Antarctic micrometeorite composed of CP and CS IDP-like material: A micro-breccia originated from a partially ice-melted comet-like small body
Abstract
Asteroids and comets are thought to form in the inner and outer solar systems, respectively. Chondritic porous and smooth interplanetary dust particles (CP IDPs and CS IDPs, respectively) in the stratosphere are regarded as dust grains from comets and hydrated asteroids, respectively. Here, we describe an Antarctic micrometeorite (AMM) composed of lithologies of both CP and CS IDPs. In addition to the CS IDP-like compact lithology that experienced severe aqueous alteration, the CP IDP-like porous lithology shows evidence of very weak aqueous alteration. The structure of the organic matter in the porous lithology varies from that in the CP IDPs to aromatic-rich organic matter. In contrast, the structure of the organic matter in the compact lithology is homogenous, which is consistent with higher degrees of aqueous alteration. Its structure is more similar to that of CP IDPs and Wild 2 samples than that of meteoritic insoluble organic matter, suggesting that the compact lithology formed from the porous lithology. Some CP IDPs are related to cometary dust streams, such as those originating from 26P/Grigg-Skjellerup. In addition, the presence of this AMM indicates an additional origin of the CP IDPs and their equivalent AMMs. The mineralogy and organic chemistry of this AMM suggest that its parent body was composed of the same building blocks as those of the comets, and later experienced incomplete aqueous alteration. The AMM probably formed as microbreccia in the regolith layer composed of materials from a CP IDP-like crust and a hydrated interior.
Introduction
The solar system was formed from a protosolar disk composed of gas and dust approximately 4.6 billion years ago (e.g., Boss & Ciesla, 2014). To date, more than 600,000 asteroids, mainly orbiting the Sun between Mars and Jupiter, have been identified (e.g., https://www.minorplanetcenter.net/iau/lists/NumberedMPs.txt). These asteroids are remnants of early solar system materials that were not able to form planets. Asteroids are classified based on their reflectance spectra, color, and albedo. The most common asteroids around 2.2–3 astronomical units (AUs) from the Sun (Rivkin et al., 2002) have reflectance spectra that evoke a genetic relationship between these asteroids and carbonaceous chondrite meteorites, and are classified as C-complex (B-, C-, and G-type) asteroids (DeMeo & Carry, 2014). Because some groups of carbonaceous chondrites, such as CI and CM chondrites, contain abundant H2O- and OH-bearing minerals (hydrated minerals), a large fraction of C-complex asteroids is expected to contain hydrated minerals. The spectral features indicative of the presence of hydrated minerals are observed in 60–70% of these (Rivkin, 2012). Hydrated minerals formed as a result of reactions between anhydrous mineral phases, organic matter, and liquid water (aqueous alteration). This means that water ice accreted as one of the building blocks when these asteroids formed. Within a few million years after accretion, the water ice melted by heating owing to the decay of short-lived radionuclides such as 26Al (Grimm & McSween, 1993).
The major spectral types of asteroids that exist in the outer asteroid belt ~3–5 AU from the Sun are the P and D types. These asteroids are ice-containing asteroids (DeMeo & Carry, 2014). Moreover, large P- and D-type asteroids may have experienced aqueous alterations, as was the case for C-complex asteroids (Yang et al., 2013). Dynamic simulations of the early solar system suggested that most asteroids orbiting >3 AU from the Sun formed in the Kuiper belt (>30 AU from the Sun), which is the source region of Jupiter-family comets (short-period comets with <20 years of orbital periods), and that they migrated to the asteroid belt owing to the orbital changes of giant planets (Levison et al., 2009; Lowry et al., 2008; Nesvorný et al., 2017; Vokrouhlický et al., 2016; Walsh et al., 2011).
Comets are typical ice-containing bodies formed far beyond the snow line. They are highly porous and composed of ice and dust (minerals and organic matter; e.g., Rotundi et al., 2018). If P- and D-type asteroids formed at >30 AU from the Sun, as suggested by dynamic simulations, P- and D-type asteroids are likely composed of building blocks similar to those of comets. If highly porous aggregates of minerals and organic matter that experienced local aqueous alteration are found, they may have been derived from partially hydrated P- or D-type asteroids.
Fine-grained particles originating from asteroids and comets distributed in the interplanetary space near the ecliptic plane may provide opportunities to investigate the materials originating from partially hydrated P- and D-type asteroids. A total of 2–4 × 104 t of these materials annually encounter the Earth (Love & Brownlee, 1993), and some survive their atmospheric entry (Rojas et al., 2021). Surviving particles are usually recovered in the stratosphere, or from Antarctic snow and ice; these are referred to as interplanetary dust particles (IDPs) and Antarctic micrometeorites (AMMs), respectively (Bradley, 2014; Maurette, 2008). IDPs, which are regarded as dust derived from comets, are highly porous aggregates mainly consisting of submicrometer-sized anhydrous minerals connected by or embedded in organic matter (Bradley, 2014; Ishii et al., 2008). The diagnostic constituent of the possible cometary material is an amorphous silicate containing nanometer-sized Fe metal and sulfides (glass with embedded metal and sulfide [GEMS]). In this regard, possible cometary dust particles are chondritic porous (CP) IDPs, CP AMMs, and ultracarbonaceous (highly enriched in carbon) AMMs (UCAMMs) (Bradley, 2014; Dobrică et al., 2012; Duprat et al., 2011; Ishii et al., 2008; Noguchi et al., 2015; Yabuta et al., 2017). IDPs and AMMs that experienced heavy aqueous alteration are also common. They are classified as chondritic smooth (CS) IDPs (Bradley, 2014) and fine-grained type C1 AMMs or hydrated fine-grained AMMs (Dobrică et al., 2019; Genge et al., 2008). These have been thought to have been derived from C-complex asteroids that experienced aqueous alteration.
In this paper, we describe an AMM composed of lithologies of both CP and CS IDPs. Because possible cometary dust grain CP IDPs are thought to have been formed in the Kuiper belt and because possible C-complex asteroidal dust grain CS IDPs are thought to have been formed in the asteroid belt, the intimate coexistence of these two lithologies in this AMM requires reconsideration of the conventional view of their origins.
