Colour vision variation in leaf-nosed bats (Phyllostomidae): Links to cave roosting and dietary specialization
Abstract
Bats are a diverse radiation of mammals of enduring interest for understanding the evolution of sensory specialization. Colour vision variation among species has previously been linked to roosting preferences and echolocation form in the suborder Yinpterochiroptera, yet questions remain about the roles of diet and habitat in shaping bat visual ecology. We sequenced OPN1SW and OPN1LW opsin genes for 20 species of leaf-nosed bats (family Phyllostomidae; suborder Yangochiroptera) with diverse roosting and dietary ecologies, along with one vespertilionid species (Myotis lavali). OPN1LW genes appear intact for all species, and predicted spectral tuning of long-wavelength opsins varied among lineages. OPN1SW genes appear intact and under purifying selection for Myotis lavali and most phyllostomid bats, with two exceptions: (a) We found evidence of ancient OPN1SW pseudogenization in the vampire bat lineage, and loss-of-function mutations in all three species of extant vampire bats; (b) we additionally found a recent, independently derived OPN1SW pseudogene in Lonchophylla mordax, a cave-roosting species. These mutations in leaf-nosed bats are independent of the OPN1SW pseudogenization events previously reported in Yinpterochiropterans. Therefore, the evolution of monochromacy (complete colour blindness) has occurred in both suborders of bats and under various evolutionary drivers; we find independent support for the hypothesis that obligate cave roosting drives colour vision loss. We additionally suggest that haematophagous dietary specialization and corresponding selection on nonvisual senses led to loss of colour vision through evolutionary sensory trade-off. Our results underscore the evolutionary plasticity of opsins among nocturnal mammals.
1 INTRODUCTION
The capacity for colour vision varies among nocturnal animals, raising numerous questions about sensory adaptation and evolution in low-light contexts (Kelber & Roth, 2006). While many nocturnal animals are monochromatic (completely colour-blind), others possess dichromacy and are capable of some chromatic distinctions (Jacobs, 2012; Melin, Danosi, McCracken, & Dominy, 2014; Veilleux, Louis, & Bolnick, 2013; Zhao, Rossiter, et al., 2009). Still others, including hawkmoths and geckos, possess three types of functional cone photoreceptors—trichromacy—and can use colour information while foraging under scotopic (extremely dark) conditions (Kelber, Balkenius, & Warrant, 2002; Kelber & Roth, 2006). Among mammals, behavioural evidence indicates that colour vision is possible under dim (mesopic) lighting, such as twilight and moonlight (Roth, Balkenius, & Kelber, 2008; Veilleux & Cummings, 2012), and may involve contributions from both rods and cones (Freitag & Pessoa, 2012). This realization has fuelled recent studies that are challenging the assertion that mammalian colour vision is useless at night (Tan, Yoder, Yamashita, & Li, 2005) and spurring efforts to understand variation in nocturnal colour vision by examining ecological and evolutionary correlates (Melin, Moritz, Fosbury, Kawamura, & Dominy, 2012; Melin, Matsushita, Moritz, Dominy, & Kawamura, 2013; Perry, Martin, & Verrelli, 2007; Veilleux et al., 2013). For example, work on euarchontan mammals (primates, colugos and treeshrews) implicates a critical role of habitat and foraging ecology in shaping opsin gene function and spectral tuning (Melin et al., 2016; Moritz, Lim, Neitz, Peichl, & Dominy, 2013; Veilleux et al., 2013).
Bats are a diverse and specialized radiation of mammals and an ideal group in which to evaluate the role of ecology on sensory evolution (Hayden et al., 2014). Extant species occupy two suborders, Yinpterochiroptera and Yangochiroptera, within the Order Chiroptera. Ecological niche divergence in diet and habitat preferences as well as pliability in sensory adaptation and evolution is present in both lineages (Lei & Dong, 2016; Teeling et al., 2002). Sensory specialization includes different forms of echolocation, diverse olfactory receptor arrangements and unique thermoreception abilities (Hayden et al., 2014; Jones, Teeling, & Rossiter, 2013). Importantly, colour vision variation within the Order is also documented (Zhao, Rossiter, et al., 2009). Among chiropterans, the leaf-nosed bats (family Phyllostomidae) display wide variation in form, physiology and ecology (Altringham, 1996; Figure 1). There are 192 described species within Phyllostomidae (Wetterer, 2000), dating back to a common ancestor ca. 52–20 million years ago (Teeling et al., 2005). Within this group alone, most diet and roost variation seen across the Order Chiroptera is represented (Gardner, 1977). Here, we explore the genetic basis of colour vision in 20 phyllostomid species and comparative outgroups, to further explore the question of how roosting and feeding ecology may have shaped the evolution of colour vision in a nocturnal niche. Like most other mammals, bats have duplex retinas populated by both rod and cone photoreceptors (Mueller, Goodman, & Peichl, 2007). Rods dominate in quantity and express rhodopsin (encoded by an RH1 opsin gene), which is highly light sensitive and responsible for low-light vision (Jones et al., 2013; Mueller et al., 2007; Shen, Liu, Irwin, & Zhang, 2010). Cone populations are, however, maintained across species and believed to play a role in chromatic discriminations (Zhao, Xu, Zhou, Flanders, & Zhang, 2009; Zhao, Rossiter, et al., 2009). In bats, as in other nonprimate placental mammals, the existence of two discrete cone opsins—conferring dichromatic vision—is the ancestral and normative character state (Davies, Collin, & Hunt, 2012; Jacobs, 2012). The long-wavelength-sensitive opsin gene (OPN1LW) encodes opsins in L-cones with peak sensitivities in the green to red range (530–563 nm), while the short-wavelength-sensitive opsin genes (OPN1SW) encode opsins in S-cones with peak sensitivities in the ultraviolet to violet range (360–440 nm) (Müller et al., 2009). Several amino acid sites are particularly important for the peak spectral sensitivity (“tuning”) of the opsins. OPN1LW and OPN1SW opsin genes feature five and ten tuning sites, respectively (Carvalho, Davies, Robinson, & Hunt, 2012; Hunt et al., 2007; Yokoyama, Starmer, Takahashi, & Tada, 2006; Yokoyama, Tada, Zhang, & Britt, 2008). A change at any of these sites may result in a change in colour perception. In addition, nucleotide insertions or deletions (indels) or other mutations (nonsense or missense) may impact protein folding or otherwise impede functionality. Despite the widespread presence of two opsins, pseudogenization of the OPN1SW gene has occurred repeatedly and independently in mammalian evolution, including some bats, leading to monochromacy (Davies et al., 2012).
