Pleiotropy in the melanocortin system: expression levels of this system are associated with melanogenesis and pigmentation in the tawny owl (Strix aluco)
Abstract
The adaptive function of melanin-based coloration is a long-standing debate. A recent genetic model suggested that pleiotropy could account for covariations between pigmentation, behaviour, morphology, physiology and life history traits. We explored whether the expression levels of genes belonging to the melanocortin system (MC1R, POMC, PC1/3, PC2 and the antagonist ASIP), which have many pleiotropic effects, are associated with melanogenesis (through variation in the expression of the genes MITF, SLC7A11, TYR, TYRP1) and in turn melanin-based coloration. We considered the tawny owl (Strix aluco) because individuals vary continuously from light to dark reddish, and thus, colour variation is likely to stem from differences in the levels of gene expression. We measured gene expression in feather bases collected in nestlings at the time of melanin production. As expected, the melanocortin system was associated with the expression of melanogenic genes and pigmentation. Offspring of darker reddish fathers expressed PC1/3 to lower levels but tended to express PC2 to higher levels. The convertase enzyme PC1/3 cleaves the POMC prohormone to obtain ACTH, while the convertase enzyme PC2 cleaves ACTH to produce α-melanin-stimulating hormone (α-MSH). ACTH regulates glucocorticoids, hormones that modulate stress responses, while α-MSH induces eumelanogenesis. We therefore conclude that the melanocortin system, through the convertase enzymes PC1/3 and PC2, may account for part of the interindividual variation in melanin-based coloration in nestling tawny owls. Pleiotropy may thus account for the covariation between phenotypic traits involved in social interactions (here pigmentation) and life history, morphology, behaviour and physiology.
Introduction
Variation in coloration is common throughout the animal and plant kingdoms with roles in camouflage, thermoregulation and social interactions (Anderson 1994; Bond 2007). In many species, coloration signals phenotypic and genetic attributes to potential mates to enhance mating success (Anderson 1994). However, the exact individual quality that a colour trait advertises often remains contentious because of restricted knowledge of the underlying mechanisms linking coloration to other phenotypic attributes. Two broad categories of mechanisms can be advocated depending on the quantitative genetics of coloration. First, the expression of coloration is condition dependent so that only individuals in prime condition can afford to pay the costs of producing the brightest colour trait. As condition is a multifactorial trait, coloration and its associated qualities are likely to be regulated by multiple genes and the environment (Rowe & Houle 1996). Second, the expression of coloration is only weakly sensitive to condition, but strongly heritable, as reported in the so-called colour polymorphic species (Roulin 2004). In this case, covariation between coloration, physiological processes and behaviours could be due to genes that pleiotropically regulate other phenotypic traits (Slominski et al. 2004; Ducrest et al. 2008). Identifying the proximate genetic mechanism underlying this type of covariation is not trivial and requires appropriate model systems.
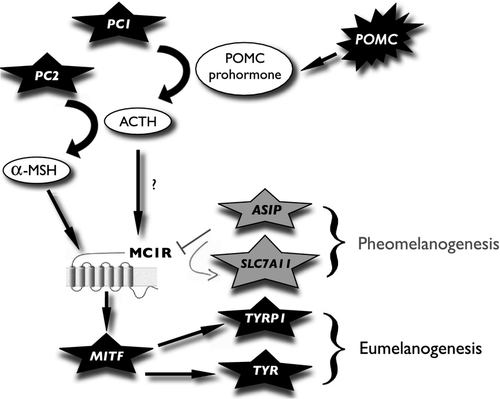
Melanin-based coloration is a promising framework to investigate the proximate basis of the adaptive function of coloration. The finding that this type of coloration frequently covaries with morphology, behaviour, physiology and life history traits raised the hypothesis that variation in coloration can signal to conspecific alternative strategies adapted to specific environmental or reproductive conditions (Roulin 2004; Forsman et al. 2008). Because these covariations are sometimes detected under specific environmental conditions (Piault et al. 2009), natural selection can locally and temporally favour alternative genotypes that differ in their phenotypic response to environmental conditions (i.e. norms of reaction (Hedrick 2006). Recently, it has been suggested that pleiotropic effects of the melanocortin system may account for these covariations (Ducrest et al. 2008; Slominski et al. 2012). In vertebrates, this conserved system includes the melanocortins, α-, β-, γ-melanocyte-stimulating hormones (MSH) and adrenocorticotropic hormone (ACTH), four post-translational bioactive peptides derived from the tissue-specific cleavage of the proopiomelanocortin prohormone (POMC) by two different convertase enzymes (PC1/3 and PC2). In human anterior lobe of the pituitary, PC1/3 cleaves POMC prohormone to ACTH, β-lipotropin (β-LPH) and N-terminal part (N-POMC), whereas in the intermediate pituitary, PC2 further processes ACTH, β-LPH and N-POMC to produce α-, β- and γ-MSH as well as β-endorphin (Wardlaw 2011). Interestingly, POMC processing by PC1/3 and PC2 also occurs in rodent and human epidermal tissues (Slominski et al. 2000; Bicknell 2008). The paracrine agouti signalling protein (ASIP), an inverse agonist and antagonist of melanocortins, is present in the skin and responsible for the yellow subapical band of mice hairs (Millar et al. 1995; Ollmann et al. 1997). Of particular interest, melanocortins (MSHs and ACTH) and ASIP can bind to five distinct melanocortin receptors (MC1-5Rs), a family of transmembrane G-protein-coupled receptors well conserved among vertebrates (Schioth et al. 2005). These tissue-specific bindings can modulate numerous physiological and behavioural functions, such as stress response, energy homeostasis, anti-inflammatory response, sexual activity, susceptibility to oxidative stress and aggressiveness (for review see Ducrest et al. 2008; Cooray & Clark 2010).
Melanin-based coloration of the hair, skin and feather is the result of a mixture of (grey black) eumelanin and (reddish brown) pheomelanin pigments. Genetic pathways leading to the production of both melanin pigments have been intensively studied (Ito & Wakamatsu 2011b). In epidermal tissues, binding of α-MSH to MC1R promotes eumelanogenesis (Walker & Gunn 2011). This activates the production of intracellular cyclic adenosine monophosphate (cAMP), a second messenger that up-regulates microphthalmia-associated transcription factor (MITF) (Cheli et al. 2010), that further activates the eumelanogenic expression and activity of tyrosinase (TYR) and tyrosinase-related protein 1 (TYRP1) within melanocytes (Ito & Wakamatsu 2011a). The rate-limiting enzyme tyrosinase catalyses the first step of melanin production: the oxidation of L-tyrosine to dopaquinone, and subsequently with the intervention of tyrosinase (TYR) in human or tyrosinase-related protein 1 (TYRP1) in mice, dopaquinone will produce eumelanin pigments (Ito & Wakamatsu 2008). In contrast, binding of ASIP to MC1R leads to the down-regulation of eumelanogenic genes such as MITF, TYR and TYRP1 and to the production of pheomelanin (Le Pape et al. 2009). SLC7A11 transports cysteine and glutamate into melanocytes, and reduction in its expression results in lowered pheomelanin but not eumelanin production (Chintala et al. 2005). Differential expression of these melanogenic genes may therefore be associated with eu- or pheomelanin-based coloration (Jeukens et al. 2009; Ekblom et al. 2012; Leskinen et al. 2012).
