Frugivory by the Crab-Eating Fox (Cerdocyon thous) and Its Seed Dispersal Potential: A Review
ABSTRACT
- Despite strong evidence supporting the role of canids as seed dispersers in the Neotropics, they have often been overlooked in seed dispersal studies. The crab-eating fox (Cerdocyon thous) stands out as a key species due to its frequent fruit consumption, generalist habitat use, and wide geographic range.
- This review evaluates current knowledge of C. thous frugivory and seed dispersal, identifying the most frequently consumed fruit species and assessing dispersal performance.
- We conducted a literature review, compiling data from 37 studies on dietary composition, fruit consumption, and seed dispersal performance across C. thous geographical distribution.
- Most studies were conducted in the Atlantic Forest, Cerrado, and Pampa. Fruits are frequent in C. thous diet (mean Frequency of Occurrence = 62.3% ± 33.1%, n = 29 studies), with 128 fruit species recorded from 81 genera and 43 families. The most frequently consumed species include Syagrus romanzoffiana, Hovenia dulcis, Psidium guajava, and Solanum lycocarpum. Consumed fruits and seeds range widely in size (4.0–296.0 mm and 0.5–125.0 mm in width, respectively).
- C. thous is a legitimate disperser: Seeds are defecated intact, remain viable post-ingestion, and gut-passage has neutral effects on germination. It disperses large seeds, such as S. romanzoffiana and Artocarpus heterophyllus, and tends to defecate in open areas, favouring long-distance dispersal.
- These features suggest that C. thous plays a key ecological role, especially in disturbed habitats lacking larger dispersers.
1 Introduction
Animal-mediated seed dispersal is a fundamental ecological process for the maintenance and recovery of biodiversity (Janzen 1970; Schupp 1993). Many plant species, particularly woody species in tropical regions, rely on frugivorous animals to disperse their seeds, facilitate reproduction and colonise new habitats (Howe and Smallwood 1982; Fleming et al. 1987). By feeding on fruits, animals transport seeds farther from the mother plant, increasing the likelihood of germination and establishment (Howe and Smallwood 1982; Schupp 1993). Some seeds even benefit from passing through a frugivore's digestive tract, which ultimately increases germination rates by scarification, seed coat removal or nutrient enrichment (Traveset and Verdú 2002; Traveset et al. 2007).
Although fruit consumption is relatively common among carnivores (Carnivora), their role as seed dispersers has been less studied compared to other frugivorous groups (e.g., birds, primates, bats), particularly in tropical habitats. Many carnivore species (e.g., bears, raccoons, foxes and civets) exhibit a varied omnivorous diet that includes fruits from many plant species (Herrera 1989; Rubalcava-Castillo et al. 2021; Draper et al. 2022). Besides the variety of fruits consumed, carnivores exhibit several key advantageous traits for seed dispersal. These include their ability to retain seeds for extended periods due to their long gut-passage time (González-Espinosa and Quintana-Ascencio 1986; Varela and Bucher 2006; Draper et al. 2022), their large home ranges (Gittleman and Harvey 1982; Sandell 1989) and their capacity to move across forests, open areas and human-modified landscapes (Šálek et al. 2015; Strampelli et al. 2022). Thus, collating available evidence on widespread Neotropical species provides an opportunity to better define and contextualise the role of carnivores as seed dispersers across diverse ecosystems.
Canids, one of the most diverse groups within Carnivora, include 14 genera and 36 species, such as wolves, foxes, coyotes, jackals and dogs (Wozencraft 1989, 1993; Sillero-Zubiri and Macdonald 2004; Castelló 2018). Although primarily meat-eaters, canids may include significant proportions of nonmeat items in their diet due to their dentition (e.g., small talonid and postcarnassial molars; Van Valkenburgh 1991). Some species, depending on ecological conditions (e.g., season, interspecific competition, resource availability), have been documented to temporarily rely predominantly on fruits (Sillero-Zubiri and Macdonald 2004). Canids, in fact, can play a significant role in seed dispersal as most of the seeds they consume remain largely intact after passing through their digestive tract due to limited mastication (Juarez and Marinho-Filho 2002).