Complete loss of colour vision occurs in some—but not all—mammalian species active in low-light environments and/or when senses other than colour vision are relied on for navigation, foraging and avoiding predators (Jacobs, 2012; Meredith, Gatesy, Emerling, York, & Springer, 2013). In the recent past, several instances of colour vision loss were documented within Chiroptera. Bats in the family Rhinolophidae have loss-of-function mutations in the OPN1SW opsin gene and are hypothesized to have lost cone-based dichromacy following the shift from ancestral, low-duty echolocation to a more sensitive form of high-duty echolocation which renders a far more complete acoustic “image” (Fenton, Faure, & Ratcliffe, 2012; Jones et al., 2013; Zhao, Rossiter, et al., 2009). Variation in diet or habitat might also shape the evolution of colour vision among bats. For example, UV-to-violet sensitive colour vision has been hypothesized to be beneficial for species that roost in trees, where twilight and moonlight may impact circadian cues by activating photosensitive pigments or facilitate visually guided navigation of obstacles or foraging under mesopic light levels (Jacobs, 2012; Walmsley et al., 2015; Zhao, Rossiter, et al., 2009). These selective constraints may be lost among species roosting in darker habitats, and independent pseudogenization of OPN1SW in several cave-roosting bats in the family Pteropodidae (Yinpterochiroptera) has been documented (Zhao, Rossiter, et al., 2009). Colour vision may also be more staunchly maintained by species foraging for fruit and nectar, for which colour cues may be more relevant to target detection and selection (Reviewed in Carvalho, Pessoa, Mountford, Davies, & Hunt, 2017), than for species that prey on insects, a task for which echolocation and visual acuity may be more important. Here, we seek to shed new light on the associations between species-specific ecology and the tuning and functionality of opsin genes underlying colour vision in the highly diverse radiation of leaf-nosed bats.
We examine relationships among roosting habits, diet and the genetic potential for colour vision by examining the OPN1LW and OPN1SW opsin genes across 20 phyllostomid species. We test the following predictions: (a) If light levels during roosting behaviour impact colour vision evolution, then tree-roosting species will show purifying selection maintaining both OPN1SW and OPN1LW opsin genes, while cave-roosting species will have OPN1SW pseudogenes. (b) If diet impacts opsin function, then frugivorous and nectarivorous species will show purifying selection maintaining colour vision in both opsins genes, while selection may be weaker or absent in OPN1SW opsins of insectivorous, carnivorous and haematophagous (vampire) bats. (c) If diet impacts opsin tuning, then shifts in the tuning of opsin genes, through amino acid variation at tuning sites, will be associated with primary food types eaten.
2 MATERIALS AND METHODS
2.1 Study species and sample collection
We isolated genomic DNA from liver and/or muscle samples from 21 species (20 phyllostomid and one vespertilionid species, Myotis lavali) (Table 1). We classified species into broad trophic categories—frugivore, nectarivore, insectivore, omnivores or haematophagous—based on the predominant food type in their diets (Table 1, Aguirre, Herrel, Van Damme, & Matthysen, 2002), although we acknowledge that while most phyllostomids specialize on a particular type of diet, they may also feed occasionally on other food items (Gardner, 1977).