The colour polymorphic tawny owl (Strix aluco) is a promising organism to investigate the pleiotropic effects of the melanocortin system. This species exhibits continuous plumage variation from light to dark reddish, which is mainly due to the deposition of pheomelanin and to a lesser extent eumelanin. Thus, reddishness is positively correlated with the ratio of pheomelanin to eumelanin and to the total amount of melanin (Gasparini et al. 2009a). Interindividual variation in tawny owl plumage coloration is highly heritable (h2 = 0.72–0.93; Brommer et al. 2005; Gasparini et al. 2009a), and there is growing evidence of colour-specific norms of reaction to reproductive and rearing conditions, food supply, pathogens and climatic conditions (Galeotti & Cesaris 1996; Galeotti & Sacchi 2003; Roulin 2004; Roulin et al. 2008; Gasparini et al. 2009b; Piault et al. 2009; Karell et al. 2011a,b). These norms of reactions may lead to differences in recruit production, probability of skipping reproduction and adult survival rate (Roulin et al. 2003; Brommer et al. 2005; Karell et al. 2011a). Moreover, it has been previously demonstrated that differently coloured female tawny owls adjust their level of circulating POMC prohormone differently according to reproductive effort and environmental conditions (Roulin et al. 2011). Dark reddish melanic females had lower circulating levels of POMC prohormone than light reddish melanic females when rearing experimentally reduced (but not enlarged) brood and when located in forest patches with high (but not low) density of beech trees, an environmental feature positively correlated with prey abundance. In this previous study, we proposed that POMC prohormone and ACTH levels needed under stressful conditions are colour specific, suggesting colour-specific post-translational modifications of POMC prohormone via alternative patterns of activity of the convertases PC1/3 and/or PC2.
These findings call for an integrative study that establishes the genetic mechanisms underlying covariation between coloration and physiological reaction norms. We thus measured in feathers of differently coloured nestling tawny owls the expression levels of the melanocortin system (MC1R, POMC, PC1/3, PC2 and ASIP) and melanogenic genes (MITF, TYRP1, TYR and SLC7A11) (Fig. 1). Our main aim is thus to examine whether variation in the expression levels of genes of the melanocortin system is correlated with the expression levels of other melanogenic genes and in turn variation in the degree of reddish plumage coloration measured in the nestlings themselves and in their parents. We also investigated whether these covariations were different in the two brood size treatments, colour morphs potentially up- or down-regulating some genes to better cope with stressful (i.e. enlarged broods) or relaxed (i.e. reduced broods) environments. This study is a necessary step in the identification of which genes of the melanocortin system could account for covariation between coloration and other phenotypic attributes. More generally, our study has the potential to highlight that pleiotropy can explain why traits used in social interactions such as pigmentation are associated with other phenotypic attributes.
Methods
Model species and brood size manipulation
In 2010, we followed the reproduction of 139 tawny owl breeding pairs reproducing in nest boxes hanged in a 911-km2 study area located in western Switzerland. Clutches were composed of 2–7 eggs (mean ± SD: 5.13 ± 0.94), which hatched between February 21 and May 31 (mean ± SD: March 31 ± 13.3 days), leading to 1–7 fledglings per brood (n = 107 successful broods, mean ± SD: 3.92 ± 1.42). On the criteria that clutches were initiated on a similar date, 94 nests were matched in pairs to perform a partial cross-fostering experiment combined with a brood size manipulation experiment. Brood sizes were systematically manipulated, leading to an exchange of 1.74 hatchlings on average (SD = 0.6) from nests E (experimentally enlarged, n = 47) and placed in nests R (experimentally reduced, n = 47), while 2.74 hatchlings on average were exchanged from nests R to nests E. Some nestlings were thus raised by randomly chosen foster parents and experienced different brood size manipulation treatment. This experiment disrupts the confounded environmental effect of being born and reared in the same ‘genetic’ nest (also referred to as ‘nest of origin’) and manipulates sibling rivalry (Roulin et al. 2008). Among the pairs of nests, treatments were randomly allocated. No differences in adult wing and tarsus lengths as well as in plumage coloration were observed between the two brood size treatments (Student's t-tests, P > 0.42).
When nestlings were 10 days of age, breeding females were captured in their nest box during daylight (8 am–6 pm, n = 94), while males were captured at night when provisioning their brood (10 pm–6 am, n = 88) to record their plumage coloration. Note that extra-pair paternity is rare in the tawny owl (Saladin et al. 2007), and thereby individuals born in a nest are assumed to be sired by the captured male. Nestlings were weighed to the nearest g, their left wing length measured to the nearest 1 mm, and we identified sex using molecular markers. For each nestling, we calculated an index of nestling body condition as the residuals of an ancova with nestling body mass as dependent variable, sex as a factor (F1,298 = 52.28, P < 0.0001) and wing length as covariate (F1,298 = 1467.41, P < 0.0001).
Assessment of feather coloration
Despite its continuous variation in the degree of reddishness, the tawny owl is considered as colour polymorphic (Galeotti 2001; Brommer et al. 2005). Each adult was thus assigned to one of five colour morphs (1 = reddish, 2 = reddish brown, 3 = brown, 4 = grey brown and 5 = grey), as described in a previous study (Roulin et al. 2003). This visual scoring method is highly repeatable between years and strongly correlated with brown chroma measured with a spectrophotometer (Gasparini et al. 2009a). However, visual colour scores better account for overall plumage coloration compared with brown chroma, which is based on the measurement of a few feathers, an approach that does not necessarily reflect entire body coloration (Brommer et al. 2005). Because birds do not change coloration with age (Roulin et al. 2003) and we assigned the same birds to colour morphs on numerous occasions between 2005 and 2011, we calculated a mean colour score for each adult based on all available measurements. Mother plumage coloration was not significantly associated with hatching date of the first egg (Pearson's correlation, r = −0.07, n = 44, P = 0.67), but in our sample of birds, there was a tendency for redder males to breed earlier in the season (r = −0.33, n = 43, P = 0.029); nevertheless, if controlling for hatching date in the statistical analyses, the associations between gene expression and father coloration remained significant. Brood size before manipulation was not associated with parental coloration (Pearson's correlation, P > 0.20). Moreover, within pairs of experimental nests, foster and biological parents did not resemble each other with respect to plumage colour scores (Pearson's correlations: −0.19 < r < −0.09, P > 0.24). Pairing with respect to male and female coloration was random in both treatments (enlarged nests: r = 0.22, n = 23, P = 0.32; reduced nests: r = 0.30, n = 21, P = 0.19).
Fledglings leave the nest box at approximately 25 days old, and at that age, feathers are still not fully grown leading to less distinctive colour patterns compared with feathers collected on the same individuals at adulthood. The lower degree of colour variation in nestling (coefficient of variation based on all 132 nestlings = 0.066) than adult feathers (CV of all fathers: 0.473 and CV of all mothers: 0.428) makes difficult the classification of nestling plumage coloration into morphs. We therefore assessed nestling plumage coloration using spectrometric measurements. To this end, we collected three downy feathers from the dorsum of each nestling at approximately 25 days of age, and we overlaid these feathers on a black paper to measure reflectance spectra at four distinct feather positions using a S2000 spectrophotometer (Ocean Optics, Dunedin, FL) and a dual deuterium and halogen 2000 light source (Mikropackan, Mikropack, Ostfildern, Germany). Based on these spectra, a mean brown chroma score was calculated for each nestling as described by Montgomerie (2006).