Materials and Methods
Collection of AMMs
The team of the 51st Japan Antarctic Research Expedition (JARE) collected ~300 kg of surface snow (up to ~10 cm below the surface) ~4 km southwest of the Dome Fuji Station in January 2010. Because the annual snowfall was ~10 cm year−1 in the collection area (Kameda et al., 2008), snow that fell within 1 year was collected. Therefore, the effects of terrestrial weathering on the AMM samples could be neglected. The snow was transferred to Japan in a frozen state, then it was melted and filtered in a Class 1000 cleanroom at Ibaraki University when the first author belonged to the university. We placed the snow in 3 L vessels composed of stainless steel and others composed of glass in a cleanroom. The snow required approximately 6 h to thaw. We filtered the water in the vessels as soon as possible after thawing. The temperature of the water was well below the dew point of the cleanroom, which we noted because the surface of the vessels quickly coated with condensation after being wiped from the surface. Because the preset temperature and relative humidity of the cleanroom were 23 °C and 45%, respectively, we calculated the dew point as 8.7 °C. The temperature of the water was probably well below ~8.7 °C. We identified approximately 400 AMMs using a scanning electron microscope equipped with an energy-dispersive spectrometer (Noguchi et al., 2017). We identified D10IB292 as an AMM.
Sample Preparation
We embedded D10IB292 AMM in molten elemental sulfur under a stereomicroscope in a clean booth at Kyushu University. We ultramicrotomed the embedded sample to ~100 nm thick ultra-thin sections, which we placed on Cu grids with an amorphous carbon supporting film for transmission electron microscopy (TEM), a Cu grid with an amorphous SiOx supporting film for C and N X-ray absorption near-edge structure analysis using a scanning transmission X-ray microscope (STXM-XANES), and on an optical grade ZnS crystal for atomic force microscopy-infrared spectroscopy (AFM-IR).
Scanning Transmission Electron Microscopy
We used scanning transmission electron microscopy (STEM; Thermo Tecnai G2-20F and JEOL JEM-ARM200F) to investigate the AMM at the Ultramicroscopy Research Center, Kyushu University. We used high-resolution TEM, selected area electron diffraction (SAED), high-angle annular dark-field scanning electron diffraction (HAADF) imaging, and energy-dispersive X-ray spectrometry (EDS) to characterize the minerals in ultra-thin sections. We quantitatively analyzed the minerals using EDS equipped on a Tecnai G2-20F. We used many minerals as standards for the k factors of the elements.
X-Ray Absorption Near-Edge Spectra Using Scanning Transmission X-Ray Microscopy
We acquired the carbon- and nitrogen-XANES spectra of the ultra-thin sections using STXM at beamline 5.3.2.2, Advanced Light Source (ALS), Lawrence Berkeley National Laboratory, USA (Kilcoyne et al., 2003), and compact STXM (cSTXM) at beamline 19A, Photon Factory (PF), High Energy Accelerator Research Organization (KEK), Tsukuba, Japan. Beamline 5.3.2.2 employs a bending magnet providing synchrotron radiation X-rays with a photon range of 250–800 eV with a flux of 107 photons per second (Kilcoyne et al., 2003). Beamline 19A employs an APPLE-II-type undulator providing synchrotron radiation X-rays with a photon range spanning 200–2000 eV with a flux of 1011 photons per second. The spatial resolution of STXM using a Fresnel zone plate (FZP) is typically approximately 30 nm (Takeichi et al., 2016). The energy resolution was ~5000. We calibrated the energy by measuring CO2 and N2 gas (ALS) or graphite (PF) before the measurements. We obtained C-XANES transmission spectra in stack scan mode, with 0.1 eV resolution across the near-edge region and 0.5 eV resolution below and above the near-edge absorption. We obtained the absorption spectra (optical density [OD]) as OD = −ln(I/I0), where I is the X-ray intensity transmitted from a sample and I0 is the X-ray intensity measured without samples (the energy of the incident X-ray). We assigned the absorption peaks of the XANES spectra according to Kilcoyne et al. (2003) and Leinweber et al. (2007).
Atomic Force Microscope Infrared Spectroscopy
We performed AFM-IR using an Analysis Instruments nanoIR2 at Nihon Thermal Consulting Co. Ltd. The instrument was a combination of AFM with a tunable IR (850–3800 cm−1) optical parametric oscillator (OPO). The absorption of the laser induced heating, which resulted in rapid expansion of the sample, which excited the coherent vibration of the cantilever of the AFM. The induced vibration exhibited a characteristic ring-down pattern. We obtained a series of ring-down signals by sweeping the wavelength of the OPO laser that illuminated the target area. We determined the relationships between the vibration amplitudes and frequency using the Fourier transform of the ring-down signals. We obtained the local IR absorption spectra as a function of the wave number of the sweeping OPO laser (pulse duration: 10 ns; pulse energy: 2 μJ; repetition rate: 1 kHz) and ring-down signals. The wave number resolution was 4 cm−1. The probing force was 0.3 V. We used an EX-nIR2 cantilever (contact point diameter: ~30 nm). We measured the amplitude maps for four wave numbers, 1460, 1730, 2850, and 2920 cm−1, of an ultra-thin foil, which we prepared by ultramicrotomy and placed on an optical-grade ZnS crystal. We set the scan rate to 0.1 Hz. The scanning area had 400 × 100 pixels, and each pixel size was 3.75 × 15 nm. These wave numbers corresponded to the vibrations of aliphatic CH2 and CH3, carbonyl (C=O), aliphatic CH3, and aliphatic CH2, respectively. We obtained the distributions of the signals corresponding to these functional groups from the amplitude maps of the thin foil sample. We measured almost the same area for each map based on the height maps of the scanned areas. From the height maps, we obtained the variation in the thickness of the thin foil sample. We measured two wavelength ranges, 900–2000 and 2600–3200 cm−1, to obtain the IR spectra of the hotspots shown in the amplitude maps.
Results
Description of D10IB292
The AMM named D10IB292 is ~30 μm across and is mainly composed of submicrometer-sized constituents (inset in Fig. 1a) and a chondritic bulk composition (Fig. S1). The bright-field (BF) transmission TEM images of the ultramicrotomed thin sections of this AMM showed that the interior of this AMM is composed of an intimate mixture of compact and porous areas (Fig. 1). The compact areas are rich in phyllosilicates, indicating that they experienced remarkable aqueous alteration. In contrast, the porous areas are composed of anhydrous phases, except for a small amount of phyllosilicate. Hereafter, we refer to the former as compact lithology and the latter as porous lithology.