| Family | Subfamily | Species | Trophic guild/Diet composition | Roost type |
|---|---|---|---|---|
| Phyllostomidae | Desmodontinae | Desmodus rotundus (n = 2) (b,d,e,g,k,l,n,o,p) | Sanguivore/blood (Greenhall, Joermann, Schmidt, & Seidel, 1983) | Caves, hollow trees (Greenhall, Joermann, Schmidt, & Seidel, 1983) |
| Phyllostomidae | Desmodontinae | Diaemus youngi (n = 1) (a,d,g,j,l,n,o,p) | Sanguivore/blood (Greenhall & Schutt, 1996) | Hollow trees, caves (Greenhall & Schutt, 1996) |
| Phyllostomidae | Desmodontinae | Diphylla ecaudata (n = 2) (d,e,h,j,m,n,o,p) | Sanguivore/blood (Greenhall, Schmidt, & Joermann 1984) | Caves, mines, hollow trees (Greenhall et al., 1984) |
| Phyllostomidae | Glossophaginae | Anoura caudifer (n = 3) (a,d,g,i,j,l,n,o,p) | Nectarivore/nectar/pollen, fruits, insects (Barros, Rui, & Fabián, 2013) | Caves, tunnels, hollow trees (Oprea, Aguiar, & Wilson, 2009), buildings (Barros et al., 2013) |
| Phyllostomidae | Glossophaginae | Glossophaga soricina (n = 7) (a,d,g,j,l,n,o,p) | Nectarivore/nectar/pollen, fruits, insects (Alvarez, Willig, Jones, & Webster, 1991) | Caves, tunnels, mines, hollow trees, buildings (Alvarez et al., 1991) |
| Phyllostomidae | Glossophaginae | Lonchophylla mordax (n = 2) (a,d,e,f,g,j,l,n,o,p) | Nectarivore/nectar/pollen, insects, fruits (Gardner, 2007) | Caves (Gregorin & Mendes, 1999) |
| Phyllostomidae | Phyllostominae | Lophostoma brasiliense (n = 5) (a,g,j,l,n,o,p) | Insectivore/insects, pollen (Munin, Fischer, & Gonçalves, 2012) | Termite nests in trees (York et al., 2008), buildings (Hice, Velazco, & Willig, 2004) |
| Phyllostomidae | Phyllostominae | Micronycteris minuta (n = 1) (a,g,l,n,o) | Insectivore/insects, fruits (López-González, 1998) | Hollow trees, caves (López-González, 1998) |
| Phyllostomidae | Phyllostominae | Micronycteris sanborni (n = 1) (d,f,g,j,m,n,o,p) | Insectivore/insects (Novaes, Laurindo, & Souza, 2015) | Hollow trees (Novaes et al., 2015), buildings (Nogueira, Pol, Pessôa, Oliveira, & Peracchi, 2015) |
| Phyllostomidae | Phyllostominae | Mimon bennettii (n = 1) (g,i,j,l,n,o,p) | Insectivore/insects, fruits, small vertebrates (Ortega & Arita, 1997) | Caves, mines, tunnels, hollow trees (Ortega & Arita, 1997) |
| Phyllostomidae | Phyllostominae | Mimon crenulatum (n = 1) (a,g,j,m,n,o,p) | Insectivore/insects, small vertebrates (Humphrey, Bonaccorso, & Zinn, 1983) | Hollow trees, buildings (Bernard & Fenton, 2003; Goodwin & Greenhall, 1961) |
| Phyllostomidae | Phyllostominae | Phyllostomus discolor (n = 9) (a,g,j,l,n,o,p) | Omnivore/nectar/pollen, fruits, insects (Kwiecinski, 2006) | Hollow trees, caves, foliage (Kwiecinski, 2006) |
| Phyllostomidae | Phyllostominae | Phyllostomus hastatus (n = 2) (a,d,g,j,l,n,o,p) | Omnivore/insects, small vertebrates, fruits, nectar/pollen (Santos, Aguirre, Vázquez, & Ortega, 2003) | Hollow trees, caves, termite nests, buildings (Santos et al., 2003) |
| Phyllostomidae | Phyllostominae | Tonatia bidens (n = 1) (a,d,g,i,j,m,n,o,p) | Omnivore/insects, small vertebrates, fruits (Esbérard & Bergallo, 2004; Martuscelli, 1995; Myers & Wetzel, 1983) | Hollow trees, caves, mines, buildings (Esbérard & Bergallo, 2004; Martuscelli, 1995) |
| Phyllostomidae | Phyllostominae | Trachops cirrhosis (n = 1) (a,d,g,j,l,n,o,p) | Omnivore/insects, small vertebrates, fruits (Cramer, Willig, & Jones, 2001) | Hollow trees, caves, tunnels, buildings (Cramer et al., 2001) |
| Phyllostomidae | Caroliinae | Carollia perspicillata (n = 2) (a,d,g,j,l,n,o,p) | Frugivore/fruits, nectar/pollen, insects (Cloutier & Thomas, 1992) | Caves, hollow trees, tunnels, foliage, buildings (Cloutier & Thomas, 1992) |
| Phyllostomidae | Stenodermatinae | Artibeus planirostris (n = 10) (a,d,g,i,j,m,n,o,p) | Frugivore/fruits, nectar/pollen, insects (Hollis, 2005; Reis, Peracchi, Pedro, & Lima, 2007) | Trees (Hollis, 2005) |
| Phyllostomidae | Stenodermatinae | Dermanura cinerea (n = 1) (a,d,g,j,l,n,o,p) | Frugivore/fruits, insects (Gardner, 1977) | Foliage (leaf tents) (Simmons & Voss, 1998) |
| Phyllostomidae | Stenodermatinae | Platyrrhinus lineatus (n = 3) (a,d,g,j,l,n,o,p) | Frugivore/fruits, nectar/pollen, insects (Willig & Hollander, 1987) | Foliage, caves (Willig & Hollander, 1987) |
| Phyllostomidae | Stenodermatinae | Sturnira lilium (n = 2) (a,d,g,i,j,m,n,o,p) | Frugivore/fruits, nectar/pollen, insects (Gannon, Willig, & Jones, 1989) | Caves, buildings, hollow trees (Gannon et al., 1989), foliage (Fenton et al., 2001) |
| Vespertilionidae | Myotinae | Myotis lavali (n = 8) (a,d,g,i,j,l,n,o,p) | Insectivore*/insects | Buildings (Moratelli & Wilson, 2013) |
- *Species recently described (Moratelli, Peracchi, Dias, & de Oliveira, 2011), for which natural history is poorly known. However, all Brazilian Myotis are insectivorous, and Marília A. S. Barros has observed insect parts in their faeces.