RNA extractions
Total RNA was extracted from one or two bases of developing feathers plucked from the dorsum of nestlings at two different ages (hereafter referred as ‘age class 1’: mean ± SD = 11.0 days old ± 2.0; ‘age class 2’: mean ± SD = 25.0 days old ± 2.2) of 151 nestlings from 45 broods. We chose to collect feathers at these two age classes in order to measure gene expression during the early stages of feather production (i.e. at 11 days of age) and close to the time when nestling leave their nest. Note that feathers are not uniform in colour, and hence, significant relationships between gene expression and nestling coloration are surely biologically relevant. In contrast, absence of significant relationships has to be cautiously interpreted, because we may have collected feathers of darkly coloured individuals just at the time when there was a reduction in pigment production (i.e. nonpigmented feather area). Mean nestling age at which developing feathers were sampled was not associated with the colour morph of the biological mother and father but also with mean nestling brown chroma (Pearson's correlations, 0.17 > r > −0.17, P > 0.29). Feather bases were immediately frozen in dry ice in the field and transferred at −80 °C within 12 h until later genetic analyses. Frozen feather bases were ground with a pestle in liquid nitrogen, passed through a Qiashredder® (Qiagen, Hombrechtikon, Switzerland). Total RNA (n = 325) were extracted using the Qiagen Rneasy Mini Kit (Qiagen) and quantified with the Qubit® 2.0 Fluorometer (Life Technologies, Zug, Switzerland). RNA quality was assessed on the Bioanalyser (Agilent Technologies, Basel, Switzerland). Prior to reverse transcription, total RNA samples were treated with DNase I. One microgram of total RNA was incubated in 10 μL with 5U DNase I (Roche diagnostics Ltd, Basel, Switzerland) in 10 mm Tris-HCl, pH 8.0, 0.5 mm MgCl2, 1 mm dithiothreitol, 20U RNasin ribonuclease inhibitor (Promega, Dübendorf, Switzerland) for 30 min at 37 °C, followed by 10 min at 75 °C to inactivate the enzyme. Finally, 100 ng of DNase I-treated total RNA was reverse-transcribed in a final volume of 20 μL, using 250 ng of random hexamer, 40U of RNasin ribonuclease inhibitor (Promega) and 200 U of Superscript III reverse transcriptase (Life Technologies). cDNA samples were 10-fold diluted in 10 mm Tris-HCl, pH 8.0, and finally stored at −20 °C.
Quantitative PCR
Specific qPCR primers were designed with the assistance of Primer Express® software 2.0 (Life Technologies). BLASTN searches were performed to control primer specificity prior being ordered from Microsynth AG (Balgach, Switzerland). Final primer pairs (Table S1, Supporting information) were selected based on amplification efficiency, dissociation curve and negative controls. Due to very low POMC gene expression in the feather base, we optimized our procedure for this gene as following. First, we increased cDNA concentrations of the diluted samples through an ethanol precipitation with one volume of 5 m ammonium acetate, pH 8.0. The resulting pellet was resuspended in 15 μL of 10 mm Tris-HCl, pH 8.0, 0.1 mm EDTA (TE 1×). Five microlitre of concentrated cDNA was then pre-amplified using 5 μL of pooled TaqMan primer and probes of the POMC, glyceraldehyde 3-phosphate dehydrogenase (GAPDH; a reference gene) and elongation factor 1-alpha 1 (EEF1A1; another reference gene) genes (Table S2, Supporting information) and 10 μL of the PreAmp Master Mix (Life Technologies) in a thermal cycler (Biometra TProfessional 96) for 14 cycles and then diluted 10-fold with TE 1×. qPCRs were performed in 384-well optical reaction plates, assembled with a Tecan Freedom Evo® liquid handler (Tecan group Ltd.) and processed on an ABI Prism® 7900HT Sequence Detection System (Life Technologies) in a final volume of 10 μL containing 2 μL of diluted cDNA mixed with primers (Tables S1 and S2, Supporting information) and 1× SYBR® Green PCR Master Mix (Life Technologies) and with 1× qPCR MasterMix plus low Rox and 0.2 μL of Rox (Eurogentec SA, Sereing, Belgium) for TaqMan assays. For each qPCR, cycling conditions were 50 °C for 2 min, 95 °C for 10 min, 45 cycles of 95 °C for 15 s and 60 °C for 1 min, and a final dissociation stage for SYBR Green. Three technical replicates were performed per cDNA sample. Quantification cycle (hereafter CT) values were recorded with SDS software 2.3. CT values larger than 35 were considered beyond the limit of detection and thus removed from further analyses, and interplate covariation was controlled according to inter-run calibrators consisting of three samples repeated on each plate.
CT scores were imported into qBasePLUS software 1.3 (Biogazelle). To correct for any variation in cDNA content and enzymatic efficiencies, CT scores of the candidate genes were normalized using the two reference genes GAPDH and EEF1A1 (showing coefficient of variation of 0.12; 99.2% of technical replicates had less than 0.5 CT variation). Mean relative quantities (RQs) for each sample were calculated and analysed with the geNorm software 3.4 (Vandesompele et al. 2002). These relative scores were finally Box–Cox-transformed before statistical procedures, to enable the use of models with a Gaussian-distributed error.
Statistical procedure
To investigate which factors accounts for variation in the levels of gene expression, we performed linear mixed models with the nest of rearing and nestling identity as two random variables. The independent variables were the age classes, nestling sex, nestling body condition index, time of the day (hour) and brood size manipulation treatment. In Table 1, we present the results by removing nonsignificant variables one after the other; note however that if we do not use a backward stepwise procedure, we obtain qualitatively similar results. Because the variable ‘age class’ explained part of the variation in gene expression levels in all but two genes, we calculated pairwise Pearson's correlations between the expression levels of the different genes in the two age classes separately.