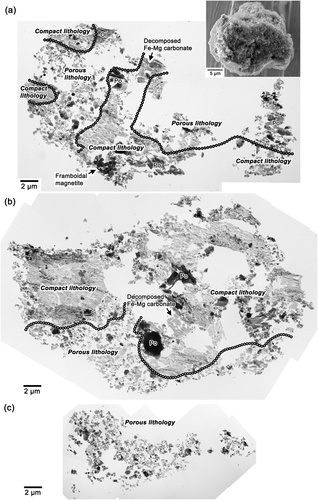
Description of Porous Lithology
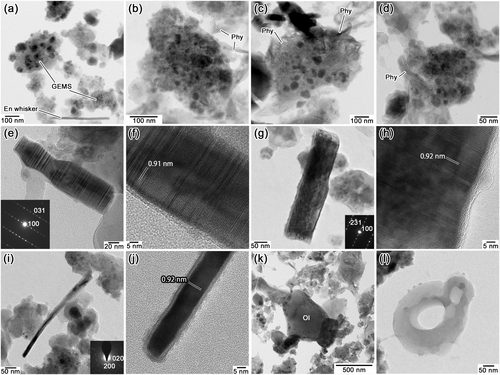
The porosity of the porous lithology is high, up to ~50% (Fig. 1). This lithology contains abundant GEMS, ranging from ~100 to ~300 nm in diameter (Fig. 2a–d), low-Ca pyroxene, olivine, and pyrrhotite (Table 1). However, in contrast to typical CP IDPs and CP micrometeorites (MMs), some grains of GEMS in the porous lithology show a very weak aqueous alteration: the presence of a minor amount of fibrous phyllosilicate on the surface of GEMS. The abundance of phyllosilicate locally varies; some grains of GEMS show no evidence of aqueous alteration (Fig. 2a); and other grains have a minor amount of fibrous phyllosilicate on their surfaces (Fig. 2b–d). The fibrous phyllosilicate is poorly crystalline but rarely shows ~1 nm lattice fringes, suggestive of saponite.
| Lithology/types of IDP and AMMs | Porous lithology | Compact lithology | CP IDPsa/CP MMsb/UCAMMsc | CS IDPs/hydrated fine-grained AMMs | The least-altered areas of matrices in the primitive chondritese-j |
|---|---|---|---|---|---|
| Porosity | High (up to ~50%) | Relatively low | High (up to ~60%, except for UCAMMs that are filled by organics) | Relatively low | Relatively low to relatively high |
| Major phases | GEMS, low-Ca pyroxene (including enstatite whisker/platelet), olivine, isolated large pyrrhotite | Saponite, pyrrhotite, carbonate, magnetite (sometimes occurs as framboidal aggregates) | GEMS, low-Ca pyroxene (including enstatite whisker/platelet), olivine, isolated large pyrrhotite | Saponite, pyrrhotite, carbonate, magnetite (sometimes occurs as framboidal aggregates) | GEMS-like amorphous silicate, olivine, low-Ca pyroxene (sometimes occurs as enstatite whisker/platelet), isolated large pyrrhotite, Fe oxide |
| Organic material | Abundant | Present | Abundant | Present | Present |
| Structure of organic material | Heterogeneous, from areas rich in aromatic C, ketone, and carboxyl to IOM-like | Homogeneous, rich in aliphatic C, ketone, and carboxyl | Heterogeneous, rich in aliphatic C, ketone, and carboxyld | Homogeneous, IOM-liked | Homogeneous, IOM-likei |
- CP = chondritic porous; IDP = interplanetary dust particles; AMMs = Antarctic micrometeorites; UCAMMs = ultracarbonaceous AMMs; GEMS = glass with embedded metal and sulfide; IOM = insoluble organic matter.
- References: aBradley (2014); bNoguchi et al. (2015); cDobrică et al. (2012); dKeller et al. (2004); eBrearley (1993); fGreshake et al. (1997); gLe Guillou & Brearley (2014); hLeroux et al. (2015); iNittler et al. (2019); jMatsumoto et al. (2019).
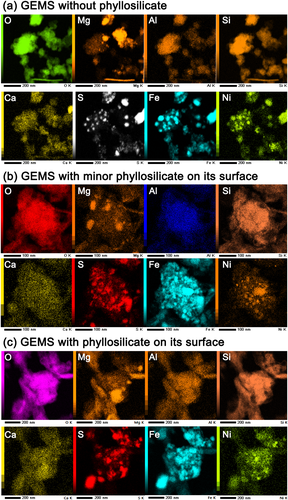
Figure 3 shows the elemental distribution maps of four representative grains of GEMS, which have different amounts of phyllosilicate. The mapped areas correspond to the whole fields in Fig. 2a, 2b, and 2d. All GEMS grains contain both nanometer-sized Fe–Ni metal and Fe sulfide, which can be understood by comparing the S, Fe, and Ni maps in Fig. 3, irrespective of the absence or presence of phyllosilicate on GEMS.
Low-Ca pyroxene is relatively abundant and sometimes occurs as platelets and whiskers (Fig. 2e–j) in this lithology. Both the enstatite platelets and whiskers elongate along the a axis. The high-resolution images of the crystals show (100) clinoenstatite lattice fringes with minor orthoenstatite lattice fringes, as well as abundant stacking disorders and twinning, as shown in the SAED patterns (insets in Fig. 2e, 2g, and 2i) and high-resolution TEM images (Fig. 2f and 2h). Although the enstatite whisker shown in Fig. 2a is straight, it slightly bent during the acquisition of the elemental distribution maps (Fig. 2i). Olivine typically occurs as subhedral to anhedral crystals (Fig. 2k). Rare hollow organic nanoglobules are present in this lithology. Owing to their high porosity, they often appear as isolated globules (Fig. 2l).
