Comparative landscape genetics of two river frog species occurring at different elevations on Mount Kilimanjaro
Abstract
Estimating population connectivity and species' abilities to disperse across the landscape is crucial for understanding the long-term persistence of species in changing environments. Surprisingly, few landscape genetic studies focused on tropical regions despite the alarming extinction rates within these ecosystems. Here, we compared the influence of landscape features on the distribution of genetic variation of an Afromontane frog, Amietia wittei, with that of its more broadly distributed lowland congener, Amietia angolensis, on Mt. Kilimanjaro, Tanzania. We predicted high gene flow in the montane species with movements enhanced through terrestrial habitats of the continuous rainforest. In contrast, dispersal might be restricted to aquatic corridors and reduced by anthropogenic disturbance in the lowland species. We found high gene flow in A. wittei relative to other montane amphibians. Nonetheless, gene flow was lower than in the lowland species which showed little population structure. Least-cost path analysis suggested that dispersal is facilitated by stream networks in both species, but different landscape features were identified to influence connectivity among populations. Contrary to a previous study, gene flow in the lowland species was negatively correlated with the presence of human settlements. Also, genetic subdivision in A. wittei did not coincide with specific physical barriers as in other landscape genetic studies, suggesting that factors other than topography may contribute to population divergence. Overall, these results highlight the importance of a comparative landscape genetic approach for assessing the influence of the landscape matrix on population connectivity, particularly because nonintuitive results can alter the course of conservation and management.
Introduction
Tropical montane species are particularly vulnerable to global climate change, as increasing temperatures will force them towards higher elevations and off the mountain tops with consequent reduction of connectivity among populations and local extinctions (Parmesan 2006; La Sorte & Jetz 2010). Upslope distribution displacement has been reported for tropical (e.g. Raxworthy et al. 2008; Forero-Medina et al. 2011) as well as temperate species (Parmesan 1996; Pauli et al. 1996; Konvicka et al. 2003). Additionally, interspecific competition and differences in dispersal abilities should increase extinction rates, as warmer-adapted species and good dispersers will tend to out-compete and eradicate cooler-adapted, slower dispersers (Urban et al. 2012). Estimating rates of gene flow and environmental correlates that influence genetic connectivity is thus crucial for understanding the long-term persistence of species in the rapidly changing global environment (Allendorf et al. 2013; Manel & Holderegger 2013).
Over the last decade, landscape genetics has emerged as an important methodology to inform conservation management as it integrates spatial and environmental data to directly test the influence of the landscape matrix on the spatial distribution of genetic variation among populations (Manel et al. 2003; Storfer et al. 2007; Holderegger & Wagner 2008). Despite tropical and montane biotas being expected to experience the highest biodiversity losses in the near future (Ricketts et al. 2005), most landscape genetic studies have been conducted in temperate regions (Storfer et al. 2010; Manel & Holderegger 2013), either on relatively widespread low-elevation species (e.g. Coulon et al. 2006; Stevens et al. 2006; Richardson 2012; Kimble et al. 2014), or on species with wide altitudinal distributions (e.g. Funk et al. 2005; Giordano et al. 2007; Spear & Storfer 2008; Murphy et al. 2010; Savage et al. 2010). In contrast, contemporary genetic patterns, dispersal capacities or even basic knowledge of ‘true’ mountain-adapted species, especially in the tropics, are still lacking (La Sorte & Jetz 2010).
In this study, we compared genetic patterns of two congeneric tropical frog species occurring at different elevations along the southern slope of Mount Kilimanjaro in Tanzania. Rising from a dry and hot savannah up to a melting glacier, Mt. Kilimanjaro is the highest free-standing mountain in the world. Its slopes are shaped by a series of parallel deep valleys and ridges that result in the formation of multiple drainage systems (Fig. 1). The large elevational range and complex topography of this volcanic mountain offer an ideal system to analyse and compare fine-scale spatial genetic patterns of lowland vs. montane species. Amietia wittei (Angel, 1924) is a high-elevation-adapted frog with a limited, patchy distribution encompassing the central highlands of Kenya and northern Tanzania, including Mt. Meru and Mt. Kilimanjaro (Lötters et al. 2004). Here, it is found from ca. 1700 m in the montane rainforest and the above shrubland up to 3500 m (Zancolli et al. 2014a). Its presence has been associated with streams in montane grasslands, but very little is known about the ecology and life history of this frog (Lötters et al. 2004; Channing & Howell 2006). From our own observations, A. wittei is generally hard to detect and it breeds in streams, in creeks and, at higher elevations, in spring pools. In the lower part of the mountain, A. wittei is replaced by its more widespread congener, Amietia angolensis (Bocage, 1866). This widespread frog species ranges throughout a variety of habitat types in eastern and southern Africa from Eritrea and Ethiopia to the southern Democratic Republic of Congo, South Africa and Angola (Poynton et al. 2004; Channing & Howell 2006). On Mt. Kilimanjaro, A. angolensis is easily found in proximity of permanent water in a wide array of habitat types, including grassland, banana forests, coffee plantations and house gardens. It is active throughout the year and breeds in slow-flowing or still water at the edge of ponds, pools, streams or rivers (Channing & Howell 2006). Tadpoles grow to a large size and have a prolonged development that may last up to 2 years until metamorphosis (Channing 2004).