| Source of variation | ASIP | PC1/3 | SLC7A11 | |||||||||||
| Nest of rearing | 14.3% | Nest of rearing | 23.6% | Nest of rearing | 24.8% | |||||||||
| Nestling identity (nest of rearing) | 0% | Nestling identity (nest of rearing) | 12.3% | Nestling identity (nest of rearing) | 46.2% | |||||||||
| n | d.f. | F | P | n | d.f. | F | P | n | d.f. | F | P | |||
| Age class (0.54 ± 0.05) | 293 | 1,165.1 | 109.54 | <0.0001 | Sex (0.25 ± 0.03) | 293 | 1,132.4 | 77.41 | <0.0001 | Age class (0.15 ± 0.02) | 293 | 1,146.1 | 57.85 | <0.0001 |
| Sex (−0.10 ± 0.04) | 1,137.5 | 5.60 | 0.019 | Age class (−0.05 ± 0.02) | 1,150.1 | 4.58 | 0.034 | |||||||
| Body condition (−0.005 ± 0.002) | 1,252 | 7.02 | 0.009 | Body condition | 1,220.1 | 0.01 | 0.93 | |||||||
| BSM | 1,40.56 | 0.21 | 0.65 | Hour | 1,177.4 | 0.02 | 0.90 | |||||||
| Hour | 1,259.5 | 0.09 | 0.77 | Hour | 1,228 | 0.55 | 0.46 | Sex | 1,138.9 | 0.20 | 0.65 | |||
| BSM | 1,42.19 | 0.76 | 0.39 | Body condition | 1,281.6 | 2.76 | 0.10 | BSM | 1,43.72 | 2.08 | 0.16 | |||
| MC1R | PC2 | TYR | ||||||||||||
| Nest of rearing | 8.8% | Nest of rearing | 14.4% | Nest of rearing | 3.6% | |||||||||
| Nestling identity (nest of rearing) | 0% | Nestling identity (nest of rearing) | 0 | Nestling identity (nest of rearing) | 0% | |||||||||
| n | d.f. | F | P | n | d.f. | F | P | n | d.f. | F | P | |||
| Age class (−0.47 ± 0.06) | 283 | 1,153.3 | 53.35 | <0.0001 | Sex (0.07 ± 0.03) | 266 | 1,132.8 | 5.50 | 0.021 | Age class (−0.54 ± 0.07) | 283 | 1,156.2 | 56.62 | <0.0001 |
| Body condition | 1,246.4 | 0.24 | 0.63 | Age class | 1,151.9 | 0.04 | 0.84 | Sex | 1,139.8 | 0.002 | 0.97 | |||
| Sex | 1,136.2 | 0.22 | 0.64 | BSM | 1,38.06 | 0.07 | 0.79 | BSM | 1,40.46 | 0.01 | 0.92 | |||
| BSM | 1,42.51 | 0.25 | 0.62 | Body condition | 1,258.1 | 1.38 | 0.24 | Hour | 1,193.4 | 0.21 | 0.65 | |||
| Hour | 1,226.4 | 0.60 | 0.44 | Hour | 1,226.4 | 1.41 | 0.24 | Body condition | 1,253.5 | 0.76 | 0.39 | |||
| MITF | POMC | TYRP1 | ||||||||||||
| Nest of rearing | 52.0% | Nest of rearing | 36.9% | Nest of rearing | 2.6% | |||||||||
| Nestling identity (nest of rearing) | 0 | Nestling identity (nest of rearing) | 1.6% | Nestling identity (nest of rearing) | 0% | |||||||||
| n | d.f. | F | P | n | d.f. | F | P | n | d.f. | F | P | |||
| Age class (−0.03 ± 0.01) | 293 | 1,153.5 | 6.39 | 0.013 | BSM | 261 | 1,44.36 | 0.04 | 0.85 | Age class (−0.88 ± 0.09) | 275 | 1,154.2 | 103.48 | <0.0001 |
| Sex (−0.03 ± 0.01) | 1,115.8 | 5.24 | 0.024 | Hour | 1,207.3 | 0.11 | 0.74 | Sex (0.26 ± 0.08) | 1,140.5 | 9.70 | 0.002 | |||
| Body condition | 1,241.4 | 0.22 | 0.64 | |||||||||||
| Body condition | 1,251.9 | 0.04 | 0.84 | Sex | 1,117.2 | 1.11 | 0.29 | Hour | 1,159.6 | 0.001 | 0.98 | |||
| BSM | 1,43.03 | 0.21 | 0.65 | Age class | 1,134.1 | 2.55 | 0.11 | BSM | 1,33.67 | 0.002 | 0.96 | |||
| Hour | 1,236.4 | 3.38 | 0.067 | Body condition | 1,245.1 | 2.06 | 0.15 | |||||||
To determine whether gene expression levels in nestling feathers were associated with parental plumage coloration, we combined the data on the different genes in a few indices through principal components analysis. This approach gave another way to study how the expression of the different genes covaried and it allowed us to reduce the number of analyses (indeed parental coloration may be differentially related to gene expression in the two brood size treatments and two age classes). Thus, for the two age classes, we performed separate linear mixed models for each of the four principal components including nest of origin as random variable. The independent variables were nestling sex, brood size manipulation and plumage coloration of the two biological parents also in interaction with the brood size manipulation experiment. We removed nonsignificant variables one after the other starting within interactions; note however that we obtain qualitatively similar results if we do not use a stepwise approach.
For the statistical analyses, we had a sample of 151 nestlings for which we measured gene expression at the two age classes. These individuals were sampled from 44 families (i.e. nests of origin) and raised in 45 nests. We could measure plumage coloration in a sample of 132 nestlings (unfortunately feathers were in poor state for some nestlings), in all 44 biological mothers and in 43 biological fathers.
Statistical analyses were performed with the software jmp 10.0. We used two-tailed tests, and P-values smaller or equal to 0.05 were considered significant.
Results
Effect of the brood size manipulation on nestling body condition
There was a significant ‘age class’ by ‘brood size manipulation experiment (BSM)’ interaction on nestling body mass (linear mixed model with nest of rearing and nestling identity as two random variables, BSM: F1,46.98 = 0.26, P = 0.61; age class: F1,291.1 = 18.55, P < 0.0001; BSM x Age class: F1,157.1 = 5.60, P = 0.019; sex: F1,141.5 = 52.11, P < 0.0001; wing length: F1,287.7 = 261.96, P < 0.0001). We thus analysed the effect of the brood size manipulation experiment in each age class.
At age class 1, nestlings were significantly heavier in reduced than enlarged broods, but nestling body mass was not associated with plumage coloration of the biological mother and father (linear mixed model with nest of origin as random variable, brood size manipulation: F1,115 = 5.79, P = 0.018; nestling sex: F1,124.9 = 18.82, P < 0.0001; father colour: F1,37.08 = 0.25, P = 0.62; mother colour: F1,37.08 = 0.29, P = 0.59; controlling for nestling wing length: F1,126.9 = 338.65, P < 0.0001). Female nestlings weighed on average 248.6 g (SE = 3.4) and males 234.5 g (SE = 3.6); nestlings raised in a reduced brood weighed on average 245.2 g (SE = 3.6) and when raised in an enlarged brood 237.8 g (SE = 3.4). Note that interactions between brood size manipulation experiment, nestling sex and parental plumage coloration were not significant (not shown). When replacing parental plumage coloration by nestling brown chroma, we obtained similar results (i.e. effect of sex and brood size manipulation but no relationship with nestling coloration, not shown).
At age class 2, nestling body mass was only associated with sex (similar model: F1,124.2 = 64.76, P < 0.0001; females weighed 365.1 ± 3.4 g and males 331.2 ± 3.8 g) but neither with the brood size manipulation experiment (F1,109.5 = 0.32, P = 0.57) nor with parental plumage coloration (father: F1,27.58 = 0.24, P = 0.63; mother: F1,25.35 = 0.41, P = 0.53; controlling for nestling wing length: F1,129.8 = 45.24, P < 0.0001).
Variation in gene expression
The levels of expression of pheomelanogenesis-related genes (ASIP and SLC7A11) were higher at 11 than 25 days of age (term ‘age class’ in Table 1); we found the opposite pattern for eumelanogenesis-related genes (TYRP1, TYR, MC1R) but also for MITF and PC1/3, with levels of expression being higher at 25 than 11 days of age (Fig. 2a). The levels of PC2 and POMC were not significantly associated with age. There were also age-related differences in the patterns of covariations between the expression levels of the different genes. At 11 compared with 25 days of age covariations were more often significant (19 against 11), and their absolute magnitude was significantly stronger at 11 than 25 days of age (paired t-test: t = 2.32, d.f. = 35, P = 0.026; mean absolute Pearson's correlations are 0.27 and 0.20, respectively) (Table 2).