Description of Compact Lithology
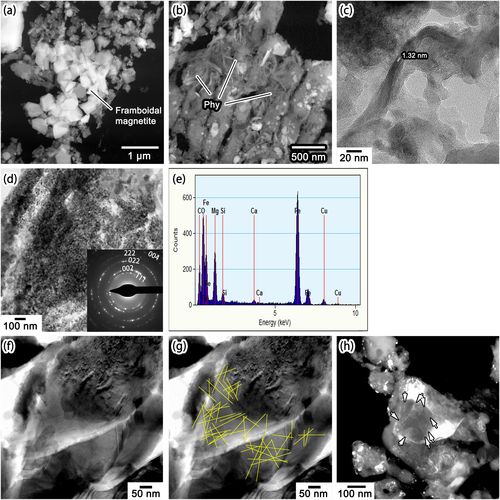
The major phases in the compact lithology are abundant phyllosilicates, a framboidal aggregate of magnetite, pyrrhotite, and very-fine-grained aggregates of Fe–Mg oxide (Table 1; Fig. 4a–d). We observed a framboidal aggregate of magnetite, composed of ~300–500 nm euhedral magnetite crystals. Figure 4a shows the aggregate in a high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) image, which corresponds to the framboidal magnetite in Fig. 1a. Fibrous phyllosilicate crystals are densely arranged and coexist with tiny (mostly <20 nm) Fe sulfide grains (bright spots in Fig. 4b). The phyllosilicate is poorly crystalline, but sometimes shows 1.0–1.3 nm basal lattice fringes, suggestive of saponite (Fig. 4c). Saponite is the predominant phyllosilicate in CS IDPs and hydrated fine-grained AMMs (Bradley, 2014; Noguchi et al., 2002). Aggregates of fine-grained (<30 nm) Fe- and Mg-rich oxide crystals (Fig. 4d and 4e) are also some of the major phases in the hydrated lithology. Such aggregates are common among hydrated fine-grained AMMs, which were originally Fe–Mg carbonates but decomposed by atmospheric entry heating (Noguchi et al., 2002; Nozaki et al., 2006). Figure 1a displays a decomposed Fe–Mg carbonate. We also observed isolated Fe sulfide and minor olivine. These minerals are common in CS IDPs (Bradley, 2014) and hydrated fine-grained AMMs (Dobrică et al., 2019; Genge et al., 2008; Noguchi et al., 2002; Sakamoto et al., 2010).
Abundant Solar Flare Tracks in Both Porous and Compact Lithologies
We found abundant solar flare tracks in olivine in both porous and compact lithologies (Fig. 4f–h). We identified solar flare tracks in olivine crystals ~2–3 μm across. Figure 4f shows a track-rich olivine crystal at the boundary between porous and hydrated lithologies. Because solar flare tracks appear as thin black lines in the BF TEM images (Fig. 4f), they are delineated as fine yellow lines in Fig. 4g for ease of recognition. The track density is greater than 5 × 1010 tracks per cm2. In the porous lithology, we observed a few olivine crystals as large as ~1 μm. Nonetheless, we identified submicrometer-sized track-rich olivine crystals. Figure 4h shows one such olivine with ~5 × 1010 tracks per cm2. The tracks appear as thin white lines in the dark-field TEM image in Fig. 4h.
Chemical Compositions of Phases
Olivine exists in both lithologies, and low-Ca pyroxene exists in the porous lithology. Most olivine and low-Ca pyroxene in all lithologies are magnesian, >Fo85, and >En90 (Fig. 5a; Table 2). The olivine crystals are rich in MnO relative to the Fa molar percentage (Fig. 5b). The most MnO-enriched samples contain Fo98 and 2.2 wt% MnO. In contrast, most olivine, except for the three plots that will be shown in Fig. 7b, has Cr2O3 contents below the detection limit. Their compositions are within the olivine range in the matrices of primitive meteorites, IDPs, CP MMs, and grains of comet 81P/Wild 2 (Frank et al., 2014; Zolensky et al., 2008). The isolated pyrrhotite crystals in both lithologies are ~2 atom% Ni, on average, and up to ~4 atom% Ni (Fig. 5c; Table 2).
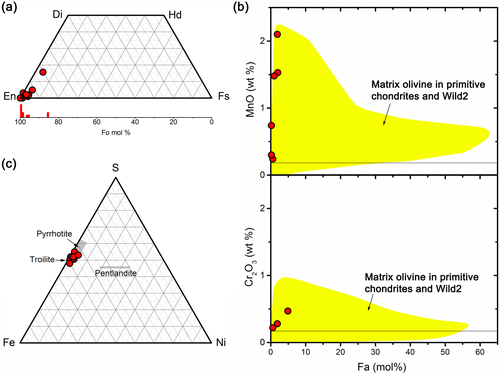
| Phase | Olivine | Olivine | Low-Ca pyroxene | GEMS | GEMS | GEMS | GEMS | GEMS | Saponite | Saponite |
|---|---|---|---|---|---|---|---|---|---|---|
| Lithology | Porous (anhydrous patch) | Compact | Porous | Porous (anhydrous patch) | Porous (anhydrous patch) | Porous (anhydrous patch) | Porous | Porous | Compact | Compact |
| SiO2 | 42.56 | 42.55 | 61.11 | 40.2 | 45.71 | 35.61 | 43.82 | 40.76 | 60.98 | 58.16 |
| TiO2 | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. |
| Al2O3 | b.d. | b.d. | b.d. | 5.09 | 4.36 | 3.17 | 2.55 | 2.38 | 5.07 | 6.32 |
| Cr2O3 | b.d. | b.d. | 0.36 | 0.49 | 1.17 | b.d. | 0.82 | 0.82 | b.d. | 0.33 |
| FeO | 0.32 | 3.08 | 0.19 | 29.82 | 27.35 | 37.42 | 30.44 | 42.42 | 12.32 | 11.59 |
| NiO | b.d. | b.d. | b.d. | 1.2 | 1.63 | 1.06 | 0.94 | 0.95 | b.d. | b.d. |
| MnO | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. |
| MgO | 57.12 | 53.99 | 38.34 | 5.23 | 5.77 | 4.67 | 4.44 | 6.98 | 19.29 | 21.81 |
| CaO | b.d. | 0.38 | b.d. | 0.93 | 0.61 | 0.79 | 0.45 | 0.62 | b.d. | 0.19 |
| Na2O | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. |
| K2O | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. |
| P2O5 | b.d. | b.d. | b.d. | b.d. | b.d. | 1.32 | b.d. | 0.93 | b.d. | b.d. |
| SO3 | b.d. | b.d. | b.d. | 17.04 | 13.39 | 15.96 | 16.54 | 4.14 | 2.34 | 1.59 |
| Total wt% | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Molar Mg/(Mg + Fe) | 0.997 | 0.969 | 0.997 | 0.238 | 0.273 | 0.182 | 0.206 | 0.227 | 0.736 | 0.770 |
| Lithology | Pyrrhotite | Pyrrhotite | Pyrrhotite |
|---|---|---|---|
| Porous (anhydrous patch) | Compact | Compact | |
| Fe | 60.96 | 61.75 | 59.61 |
| Ni | 0.68 | 1.69 | 1.73 |
| S | 38.36 | 36.6 | 38.66 |
| Total wt% | 100.00 | 100.00 | 100.00 |
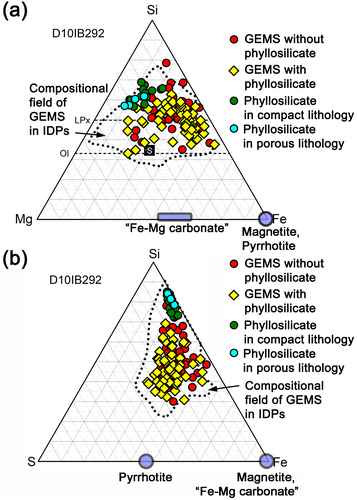
The major elemental composition of GEMS in the porous lithology is within the compositional field of GEMS in CP IDPs (Fig. 6a and 6b) (Keller & Messenger, 2011), irrespective of the absence or presence of phyllosilicates on GEMS. The chemical compositions of phyllosilicates in both the porous and compact lithologies are plotted in Fig. 6a and 6b. Their chemical compositions overlap with those of phyllosilicates in CS IDPs and hydrated fine-grained AMMs (Dobrică et al., 2019; Germani et al., 1990; Nakamura-Messenger et al., 2011; Noguchi et al., 2002).
