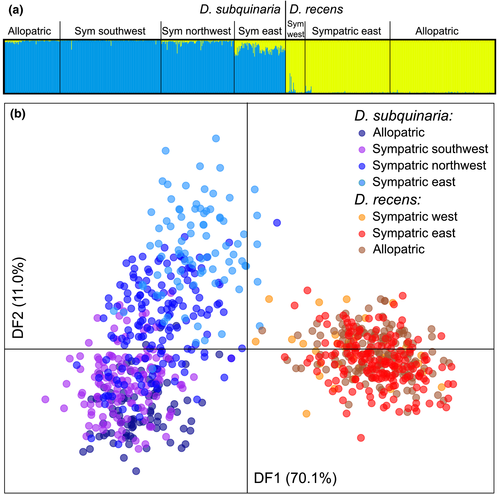
The direction of introgression between species is biased, with more admixture from D. recens into D. subquinaria than vice versa (Figure 4a; Wilcoxon Z = −8.9, p = 0.0001). Furthermore, within each species there is significant variation among geographic regions in the amount of admixture from the other species (Supporting Information Figure S9; D. subquinaria: 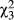 = 878, p < 0.0001; D. recens:
= 878, p < 0.0001; D. recens: 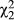 = 87, p < 0.0001). The geographic region where each species occurs at lowest relative abundance is also where the highest amount of admixture from the other species is found. In D. subquinaria, this is the eastern sympatric region and in D. recens this is the western sympatric region (both species: steel method using this region as a control, all comparisons p < 0.0001). When the X-linked and autosomal loci are considered separately, there is significantly reduced introgression of the X-chromosome loci compared to the autosomes within every geographic region of D. subquinaria (Supporting Information Figure S9; all p < 0.0001 with Wilcoxon rank sum test). In contrast, within D. recens the allopatric region shows significantly reduced admixture of X-linked loci relative to autosomal loci (Wilcoxon Z = −3.86, p < 0.0001), whereas the eastern sympatric region shows more introgression at X-linked loci (Z = 9.28, p < 0.0001) and the western sympatric region does not show a significant difference (Supporting Information Figure S9; Z = −1.2, p = 0.22).
= 87, p < 0.0001). The geographic region where each species occurs at lowest relative abundance is also where the highest amount of admixture from the other species is found. In D. subquinaria, this is the eastern sympatric region and in D. recens this is the western sympatric region (both species: steel method using this region as a control, all comparisons p < 0.0001). When the X-linked and autosomal loci are considered separately, there is significantly reduced introgression of the X-chromosome loci compared to the autosomes within every geographic region of D. subquinaria (Supporting Information Figure S9; all p < 0.0001 with Wilcoxon rank sum test). In contrast, within D. recens the allopatric region shows significantly reduced admixture of X-linked loci relative to autosomal loci (Wilcoxon Z = −3.86, p < 0.0001), whereas the eastern sympatric region shows more introgression at X-linked loci (Z = 9.28, p < 0.0001) and the western sympatric region does not show a significant difference (Supporting Information Figure S9; Z = −1.2, p = 0.22).
3.4 Patterns of mate discrimination by D. recens females
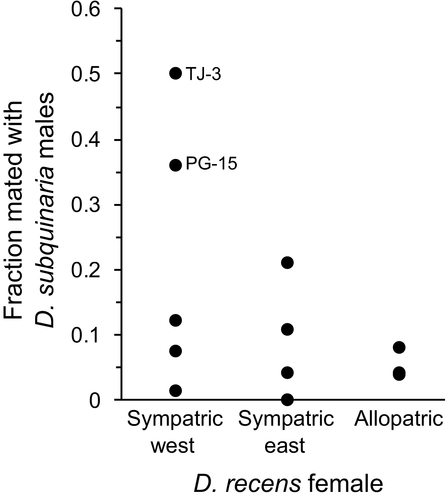
We first consider mate discrimination by D. recens females from the western sympatric populations where they are at low frequency relative to D. subquinaria. We do not find any premating isolation between these females and conspecific males from the eastern side of the Rockies, nor do we find that these females discriminate against D. subquinaria males from their same population more than D. subquinaria males from other geographic regions (male strain within male species: p > 0.05 for every line). Furthermore, all but one line (NB-8) shows significantly reduced mating with D. subquinaria relative to D. recens (Supporting Information Figure S11; male type: p < 0.0024 except NB-8, in which p = 0.494). Comparing across the geographic regions, females from the western sympatric region mate more with D. subquinaria than females from the rest of the geographic range (Figure 5; region effect: LRT 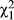 = 8.6; p = 0.014). Only considering the western vs. eastern sympatric regions this effect is also significant (region effect: LRT
= 8.6; p = 0.014). Only considering the western vs. eastern sympatric regions this effect is also significant (region effect: LRT 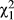 = 8.4; p = 0.004). Both of these patterns are driven by two lines in the western sympatric region that mate substantially more than the others with D. subquinaria (Figure 5). With RCD, we would predict that where D. recens is the rarer species premating isolation would be stronger; however, this is the opposite of what we observe.