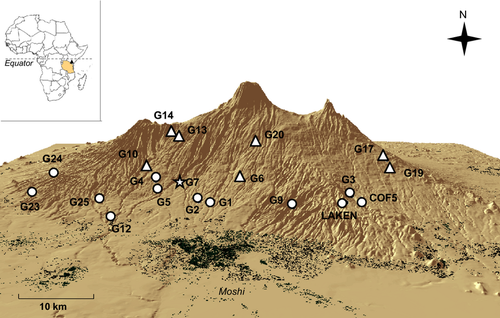
The lower part of Mt. Kilimanjaro is intensively populated by humans and agriculture dominated; the prevailing climate is dry with a main rainy season in March–May and few rains in November. Considering the association of A. angolensis with permanent water, we hypothesized that gene flow might be reduced across terrestrial habitats and likely restricted to aquatic corridors. Moreover, the presence of human settlements and topography might restrict gene flow among populations. Given that A. wittei is a montane specialist found in moist, continuous rainforests, we predicted high terrestrial dispersal and relatively high gene flow. Alternatively, it is possible that increased topographic complexity (e.g. more ridges and steeper slopes) at the higher elevations of Mt. Kilimanjaro might promote greater genetic differentiation among populations of A. wittei relative to A. angolensis.
Based on the hypotheses above, our objectives for this study were to (i) test whether A. wittei and A. angolensis show different patterns of genetic connectivity and population structure and (ii) identify which landscape features influence current gene flow.
Materials and methods
Study area
Mount Kilimanjaro is the remnant of a stratovolcano located in northern Tanzania (2°45′ to 3°25′S; 37°00′ to 37°43′E). At the foothills (700 m), the original vegetation has been almost entirely converted into croplands (maize and sunflowers), while different degrees of an agroforestry system called ‘homegarden’, with banana and coffee trees as main crops, are found between 1100 and 1700 m. Above, the mountain's ecosystems are protected by the Kilimanjaro National Park; however, the montane rainforest is regularly exposed to illegal logging, grazing, small-scale farming and fires (Lambrechts et al. 2002). Moreover, the high number of tourists attempting to climb the mountain (35 000 climbers per year; Peaty 2012) is an important source of disturbance and pollution.
Sampling and DNA extraction
We searched for tadpoles of Amietia wittei and Amietia angolensis at potential breeding sites from April to June and October to November in 2011. Geographic position of each site was recorded with a Garmin® GPSMAP 62S. In total, we sampled 20 sites: seven for A. wittei, 12 for A. angolensis and one site, G7, where both species were present (Fig. 1). Even though Amietia species are hard to tell apart based on gross morphology, we can exclude the possibility that we sampled frogs other than A. wittei and A. angolensis because no other species in this genus are known to occur in the area (Zancolli et al. 2014a,b). Nonetheless, species identity was verified by means of DNA barcoding (see below). Tissue samples were collected by tail clipping (<0.5 cm) of larvae at different developmental stages (Gosner 1960) to reduce sampling siblings. Considering the slow larval development in these anurans (Channing 2004), we assumed that tadpoles at distinct stages should have hatched at different times and were thus from different egg clutches.
Total genomic DNA was extracted using the Roche High Pure PCR Template Preparation Kit following the manufacturer's instructions, except for an extra step after the digestion by proteinase K aiming to discard residual pigments.
Genotyping and AFLP scoring
Amplified fragment length polymorphism (AFLP) markers were obtained using a modified version of Vos et al. (1995) (Appendix S1, Supporting Information). After an initial screening, we selected six primer combinations (Table S1, Supporting information). Fragments were separated in an ABI 3130xl automatic capillary sequencer (Applied Biosystems) with an internal GeneScan 500 ROX size standard. Bin positions and fluorescent intensity of any peaks occurring between 50 and 500 bp and higher than 50 relative fluorescent units (rfu) were determined using GeneMapper 4.1 (Applied Biosystems) and adjusted manually following the semi-automated scoring procedure described in Whitlock et al. (2008). For each primer combination, electropherograms with the sum of all peaks lower than the median fingerprint intensity were removed. Individuals with two or more missing combinations were excluded, whereas individuals with one missing combination were kept and loci were treated as missing value in further statistical analyses. Final binary matrices were generated with AFLPscore (Whitlock et al. 2008). All fingerprints were normalized and filtered using a locus selection threshold of 130 rfu and a phenotype-calling threshold of 100 rfu. All rejected loci were removed, and the peak intensity matrices were rerun so that normalization was based only on the retained loci. To assess the reliability of our AFLP markers, mismatch error rate was calculated using 39 randomly replicated samples which were distributed between sample plates. Markers with more than 5% mismatches were discarded. We also removed loci with band frequencies ≤3/N, which may be spurious peaks or contamination, and ≥ (1–3/N) which may be null alleles (Lynch & Milligan 1994).