C- and N-XANES and AFM-IR Analyses of Organic Matter
Figure 7a–c shows the organic matter in the compact lithology. Compared with the C and N distribution maps and BF TEM images in Fig. 7a, abundant organic matter is homogeneously distributed in the phyllosilicate-rich target area, as indicated by the red open square. The C-XANES spectrum of the target area shows a remarkable peak, which we assigned to the 1s–π* transition of aromatic/olefinic carbon (C=C) around 285.1 eV; a shoulder, which we assigned to the 1s–π* transition of N heterocycles (C–N=C), nitrile (C≡N), and/or aromatic ketone (C=C–C=O) at 286.7 eV; a remarkable peak that we assigned to the 1s–3p/s* transition of aliphatic carbon (CHx) around 287.4 eV; and a small peak, assigned to the 1s–π* transition of ester (COOR) and/or carboxylic (COOH) groups at around 288.5 eV, and the 1s–π* transition of alcohol (C–OH) and ether (C–O–C) at 289.7 eV (Fig. 7b). The N-XANES spectrum of the target area shows peaks that we assigned to the 1s–π* transition of imine (C=N) at 398.9 eV and the 1s–π* transition of N heterocycles (C–N=C) and/or nitrile (C≡N) at 401.1–401.5 eV.
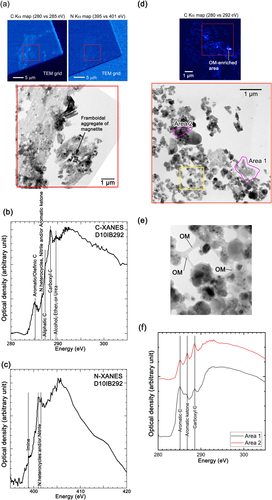
Figure 7d–f shows the organic matter in the porous lithology. Most of the organic matter in the compact lithology covers the small constituents as a thin film, and the constituents are attached by thin organic films and rare concentrated patches (Fig. 7d and 7e). The structure of organic matter differs according to its location. Figure 7e shows the local structural variation in the organic matter. The C-XANES spectrum of Area 1, which corresponds to the organic matter-enriched area in Fig. 7d, is similar to that of insoluble organic matter (IOM) in carbonaceous chondrites (e.g., Cody & Alexander, 2005). Contrastingly, the XANES spectrum of Area 2 has three remarkable peaks, which we assigned to the 1s–π* transition of aromatic/olefinic carbon (C=C) at approximately 285.1 eV, aromatic ketone (C=C–C=O) at 286.7 eV, and 1s–π* transition of carboxyl carbon (COOH) at approximately 288.5 eV, which is similar to that of the organic matter in anhydrous IDPs (e.g., Flynn et al., 2004). We did not obtain a remarkable N edge in the analyzed area because of the low N concentration.
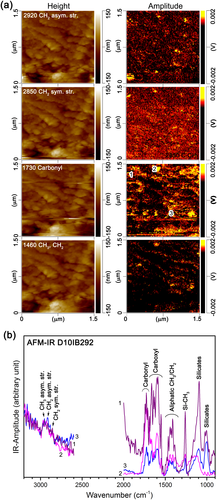
We obtained height and amplitude maps of the four functional groups (CH2, CH3, C=O, and CH3/CH2) in a 1.5 × 1.5 μm area of an ultrathin section that we placed on a ZnS crystal using AFM-IR (Fig. 8a). The peaks at 1460, 1730, 2850, and 2920 cm−1 correspond to CH2 and CH3 angular vibrations, carbonyl C=O stretching vibration, CH2 asymmetric stretching vibration, and CH3 asymmetric stretching vibration, respectively. The maps in the right column of Fig. 8a show the amplitude distribution of these peaks. This area shown in Fig. 8a belongs to the compact lithology. Figure 8b shows the AFM-IR spectra of the small spots (~30 nm in diameter), indicated in the 1730 cm−1 amplitude map in Fig. 8a. These spots show remarkable peaks of carboxyl, ketone, and scissoring vibrations of CH2 and CH3.
Discussion
Porous Lithology as CP IDP Experienced Very Weak Aqueous Alteration
The major components of the porous lithology (Figs. 1-3 and 5) are GEMS, low-Ca pyroxene, olivine, and pyrrhotite, which are similar to those in CP IDPs (Bradley, 2014) as well as those in CP MMs and UCAMMs (Dobrică et al., 2012; Noguchi et al., 2015). However, minor to trace amounts of phyllosilicate on the surface of GEMS (Fig. 2b–d) indicate that this lithology experienced very weak aqueous alteration.