= 8.4; p = 0.004). Both of these patterns are driven by two lines in the western sympatric region that mate substantially more than the others with D. subquinaria (Figure 5). With RCD, we would predict that where D. recens is the rarer species premating isolation would be stronger; however, this is the opposite of what we observe.
3.5 Patterns of mate discrimination in D. subquinaria
In stark contrast to previous work that showed that females from the eastern sympatric region never mate with D. recens males (Bewick & Dyer, 2014), we find that D. subquinaria from the western sympatric region show substantial variation in willingness to mate with D. recens (Supporting Information Figure S12). There is no significant difference in mating rate of females from the northern and southern transects with D. recens males (LRT 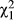 = 0.00002; p = 0.99), but there is significant variation in mating rate among populations and lines (both model effects p < 0.0001). Females mate less with D. recens males than with conspecific males from the same line in 37 of 41 lines (p < 0.05), and 31 lines remained significant at a Bonferroni corrected level (p < 0.013). Four lines (MT-11, CO-11, CO-12 and TA-5) mate with D. recens about the same amount as they mate with conspecific males. Considering mating rate with coastal allopatric D. subquinaria males, there is significant variation between the northern and southern transects, among populations, and among lines (every model effect p < 0.0001; Supporting Information Figure S12). Fifteen of the 41 lines had a reduced mating rate with conspecific allopatric D. subquinaria males relative to conspecific controls considering an uncorrected p < 0.05 (Supporting Information Figure S12), and five of these lines, which are all from different populations, remained significant after applying a Bonferroni correction (p < 0.013).
= 0.00002; p = 0.99), but there is significant variation in mating rate among populations and lines (both model effects p < 0.0001). Females mate less with D. recens males than with conspecific males from the same line in 37 of 41 lines (p < 0.05), and 31 lines remained significant at a Bonferroni corrected level (p < 0.013). Four lines (MT-11, CO-11, CO-12 and TA-5) mate with D. recens about the same amount as they mate with conspecific males. Considering mating rate with coastal allopatric D. subquinaria males, there is significant variation between the northern and southern transects, among populations, and among lines (every model effect p < 0.0001; Supporting Information Figure S12). Fifteen of the 41 lines had a reduced mating rate with conspecific allopatric D. subquinaria males relative to conspecific controls considering an uncorrected p < 0.05 (Supporting Information Figure S12), and five of these lines, which are all from different populations, remained significant after applying a Bonferroni correction (p < 0.013).
To investigate the broader geographic patterns of mate discrimination, we included additional mate discrimination data from the allopatric region to the west of these populations and from the sympatric region east of these populations (Supporting Information Figure S13; Bewick & Dyer, 2014). In general, as one moves east females are less likely to mate with either D. recens or D. subquinaria coastal males (Figure 6). We found significant variation across regions for mating with both D. recens (Wilcoxon rank sum test, 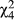 = 52; p < 0.0001) and coastal D. subquinaria males (
= 52; p < 0.0001) and coastal D. subquinaria males (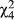 = 62; p < 0.0001). Considering mating with D. recens males, females from the eastern sympatric region mated less with these males than females from any other region (p < 0.0002), and northwestern sympatric females mated somewhat less with D. recens males than coastal allopatric (Z = −2.92, p = 0.029) and inland allopatric females (Z = −2.76, p = 0.051). All other regional pairwise comparisons had a p-value of >0.3. A similar pattern emerges for mating with coastal allopatric D. subquinaria males: eastern sympatric females mate less with these males than females from every other region (all p < 0.0001), and northwestern sympatric females show reduced mating compared both to coastal allopatric (Z = −3.78, p = 0.001) and inland allopatric females (Z = −3.52, p = 0.004).