DNA barcoding
Because Amietia species, especially at the larval stage, are cryptic and difficult to identify, we sequenced a fragment (mean length of 470 bp) of the 16S mitochondrial rRNA gene from 14 randomly selected individuals (GenBank Accession Nos.: KJ469265-KJ469278) to confirm species identity. For primer sequences and PCR protocol, refer to Barej et al. (2014). Sequences were aligned and edited with ClustalW2 (Larkin et al. 2007; default parameters), and MEGA 5.2 (Tamura et al. 2011) was used to compute uncorrected pairwise distances. To further distinguish the two species and possible hybrids from our AFLP data, we used the program NewHybrids 1.1 beta (Anderson & Thompson 2002), which uses the framework of Bayesian clustering to compute, using Markov chain Monte Carlo (MCMC) sampling, the posterior probability that individuals belong to distinct categories, namely pure (i.e. parental species), F1, F2 and backcrosses. This method does not require that allele frequencies be known in the parental species nor that separate, pure samples of the parental species be available.
Population genetic analysis
Summary statistics of genetic diversity for the two species and for each sample site were calculated with AFLP-SURV 1.0 (Vekemans et al. 2002), using a Bayesian method with nonuniform prior distribution of allele frequencies (Zhivotovsky 1999). We chose this method because it gave the most accurate results in a study using simulated and real AFLP data (Bonin et al. 2007). Allele frequencies were used to calculate FST following Lynch & Milligan (1994). FST has been commonly used to estimate levels of genetic differentiation among populations and therefore is useful for comparison with other landscape genetic studies. In addition to frequency-based approaches such as FST, we also estimated ΦPT via an analysis of molecular variance (AMOVA; Excoffier et al. 1992) implemented in GenAlEx 6.5 (Peakall & Smouse 2012). A pairwise individual-by-individual genetic distance matrix was generated following the method of Huff et al. (1993) based on shared band presence or absence and used as input matrix in AMOVA. A band-based method is preferred with dominant markers such as AFLP because it does not require additional assumptions (e.g. Hardy–Weinberg equilibrium) or information about population genotypic structure (e.g. FIS) to estimate allele frequencies; however, ΦPT considerably gives higher estimates of differentiation (Bonin et al. 2007).
We estimated genetic structure with the Bayesian clustering method implemented in the program STRUCTURE 2.3.4 (Pritchard et al. 2000; Falush et al. 2007), assuming admixture and correlated allele frequencies. Ten independent runs with a burn-in period of 100 000 replications and 1 000 000 MCMC iterations were performed for a number of populations K = 1–10. To improve the clustering, we also ran STRUCTURE using sampling locations as prior information. The LOCPRIOR model is recommended in most situations, especially in case of very weak structure, but it will ignore the location information when the ancestry of individuals is uncorrelated with sampling locations (Hubisz et al. 2009; Pritchard et al. 2010). Appropriate K values were selected by plotting the average values of the ln Pr(X/K) and evaluating the smallest K at which the curve plateaus and by means of the ΔK criterion (Evanno et al. 2005).
Least-cost path analysis
To test the influence of different landscape features on population structure, we developed ten different potential least-cost paths in ArcGIS 10 (ESRI) for the two species separately. Least-cost analysis was conducted with the “path distance” (which already corrects for topography) and “cost path” functions. The first model was a straight-line (Euclidean) distance between sites as a null model, which would be expected if there was no landscape influence on gene flow but merely isolation by distance (IBD; Wright 1942). The first least-cost path (LCP) was a modification of the null model, in which we calculated topographically corrected straight lines among all site pairs using a 30 × 30 m digital elevation model (DEM, J.A. Ong'injo, C. Lambrechts & A. Hemp, unpublished). All further resistance surfaces were calculated from the DEM.
The next LCPs represent hypotheses that gene flow is enhanced when minimizing slope, Roughness (rough) and compound topographic index (cti). The roughness index expresses the amount of elevation changes between adjacent cells, with low values indicating more flat areas (Riley et al. 1999). Cti is a steady-state wetness index, which is a function of both slope and upstream contributing area per unit width orthogonal to the flow direction; thus, it is strongly correlated with soil moisture and water catchment (Gessler et al. 1995). Areas with high cti values represent drainage depressions or plains, whereas low cti values correspond to areas with small catchments such as steep slopes, crests or ridges. To test whether frogs appear to preferentially move through areas with high ground moisture, we inverted the raster values so that low cti values (i.e. low cost) correspond to wet areas. These indices were calculated with the Geomorphometric and Gradient Metrics toolbox (available at http://evansmurphy.wix.com/evansspatial) in ArcGIS 10 (ESRI). Furthermore, we standardized the slope, roughness and cti surfaces to a specific range (0–10) using the “stretch” function which redistributes values of an input raster over a wider or narrower range of values in an output raster. We did not assign cost values to these continuous surfaces, but we used them directly into the least-cost analysis. Our 5th and 6th LCPs were based on the presence of streams. Because an exhaustive stream surface was not available for the Kilimanjaro region, we created a stream network surface using the ‘flow accumulation’ tool (Hydrology toolset, ArcGIS 10) which in its simplest form calculates the number of upslope cells that flow into each cell. The stream network can then be delineated by applying a threshold value to the flow accumulation output raster. We applied a threshold of 500, as it seemed to best capture the stream network in the area and as suggested in the ArcGIS tool resources. We then assigned a value of 1 to the stream cells and cost value of 2 or 10 to nonstream, upland cells. Thus, we obtained two stream-based resistance surfaces with cost ratios of 1:2 and 1:10, respectively. The last four models were based on multiplied effects of topography and stream network. These multivariate resistance surfaces were calculated by combining one topographic feature (slope or roughness) with the stream surface (1:2 or 1:10) for a total of four different combinations: roughness and stream 1:2, roughness and stream 1:10, slope and stream 1:2, and slope and stream 1:10. The rationale behind a multivariate approach is that we hypothesized that river frogs disperse along riverine habitats but, at the same time, try to avoid steep slopes, or crests and ridges (i.e. high roughness) when crossing terrestrial areas. Because some routes were very unlikely (e.g. passing through the glacier on top of Kibo, the highest peak), we assigned to all resistance surfaces a cost value of 5000 to the cells above 3500 m for A. wittei and those above 1800 m for A. angolensis. We chose these constraints based on the observed elevational limits of the two species.