The phyllosilicates on the GEMS in this AMM are unlikely to have formed by terrestrial weathering. The AMM was recovered within ~1 year after falling on the ground with uncompressed surface snow near Dome Fuji Station. The maximum temperature in the Dome Fuji area never exceeds −30 °C due to its high elevation of 3810 m above sea level. Thus, liquid water is unlikely to form under these conditions. Another possibility is that phyllosilicate on the GEMS formed during the melting of snow and filtering of water in the cleanroom. As described in the Materials and Methods section, the temperature of the water formed by the melting of the snow was well below 9.3 °C (dew point at 20 °C, 50% relative humidity). When we filtered the water soon after the snow had completely melted, the temperature of the water was ~0 °C. Therefore, we think that the actual temperature of the water was well below the dew point. The total duration of the melting and filtering process was 3–6 h. The typical concentration of hydrogen ions (pH) of inland Antarctic snow is 5.14 ± 0.25 (1σ), which results from the dissolution of atmospheric CO2 into water (e.g., Kamiyama et al., 1990). Mildly acidic conditions are common in natural, clean rainwater. Therefore, we performed the melting and filtering processes at temperatures lower than 8.7 °C and for relatively short periods (3–6 h) under mildly acidic conditions. These conditions are different from those used in the aqueous alteration experiments with ultra-thin sections of anhydrous IDPs by Nakamura-Messenger et al. (2011). Their alteration conditions were highly alkaline (pH 12–13); involved higher temperatures of 24, 120, and 160 °C, and longer periods (12 and 24 h); or involved neutral (pH 7), but high temperatures of 120 and 160 °C and longer periods (24 or 48 h). Ultra-thin sections were used in their experiments. Because the relative surface area of the ultra-thin sections was much larger than that of the bulk AMM samples, the effective reaction rate of the AMM was probably much lower than that of the ultra-thin sections. Therefore, we think that the melting and filtering processes did not form phyllosilicate on the surface of GEMS in the AMM and that phyllosilicate formed on its parent body. This hypothesis is supported by the TEM observations of many GEMS-bearing AMMs (CP MMs; Noguchi et al., 2015). We did not find phyllosilicate on the surface of GEMS in the CP MMs, which we also observed in the same AMM collection. Because of the small amounts of Fe–Ni metal in the GEMS on which phyllosilicate is attached, we think that the leaching of Fe–Ni metal was highly limited and that the formation of phyllosilicate was restricted to the surface of GEMS by consuming amorphous silicate on the surfaces of GEMS during aqueous alteration of the parent body.
Heterogeneous and very weak aqueous alteration can be achieved by melting a small amount of ice. The highly limited supply of liquid water during aqueous alteration is consistent with the presence of both small amounts of Fe–Ni metal and Fe sulfide in the GEMS in the porous lithology, as shown in Fig. 3, because Fe–Ni metals were likely susceptible to aqueous alteration. The slightly lower abundance of small amounts of Fe–Ni metal in GEMS shown in Fig. 3c may have resulted from the leaching of a trace amount of Fe–Ni metal. The high porosity of the entire porous lithology also suggests that pore-filling ice remained during the weak aqueous alterations. During the mixing of ice-containing material and hydrated material, probably by regolith gardening, ice melting may not have occurred, which is discussed below.
Rare, partially hydrated, GEMS-bearing IDPs, called hybrid IDPs, have been reported (Germani et al., 1990; Nakamura-Messenger et al., 2011). These IDPs are not as porous as the porous lithology of this AMM, and the phyllosilicate in these IDPs is much more abundant than that in the AMM. Therefore, the degrees of aqueous alteration of the hybrid IDPs are higher than the degree of aqueous alteration of the porous lithology.
Effects of Atmospheric Entry Heating on Structure of Organic Matter in this AMM
Next, we assessed the effects of atmospheric-entry heating on the structure of the organic matter in the AMM. Riebe et al. (2020) reported that organic matter in IDPs and AMMs was affected by atmospheric entry heating because most of them experienced >500 °C heating, according to the results of heating experiments. Because the Fe–Mg carbonate in this AMM was decomposed during atmospheric entry, the maximum temperature during entry may have been ~600 °C based on the results of heating experiments (Nozaki et al., 2006). However, solar flare tracks are well preserved in the ferromagnesian silicates in this AMM (Fig. 4), and detecting tracks in ferromagnesian silicate pulses heated above 550 °C is difficult (Fraundorf et al., 1982). Therefore, the AMM likely experienced temperatures <550 °C during atmospheric heating. Although such pulse heating would have increased the ordering of all the organic matter in this AMM, meteoritic IOM heated to 600 °C preserved the isotopic heterogeneity of an unheated sample (Riebe et al., 2020). Therefore, if systematic structural differences are present in the organic matter between the two lithologies before entering the Earth's atmosphere, the systematic differences would be inherited from the present organic matter in both lithologies.
Comparison with GEMS in D10IB292 AMM and GEMS-Like Amorphous Silicate in Primitive Meteorites
Recently, amorphous silicate embedding Fe sulfide with rare Fe–Ni metal has been discovered in the fine-grained matrices of primitive chondritic meteorites (Le Guillou & Brearley, 2014; Leroux et al., 2015; Matsumoto et al., 2019; Nittler et al., 2019). The mineralogy of such amorphous silicates in primitive chondritic meteorites is similar to that of GEMS in the IDPs and AMMs. Amorphous silicate appears as relatively less porous and continuous matrices in such meteorites, but some occur in highly porous clasts (Matsumoto et al., 2019; Nittler et al., 2019). The amorphous silicates in primitive meteorites are Fe-rich (e.g., fig. 3 in Matsumoto et al., 2019) in contrast to those in GEMS, which are essentially Fe-free. In addition, metals are rare or absent in amorphous silicates in primitive meteorites (Bradley, 2019; Ohtaki et al., 2021; Villalon et al., 2021).
Based on the abundance of excess O in amorphous silicate in MET 00426 CR 3, Le Guillou and Brearley (2014) suggested that the hydration of amorphous silicate occurred before the formation of abundant phyllosilicate. Keller et al. (2012) reported that amorphous silicates in Paris and Acfer 094 have Fe-rich and more homogeneous chemical compositions than GEMS in IDPs, and that their compositions resulted from homogenization during aqueous alteration. Because the chemical compositions of amorphous silicate in other primitive chondrites are also more homogeneous than GEMS in CP IDPs, the former was likely also hydrated and homogenized by similarly weak aqueous alterations. Leaching of small amounts of embedded Fe–Ni metal from amorphous silicate may also have occurred with hydration and homogenization before the formation of abundant phyllosilicate. Poorly crystalline fibrous phyllosilicate has been found in the pores of GEMS-like amorphous silicate in Paris (Leroux et al., 2015) and GEMS-like amorphous silicate in other primitive chondrites (Brearley, 1993; Greshake et al., 1997; Le Guillou & Brearley, 2014).
The chemical compositions of GEMS in the AMM are comparable to those in IDPs (Fig. 6) and are more heterogeneous than GEMS-like amorphous silicates in primitive meteorites (e.g., Keller et al., 2012; Le Guillou & Brearley, 2014; Leroux et al., 2015; Matsumoto et al., 2019). These differences are probably related to the different water/rock ratios, as well as different temperatures, periods, and compositions of the aqueous solutions during aqueous alteration. Therefore, ice likely melted more broadly in the parent bodies of these meteorites than in the parent body of the AMM (Bradley, 2019; Ohtaki et al., 2021; Villalon et al., 2021) and that the ice in the parent bodies of these meteorites was only locally preserved (Matsumoto et al., 2019; Nittler et al., 2019).