= 62; p < 0.0001). Considering mating with D. recens males, females from the eastern sympatric region mated less with these males than females from any other region (p < 0.0002), and northwestern sympatric females mated somewhat less with D. recens males than coastal allopatric (Z = −2.92, p = 0.029) and inland allopatric females (Z = −2.76, p = 0.051). All other regional pairwise comparisons had a p-value of >0.3. A similar pattern emerges for mating with coastal allopatric D. subquinaria males: eastern sympatric females mate less with these males than females from every other region (all p < 0.0001), and northwestern sympatric females show reduced mating compared both to coastal allopatric (Z = −3.78, p = 0.001) and inland allopatric females (Z = −3.52, p = 0.004).
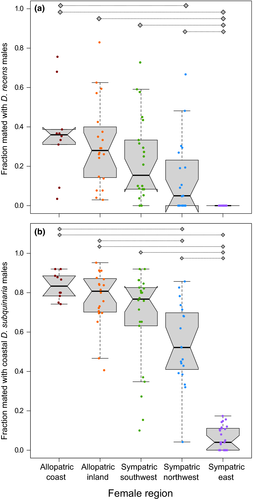
Consistent with these results, we found that the higher the relative prevalence of D. recens at a location, the stronger D. subquinaria females discriminate against both D. recens and D. subquinaria coastal allopatric males (Supporting Information Figure S14ab; both p < 0.0001). We repeated these analyses removing the eastern sympatric lines and the same pattern emerged (D. recens: r2 = 0.09, F1,69 = 6.5, p = 0.013; D. subquinaria coastal: r2 = 0.13, F1,69 = 9.9, p = 0.002). We also completed these analyses using population averages of the line means, and the results were consistent (Supporting Information Figure S14cd; p < 0.05). Interestingly, the two populations outside of the eastern sympatric region with the overall strongest level of discrimination (PG and TJ) and the highest proportion of D. recens are not the most proximate populations to the eastern sympatric region. This suggests there is small scale geographic variation in the effectiveness of reinforcing selection.
3.6 Cline analyses within D. subquinaria
We inferred the best-fitting cline for mate discrimination against allopatric D. subquinaria males, mate discrimination again D. recens males, population structure within D. subquinaria, admixture with D. recens and the prevalence of D. subquinaria relative to D. recens (Supporting Information Table S9; Figure 7 and Supporting Information Figure S15). The five clines are concordant, with all of them centred just to the east of the continental divide (c = 773–819 km on our transect, where the continental divide is at 765 km). The clines for mating with D. recens, population structure within D. subquinaria and admixture with D. recens are centred within 3 km of each other between Lake Louise (LL, 779 km) and Canmore (CA, 797 km) and are extremely narrow (w = 3.8–18 km). The clines for discrimination against allopatric D. subquinaria and the prevalence of D. subquinaria are wider (63 and 109 km, respectively) but overlap with the others.