For each resistance surface, we measured route lengths (i.e. topographic distances); in addition, we calculated the weighted average of the other landscape variables (i.e. cti, slope and roughness) along each route. We produced a weighted average by first multiplying each individual value by the percentage of the overall route that passed through pixels with that value and then adding individual calculations together (Spear & Storfer 2010). To test whether genetic differentiation was affected by land cover, we calculated the proportion of each cover type along the routes. The land cover surface (T. Appelhans & T. Nauss, unpublished) consisted of eight discrete habitat types, namely cropland, homegarden, human settlements, shrubland, forest, disturbed forest, reforestation and grassland. We did not develop LCPs based on habitat types because we lacked the empirical data necessary to parameterize accurately cost assignments when either species moves through specific cover types. Instead, we included the proportions of land cover as explanatory variables in the regression models (see Statistical Analyses below).
Statistical analyses
All statistical analyses were run with the software R (R Development Core Team 2012). The correlations between A. wittei and A. angolensis gene flow (estimated by FST and ΦPT) and landscape features were assessed using two methods. First, we employed a new mixed modelling approach (Van Strien et al. 2012). Methods such as linear regression or partial Mantel tests are most often applied in landscape genetic studies (Storfer et al. 2010); however, they do not take into account the dependency of pairwise genetic distances (Yang 2004). Clarke et al. (2002) described the maximum-likelihood population-effects (MLPE) model, in which the covariate structure is tailored for the specific dependency between values in a distance matrix. We followed the procedure described in Van Strien et al. (2012), in which the authors used Clarke's MLPE method to fit the regression models between genetic distances and a set of landscape variables. Our explanatory variables included geographic distances, topographic features (i.e. weighted average of cti, slope and roughness) and percentages of each cover type. Because we were interested in which landscape features best explained genetic variation, we calculated all possible combinations of explanatory variables for each LCP for a total of 10 240 models (both species). To select the best LCP models, we applied a suite of criteria and measures of fit. Although some authors did not recommend Akaike's Information Criterion (AIC) calculated from restricted maximum likelihood (REML) in linear mixed models with different fixed effects (Verbeke & Molenberghs 2000; Orelien & Edwards 2008), Gurka (2006) demonstrated that information criteria such as AIC, AICc, CAIC and BIC can be employed for REML mixed model selection and that, in many cases, the criteria actually performed better in choosing the proper set of fixed effects under REML compared with when using the maximum-likelihood (ML) estimation method. We thus used AICc and BIC to select the best models, and we employed measures of the fit such as log likelihood (logLik) and deviance for the REML criterion (REMLdev) to validate selection based on the information criteria.
To compare with the MLPE results, we also used the BIOENV procedure (Clarke & Ainsworth 1993) in the R package vegan (Oksanen et al. 2010). BIOENV tests all possible combinations of independent variables to find the best subset using a Spearman rank correlation (ρW). The function calculates Euclidean distances for all possible subsets of scaled environmental variables and finds the maximum (rank) correlation with the genetic distance matrix. Although BIOENV is an exploratory procedure, it has been successfully applied in other landscape genetic studies (e.g. Spear et al. 2005; Trumbo et al. 2013) to corroborate the most supported models from other statistical analyses (e.g. multiple linear regression on distance matrices; Smouse et al. 1986). For both MLPE and BIOENV analyses, we selected the best model for each LCP and reported the top two LCPs explaining most of the genetic differentiation.
Results
AFLP analysis
In total, we collected 502 samples from 20 sites. After AFLP data refinement, 180 samples of Amietia wittei and 301 of Amietia angolensis were successfully scored and analysed. The six primer combinations produced 235 polymorphic AFLP loci in A. wittei and 165 in A. angolensis. Final mismatch error rate was 3.45%, within the typical range for AFLPs (Bonin et al. 2007).
DNA barcoding
Genetic distances based on the 16S sequences ranged from 0–0.5% among individuals of the same species to 8% between putative A. wittei and A. angolensis, confirming the assignment into two separate taxa (Table S2, Supporting Information).
For each site, NewHybrids assigned all individuals to one of either species except for site G7 where we found both species and backcrossed individuals. In turn, possible hybrids were excluded from the data set.