Coexistence of Two Lithologies and Common Origin of CP and CS IDPs
The three possibilities for forming this AMM are in situ formation, a mixture of an exogenous projectile and a parent body, and a mixture of materials formed at different parent body locations.
To discuss the first possibility, we begin by considering the aqueous alteration conditions of both lithologies. The compact lithology of this AMM is indistinguishable from that of CS IDPs (e.g., Bradley, 2014) and hydrated fine-grained AMMs (Dobrică et al., 2019; Noguchi et al., 2002). The major minerals in the IDPs and AMMs are saponite, magnetite, Fe–Ni sulfides, dolomite, and Fe–Mg carbonate. Their mineralogy is similar to those of CI chondrites and asteroid Ryugu samples, although they also contain abundant serpentine (e.g., Brearley & Jones, 1998; Nakamura et al., 2022; Yokoyama et al., 2022). For the AMMs, CI chondrites, and Ryugu samples containing both magnetite and dolomite, the precipitation temperatures of these phases were inferred based on the oxygen isotopic fractionation of these phases: 160–280 °C for a hydrated fine-grained AMM (Dobrică et al., 2019), 10–150 °C for CI chondrites (Alexander et al., 2015; Berger et al., 2015; Clayton & Mayeda, 1999; Guo & Eiler, 2007; Leshin et al., 1997), and 37 ± 10 to 104 ± 22 °C for Ryugu samples (Nagashima et al., 2022; Yokoyama et al., 2022). The temperature of aqueous alteration might overlap the inferred temperatures described above because of mineralogical similarity. However, we were unable to calculate the inferred precipitation temperatures of the magnetite and dolomite assemblage for the compact lithology because dolomite was not found in the lithology.
The results of closed-system chemical equilibrium calculations of CI chondritic materials suggested that the CI-like mineralogy formed from ~50 to 150 °C under water-to-rock ratios of 1 (Zolensky et al., 1989; Zolotov, 2012). Saponite crystallizes at temperatures higher than ~50–60 °C in these calculations. The formation of saponite from gels with saponite compositions of oxalate or aluminosilicate gel and magnesium chloride has been recently investigated (e.g., Besselink et al., 2020; Schumann et al., 2012); the results showed that saponite formed from gels at 60 and 95 °C, respectively. These calculations and experiments suggested that abundant saponite in the compact lithology formed at temperatures 60 °C.
In contrast, the aqueous alteration of the porous lithology is highly limited. Only a minute amount of saponite formed on the surfaces of GEMS, and Fe–Ni metal and sulfide were preserved in GEMS (Figs. 2 and 3). The porosity of up to ~50% in the porous lithology suggests that a minute amount of aqueous solution coexisted with ice, which means that aqueous alteration occurred at the freezing point of ice. A gel with a saponite composition sluggishly reacted with water to form a small amount of saponite at 25 °C based on the results of experiments (Besselink et al., 2020). If the ice is a mixture of H2O and NH3, olivine can be altered at subfreezing temperatures as low as −20 °C (Zandanel et al., 2022). A minute amount of saponite can form at or below the freezing point of ice, although further studies are needed to confirm this finding.
Based on the above discussion, the temperature of aqueous alteration for the compact lithologies is likely quite different from that of the porous lithology. If this AMM was formed by in situ aqueous alteration, local temperature heterogeneity would have occurred in the location. Because the boundaries between both lithologies are quite sharp (Fig. 1), the overall temperature at which the precursor of this AMM formed would have rapidly decreased before the melting of ice in the porous lithology. Although denying that such a circumstance might have been accomplished very locally during impact crater formation on an icy small body may be difficult, this AMM was not likely derived from such an extremely limited location.
The second possibility is that the AMM is an accidental mechanical mixture of the host (hydrated asteroidal) material and exogenous (cometary) material created by the impact of a small body on the parent body of the AMM. Small pieces of CS material are occasionally found in anhydrous CP IDPs. Such phyllosilicates are assumed to have originated from CS-IDP-like parent bodies that occurred in the location where the CP-IDP-like material accreted (D.E. Brownlee, personal communication). If the accidental mechanical mixture is the case, no genetic relationship would exist between the impactor and host. The organic matter in the compact lithology would be more abundant in carboxyls relative to the IOM in hydrated carbonaceous chondrites, and that in the porous lithology would be similar to the IOM in hydrated carbonaceous chondrites and/or comet 81P/Wild 2 dust particles.
The organic matter in the compact lithology of this AMM is homogeneously distributed, based on the results of C- and N-XANES mapping analysis (Fig. 7a); the C- and N-XANES spectra obtained in the target area shown in Fig. 7a are homogeneous. Organic matter in the compact lithology likely homogenized during aqueous alteration. The organic matter is rich in aliphatic C and carboxyl C relative to aromatic C (Fig. 7b). The high abundance of carboxyls and aliphatic C is also consistent with the AFM-IR spectra of the AMM (Fig. 8b). This feature is similar to the diffuse carbon in Murchison CM and Orgueil CI chondrites (Le Guillou & Brearley, 2014) and the oxygen-rich rims with higher carbonyl contents than in the globule cores (Keller et al., 2019).
The organic matter in the porous lithology shows variation (Fig. 7d and 7f), which may reflect weak but variable degrees of weak aqueous alteration in this lithology and/or primary heterogeneity. The BF TEM image in Fig. 7d reveals that the organic matter-enriched area is ~2 μm across the carbonaceous inclusions. The C-XANES spectrum in this region is aromatic-rich (Area 1 in Fig. 7f), which is similar to the highly aromatic organic nanoglobules that are thought to be of cold environmental origin (De Gregorio et al., 2013; Nakamura-Messenger et al., 2006). In contrast, the organic matter in other areas is similar to the IOM in primitive carbonaceous chondrites (e.g., Cody et al., 2008), comet Wild 2 dust particles (De Gregorio et al., 2011), and CP IDPs (e.g., Flynn et al., 2004; Keller et al., 2004). These C-XANES features suggest that the organic matter in the porous lithology is similar to that of CP IDPs and that the organic matter in the compact lithology may have been formed by oxidation or hydrolysis of the CP IDP-like organic matter. Therefore, the compact lithology likely formed from the porous lithology by a more developed aqueous alteration.