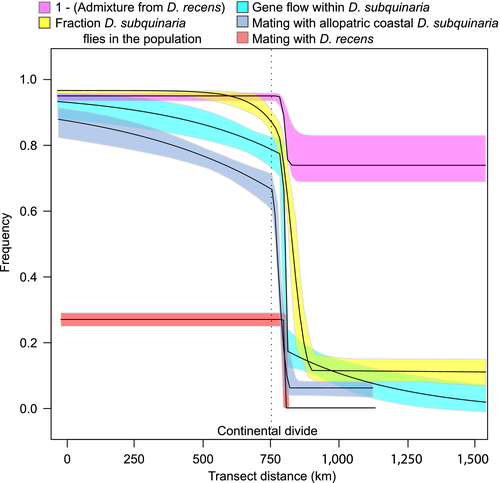
The strength of selection (s) that we infer from the cline widths is extremely strong for mating with D. recens (Supporting Information Table S9) and ranges from s = 0.13 to 1.0 as dispersal distance increases. In contrast, the highest s we estimate for mating with D. subquinaria allopatric males is 0.0023, and the population structure within D. subquinaria is much stronger than this, with s ranging from 0.01 to 0.05. The difference in these clines may indicate that genetic differentiation within D. subquinaria contributes to incipient premating isolation among conspecific populations. The transition in species composition from allopatry to sympatry is relatively weak (s = 0.0001–0.0008) especially compared to selection inferred from the amount of admixture in D. subquinaria from D. recens (s = 0.003–0.03). Thus, selection for discrimination against mating with D. recens is extremely effective even in the face of some admixture from D. recens.
4 DISCUSSION
Even when hybrid fitness is low, reinforcement may not always be the outcome of sympatry between hybridizing species. Determining the conditions under which reinforcement occurs is an important part of understanding its contribution to the speciation process. Here, we study natural populations of two closely related species, Drosophila subquinaria and Drosophila recens, to investigate how migration and selection interact to shape patterns of premating isolation. Overall, we find that reinforcing selection can be extremely strong, with differences in mate discrimination occurring on the scale of a few kilometres and in the face of gene flow both from allopatric populations and from introgression from the other species.
4.1 Drosophila recens
We discovered D. recens at low-to-moderate prevalence to the west of the Canadian Rockies (Figure 1). These populations appear to be stable rather than one-off long-distance migration events, as we found them in several locations and during different years. These western populations are not genetically differentiated from the sympatric populations to the east of the Rockies (Figure 2), and thus, this is probably an evolutionarily recent range expansion. Perhaps flies migrated across the Rockies to the north of our northern transect, where the Rockies are at their narrowest and there is a lake that spans across the range (Supporting Information Figure S16). Considering the entire geographic range of D. recens, there is a general pattern of moderate east–west genetic differentiation, and we find two higher levels of structure among populations. The first is between the Smoky Mountains TN population and the main part of the range (Figure 2); this population is geographically isolated at the southern edge of the Appalachian Mountains (Figure 1), and the mtDNA shows a similar pattern of differentiation (Jaenike et al., 2006). The second is between populations to the west of the Great Lakes and populations near and to the east of the Great Lakes (Figure 2), a pattern which does not occur in the mtDNA. There is not a difference in either female discrimination against D. subquinaria or in male signal traits between these regions (Figure 5; Dyer, White, Sztepanacz, Bewick, & Rundle, 2014; Rundle & Dyer, 2015), and there are no geological breaks at this boundary, thus it is unknown what drives this pattern.
We expect reinforcing selection on D. recens to be strongest in the very western region where it is at a lowest prevalence relative to D. subquinaria. However, females from this region do not show enhanced mate discrimination (Figure 5). D. recens segregates genetic variation for discrimination (Figure 5; Jaenike et al., 2006), and experimental evolution studies in other species indicate that premating isolation due to reinforcing selection can increase in under ten generations (Higgie, Chenoweth, & Blows, 2000; Matute, 2010b, 2015; Rice & Hostert, 1993). Thus, even if these western populations are a recent range expansion they probably have had sufficient time to respond to selection.
The lack of a detectable pattern of RCD in the western region and more generally across the range of D. recens may be a result of gene flow. Sufficient migration can homogenize populations to eliminate a signature of character displacement, even if reinforcing selection is strong (Servedio & Kirkpatrick, 1997). It is also possible that hybrid male sterility alone may not be sufficient to select for reinforced mate discrimination unless combined with other demographic or ecological forces that drive disrupting selection between populations. We note that our use of no-choice mate trials may underestimate RCD that may be uncovered by more sensitive choice experiments (Dougherty & Shuker, 2015), and character displacement may occur in a reproductive isolating barrier or an ecological trait that has not been studied. For instance, Drosophila yakuba shows RCD in postmating gametic isolation but not in behavioural isolation against the sympatric species Drosophila santomea (Comeault, Venkat, & Matute, 2016; Matute, 2010b).