| MC1R | POMC | PC1/3 | PC2 | ASIP | MITF | SLC7A11 | TYR | |
|---|---|---|---|---|---|---|---|---|
| (A) Age class 1 | ||||||||
| MC1R | ||||||||
| POMC | −0.08, 0.45 | |||||||
| PC1/3 | −0.26, 0.0021 | 0.06, 0.47 | ||||||
| PC2 | 0.24, 0.008 | −0.01, 0.89 | 0.02, 0.83 | |||||
| ASIP | −0.21, 0.01 | 0.07, 0.44 | 0.10, 0.22 | 0.42, <0.0001 | ||||
| MITF | −0.08, 0.37 | −0.37, <0.001 | 0.07, 0.42 | 0.29, 0.0007 | 0.15, 0.07 | |||
| SLC7A11 | −0.26, 0.0019 | −0.06, 0.50 | 0.13, 0.13 | 0.42, <0.0001 | 0.45, <0.0001 | 0.25, 0.0016 | ||
| TYR | 0.93, <0.0001 | −0.14, 0.11 | −0.26, 0.0017 | 0.22, 0.012 | −0.24, 0.005 | −0.15, 0.08 | −0.25, 0.003 | |
| TYRP1 | 0.80, <0.0001 | −0.13, 0.14 | −0.05, 0.55 | 0.14, 0.14 | −0.27, 0.0016 | −0.14, 0.12 | −0.33, 0.0001 | 0.85, <0.0001 |
| (B) Age class 2 | ||||||||
| MC1R | ||||||||
| POMC | −0.02, 0.81 | |||||||
| PC1/3 | −0.05, 0.54 | −0.06, 0.54 | ||||||
| PC2 | 0.45, <0.0001 | −0.10, 0.30 | −0.03, 0.71 | |||||
| ASIP | 0.04, 0.63 | −0.17, 0.06 | −0.14, 0.10 | −0.07, 0.43 | ||||
| MITF | 0.11, 0.20 | −0.22, 0.015 | 0.03, 0.76 | 0.23, 0.007 | 0.10, 0.23 | |||
| SLC7A11 | −0.08, 0.32 | −0.13, 0.15 | 0.17, 0.048 | −0.07, 0.46 | 0.18, 0.029 | 0.18, 0.036 | ||
| TYR | 0.89, <0.0001 | −0.09, 0.33 | −0.04, 0.63 | 0.43, <0.0001 | 0.12, 0.17 | 0.09, 0.28 | −0.10, 0.23 | |
| TYRP1 | 0.85, <0.0001 | −0.06, 0.49 | 0.12, 0.16 | 0.46, <0.0001 | −0.12, 0.16 | 0.07, 0.38 | −0.08, 0.37 | 0.89, <0.0001 |
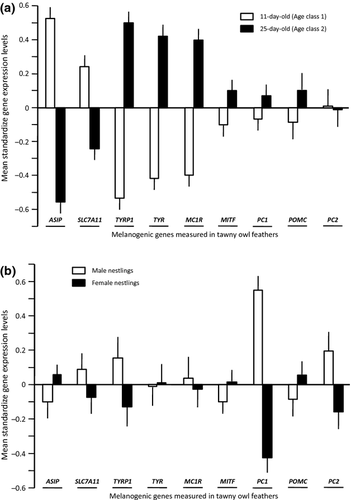
We detected significant sex-specific patterns of the expression of five of the nine genes (Table 1). Males expressed PC1/3, TYRP1 and PC2 to higher levels than females, whereas females expressed MITF and ASIP to higher levels than males (Fig. 2b).
We found little evidence that gene expression is associated with the brood size manipulation experiment, body condition and time of the day. Only ASIP was less expressed when nestlings were heavier (Table 1). The nest of rearing explained up to 52.0% of the variation in gene expression, indicating that nestlings that are sharing the same nest (or the same origin) expressed genes to relatively similar levels. Also, nestling identity explained up to 46.2% of the variation, indicating that an individual expressed a given gene to similar levels at the age classes 1 and 2. To further investigate this aspect, we performed repeatability analyses (Lessells & Boag 1987). The two measures performed per individual at the two age classes were significantly repeatable for SLC7A11 (0.61 ± 0.06; F142,150 = 4.17, P < 0.0001), PC1/3 (0.49 ± 0.07; F142,150 = 2.91, P < 0.0001), MITF (0.48 ± 0.07; F142,150 = 2.82, P < 0.0001) and POMC (0.39 ± 0.08; F117,143 = 2.25, P < 0.0001). PC2 was close to significance (0.12 ± 0.09; F119,146 = 1.27, P = 0.087), whereas the other genes (TYRP1, TYR, MC1R and ASIP) were not repeatable (r = 0, P-values > 0.83).
Covariation between gene expression levels
As shown in Table 2, genes typically involved in eumelanogenesis (TYR, TYRP1 and MC1R) were strongly correlated and negatively associated with genes typically involved in pheomelanogenesis (ASIP and SLC7A11). These three eumelanogenic genes were positively correlated with PC2 expression levels and not or negatively correlated with PC1/3 expression levels. The two above-mentioned genes involved in pheomelanogenesis were correlated and positively associated with the two convertases PC1/3 and PC2. The expression levels of MITF were positively correlated with expression levels of PC2 and SLC7A11 (but not with the pheomelanogenic gene ASIP) but negatively correlated with POMC (the only significant association between POMC and another gene).
Covariation between gene expression and coloration of biological parents
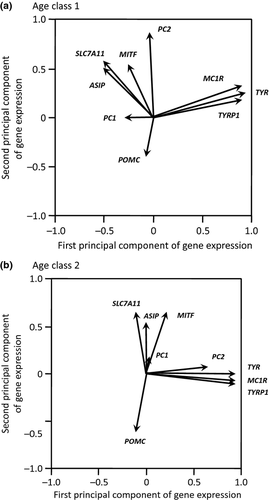
We kept the four principal components because the expression of most genes were not independent from each other (Table 2) and was strongly associated with age (Tables 1 and 2, Fig. 2a), we performed separate principal components analyses for the two age classes. We extracted the first four components for the two age classes (Table 3; Fig. 3). We kept the fourth principal component because their eigenvalues were only slightly smaller than 1 (0.98), and this was the only principal component associated with PC1/3.