Genetic diversity
Genetic diversity was generally low in both species (Table 1). The site with both species as well as hybrids (G7) had the highest genetic diversity (0.294 in A. wittei, 0.343 in A. angolensis) and an exceptional number of private bands (20–24). With NewHybrids, we detected backcrosses, and it could be that individuals assigned in the pure species category may not be completely pure but admixed individuals derived from consecutive intercrossing. To avoid bias or errors in estimates of population genetic structure, we consequently removed this site (G7) from further statistical analyses. We excluded the possibility of finding similar admixed individuals in other sites as there is no overlap in the geographic distributions of the two species (except in site G7). Expected heterozygosity (Hj) across A. wittei sites was similar to A. angolensis, whereas the proportion of polymorphic loci (%P) was significantly lower (Tables 1 and 2). Hj and %P were similar across sites for A. angolensis, except for G5 and G4 with the lowest and highest Hj values, respectively (0.223 and 0.287). Contrary to A. angolensis where no private bands were detected, in A. wittei, private bands were identified in four sites (Table 1). This difference may be partially due to the lower sample size of A. wittei compared with A. angolensis.
| Species | Pop | Elevation (m) | N | No. P loci | % P | Hj | SE (Hj) | Private bands |
|---|---|---|---|---|---|---|---|---|
| Aw | G13 | 3023 | 22 | 166 | 70.6 | 0.268 | 0.012 | 0 |
| G20 | 3012 | 26 | 152 | 64.7 | 0.242 | 0.012 | 1 | |
| G14 | 2978 | 18 | 160 | 68.1 | 0.261 | 0.012 | 0 | |
| G17 | 2527 | 19 | 159 | 67.7 | 0.228 | 0.012 | 1 | |
| G19 | 2359 | 35 | 174 | 74 | 0.255 | 0.012 | 6 | |
| G10 | 2027 | 20 | 153 | 65.1 | 0.229 | 0.012 | 0 | |
| G6 | 1992 | 26 | 145 | 61.7 | 0.234 | 0.013 | 1 | |
| G7 | 1705 | 14 | 176 | 74.9 | 0.294 | 0.012 | 20 | |
| Aa | G24 | 1708 | 19 | 119 | 72.1 | 0.258 | 0.015 | 0 |
| G7 | 1705 | 16 | 148 | 89.7 | 0.343 | 0.015 | 24 | |
| G4 | 1692 | 34 | 127 | 77 | 0.287 | 0.015 | 0 | |
| G3 | 1679 | 35 | 120 | 72.7 | 0.263 | 0.014 | 0 | |
| COF5 | 1658 | 25 | 119 | 72.1 | 0.266 | 0.015 | 0 | |
| LAKEN | 1658 | 18 | 116 | 70.3 | 0.268 | 0.015 | 0 | |
| G5 | 1532 | 20 | 122 | 73.9 | 0.223 | 0.014 | 0 | |
| G9 | 1416 | 15 | 114 | 69.1 | 0.263 | 0.012 | 0 | |
| G2 | 1321 | 24 | 118 | 71.5 | 0.266 | 0.015 | 0 | |
| G23 | 1306 | 20 | 116 | 70.3 | 0.234 | 0.014 | 0 | |
| G25 | 1276 | 36 | 122 | 73.9 | 0.272 | 0.015 | 0 | |
| G1 | 1270 | 28 | 118 | 71.5 | 0.270 | 0.015 | 0 | |
| G12 | 1097 | 11 | 119 | 72.1 | 0.253 | 0.015 | 0 |
| Species | N | Tot. markers | Tot. P | Ht | Mean % P | Mean Hj ± SD | Mean FST ± SD | Mean ΦPT ± SD |
|---|---|---|---|---|---|---|---|---|
| Aw | 8 | 395 | 235 | 0.285 | 67.4 ± 4.07 | 0.245 ± 0.02 | 0.106 ± 0.04 | 0.170 ± 0.06 |
| Aa | 13 | 351 | 165 | 0.295 | 72.2 ± 2.07 | 0.260 ± 0.02 | 0.086 ± 0.04 | 0.123 ± 0.06 |
| U-test | * | n.s. | * | ** |
- All statistical analyses were calculated excluding site G7 where hybridization occurs.
- Differences in mean estimates were calculated with Mann–Whitney U-tests, and the asterisks below each genetic estimate represent the significant level (**P < 0.01, *P < 0.05, n.s. = non significant).
Population structure
The overall level of genetic differentiation differed significantly between the two species (Table 2; Table S3 and S4, Supporting Information). Average FST values estimated across all loci and sample sites were 0.106 in A. wittei and 0.086 in A. angolensis (U = 905, P = 0.03). As expected, estimates of ΦPT were higher, with mean values of 0.170 in A. wittei and 0.123 in A. angolensis (U = 1003, P = 0.002). Higher levels of genetic differentiation in the montane species suggest lower gene flow among populations and higher degree of isolation compared with the lowland species. However, pairwise FST values between some sites were quite low, considering their Euclidean distances. For example, G14 and G20 are approximately 13.5 km apart, and the pairwise FST was 0.048. Notably, G13 and G19 are 30.2 km apart, and their FST value was 0.098. Pairwise FST values among populations of the lowland species were even lower among sites which are the same or even further apart, for example between G25 and G3 (approximately 30 km, FST = 0.075) and between G23 and G9 (~ 40 km, FST = 0.084).