Bat tissue samples were obtained from specimens collected as vouchers during studies on bat occurrence, biology and ecology, and permission to use tissue samples for the present research was granted by the Brazilian Institute of the Environment and Renewable Natural Resources (IBAMA) (export permits # 13BR010191/DF and 16BR022149/DF). Bats were collected under Brazilian federal permissions given by the Chico Mendes Institute for Biodiversity Conservation (ICMBio) through the System for Authorization and Information on Biodiversity (SISBIO), between 2009 and 2015, at eleven Brazilian municipalities: four located in the state of Rio Grande do Norte (Natal, Nísia Floresta, Caraúbas and João Câmara; SISBIO Permits # 25233, 30730, 48325 and 53169), two in the state of Paraíba (Rio Tinto and João Pessoa; SISBIO Permits # 35846 and 45168), one in the state of Bahia (Igrapiúna; SISBIO Permit # 26934), one in the state of Minas Gerais (Januária; SISBIO Permit # 14875), one in the state of Santa Catarina (Florianópolis; SISBIO Permit # 17131) and two in the state of Rio Grande do Sul (Maquiné and Barracão; SISBIO Permit # 21438). Bats were captured in mist nets at the ground level and were euthanized using sulphuric ether or cervical dislocation following established protocols (Kunz & Parsons, 2009; Sikes & Gannon, 2011). Liver or muscle samples were preserved in absolute ethanol and kept frozen at ca. −18°C.
2.2 Isolation, amplification and sequencing of opsin genes
We isolated genomic DNA from tissue samples using a Qiagen DNeasy Blood and Tissue Kit, following the manufacturer's protocol. We amplified and sequenced partial exons 1 to 4, along with introns 1 and 3, of the short-wavelength-sensitive opsin gene (SWS1; OPN1SW). We also amplified and sequenced partial exons 1 to 5 of the long–wavelength-sensitive opsin gene (OPN1LW). Five sites of the OPN1LW are known to impact spectral tuning and are found on exon 3 (positions 180), exon 4 (position 197) and exon 5 of the OPN1LW (positions 277, 285, 308); the ten tuning sites of the OPN1SW gene are located on exon 1 (positions 46, 49, 52, 86, 90, 93, 97, 114, 116, 118; Yokoyama et al., 2006, 2008; Hunt et al., 2007; Carvalho et al., 2012). We note that OPN1LW amino acid numbering follows nomenclature for the human opsin and OPN1SW sequences follow bovine nomenclature.
We designed primer pairs for OPN1SW and OPN1LW opsin genes based on an alignment of sequences published in GenBank for related bat species using Primer3 (Supporting Information Tables S1 and S2). In addition, we used previously described OPN1SW (exons 1–3) and OPN1LW primers (Zhao, Rossiter, et al., 2009). In several cases, some species failed to amplify with our original primers and we designed new ones as needed (Table 1, Supporting Information Table S1). We amplified the target sequence by PCR using a BioRad C1000 Touch Thermocycler, as follows: 2 μl of template DNA, 2.5 μl of 10× DreamTaq buffer, 1.25 μl of each primer (10 μM), 2.5 μl dNTPs (2 mM each), 2 μl MgCl2 (25 mM) 13.38 μl ddH20 and 0.125 μl DreamTaq (5 U/μl). PCR conditions were optimized for each primer set (Supporting Information Table S1).
We visualized PCR products on 1% agarose gels against a 100-bp ladder (GeneRuler) to verify PCR success. PCR products that showed a single band in the gel were purified using ExoSAP-IT (Applied Biosystems) protocol. If multiple bands were present, we used the Promega Wizard SV Gel and PCR Clean-Up kit to isolate the band of interest, following the manufacturer's protocol. Purified products were directly Sanger-sequenced (ABI platform via Eurofins’ SimpleSeq service) with the same primer sequences used during amplification, with one exception. For OPN1SW exons 3–4 (primer set g), we designed new sequencing primers slightly offset from the PCR primers to increase specificity (Forward: 5′ TGCARTGTTCCTGTGGCCCCG 3′; Reverse 5′ GTATGGGATTGTAGACAC 3′).
We assembled the forward and reverse sequences into contigs using geneious version 10.0.2. The consensus sequences were aligned against other species from our experiment as well as additional reference sequences obtained from GenBank (Supporting Information Table S2) using the ClustalW function in Geneious. Alignments were manually adjusted as needed.
2.3 Phylogeny and selection analyses
For tests of selection, we aligned the opsin gene sequences we generated in addition to those from six additional species of interest (Microcebus murinus, Pteropus vampyrus, Pteropus alecto, Miniopterus natalensis, Rousettus aegyptiacus and Artibeus jamaicensis; Figure 2, accession numbers listed in Supporting Information Table S2). Relationships among species were inferred using divergence dates calculated with TimeTree ( www.timetree.org, accessed February 2018).