Therefore, the third possibility that the AMM formed as a microbreccia by regolith gardening between an ice-preserved surface layer and a hydrated interior is the most plausible among the three interpretations. In this scenario, large impacts formed craters that excavated a hydrated interior and scattered hydrated material on the surface of an icy body. Regolith gardening formed an intimate mixture of almost anhydrous and hydrated materials, which were the precursors of this AMM. Small amounts of water ice may have melted during regolith gardening, and its melting degree may have also been locally variable.
CP IDPs are known to originate in comets because such CP IDPs seem to be related to some dust streams of comets, such as 26P/Grigg-Skjellerup (Busemann et al., 2009). Therefore, the presence of this AMM indicates an additional origin for the CP IDPs and their counterpart AMMs. This means that the presence of this AMM requires reconsideration of the conventional view of its origins: CP IDPs and CS IDPs were derived from comets and hydrated asteroids, respectively.
Structure of Parent Body of this AMM
As discussed in the previous section, the parent body of this AMM has a hydrated interior and an ice-preserved surface layer, and the building blocks of its parent body are indistinguishable from the comets during accretion. Aqueous activity on the cometary nucleus has not been confirmed because phyllosilicate is absent in the stardust samples recovered from 81P/Wild 2, whereas rare magnetite, Mg–carbonate, and some sulfides may be related to aqueous alteration (Wooden et al., 2017). If short-lived radionuclides, such as 26Al, were the heat sources of the aqueous activity of the parent body, this creates limitations on the timing of accretion, water/rock ratio (ice/rock ratio when the parent body was accreted), and size of the body required to maintain its surface in a frozen state even during aqueous alteration. Later accretion, which decreased the abundance of short-lived radionuclides incorporated in the bodies and smaller parent body size, suppressed the peak temperature during aqueous alteration. A higher ice/rock ratio maintained the melting temperature of ice for a longer period during aqueous alteration. Fujiya et al. (2012) showed that the surface layer shallower than ~3 km of an ~60 km body that accreted 3.0–3.5 Ma after the formation of CAIs would not have reached temperatures above the melting point of ice. Because the Ca and Al-rich chondrule Iris that was recovered from the 26P/Wild 2 comet does not have excess 26Mg, the nucleus of the 81P/Wild 2 comet accreted >3 Myr after CAI formation (Matzel et al., 2010; Nakashima et al., 2015; Ogliore et al., 2012). If an ~60 km body accreted far beyond the snow line as late as 3.0–3.5 Ma after CAI formation, the temperature near the surface probably did not exceed the melting temperature of ice during aqueous alteration.
Asteroids with a pyroxene-rich CP IDP-like surface layer and a hydrated interior, analogous to CI chondrites, Tagish Lake ungrouped C chondrite, and CS IDPs were proposed as a possible structure of small (<200 km in diameter), low-density (<1.5 g cm−3) C-complex asteroids such as Eugenia, which does not show the features of hydrated phases on its near-infrared spectra (Vernazza et al., 2015, 2017). The parent body of this AMM probably had a structure similar to that of asteroids. A similar structure with an ice-bearing surface and ice-melted interior was also proposed as explaining the presence of comet-like porous material in the Acfer 094 primitive chondritic meteorite (Matsumoto et al., 2019).
Possible Parent Body
Based on the mid-infrared (MIR) spectra of B-, C-, and G-type asteroids; P- and D-type asteroids; and comets (Vernazza et al., 2015), the crystalline olivine/(olivine + low-Ca pyroxene) ratios of these bodies are 0–0.12, 0.36–0.52, and 0.50–0.82, respectively. The ratio in the porous lithology of this AMM is 0.55 (N = 22), which is at the boundary between the range of P- and D-type asteroids and that of comets (Vernazza et al., 2015). If this comparison is correct, the parent body of this AMM was a partially hydrated P- or D-type asteroid.
The number density of solar flare tracks preserved in minerals is useful for estimating the period of exposure in interplanetary space. Solar flare tracks are fine linear damages produced by 1–100 MeV energetic particles emitted by solar flare events, which proportionally accumulate during the exposure period in interplanetary space. The track density of this AMM is at least as high as ~5 × 1010 cm−2, irrespective of lithology. Therefore, we expect that the AMM was exposed to solar activity for a long period.
Keller and Flynn (2022) reported that IDPs containing silicates with a high number density (>1010 cm−2) of solar flares may have originated from hydrated Kuiper belt objects, based on the results of the calculation of track density accumulated in silicate during transportation from the Kuiper belt to 1 AU by Poynting–Robertson drag. They also calculated the exposure period of regolith grains with >1010 tracks per cm2 on the Main Belt asteroids to solar activity. They found that approximately 6 × 107 years were required to accomplish track densities of >1010 tracks per cm2. Further studies are needed to assess the possibility of the Kuiper belt origin of this AMM.
Conclusions
In this study, we found a unique AMM, D10IB292, composed of both porous and compact lithologies. The former lithology is indistinguishable from those of the CP IDPs and CP MMs. Unlike the typical CP IDPs and CP MMs, the lithology shows evidence of very weak aqueous alteration. In contrast, the compact lithology is indistinguishable from those of CS IDPs and hydrated fine-grained AMMs. The structure of the organic matter in the compact lithology of the AMM suggests that the CP IDP-like material was altered to form a CS IDP-like material via aqueous alteration. This AMM probably formed as a microbreccia by regolith gardening of a partially hydrated comet-like small body with an ice-bearing CP IDP-like surface layer and a hydrated interior. Because both AMM lithologies are genetically related, we derived a subset of CP IDPs and CS IDPs from the same partially hydrated parent bodies. Because the olivine/pyroxene ratio of this AMM is comparable to the ratios of these minerals in P- and D-type asteroids and comets obtained from MIR spectra, its parent body may be a partially hydrated P- or D-type asteroid.
Acknowledgments
We acknowledge H. Motoyama and the 51st Japanese Antarctic Research Expedition team for collecting the surface snow near the Dome Fuji Station, Antarctica. The STXM facility at beamline 5.3.2.2, ALS, was supported by the Department of Energy, Basic Energy Sciences program. This work was supported by the Astrobiology Center Program of the National Institutes of Natural Sciences (NINS) (AB281011, AB291008, and AB301004), JSPS KAKENHI grant number 19H00725, and NIPR through Project Research KP307 and General Collaboration Project No. 28-30.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