| Age class 1 | Age class 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | 1st | 2nd | 3rd | 4th | |
| Eigenvalues | 3.17 | 1.95 | 1.24 | 0.98 | 3.11 | 1.51 | 1.18 | 0.98 |
| % Variance | 35.2 | 21.6 | 13.8 | 10.9 | 34.6 | 16.7 | 13.1 | 10.9 |
| Eigenvectors | ||||||||
| TYR | 0.52 | 0.18 | 0.06 | 0.01 | 0.53 | −0.01 | −0.10 | 0.19 |
| TYRP1 | 0.51 | 0.12 | 0.06 | 0.26 | 0.53 | −0.08 | 0.13 | 0.14 |
| MC1R | 0.50 | 0.23 | 0.11 | −0.01 | 0.53 | −0.06 | −0.09 | 0.15 |
| MITF | −0.15 | 0.40 | −0.50 | 0.10 | 0.11 | 0.50 | 0.06 | −0.51 |
| PC1/3 | −0.17 | −0.01 | 0.09 | 0.95 | 0.004 | 0.12 | 0.78 | 0.28 |
| PC2 | −0.01 | 0.61 | 0.24 | −0.01 | 0.37 | 0.05 | 0.08 | −0.43 |
| POMC | −0.04 | −0.27 | 0.70 | −0.003 | −0.07 | −0.51 | −0.001 | 0.24 |
| ASIP | −0.27 | 0.36 | 0.38 | −0.10 | −0.003 | 0.43 | −0.55 | 0.41 |
| SLC7A11 | −0.29 | 0.41 | 0.15 | −0.04 | −0.05 | 0.52 | 0.21 | 0.41 |
At age class 1, the first principal component was positively associated with mother coloration in interaction with the brood size manipulation experiment (Table 4). This interaction is explained by a significant effect in offspring raised in an enlarged brood (linear mixed model with nest of origin as random variable: F1,30.3 = 10.20, P = 0.0033, Fig. 4a) but not in a reduced brood (similar model: F1,27.65 = 0.005, P = 0.95). Because this component reflects higher expression levels of three eumelanogenic genes (Table 3), we performed similar analyses as in Table 4 for each of them. In the enlarged treatment, offspring of darker reddish biological mothers expressed to higher levels TYR (F1,38.92 = 12.41, P = 0.0011), MC1R (F1,38.6 = 14.38, P = 0.0005) and TYRP1 (F1,36.32 = 5.84, P = 0.021). The second principal component was positively associated with father coloration independently of the brood size manipulation experiment (Table 4; Fig. 4b). This second principal component is mainly related to PC2 (Table 3), and in a similar analysis, offspring of darker reddish fathers tended to express PC2 to higher levels (F1,43.91 = 3.13, P = 0.08; nestling sex: F1,124.5 = 6.34, P = 0.013). The third principal component was related to the interaction between father coloration and brood size manipulation (Table 4) because in the reduced treatment, this component was lower when the father was darker reddish (F1,28.88 = 4.21, P = 0.049), a relationship that did not apply to the enlarged treatment (F1,29.19 = 0.25, P = 0.62). This third component was mainly associated with POMC (Table 3), and accordingly in the reduced treatment, nestlings expressed the POMC gene to lower levels when their father was darker reddish (F1,35.66 = 5.46, P = 0.025, Fig. 4c). The fourth principal component was negatively associated with father coloration (Table 4), a component related to PC1/3 (Table 3). Accordingly, PC1/3 was less expressed in offspring sired by darker reddish fathers (F1,45.82 = 4.41, P = 0.04; nestling sex: F1,142.4 = 47.28, P < 0.0001; Fig. 4d).
| Nestling sex | Brood size manipulation (BSM) | Colour of biological mother (Mother) | Colour of biological father (Father) | Interaction Mother × BSM | Interaction Father × BSM | |
|---|---|---|---|---|---|---|
| Age class 1 | ||||||
| 1st component | F 1,99.57 = 0.22, P = 0.64 | F 1,100.5 = 0.19, P = 0.66 | F 1,32.11 = 4.75, P = 0.037 | F1,42.77 = 0.29, P = 0.59 | F 1,97.36 = 4.06, P = 0.047 | F1,95.98 = 0.002, P = 0.96 |
| 2nd component | F1,81.83 = 1.20, P = 0.28 | F1,76.2 = 0.16, P = 0.69 | F1,31.88 = 0.02, P = 0.88 | F 1,37.12 = 5.36, P = 0.026 | F1,74.1 = 1.24, P = 0.27 | F1,84.97 = 0.33, P = 0.57 |
| 3rd component | F1,91.2 = 0.38, P = 0.54 | F 1,88.13 = 2.07, P = 0.15 | F1,31.53 = 0.01, P = 0.93 | F 1,37.44 = 0.92, P = 0.34 | F1,81.01 = 0.46, P = 0.50 | F 1,96.51 = 6.00, P = 0.016 |
| 4th component | F 1,96.97 = 52.98, P < 0.0001 | F1,89.03 = 1.32, P = 0.25 | F1,33.87 = 0.44, P = 0.51 | F 1,43.36 = 3.94, P = 0.05 | F1,84.81 = 0.78, P = 0.38 | F1,93.98 = 0.81, P = 0.37 |
| Age class 2 | ||||||
| 1st component | F1,108.4 = 0.002, P = 0.96 | F 1,110 = 0.05, P = 0.83 | F 1,27.76 = 0.92, P = 0.35 | F1,37.4 = 0.16, P = 0.69 | F 1,108.7 = 4.96, P = 0.028 | F1,101.5 = 0.008, P = 0.93 |
| 2nd component | F1,94.66 = 0.20, P = 0.66 | F1,96.75 = 0.71, P = 0.40 | F1,38.5 = 0.39, P = 0.53 | F1,44.2 = 0.07, P = 0.79 | F1,89.09 = 0.55, P = 0.46 | F1,94.48 = 2.71, P = 0.10 |
| 3rd component | F 1,102.7 = 41.08, P < 0.0001 | F1,98.87 = 0.002, P = 0.96 | F1,37.04 = 0.15, P = 0.70 | F1,39.11 = 0.37, P = 0.55 | F1,95.43 = 0.63, P = 0.43 | F1,92.17 = 0.46, P = 0.50 |
| 4th component | F1,104.7 = 0.50, P = 0.48 | F1,106.9 = 3.21, P = 0.08 | F1,37.4 = 2.16, P = 0.15 | F1,47.43 = 1.63, P = 0.21 | F1,98.51 = 1.57, P = 0.21 | F1,102.6 = 2.30, P = 0.13 |
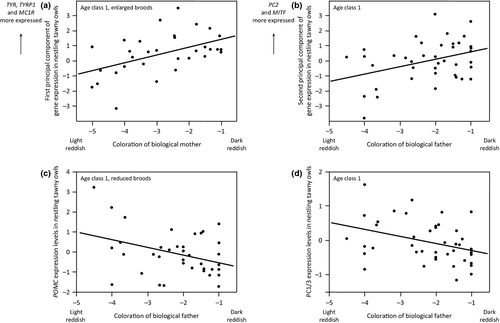
At age class 2, the first principal component was associated with mother coloration in interaction with the brood size manipulation experiment (Table 4). In the reduced treatment, offspring of darker reddish mothers tended to express eumelanic genes to higher levels (F1,20.36 = 4.05, P = 0.058; enlarged treatment: F1,31.55 = 1.18, P = 0.29). The three other principal components were not significantly associated with parental coloration alone or in interaction with the brood size manipulation treatments (Table 4).
Quantitative genetics of nestling plumage coloration
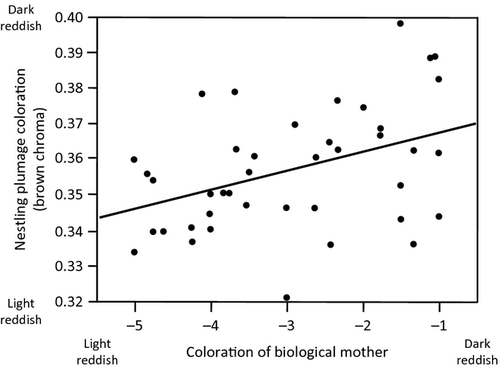
Before investigating the relationship between nestling plumage coloration and gene expression, we analysed the quantitative genetics of plumage coloration to have a better idea about the relative impact of the rearing environment and genes on the expression of melanin-based coloration in nestlings. We first found that nestlings were similarly coloured when raised in experimentally enlarged and reduced broods (linear mixed model with nest of rearing as random variable: F1,41.02 = 0.55, P = 0.46). Then, we correlated mean coloration of siblings raised in a foster nest with coloration of the foster father (r = −0.06, n = 34, P = 0.73) and mother (r = −0.27, n = 35, P = 0.12), which were negative and not significant. Thus, the rearing environment does not inflate the resemblance between related individuals. To examine whether offspring resemble their biological parents, we considered the entire sample of cross-fostered and non-cross-fostered nestlings and calculated mean sibling values. Offspring resembled significantly their biological mother (multiple regression analysis on mean sibling values: F1,36 = 5.78, P = 0.02; Fig. 5) but not their biological father (F1,36 = 0.34, P = 0.56). Finally, siblings resembled each other significantly (repeatability, r = 0.38 ± 0.05, F39,92 = 3.15, P < 0.0001). In contrast, siblings raised in a foster nest did not resemble their unrelated non-cross-fostered nestmates (analysis based on mean sibling values, repeatability = 0, F29,30 = 0.98, P = 0.52).