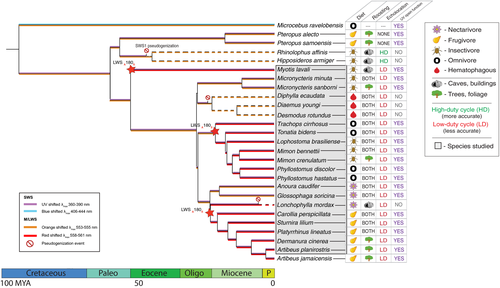
We tested for evidence of purifying or relaxed selection acting on OPN1SW and OPN1LW using codeml branch models implemented in paml (Yang, 2007) and the program relax (Wertheim, Murrell, Smith, Kosakovsky Pond, & Scheffler, 2015). In both programs, we tested whether there was a difference in selection acting on branches with putative pseudogenes vs. functional genes (OPN1SW) or with regard to roosting or diet style (OPN1LW) and compared branches. In both paml and relax analyses of OPN1SW, we compared the evolutionary scenarios of pseudogenization as one ancestral event vs. convergent events against null models in an effort to illuminate the evolutionary history of colour vision loss in the lineage. We removed premature stop codons present in the OPN1SW alignment by replacing the deletions leading to them with ambiguous characters (Ns). In addition to branch models, for OPN1LW, we also used codeml site models to test whether any of the opsin tuning sites are under positive selection. When interspecific variation at spectral tuning sites was found, we inferred the ancestral state using maximum likelihood (JTT model) in MEGA v7.0 (Jones, Taylor, & Thornton, 1992; Kumar, Stecher, & Tamura, 2016; Nei & Kumar, 2000). For ancestral state reconstruction analyses, we included OPN1LW sequences from 18 additional outgroups within Chiroptera to resolve ambiguities at some nodes (accession numbers listed in Supporting Information Table S2).
3 RESULTS
3.1 Short-wavelength-sensitive opsin gene (OPN1SW)
We sequenced partial exons 1–3 of the OPN1SW opsin gene for 20 of the 21 species newly examined in this study. Our repeated attempts to amplify exons 1–3 of the OPN1SW opsin gene for Mimon bennettii were unsuccessful, which we attribute to low-quality DNA as this sample was difficult to work with for all analyses. We successfully obtained sequences for partial exons 3–4 of the OPN1SW opsin gene for all 21 species examined. We found variation in the persistence of a functional OPN1SW gene across phyllostomid species (Figure 2).
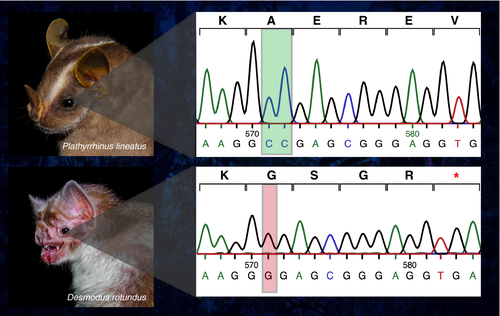
The OPN1SW gene for each of the three extant species of vampire bats, Desmodus rotundus, Diaemus youngi and Diphylla ecaudata, showed strong evidence of pseudogenization. Each of the three species possessed at least one deletion leading to a premature stop codon in exon 1. Diaemus youngi and Desmodus rotundus share one of the deletions in exon 1, suggesting that this is a shared mutation that occurred in the common ancestor of these two species. Several unique deletions were also present in exon 4 of D. youngi and in D. rotundus, resulting in other premature stop codons (Figure 3; Supporting Information Figure S1). None of the indels leading to a premature stop codon that we identified were shared between all three vampire bat species; however, the large number of single nucleotide polymorphisms and deletions that were present in each species may be obscuring a previously shared mutation. In addition, a mutation to the start codon is evident in the whole-genome sequence of Desmodus rotundus (Zepeda Mendoza et al., 2018). However, we were missing this sequence in D. youngi and D. ecaudata, as our primer design necessarily included the conserved regions leading up to the start codon and we cannot know whether this mutation is shared. We found evidence of a comparatively recent and independent pseudogenization of the OPN1SW gene of Lonchophylla mordax. A single nucleotide deletion has occurred in this species, leading to a premature stop codon in exon 1 (Supporting Information Figure S1). In addition, a unique Phe46Cys substitution in L. mordax was found; this site is known to affect the spectral tuning of the opsin, however, the substitution may have occurred after the loss of gene function (Supporting Information Figure S1).
The OPN1SW gene appeared to be intact and presumed functional for the remaining 16 phyllostomid bats and M. lavali, as we observed no indel, frameshift or nonsense mutations in the opsin coding regions (Figure 2, Supporting Information Figure S1). For all species with putatively functional OPN1SW genes, amino acids at the 10 sites known to impact the spectral tuning of the cone photopigment were invariant in the species we sequenced. This suggests that all species with a functional OPN1SW gene maintain a UV-sensitive OPN1SW opsin.
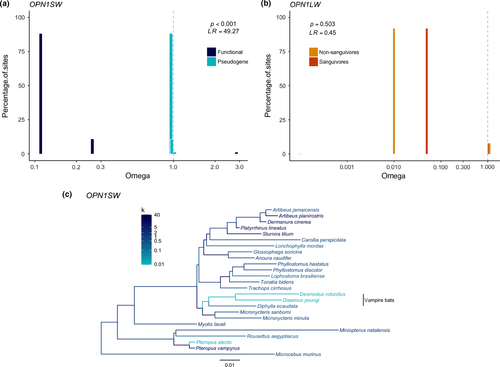
Within the OPN1SW gene of Phyllostomidae, we found a total of 101 variable sites of 310 examined. If we exclude vampire bats and L. mordax and include only the 16 species with seemingly intact OPN1SW genes, 52 amino acids of 310 positions are variable. Results of the paml analysis strongly favoured the two-branch models (M2) over the null model of a single ω value for all branches in the tree (M2 Hyp. 1: p < 0.00001, LR = 47.69; M2 Hyp. 2: p < 0.00001, LR = 45.14), indicating that branches with putative pseudogenes are under relaxed selection (ω = 0.80–0.81), while branches with functional genes are under purifying selection (ω = 0.13). We attempted to elucidate the evolutionary history of OPN1SW pseudogenization in the vampire bats by modelling the two hypotheses (ancestral vs. convergent pseudogenization) separately; however, the log likelihood scores for both models were very similar and could not be distinguished statistically.