The crab-eating fox Cerdocyon thous (Linnaeus, 1766) is an example of a Neotropical canid with an omnivorous diet (Motta-Junior et al. 1994; Courtenay and Maffei 2004). C. thous is a midsized ground-dwelling canid, weighing between 5 and 8 kg (Hover 2003; Courtenay and Maffei 2004) and is widely distributed in South America, occurring from Colombia to northern Argentina (Berta 1982; Beisiegel et al. 2013). C. thous is primarily crepuscular and nocturnal (Courtenay and Maffei 2004; Faria-Corrêa et al. 2009) and occupies most habitat types, including forests, savannas, grasslands, marshlands and ecotones (Courtenay and Maffei 2004; Lucherini 2015). This species can also be found in human-disturbed areas, tolerating deforestation, agricultural development and habitat degradation (Courtenay and Maffei 2004; Lemos et al. 2011; Lucherini 2015). Its distribution has been expanding, including into the Brazilian Amazon, probably due to the large-scale conversion of forests into pasture and agricultural lands (Beisiegel et al. 2013). This species' diet includes small mammals, birds, amphibians, insects, crustaceans, fruits and carrion (Berta 1982; Motta-Junior et al. 1994; Courtenay and Maffei 2004). The frugivorous component of C. thous' diet is influenced by the temporal availability and seasonality of fruits, resulting in the consumption of a diverse array of fruits across various phenological periods (Courtenay and Maffei 2004; Kuester et al. 2020).
Although multiple studies have documented the frugivorous habits of C. thous, a comprehensive synthesis of its frugivory and seed dispersal potential is still lacking. C. thous has been reported as an important seed disperser across different Neotropical biomes, including the Atlantic Forest (Rocha et al. 2004; Cazetta and Galetti 2009; Raíces and Bergallo 2010), Cerrado (Motta-Junior et al. 1994; Juarez and Marinho-Filho 2002), Caatinga (Silva et al. 2020; Henriques e Souza et al. 2021) and Pampas (Bossi et al. 2019; Kuester et al. 2020). However, most studies providing information on its seed dispersal role have been limited in scope (e.g., diet analysis), geographically localised (e.g., São Paulo or Paraná states in Brazil) or diluted in local languages (e.g., Portuguese; Spanish) and grey sources.
Consolidating this scattered information is essential to fully characterise the role of this widespread, adaptable species as a seed disperser across the diverse habitats it occupies. Also, such a synthesis might support a trait-based approach to understanding how the dietary preference of C. thous influences the plant species it disperses, enabling predictions about its ecological role across different habitats in the Neotropics. Lastly, understanding the potential role of C. thous as a seed disperser is critical to inform conservation efforts, especially considering its commonness in many Neotropical habitats, including disturbed areas that often experience an absence of large-sized seed dispersers (Dirzo et al. 2014). As a midsized frugivore, C. thous may partially compensate for the loss of larger seed dispersers, likely playing a role in maintaining seed dispersal processes in such environments. In this work, we performed a literature review aiming to (i) describe the frugivorous diet of C. thous, showing the frequency of frugivory and the fruit species most frequently consumed, and (ii) analyse the performance of the crab-eating fox as a seed disperser.
2 Materials and Methods
2.1 Literature Search
We conducted a literature review using the search string ‘(“Cerdocyon thous” OR “crab-eating fox”) AND “diet” AND “fruit” AND (“seed dispers*” OR “frugivor*”)’ in databases of Google Scholar, Science Direct, Scopus and Web of Science during the period of search from August 2022 to May 2023. To be included in the review, studies needed to (i) present any of the search string terms in their title, abstract or keywords, and (ii) convey information about C. thous diet and its potential for seed dispersal (e.g., fruit species consumed, foraging habits, habitats where seeds are dispersed).
Besides English, we included studies in Portuguese and Spanish, the primary languages of the countries where C. thous occurs, to ensure broad coverage of relevant sources and capture regionally significant studies that might otherwise be overlooked. Additionally, we did not restrict our search by publication year, allowing the inclusion of older or less widely known studies that could contribute valuable local knowledge. Duplicated studies were excluded. Dissertations and theses were also considered as long as they had not generated similar-content papers already in our search. A complete list of the 37 finalist studies included in our study is available in Table S1.
2.2 Frugivory
Based on the studies obtained in the literature review, we described the frugivorous diet of C. thous by retrieving all fruit species consumed by the canid. Plant species whose parts were consumed for reasons other than frugivory, for example, digestive aid, accidentally—mainly Poaceae species (Motta-Junior et al. 1994; Rocha et al. 2004, 2008)—were not considered as part of the fruit diet of C. thous. Unidentified fruits or generic plant groups (e.g., grasses, miscellaneous) were also not considered. All plant species names were verified for accuracy and current taxonomy using Plants of the World Online (POWO) and List of Species of the Flora of Brazil—REFLORA to ensure correct spelling and valid nomenclature.
We considered studies performed in native habitats (i.e., those mainly composed of natural vegetation), modified areas (i.e., anthropised habitats to some degree but still with some natural vegetation) and controlled environments (e.g., laboratory, captivity). We also recorded the seasons covered in the reviewed studies to assess the seasonality of fruit consumption by C. thous. These features provided thorough data across various timeframes and ecological contexts.