Covariation between gene expression and nestling coloration
We ran Pearson's correlations between mean gene expression levels and mean nestmate coloration. At age class 1, dark melanic nestlings expressed at lower levels, PC1/3 (r = −0.37, n = 41 families, P = 0.017, Fig. 6a) and SLC7A11 (r = −0.36, P = 0.021, Fig. 6b), but MC1R at higher levels (r = 0.32, P = 0.046, Fig. 6c). In contrast, the association between nestling plumage coloration and ASIP was not significant (r = −0.26, P = 0.11). A similar conclusion applies to PC2 (r = −0.15, P = 0.38), TYR (r = 0.22, P = 0.18), POMC (r = 0.10, P = 0.55), MITF (r = −0.04, P = 0.80) and TYRP1 (r = 0.07, P = 0.67).
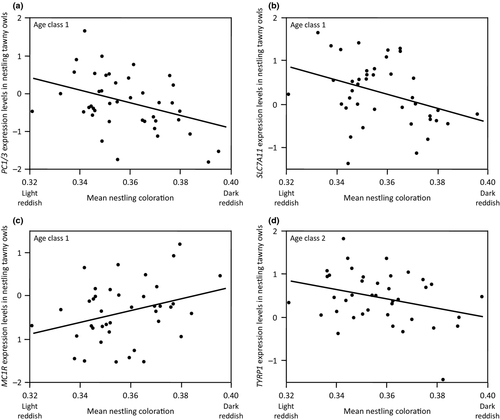
Based on mean sibling values at age class 2, darker reddish nestlings expressed TYRP1 to lower levels (r = −0.31, n = 40 families, P = 0.049, Fig. 6d). The expression levels of the other genes were not significantly associated with nestling plumage coloration (ASIP: r = 0.25, P = 0.12; PC1/3: r = −0.16, P = 0.34; SLC7A11: r = −0.22, P = 0.18; MC1R: r = −0.30, P = 0.06; PC2: r = 0.16, P = 0.34; TYR: r = −0.21, P = 0.18; POMC: r = 0.19, P = 0.24; MITF: r = 0.18, P = 0.26).
Discussion
We recently proposed that covariation between traits used in social interactions such as pigmentation and other phenotypic attributes such as immunity can be generated by genes that pleiotropically regulate all these traits (Ducrest et al. 2008). Based on a review of the genetic and pharmacological literature, we proposed that the melanocortin system is a potential candidate. In line with this model, we report here that the expression levels of genes belonging to the melanocortin system are associated with both the expression levels of melanogenic genes and melanin-based coloration in the tawny owl. This result has important implications not only to understand which genes participate in the production of eumelanin and pheomelanin pigments but also to identify the proximate mechanism underlying colour-specific reaction norms.
Covariation between the expression levels of genes of the melanocortin system and those involved in melanogenesis
In human and murine cells, the melanocortin α-MSH up-regulates the eumelanogenic genes MC1R, MITF, TYR and TYRP1, implying that the expression levels of these genes should be positively correlated (Abdel-Malek et al. 1995; Le Pape et al. 2009). Conversely, ASIP and SLC7A11 are regulators of pheomelanogenesis (Sakai et al. 1997; Suzuki et al. 1997; Chintala et al. 2005), and hence, their expression levels may be negatively correlated with the levels of eumelanogenic genes. These predictions were fulfilled except that MITF was positively correlated only with the pheomelanic gene SLC7A11. This relationship might be explained by the fact SLC7A11 can be up-regulated by MITF (Hoek et al. 2008). The role of MITF in the production of melanin-based coloration is therefore unclear in the tawny owl. Another potential explanation is that only the MITF isoform M is up-regulated during eumelanogenesis (Li et al. 2012), and as we measured expression levels of both isoforms B and M, we did not obtain comparable results. Nevertheless, the negative correlations between the expression levels of eumelanogenic and pheomelanogenic genes indicate that the production of one type of melanin pigment is made at the expense of the other. This finding may, however, be specific to nestlings for which feathers are relatively darkly coloured suggesting overexpression of eumelanins compared with pheomelanins, suggesting that the ratio of eumelanin/pheomelanin is higher in nestlings compared with adults (Gasparini et al. 2009a).
Turning to the genes belonging to the melanocortin system, we found that expression levels of POMC covaried only with MITF (negative correlations at the two age classes) but with none of the other melanogenic genes (MC1R, TYR, TYRP1, SLC7A11 and ASIP). This suggests that variation in the expression levels of the POMC gene is hardly associated with melanogenesis. Similar results were observed in pomc knockout mice on a nonagouti background. These mice were black with no expression of the POMC gene, suggesting that the constitutive activity of the MC1R was sufficient to produce eumelanin in the absence of melanocortins or that other molecules may control eumelanin synthesis (Slominski et al. 2005). In dogs, the beta-defensin was shown to bind to MC1R and to induce eumelanin synthesis (Candille et al. 2007). Therefore, although the POMC gene produces α-MSH, an important component of melanogenesis, production of darker eumelanic feathers may not be achieved through higher POMC expression levels. Post-translational modification of the POMC prohormone may therefore be key to explain variation in melanogenesis. This is why we measured the convertases PC1/3 and PC2.
A first result regarding these two convertases is that their expression levels were not correlated with POMC expression levels implying an independent regulation. Therefore, production of the POMC prohormone (through differential expression of the POMC gene), ACTH (through cleavage of the POMC prohormone by PC1/3) and α-MSH (through cleavage of ACTH by PC2) involves different mechanisms that can be independently activated depending on the specific requirements. The two convertases PC1/3 and PC2 have numerous physiological functions beyond the melanocortin system (Seidah & Prat 2012). To get insights into the regulatory effects of these two convertases on melanogenesis, we can discuss their patterns of covariations with the other genes. With respect to PC1/3, we detected only three significant correlations of 16 Pearson's correlation analyses, and these significant associations were relatively weak (mean absolute coefficient is 0.10). This indicates that variation in the expression levels of PC1/3 is weakly associated with melanogenesis. This is an interesting result because PC1/3 gives ACTH and only indirectly α-MSH, which is involved in melanogenesis in contrast to ACTH (but see Hunt et al. 1994; Ling et al. 2004). It should be, however, emphasized that the few significant associations with PC1/3 indicate that the expression levels of this gene is negatively related to eumelanogenesis (negative correlations with MC1R and TYR at age class 1) and positively with pheomelanogenesis (positive correlation with SLC7A11 at age class 2). This suggests that in the tawny owl when ACTH is produced in feather bases, eumelanin production is reduced in contrast to pheomelanin, which is enhanced. Compared to PC1/3, the expression levels of PC2 were more often associated with the other genes (9 significant Pearson's correlations of 16) and more strongly (mean absolute Pearson's correlation coefficient is 0.23). PC2, which cleaves ACTH to generate α-MSH, was positively correlated with the expression levels of both eumelanin-related (MC1R, TYR, TYRP1) and pheomelanin-related genes (ASIP and SLC7A11) (Table 2). If the association between expression levels of PC2 and eumelanogenic genes is expected given that PC2 gives α-MSH, the positive association with pheomelanogenic genes indicates that PC2 is not responsible for the negative association between the expression levels of eumelanogenic and pheomelanogenic genes.