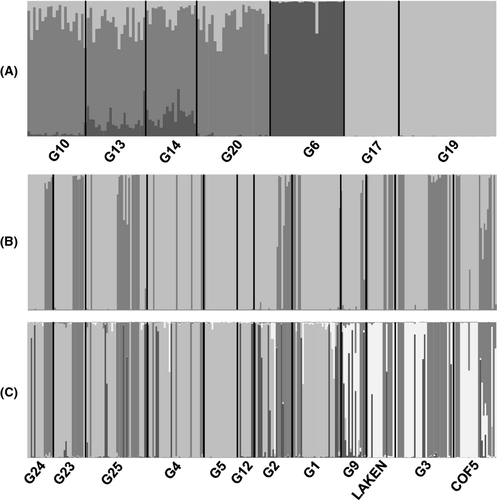
In A. wittei, the Bayesian clustering method used in STRUCTURE identified three well-defined groups (Fig. 2A). One group incorporated the central sites (G10, G13, G14 and G20), excluding G6 which constituted a single genetic cluster. The third group included the eastern sites (G17 and G19; Fig. 2A). In A. angolensis, the standard Bayesian clustering analysis did not reveal any spatially detectable grouping of individuals. When using prior location information, we noticed a slight improvement in the detection of population structure with K = 2 and K = 5 as the best supported values. When K = 2, individuals from each site were assigned to both clusters except from some central sites (G4, G5, G12, G1 and G9) which were strongly assigned to one cluster (Fig. 2B). At K = 5, the eastern sites revealed hints of distinctiveness, although large proportions of individuals showed substantial admixture across all sampling locations (Fig. 2C).
Landscape genetic analysis
Overall, all selection criteria employed in the MLPE method for detecting the best models among all possible combinations were consistent; that is, models with the lowest AICc and BIC values had also the highest logLik and the lowest REMLdev values (Table 3). Moreover, the top models within each and across all the LCPs were similar, and their AIC values did not differ by more than a value of 3, except for ΦPT for A. wittei (which differed by six). These data suggest no differences between the top two models as reported (Table 3). The null IBD model was never selected, suggesting that the pattern of genetic differentiation can be explained by landscape variables (and are not simply a result of IBD). In both species, LCPs based on streams alone or in combination with either slope or roughness had the highest support. However, the variables included in these models were different between the two species. In A. wittei, roughness and slope were included in the best models selected with the MLPE method and they were both negatively correlated with genetic distances (Table 3). In A. angolensis, variables included in the best MLPE models were topographic distance and presence of human settlements, both positively correlated with genetic distances (Table 3). The variables selected with the BIOENV procedure in A. wittei were cti, slope, rough, distance and some habitat types (i.e. shrubland, forest and reforestation; Table 4). However, some of these variables were selected only once suggesting minor influence or spurious results. Single scatterplots and correlations with genetic distances revealed nonsignificant relationships with cti and forest and positive correlation with reforestation and distance, whereas slope, rough and shrubland were significantly negatively associated with genetic distances (Table S5, Supporting information). Moreover, the MLPE method selected LCPs with stream surface cost ratio of 1:10 (similar to A. angolensis; Table 3), whereas the BIOENV selected the lower cost ratio (1:2) and also the roughness resistance surface alone, suggesting terrestrial dispersal (Table 4). In A. angolensis, proportion of human settlements and topographic distances were steadily included and positively correlated with genetic distances also in the BIOENV method (Table 4).
| Species | Genetic distance | LCP | Variables | AICc | AICc weight | BIC | logLik | REML dev |
|---|---|---|---|---|---|---|---|---|
| Aw | F ST | Slope/stream 1:10 | Rough (−) | −74.79 | 0.86 | −73.11 | 42.6 | −85.3 |
| Stream 1:10 | Rough (−) | −73.04 | 0.84 | −71.36 | 41.8 | −83.5 | ||
| Φ PT | Stream 1:10 | Rough (−) | −61.49 | 0.81 | −59.81 | 36.0 | −72.0 | |
| Rough/stream 1:10 | Slope (−) | −57.29 | 0.11 | −55.62 | 33.9 | −67.8 | ||
| Aa | F ST | Stream 1:10 | Settlement (+) | −269.31 | 0.91 | −261.21 | 139.0 | −278.0 |
| Slope/stream 1:10 | Settlement (+) | −266.45 | 0.97 | −258.34 | 137.6 | −275.1 | ||
| Φ PT | Stream 1:10 | Distance (+) | −235.47 | 0.63 | −227.37 | 122.1 | −244.1 | |
| Stream 1:2 | Distance (+) | −232.93 | 0.82 | −224.83 | 120.8 | −241.6 |
- For model selection, we used a suite of criteria and measures of fit including AICc, BIC, log likelihood (logLik) and deviance for the REML criterion (REMLdev). AICc weights represent the values of the corrected Akaike weights.