Results of the relax analyses likewise supported the hypothesis that lineages with putative pseudogenes are under relaxed selection compared to those with functional genes (p < 0.0001, LR = 49.27) but could not distinguish between the two evolutionary scenarios of OPN1SW pseudogenization in vampire bats. In both relax models, ω values for all sites along pseudogenes were significantly closer to neutrality than ω values for sites along functional genes (Figure 4a). Estimates of the selection intensity parameter k further indicated relaxed selection in extant vampire bats, as well as in the ancestral branch of Desmodontinae (Figure 4c).
3.2 Long-wavelength-sensitive opsin gene (OPN1LW)
We successfully sequenced partial exons 1–5 of the OPN1LW opsin gene for all but one of the 21 sampled species. We failed to amplify exons 2, 3 and 4 of the OPN1LW of M. minuta and thus excluded this species from paml and relax analyses. The OPN1LW gene was intact and presumed to be functional for all species examined as we found no frameshift, indel or nonsense mutations (Supporting Information Figure S2). Within the 20 phyllostomid species, we found a total of 37 variable sites of 328 examined. Using the relax test, we found no difference in the intensity of selection acting on the different branches (p = 0.5, LR = 0.45; Figure 4b), indicating purifying selection is present across species, regardless of diet or roosting category (Supporting Information Figure S3). Results of the paml analysis of OPN1LW did not find the tuning sites to be under positive selection, but did indicate that three other sites (4, 20, 171) are under positive selection (p < 0.001, LR = 20.59). Despite this, we noticed substantial amino acid variation at one of critical tuning sites of the OPN1LW.
Tuning site 180 (exon 3) was dynamic among chiropteran species. To assign the ancestral state at this site for Phyllostomidae, and track patterns of substitutions occurring across the radiation of species, we examined exon 3 of our OPN1LW sequences and those of 18 additional bat species downloaded from GenBank (Supporting Information Figure S4). Across the Order Chiroptera, we found 18 species along four separate taxonomic branches possessed serine at site 180, while 21 species along six branches retained alanine as the ancestral character state (Figure 2, Supporting Information Figure S4). In addition, we found a substitution at a different tuning site, Ala285Tyr, in one of the reference species, Myotis ricketti (EU912345.1).
4 DISCUSSION
We examine the colour vision plasticity of leaf-nosed bats by sequencing the short-wavelength-sensitive (OPN1SW) and long-wavelength-sensitive (OPN1LW) opsin genes of 20 species with diverse dietary and roosting ecologies. Our principle results are threefold: (a) We find evidence of purifying selection for the OPN1SW and the OPN1LW opsin genes of most species of leaf-nosed bats, despite considerable interspecific variation in diet; (b) loss-of-function mutations have occurred in the OPN1SW gene of vampire bats and independently in a nectarivorous bat, L. mordax, a cave rooster. These events are phylogenetically independent from the pseudogenization of the OPN1SW gene previously reported in the other major radiation of bats, the Yinpterochiroptera (Zhao, Rossiter, et al., 2009); (c) We find considerable variation in the spectral tuning sites of the OPN1LW gene in the suborder Yangochiroptera. We are the first to report extensive variation at OPN1LW tuning sites within chiropterans.
4.1 OPN1SW variation
The lack of missense or nonsense mutations in sixteen of the twenty leaf-nosed bat species we sequenced, along with low substitution rates, suggests that the OPN1SW gene is under purifying selection in most phyllostomid bats. We did, however, find that Lonchophylla mordax has a premature stop codon in exon 1. Although we did not directly examine opsin function, a premature stop codon this early in the opsin gene—together with the absence of a resurrecting start codon nearby—strongly suggests lost function of the OPN1SW gene. Bats from the genus Lonchophylla are well known to use caves as roosts (Coelho & Marinho-Filho, 2002; Graham, 1988), and some species are considered highly dependent on cave habitats (e.g., Lonchophylla dekeyseri, Aguiar & Bernard, 2016). Although the roosting preferences of Lonchophylla mordax have not been studied extensively, the sparse available records indicate that this species, like other members of its genus, roosts mainly in caves (Gregorin & Ditchfield, 2005; Gregorin & Mendes, 1999; McCarthy, Albuja, & Manzano, 2000) or cavelike structures (Auler et al., 2006). Our results may therefore provide support for the hypothesis that cave-roosting relaxes selection on OPN1SW. Multiple cases of colour vision loss in the suborder Yinpterochiroptera linked to cave roosting (e.g., Genera Dobsonia and Rousettus), led Zhao, Rossiter, et al., (2009) to hypothesize that the behavioural innovation of cave roosting released OPN1SW from selective constraint due to the scotopic light environment. S-cones are involved in nonimage forming biological roles under mesopic conditions, and constraints linked with photoentrainment to day–night cycles may explain the persistence of the S opsin among mammals that are active in the canopy (e.g., tree roosting) at dawn and dusk (Allen, Brown, & Lucas, 2011). Additional evidence from other species of a link between scotopic roosting environments and evolutionary loss of colour vision would be instructive. For example, Lonchorhina aurita, thought to be closely related to the genus Lonchophylla, is reported to be strictly cave dwelling (Baker, Solari, Cirranello, & Simmons, 2016; Nowak, 1994; Rojas, Warsi, & Dávalos, 2016). Future work examining the opsin genes of Lonchorhina aurita and other neotropical cave specialists (e.g., families Natalidae, Furiperidae and Mormoopidae) as well as optionally cave-roosting bats (e.g., families Phyllostomidae, Molossidae and Vespertilionidae; Eisenberg & Redford, 1999) would add to our growing understanding of the link between roosting environment and colour vision evolution.