Age-specific patterns of gene expression
Pheomelanogenesis-related genes (ASIP and SLC7A11) were more expressed at 11 than 25 days of age, whereas the opposite pattern was detected in eumelanogenesis-related genes (MC1R, TYR and TYRP1). Thus, in young tawny owls, melanogenesis may proceed in waves starting mainly with pheomelanogenesis followed by eumelanogenesis. Similarly to these three eumelanogenic genes, MITF and PC1/3 were more expressed at 25 than 11 days of age, while the expression levels of POMC and PC2 did not differ between the two age classes. The stronger covariations between gene expression levels at 11 than 25 days of age suggest a more coordinated control of the expression of different genes participating in melanogenesis at younger ages. A nonmutually exclusive explanation for the age-specific pattern of gene expression is that most colour pigments are produced at 11 days of age, whereas the less pigmented basal part of the feather is produced at 25 days of age.
Sex-specific patterns of gene expression
Laboratory studies highlighted that the expression of melanogenic genes can differ between males and females. Although PC1/3 and PC2 are not located on sex chromosomes in mammals (chromosomes 13 and 2 in Mus musculus, respectively), female mice frequently have higher expression of PC1/3 and PC2 than males in the brain (Beinfeld et al. 2005). Sexual dimorphism in gene expression may be the consequence of trans-regulatory elements mapped along mammalian X chromosome, an issue that needs to be tackled further. In birds, PC1/3 and TYRP1 genes are located on the sex Z chromosome potentially explaining why males (homogametic ZZ) expressed higher levels of PC1/3 and TYRP1 than females (heterogametic ZW). Even if plumage coloration is not sexually dimorphic in the tawny owl, males may, nevertheless, have a higher eumelanogenic potential than females (through TYRP1 expression) but a lower pheomelanogenic potential (through ASIP expression). They may also have a greater capacity of POMC processing (through PC1/3 and PC2 activities) to give rise to ACTH and α-MSH (Wardlaw 2011), key peptides of the melanocortin system responsible for numerous pleiotropic effects beyond melanogenesis including stress response, energy homeostasis, anti-inflammatory response, sexual activity, susceptibility to oxidative stress and aggressiveness (Ducrest et al. 2008). This is plausible because the melanocortin system plays a key role in the interplay between local (i.e. skin and hence feather base) and systemic neuroendocrine system (Slominski & Wortsman 2000; Slominski 2009; Slominski et al. 2012).
The higher male eumelanogenic capacity and POMC processing raises a number of issues. First, it would be interesting to test whether reddish coloration in male and female tawny owls is due to different mix of eumelanin and pheomelanin feather contents. Second, although hypothetical, the higher male than female potential to produce ACTH or α-MSH (but also other MSHs) to bind the various melanocortin receptors may enable them to increase their energy expenditure, sexual activity and aggressiveness (Ducrest et al. 2008). Moreover, higher amount of the PC1/3 convertase will be also available for other potential targets in feathers, such as proinsulin, proglucagon, prothyroid hormones (Seidah & Prat 2012). Accordingly, sex-specific expression of PC1/3 and PC2 genes may participate in the strong partition of reproductive tasks between males and females in the tawny owl.
Colour-specific patterns of gene expression
The degree of nestling melanin-based coloration was significantly positively associated with the expression levels of two genes involved in the production of eumelanin (MC1R and TYRP1) but negatively with the levels of a gene associated with the production of pheomelanin (SLC7A11). We further found that when the biological parents were darker reddish, their offspring expressed eumelanogenic genes (TYR, TYRP1 and MC1R) to higher levels. In the tawny owl, colour variation is less pronounced in nestlings than in adults. Although at the nestling stage feathers also vary from pale to dark reddish, the intensity of reddish coloration is less pronounced than in adults, potentially suggesting that the eumelanin-to-pheomelanin ratio may be higher in nestlings than in adults. In line with this suggestion, we found that parental coloration was more strongly associated with the offspring expression of eumelanogenic than pheomelanogenic genes.
We found that offspring born from darker melanic parents tended to express PC2 at higher levels, expressed POMC to higher levels (when raised in experimentally reduced but not enlarged broods) but expressed PC1/3 to lower levels. The expression of PC1/3 was also negatively correlated with nestling plumage coloration further emphasizing the potential key role of this convertase in the production of melanin-based coloration (higher expression levels of PC1/3 were negatively correlated with the expression levels of eumelanogenic genes, Table 2). The PC1/3 cleaving enzyme is responsible for the tissue-specific post-translational processing of POMC prohormone into mature ACTH, β-lipotropin (β-LPH) and N-terminal peptide (N-POMC) (Pritchard & White 2007). Furthermore, offspring born from light melanic parents are likely to have a greater capacity of POMC processing to obtain more ACTH through their higher PC1/3 gene expression. ACTH is a key regulator of the hypothalamic–pituitary–adrenal (HPA) axis, mainly involved in stress response. Upon release into the systemic circulation, ACTH reaches the adrenal gland and binds to MC2R to stimulate the production of glucocorticoids (corticosterone or cortisol) that regulate stress responses (Papadimitriou & Priftis 2009) and modulate the functioning of a large number of tissues, including the epidermis (Slominski et al. 2007). Because glucocorticoids have numerous effects on physiology including energy homeostasis (Sapolsky et al. 2000), colour-specific expression of PC1/3 genes and its cascading effects on the production of glucocorticoids could potentially explain previously published results. Indeed, nestlings born from light melanic females converted food less efficiently into body mass when fed ad libitum than nestlings born from dark melanic mothers, but suffered less from food restriction (Piault et al. 2009).
Altogether, our results raise the hypothesis that regulation of PC1/3 gene may be a potential proximate mechanism modulating the pleiotropic effects of melanocortins in the tawny owl and account for colour-specific norms of reactions. A first step to examine this hypothesis would be to compare the expression levels of convertase enzymes measured in growing feathers with the levels in other organs and tissues. The prediction is that expression levels of convertases are similar across tissues, implying that if in feathers the expression levels of convertases are related to melanogenesis, in other tissues these convertases may have other effects generating covariation between coloration and other phenotypic traits. To conclude, our study suggests that indeed pleiotropy could explain why strongly heritable colour traits are associated with many other phenotypic attributes.
Acknowledgements
We are grateful to the Swiss National Science Foundation for financing the study and Louis Bernatchez and five anonymous reviewers for useful comments.
References
G.E., A.-L.D., P.B. and A.R. designed the study. G.E., P.B. and A.R. contributed to field work and field data collection. G.E., A.-L.D., H.R. and C.S. performed the molecular work. A.R. and G.E. performed the statistical analyses and wrote the article. A.R., G.E., A.-L.D. and P.B. edited the manuscript.
Data accessibility
cDNA sequences: GenBank Accession nos: ASIP: KF201574, GAPDH: KF201575, EEF1A: KF201576, MC1R:KF201577, MITF: KF201578, PC1: KF201579, PC2: KF201580, POMC: KF201581, SLC7A11 variant 1: KF201582, SLC7A11 variant 2: KF201587, TYR: KF201583, TYRP1: KF201584.
Sample information including age, brood details, sex, weight, coloration data, clutch size, body condition, and four components of gene expression: Dryad doi:10.5061/dryad.6f4 g4