4.2 Drosophila subquinaria
In contrast to D. recens, D. subquinaria exhibits a strong pattern of RCD consistent with both classical and cascade reinforcing selection (Bewick & Dyer, 2014; Jaenike et al., 2006). All previously characterized sympatric populations were to the east of the Rockies where D. subquinaria is less abundant than D. recens. In this study, we find that D. subquinaria females from the newly discovered sympatric region west of the Rockies also show RCD against mating with both D. recens males as well as with conspecific allopatric males, although discrimination is substantially weaker than the eastern sympatric populations (Figure 6a). Our analysis of genetic differentiation suggests that populations in the northern transect of the western sympatric region are much less differentiated from the eastern sympatric populations than those in the southern transect (Figure 3). The overall pattern of genetic differentiation is more gradual than step-like at the Rocky Mountains, as was previously characterized for this species (Bewick & Dyer, 2014), and there is much more gene flow across the range than previously appreciated.
We identify two lines of evidence that suggest that reinforcing selection is strong and can vary on small spatial scales. First, among the western populations discrimination against D. recens males is stronger where D. recens is more abundant (Supporting Information Figure S14). This variation occurs in the face of substantial gene flow among populations (Figure 3) and thus indicates that the strength of reinforcement (and the response to this source of selection) can vary over small geographic scales. It is surprising that discrimination by D. subquinaria females against D. recens males is not even stronger in these western sympatric populations. There is ample genetic diversity for mate discrimination against D. recens, and the cost of heterospecific mating is very high. One possibility is that matings with D. recens males are not common, perhaps due to their low abundance, which can effectively reduce the strength of reinforcing selection if the “choosy” alleles are rarely are exposed to selection (Nosil, 2013; Yukilevich, 2012). In addition, genetic differentiation from allopatric populations of D. subquinaria is relatively small (Figure 3) and migration from nearby allopatric populations may move low discrimination alleles into these populations.
Second, we find that the species-wide cline in mating with D. recens males is extremely narrow at less than 3 km (Figure 7). For example, flies from Canmore, AB, strongly discriminate against D. recens, whereas flies from just tens of kilometres up the Bow River from Lake Louise, AB, do not. Both of these populations are to the east of the continental divide and the ridge of the Rocky Mountains, and thus the cline in mate discrimination against D. recens males is not maintained by current geological barriers. The inferred selection coefficient on mating with D. recens is upwards of lethality (s = 1), as one would expect from the strong reduction in hybrid fitness due to the combined effects of Wolbachia infection and hybrid male sterility. The species prevalence also changes substantially in this region, although the transition is not nearly as sharp. The narrowness of this transition in mate discrimination is remarkable given the geographic range of this species spans thousands of km and the inherent ability of flies to migrate.
Other systems where reinforcement has been studied on a fine scale also show a rapid transition in the reinforced phenotype. For instance, D. yakuba shows variation in gametic isolation against D. santomea over an altitudinal transect on Sao Tome island (Comeault et al., 2016). In the only other instance we are aware of where the strength of selection was inferred from a cline in a reinforced phenotype, Phlox plants show clines in flower colour due to reinforcement that are under 4 km wide and with selection coefficients up to 0.22 (Hopkins, Guerrero, Rausher, & Kirkpatrick, 2014). At the broader level, few traits have as high a selection coefficient as we find in this study, suggesting that classical reinforcement in D. subquinaria (as well as the other cases of reinforcement discussed here) are some of the strongest instances of local adaptation known (Hoekstra et al., 2001; Linnen & Hoekstra, 2009).