| Species | Genetic distance | LCP | ρw | Variables |
|---|---|---|---|---|
| Aw | F ST | Rough/stream 1:2 | 0.5331 | Cti |
| Slope | ||||
| Shrubland | ||||
| Stream 1:2 | 0.486 | Cti | ||
| Rough | ||||
| Φ PT | Rough | 0.5334 | Distance | |
| Slope | ||||
| Forest | ||||
| Reforestation | ||||
| Rough/stream 1:2 | 0.4878 | Cti | ||
| Shrubland | ||||
| Aa | F ST | Stream 1:10 | 0.3255 | Distance |
| Settlement | ||||
| Slope/stream 1:10 | 0.3095 | Distance | ||
| Settlement | ||||
| Φ PT | Stream 1:10 | 0.4876 | Distance | |
| Settlement | ||||
| Slope/stream 1:10 | 0.4515 | Distance | ||
| Settlement |
Discussion
Genetic differentiation and population structure
Understanding dispersal and population differentiation is crucial for identifying conservation genetic units and estimating the potential of a species to colonize new habitats (Allendorf et al. 2013). Empirical studies on current rates of gene flow in montane taxa often show restricted connectivity primarily reduced by topographic relief (Monsen & Blouin 2004; Dubey & Shine 2010; Velo-Antón et al. 2013; Castillo et al. 2014). Elevation also explains variation in life history traits, such as flowering time and consequent genetic divergence among three species of alpine snowbed plants (Hirao & Kudo 2004). Our fine-scale analysis of the montane frog specialist, Amietia wittei, revealed relatively high rates of gene flow (FST = 0.01–0.16). Greater population differentiation and isolation across smaller geographic scales have been reported for other high-elevation amphibian species, such as the cascades frog, Rana cascadae (Monsen & Blouin 2004), the long-toed salamander, Ambystoma macrodactylum (Tallmon et al. 2000; Giordano et al. 2007; Savage et al. 2010), the spotted frog, Rana luteiventris (Funk et al. 2005), the tree frog Bokermannohyla saxicola (Eterovick et al. 2009) and the leaf-litter frog, Arthroleptis xenodactyloides (Measey et al. 2007). Also, landscape features that commonly limit gene flow in other high-elevation species such as topographic roughness (e.g. Murphy et al. 2010) were surprisingly positively associated with gene flow in A. wittei.
Nonetheless, the montane species showed greater genetic differentiation among populations as compared with its lowland congener. Mean values of FST and ΦPT were significantly lower in Amietia angolensis, suggesting higher gene flow in the lowland species. Bayesian clustering also supported higher genetic structure in A. wittei relative to A. angolensis. Populations of A. wittei were grouped into three discrete spatial genetic clusters (see Fig. 2A), and several private AFLP bands were present in the eastern sites. It is yet possible that we missed some populations in between that might reduce the number of private bands and decrease the overall level of differentiation (Slatkin 2005). In contrast, populations of A. angolensis were highly admixed, and no genetic clusters were clearly defined. Several factors might contribute to the lack of a well-defined population structure. First, the six primer pairs produced a relatively low number of polymorphic AFLP markers (165) compared with A. wittei (235); as a consequence, the signal in the data set might not have been strong enough for correct assignment of some individuals. Second, there could be isolation by distance, and the inclusion of geographic distance in the best LCP models may support this hypothesis. When allele frequencies vary gradually across the study area (as under IBD model), the inferred value of K can be arbitrary, and most individuals may have mixed membership in multiple groups (Pritchard et al. 2010). Finally, the high degree of admixture can be due to ongoing gene flow among populations and/or retained ancestral polymorphism (i.e. if isolation is recent and population sizes are large enough).
Landscape features affecting gene flow
Considering the association of Amietia species with aquatic habitats, we hypothesized that gene flow would preferentially occur along water bodies. The least-cost path analysis supported stream-based movements, corroborating the relationship of these African amphibians with riverine habitats. However, different landscape variables further influenced each of the two species. In the montane species, only topographic variables, namely roughness and slope, were included in models that explained interdeme genetic distances best and both were positively correlated with genetic differentiation. This finding has two important considerations. First, the inclusion of topographic features suggests that dispersal is not limited to stream corridors but may occur overland. Second, the direction of the correlation suggests that topographic complexity enhances, rather than restricts gene flow. This is a counterintuitive result and in contrast with other amphibian studies (Funk et al. 2005; Giordano et al. 2007; Murphy et al. 2010). Nonetheless, considering that A. wittei occurs in the highlands and mountain tops of East Africa, we expected some degree of adaptation to rugged terrain. Within the National Park, streams are isolated in deep gorges; consequently, A. wittei is more likely to encounter steep slopes when leaving a breeding site, resulting in higher exposure to rough areas during terrestrial dispersal. Low genetic differentiation across areas characterized by rough terrain has been also observed in another anuran, the tailed frog (Ascaphus truei) (Spear & Storfer 2008).
Although landscape genetic analysis revealed several insights into the landscape variables affecting genetic connectivity, no specific physical barrier seemed to coincide with the observed genetic breaks in A. wittei. Comparably, Zhao et al. (2009) observed strong population structure in a high-elevation frog (Rana kukunoris) in China, but no single landscape feature seemed to explain the clustering. It appears that factors other than topography contribute to genetic isolation, such as selection against dispersal or local adaptation (Dobzhansky 1937; Nosil et al. 2005). Across the southern slope of Mt. Kilimanjaro, environmental conditions are not uniform; for instance, precipitation is patchy and some areas receive more annual rainfall than others (Hemp 2006). Water availability and, consequently, mating time also appear asynchronous across sites as we observed a temporal variation in the presence of tadpoles. Few and scattered breeding sites, selection against dispersal and changes in environmental conditions over short distances could contribute to the spatial genetic structure that lacks obvious physical dispersal barriers. Further studies on the life history and ecology of this and other high-elevation species could help elucidate which specific attributes contribute to the genetic divergence in montane specialists.