4.2 Loss of OPN1SW opsin function in Desmodontinae
All three species within Desmodontinae have a highly specialized diet—they feed exclusively on blood from mammals and birds (Greenhall, Joermann, Schmidt, & Seidel, 1983; Greenhall & Schutt, 1996; Ito, Bernard, & Torres, 2016). Desmodus rotundus shows a preference for mammalian prey (Bobrowiec, Lemes, & Gribel, 2015; Voigt & Kelm, 2006), while Diphylla ecaudata and Diaemus youngi preferentially prey on birds (Costa, Oliveira, Fernandes, & Esberard, 2008; Greenhall, Schmidt, & Joermann, 1984). Our results suggest OPN1SW pseudogenization and monochromacy occurred either: 1) in the last common ancestor of all vampire bats prior to their divergence ca. 23 mya, or 2) in the common ancestor of Desmodus rotundus and Diaemus youngi before their split ca. 14 mya, and independently in Diphylla ecaudata. The former explanation is more parsimonious, but could not conclusively be demonstrated. Either scenario suggests that changes linked to a haematophagous diet correlate with the loss of colour vision in the evolutionary radiation of this clade.
In contrast to many bats, which prefer to be more active under dim (mesopic) conditions early in the evening when colour cues may still be salient (Aguiar & Marinho-Filho, 2004; La Val, 1970; Melin et al., 2012; Roth et al., 2008), vampire bats are usually more active during the darkest (scotopic) hours and on evenings with reduced moonlight (Flores Crespo, Linhart, Burns, & Mitchell, 1972; Mitchell, Burns, & Kolz, 1973; Uieda, 1992). For example, common vampire bats (Desmodus rotundus) forage only after dark (Wimsatt 1969), and emerge from their roosts about 75–90 min after sunset, which is later than other species roosting in the same cave (Trajano, 1984). The change in diet and shift to more scotopic foraging may have rendered UV sensitivity and colour vision obsolete.
Vampire bats are known to rely heavily on nonvisual senses to locate prey. Desmodus rotundus has highly acute passive sound localization and the most sensitive hearing among bat species so far tested (Heffner, Koay, & Heffner, 2013). Due to specialized noise-sensitive neurons, common vampire bats are even able to detect and recognize breathing sounds of individual animals (Gröger & Wiegrebe, 2006; Schmidt, Schlegel, Schweizer, & Neuweiler, 1991). In addition to hearing, D. rotundus uses olfaction to locate prey (Bahlman & Kelt, 2007), and over short distances, it also relies on a thermoreception system that is unique among mammals and enables them to detect more vascularized areas on the prey's body surface (Gracheva et al., 2011; Jones et al., 2013; Kürten & Schmidt, 1982). Due to the high metabolic energy costs of sensory systems (Moran, Softley, & Warrant, 2015), an energetic prioritization may have been placed on the development of the nonvisual senses at the expense of the OPN1SW opsin protein. Loss of function in UV-sensitive opsins among bats has previously been linked to this form of sensory trade-off in Yinpterochiropteran bats (Zhao, Rossiter, et al., 2009). Vision is costly, and instances of cost–performance trade-offs for sensory information processing have been implicated in a wide variety of systems (Lan, Sartori, Neumann, Sourjik, & Tu, 2012; Moran et al., 2015; Niven & Laughlin, 2008). Deciphering whether the loss of UV vision among vampire bats was driven by an energetic trade-off or simply due to neutral evolution due to disuse in leaf-nosed bats (Yangochiroptera) would shed light on the mechanisms shaping the sensory evolution of these unique mammals.
4.3 Persistence of colour vision in phyllostomid bats, links with echolocation mechanisms
Setting vampire bats and L. mordax aside, other leaf-nosed bats with diverse ecological habits (Table 1) appear to have retained a functional OPN1SW gene, indicating that variation in diet (fruit, nectar or insects) does not affect UV perception significantly. This is perhaps surprising given the loss of the OPN1SW opsin functionality in insectivorous species, including rhinolophoid bats (in Figure 2, represented by Hipposideros armiger and Rhinolophus affinis) (Zhao, Rossiter, et al., 2009); however, there is at least one fundamental difference between these bats and leaf-nosed bats. Rhinolophoids have a highly acute, derived form of echolocation, known as “high-duty cycle echolocation” (Ho, Fang, Chou, Cheng, & Chang, 2013). Ultraviolet light sensitivity may be of relatively higher importance for the phyllostomids, which rely on low-duty cycle echolocation (Shen, Fang, Dai, Jones, & Zhang, 2013). It is plausible that colour vision and other senses are more important to species with less acute sonic perceptions. Most bats that use high-duty cycle echolocation are insectivorous (Fenton et al., 2012), and acute sonic perception appears to be sufficient to locate prey. Several of the leaf-nosed bat species in this study are also insectivorous, yet all possess the capacity for UV light perception. Our results therefore are also consistent with the hypothesis that sensory trade-off has occurred between high-duty echolocation and functional ultraviolet perception (Zhao, Rossiter, et al. 2009).