We also find that female D. subquinaria from the western sympatric populations discriminate moderately against allopatric coastal D. subquinaria males (Figure 6b). Like for discrimination against D. recens males, RCD is not nearly as strong as in the eastern sympatric region. Similar to mating with D. recens, we find two patterns consistent with cascade reinforcement responding to selection at a fine geographic scale. First, as with discrimination against D. recens males, female discrimination among the western populations against coastal allopatric D. subquinaria increases with the prevalence of D. recens (Supporting Information Figure S14). A similar pattern has been observed in D. yakuba: along the same altitudinal transect as females vary in fertility with D. santomea males, they also differed in fertility when mated with conspecific males from allopatry vs. sympatry (Comeault et al., 2016). Second, the inferred species-wide cline in mate discrimination against coastal allopatric D. subquinaria males has a width of 66 km, indicating substantial geographic variation. This cline is thus much wider than for mating with D. recens and also much wider than the cline inferred using intraspecific population genetic differentiation, which has a width of 15 km. Thus, genetic differentiation among populations may strengthen incipient isolation among populations of D. subquinaria, especially in the eastern sympatric region.
Cascade reinforcement is expected to evolve a byproduct of classical reinforcement and not as a result of direct selection (Hoskin & Higgie, 2010; Howard, 1993; Ortiz-Barrientos et al., 2009). Furthermore, theory suggests that for cascade reinforcement to evolve, there must either be no gene flow among sympatric and allopatric populations or with gene flow there must be substantial ecological divergence (McPeek & Gavrilets, 2006; Pfennig & Ryan, 2006; Yukilevich & Aoki, 2016). While cascade reinforcement may occur in the eastern sympatric populations in part because gene flow from the western populations is low, our finding of variation in conspecific mate discrimination among western sympatric populations is surprising given that these populations are only weakly genetically differentiated from allopatric populations. We would not expect any selection for discrimination against conspecific males in these populations given there is no known postzygotic fitness consequence to these matings, and there also is no known ecological divergence among them. Instead, an alternate hypothesis for this observation is that the genetic basis of discrimination against D. recens and allopatric D. subquinaria males may be in linkage disequilibrium or pleiotropic, such that selecting on one trait confers the other. Combined with this, perhaps the western populations with relatively more D. recens (e.g., Prince George BC) have more recent migrants of both species from the eastern sympatric region. This input of high discrimination alleles could increase the overall level of discrimination in these populations even if reinforcing selection was weak. If these alleles reduce the overall fitness of their carriers in allopatry because they have a reduced mating rate with local conspecifics then they would be expected to be slightly deleterious and not spread despite otherwise abundant gene flow with nearby populations.
4.3 Hybridization and subsequent introgression between species
Some interspecific hybridization is necessary for reinforcing selection to occur (Coyne & Orr, 2004). There must be occasional introgression from D. recens into D. subquinaria, as ~3% of D. subquinaria harbour a D. recens mtDNA haplotype (Bewick & Dyer, 2014; Jaenike et al., 2006). Our analyses of nuclear loci suggest that introgression between species occurs throughout the genome (Figure 4), and, like for the mtDNA it is biased from D. recens into D. subquinaria. When D. recens females and D. subquinaria males mate, the hybrid daughters are infected with Wolbachia and can produce viable offspring when backcrossed to males of either species. As a result, nuclear loci have the potential to introgress in either direction. If this is the primary way that hybrids are formed, then our observation of asymmetric introgression from D. recens into D. subquinaria is consistent with these F1 hybrid females backcrossing more often with D. subquinaria males. However, this asymmetric pattern could also result from the reciprocal cross in which D. subquinaria females mate with D. recens males. In this case, the F1 hybrid daughters would not be infected with Wolbachia and only backcrosses to D. subquinaria males would produce viable offspring (most offspring from backcrosses with D. recens die due to CI). While “choosy” sympatric D. subquinaria females never mate with D. recens males in the laboratory, in nature this cross could occur with a recent migrant “nonchoosy” female from allopatry.