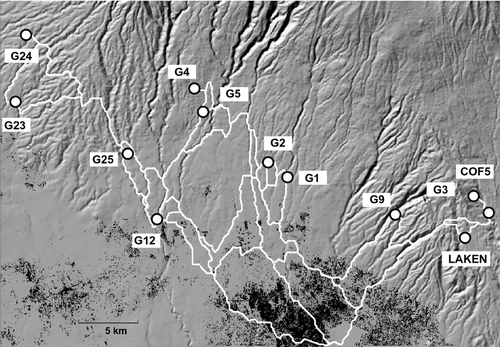
Consistent with our hypothesis, the lowland species seems to avoid terrestrial dispersal and disturbed habitats as the presence of human settlements was negatively correlated with gene flow. Lynn & Lindle (2002) investigated the influence of anthropogenic disturbance on habitat use by A. angolensis but did not find a significant effect. However, using a landscape genetic approach, we were able to detect a negative correlation between the presence of human settlements and gene flow. By following water courses, tadpoles or adult frogs will eventually cross Moshi, the main town at the foothills of Mt. Kilimanjaro, or the surrounding areas. Several of the possible stream-based LCPs (see Fig. 3), especially those connecting the central populations with the eastern ones, cross this urban area. For instance, the route between G1 and G3 crosses areas with intense settlements, and the two populations are moderately differentiated (FST = 0.103). On the contrary, the path between G1 and G23 does not cross urban patches, and the two populations are genetically more similar (FST = 0.063) even if they are farther away (see Figs 1 and 3). As such, Moshi and the surrounding settlements seem to coincide with the genetic break between the central and eastern populations identified by the cluster analysis. Considering that A. angolensis is a generalist, abundant species able to successfully reproduce in a wide array of water bodies, we might expect a more deleterious effect on less mobile and more vulnerable species.
Conclusions
Landscape matrix configuration and geographic distances are key factors affecting dispersal and population connectivity (Manel et al. 2003; Storfer et al. 2007, 2010; Holderegger & Wagner 2008). Topographic relief and linear features such as rivers or mountain ridges have been frequently identified as barriers to gene flow in several taxonomic groups (e.g. Berthier et al. 2005; Pérez-Espona et al. 2008; Radespiel et al. 2008; Castillo et al. 2014), including the majority of amphibian species (Funk et al. 2005; Giordano et al. 2007; Murphy et al. 2010; Guarnizo & Cannatella 2013, 2014; Kershenbaum et al. 2014). In this study, gene flow among populations of the montane frog Amietia wittei was, however, relatively high across rough terrains and positively correlated with topographic variables. Our counterintuitive findings suggest that it may be difficult to make generalizations about the effects of high altitudes on gene flow, and the inclusion of landscape genetic techniques represents a powerful tool to elucidate case-specific patterns and processes. Our results also suggest that montane species can be adapted to disperse through areas of high topographic relief. Furthermore, we found that genetic breaks did not correspond to topographic barriers, and we speculate that they could be generated by cryptic, nonphysical barriers such as local environmental conditions. Additional noteworthy results were found for the lowland species, which was previously thought to be uninfluenced by anthropogenic disturbance based on ecological data. Our genetic study showed that human settlements negatively affected gene flow among populations of this species. These data, taken together, show the importance for landscape genetic studies as a tool for understanding population genetic processes. Given that an increasing number of tropical faunas are becoming threatened (especially amphibians), our data have provided important information for conservation and management, for instance by stressing the role of stream networks in ensuring population connectivity. As reported by two recent reviews on landscape genetic research (Storfer et al. 2010; Manel & Holderegger 2013), empirical studies in tropical regions are enormously underrepresented compared with temperate areas. We hence want to emphasize the urgent need to increase research efforts in these biologically important and vulnerable regions.
Acknowledgements
This study was supported by a grant of the German Excellence Initiative to the Graduate School of Life Sciences, University of Würzburg. We thank the Tanzanian Commission for Science and Technology (COSTECH research permit 2010-363-NA-96-44) and other Tanzanian authorities for issuing the necessary permits. The DFG-Research Unit FOR1246 (Kilimanjaro ecosystems under global change: Linking biodiversity, biotic interactions and biogeochemical ecosystem processes (KiLi)) provided infrastructure, support and assistance in the field. M. Barej performed the DNA barcoding. A. Channing helped with species identification and provided valuable information on the studied species. We are grateful to Prof. Müller-Reible and the personnel of the Human Genetics department at the University of Würzburg for paramount assistance in the laboratory. We thank M. van Strien for crucial support with the mixed model analysis. Finally, we acknowledge anonymous reviewers for their comments on previous versions of the manuscript.
References
G.Z., M.-O.R. and I.S.-D. conceived the study. G.Z. collected field data, performed laboratory and landscape genetic analyses and wrote the manuscript. A.S. provided support with data analysis, interpretation and authorship of the manuscript. All authors read, commented and approved the final version of the manuscript.
Data accessibility
Amietia wittei and Amietia angolensis 16S aligned sequences, AFLP data sets and landscape distance data from least-cost path analyses: Dryad Digital Repository, doi:10.5061/dryad.9pb01.