4.4 Functionality of OPN1LW
Our data indicate that the gene for the long-wavelength-sensitive opsin (OPN1LW) is functional and under purifying selection in all the species we investigated in our study. This suggests that sensitivity to longer wavelengths of light is largely maintained across the family Phyllostomidae. This retention of a functional long-wavelength-sensitive opsin is consistent with patterns observed across bats and all other nonmarine mammals examined to date (Jacobs, 2009; Meredith et al., 2013; Zhao, Rossiter, et al., 2009).
4.5 Spectral tuning of opsin genes
Regarding the hypothesis that diet or roosting behaviour will impact the tuning of opsins to different wavelengths of light, we do not find clear support. We found amino acid consistency at all known tuning sites of the OPN1SW, indicating uniform UV vision in phyllostomid bats with a functional OPN1SW regardless of the species-specific ecology. However, we report for the first time considerable variation in tuning of the OPN1LW opsin of Phyllostomids and other Yangochiropterans (Figure 2). To our knowledge, OPN1LW variation among closely related mammalian species (within the same family) is only known among treeshrews and primates, the latter group well known for polymorphic colour vision and high levels of inter- and intraspecific variation (Jacobs, 2009; Jacobs et al. 2017, Kawamura & Melin, 2018). Here, we report numerous shifts in amino acids at site 180 among taxa (Figure 2, Supporting Information Figure S4). Bat species with alanine at site 180 are predicted to be maximally sensitive to wavelengths of light ca. 553–558 nm; the λmax for species with serine at site 180 is red-shifted and predicted to be sensitive to light ca. 560–563 nm (Neitz, Neitz, & Jacobs, 1991; Yokoyama et al., 2008). While the spectral tuning of OPN1LW at site 180 is not clearly correlated with any broad dietary category we consider here (classification as fruit, nectar or insect-based diets, Figure 2), our results underscore the evolutionary plasticity of opsins among nocturnal mammals and may reflect habitat or finer-scale diet heterogeneity. Intriguingly, we also noted a shift at site 285 from Tyr to Ala in one of the reference sequences, Myotis ricketti. This substitution is predicted to reduce the spectral sensitivity of this species by 16 nm (Yokoyama et al., 2008). Taken together with previously reported OPN1LW tuning variation for Myotis velifer (Wang et al., 2004), the visual ecology of this genus may be of special interest in the future. Future study of the reflectance properties of foods together with the irradiance spectra of nocturnal illumination in the habitats of these species may provide insight into the pressures shaping variation in long-wavelength colour perception among bats.
5 SUMMARY AND CONCLUSIONS
We report the first evidence of colour vision loss in the suborder Yangochiroptera. Our results provide new support for the hypothesis that obligate cave roosting coincides with the loss of colour vision in bats due to relaxation of selective constraints imposed by tree roosting (Zhao, Rossiter, et al., 2009). We additionally reveal a new way that diet shapes colour vision evolution. Blood-feeding specialists have lost functional colour vision; their heavy reliance on thermoreception, olfaction or other senses, combined with vision being of little use for detecting large animal prey under scotopic conditions, may explain the cone-based monochromacy in this group. The ability for a bat species to persist without colour vision (or UV sensitivity more generally) may then be attributable to a combination of several ecological factors. In the case of the subfamily Desmodontinae, a change in diet and occupation of a very dark nocturnal niche, along with enhancement of other senses, may have led to the loss of violet and UV light sensitivity. In other lineages, a shift to high-duty echolocation might have set the stage for the eventual loss of colour vision and UV sensitivity. Finally, the evolutionary plasticity of the OPN1LW gene in nocturnal species is intriguing and somewhat surprising; future study may reveal how diet, habitat and other social or ecological pressures shape the colour vision of leaf-nosed bats.
ACKNOWLEDGEMENTS
We thank Drs. Carrie Veilleux, Shoji Kawamura, Nathaniel Dominy, Mrinalini Watsa and Eva Garrett for valuable comments and assistance; Clever Gustavo de Carvalho Pinto, Emmanuel Messias Vilar, Hannah Nunes and Juan Carlos Vargas-Mena for providing tissue samples; and Roberto Leonan Morim Novaes for the photographs of phyllostomid bats. We thank Dr. Valdir Pessoa who provided insight and expertise that greatly inspired the research. This work was supported by The Natural Sciences and Engineering Research Council of Canada (ADM 2017-03782), The Canada Research Chairs program (ADM) and a Summer Undergraduate Research Fellowship from Washington University in St. Louis (KK).
AUTHOR CONTRIBUTION
A.D.M. and D.M.A.P. designed the study. M.A.S.B. collected the samples. K.K. and M.A.S.B. assembled ecological data. G.D., K.K. and A.D.M. designed primers and performed the laboratory experiments. G.D., A.D.M., K.K., J.D.O. and M.C.J. analysed the data. A.D.M. and K.K. wrote the manuscript. All authors contributed to manuscript revisions and approved the final submission.
DATA ACCESSIBILITY
Nucleotide and amino acid sequences of OPN1SW (exons 1–4) and OPN1LW exons 1–5 are provided in Supporting Information Figures S2 and S3. Sequences generated in this study have been submitted to GenBank (Accession nos. MH179206–MH179247). Data files used for analyses in this study are available on DRYAD ( https://doi.org/doi:10.5061/dryad.fr68r1q).