Even with some introgression, the overall amount of admixture is very low, suggesting that the combined effects of low fitness hybrids and reinforcement are effective in maintaining species boundaries. Within each species, the signature of admixture is strongest where it is rarest relative to the other species; in D. subquinaria this is in the eastern sympatric region and in D. recens this is in the western sympatric region (Supporting Information Figure S9). We note that caution should be used in inferring admixture from clustering methods such as structure (Lawson, Dorp, & Falush, 2018). However, we note that a similar pattern emerges using FST between populations of the two species, where the lowest genetic differentiation is found between D. recens and the eastern sympatric D. subquinaria populations (Supporting Information Table S10; Supporting Information Figure S17). In addition, synonymous nucleotide divergence (Ks) using DNA sequences mirrors this pattern, with lower divergence between D. recens and eastern sympatric D. subquinaria (Ks = 0.069) than between D. recens and allopatric D. subquinaria (Ks = 0.079; Humphreys, Rundle, & Dyer, 2016). The lower differentiation in the sympatric region suggests that most of the introgressed genetic material is removed by selection, although asymmetric migration within species may also contribute to this pattern. It is surprising that the signature of admixture in the western sympatric region is not higher, even though both species co-occur and neither exhibits strong mate discrimination against the other. This suggests that hybridization is rare in nature and/or that when hybrids are produced they have very low fitness.
The signature of admixture between species has been tested in some other systems undergoing reinforcement, and results vary in the magnitude and direction of introgression (e.g., Burri et al., 2015; Kulathinal, Stevison, & Noor, 2009; Lemmon & Juenger, 2017; Roda, Mendes, Hahn, & Hopkins, 2017; Turissini & Matute, 2017). A similar pattern to our system is seen in Phlox plants, where introgression between species occurs in sympatric but not in allopatric populations and the direction of introgression is asymmetric (Roda et al., 2017). In contrast, collared and pied Ficedula flycatchers are not more differentiated in sympatric vs. allopatric populations (Burri et al., 2015), and in D. yakuba and D. santomea introgression is rare in the genome and tracts appear to be old (Turissini & Matute, 2017).
Finally, we observe a pattern of reduced X-chromosome introgression compared to the autosomes that is specific to D. subquinaria (Supporting Information Figure S9). Because introgression is through females (hybrid males are sterile for several generations of backcrossing), this suggests that selection acts against the D. recens X-chromosome in the D. subquinaria genetic background. The X(Z) chromosome often shows increased differentiation relative to the autosomes (Muirhead & Presgraves, 2016; Payseur & Rieseberg, 2016), and both “Large-X” and “Faster-X” patterns have been found in many species (Charlesworth, Campos, & Jackson, 2018; Charlesworth, Coyne, & Barton, 1987; Meisel & Connallon, 2013; Presgraves, 2008). In particular, the X(Z)-chromosome is known to play a role in reinforcement in Ficedula flycatchers (Saether et al., 2007; Saetre & Saether, 2010) as well as other Drosophila (Noor et al., 2001; Ortiz-Barrientos, Counterman, & Noor, 2004). Further studies are necessary to determine the cause of this pattern in our system.
5 CONCLUSION
A major goal of speciation research is to characterize the selective processes that generate reproductive isolation as well as how demographic forces promote or hinder this divergence. Here, we build upon previous knowledge of a unique Drosophila system to investigate variation in both classical and cascade reinforcement among natural populations. We show that reinforcing selection can be extremely strong and can occur in the face of gene flow within species and hybridization between species. Ongoing and future studies investigate the genetic basis of both reinforced phenotypes that occur in Drosophila subquinaria and will use whole genome data sets to quantify the timing and magnitude of gene flow within species and introgression between species. This will also allow us to identify which regions of the genome freely cross these barriers and those that do not, the latter of which may contain candidates for reproductive isolation within and/or between species.
ACKNOWLEDGEMENTS
We are grateful to J. Jaenike for sharing DNA samples, D. Hall for useful discussion, and three anonymous reviewers for comments that improved the paper. This work was funded by National Science Foundation grants DEB-1149350 to KAD and DEB-1110462 to ERB and KAD, and by the NIGMS of the NIH under award T32GM007103 to ERB.
AUTHOR CONTRIBUTIONS
K.A.D. and E.R.B. conceived of the study; K.A.D., E.R.B., B.E.W., M.J.B. and D.P.H. collected data; K.A.D. and E.R.B. performed data analyses and wrote the manuscript.
DATA ACCESSIBILITY
Behavioural data, microsatellite genotypes, and the code for running analyses are available in Dryad (https://doi.org/10.5061/dryad.80c3q54).