We used the Frequency of Occurrence (FO) to quantify the importance of fruits in C. thous diet (Klare et al. 2011). Typically, FO is expressed as a count of the number of samples (e.g., scats, stomachs) that contain the food item (e.g., fruits, insects, birds, small mammals) out of the total number of samples examined, indicating whether such an item is relatively common in the species' diet (Dietz 1984; Motta-Junior et al. 1994; Morin et al. 2019). FO is one of the most widely adopted techniques for determining carnivores' diet (Klare et al. 2011), enabling comparisons among studies, particularly relative to diet changes in time and space (Brzeziński and Marzec 2003). We obtained FO from 29 studies of the literature review.
FO only accounts for the presence of a certain food item among samples, disregarding its biomass (Porto and Rui 2019). It is preferable to use biomass calculations for better evaluating diet composition in carnivores (Klare et al. 2011). Nonetheless, just a few studies in the literature (n = 11) used methods besides FO while describing C. thous diet, let alone appraising biomass (n = 3/11) or volume (n = 4/11). Thus, due to the low sample size, we opted not to present analyses with the alternative metrics of Percentage of Biomass and Percentage of Volume found in the literature.
As an additional diet metric, we included data solely on the Percentage of Occurrence (PO), a derivative metric of FO. PO shows the proportion of samples in which a food item is present relative to the total number of samples, expressed as a percentage (Pedó et al. 2006; Bianchi et al. 2014). As samples often contain remains of multiple dietary items, FO expressed in percentages can sometimes exceed 100% (Newsome et al. 1983; Morin et al. 2019). While FO gives a raw count of how often an item is found in the samples, PO standardises this measure, facilitating the comparison across different studies or food items. To estimate the frequency of a given fruit species in C. thous' diet, we compared the number of its records in the literature review with the total number of studies analysed.
2.3 Seed Dispersal
For all consumed species, we compiled information on fruit and seed size (both measured as width) from the literature (M. A. Pizo unpubl. data). Fruits that were only identified at the genus level were assigned a size by averaging across all congeneric species available in the database. Whenever available, we compiled information regarding the seed dispersal performance of C. thous (e.g., foraging behaviours, deposition sites, dispersal distance, seed viability and effects on germination). From studies that evaluated seed germination (n = 8), we considered whether C. thous exhibited any effect on germinability (i.e., the ability of seeds to germinate) after seed ingestion and the effect (positive, negative or neutral) on germination percentages compared to a control test (seeds collected directly from fruit).
3 Results
3.1 Literature Review
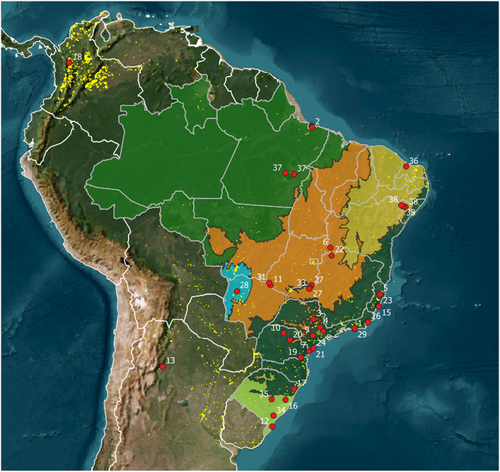
We retrieved 37 studies from the literature addressing aspects of the C. thous frugivory (Table S1). Studies varied widely in duration, ranging from short-term observations lasting 4 days to extensive, long-term studies spanning up to 11 years (132 months; Table S2). The average study length was 34.7 months (±37.6). Although studies covered different Neotropical biomes (Atlantic Forest, Cerrado, Pampa, Caatinga, Amazon, Pantanal, Chaco, Páramo; Table S2), they were predominantly conducted in Brazil's central-southern region (Figure S1), most of them from the Atlantic Forest (44.7%), Cerrado (21.1%) and Pampa (13.2%) biomes (Figure 1). The dominant study site type was native habitats (52.6%), followed by modified areas (42.1%) and controlled environments (e.g., captivity; 5.3%). Our analysis, therefore, encompasses a wide variety of environments with distinct levels of disturbance (e.g., native vegetation, crops, pastures, highways) and physiognomies (e.g., forests, savannas, marshes, grasslands).
To determine the C. thous diet, a large proportion of studies used faecal analysis (62.8% studies; sample sizes: 5–301 scats), while under a fifth sampled stomachs (16.3% studies; sample sizes: 2–46 roadkill foxes). Other studies adopted distinct methods, such as germination tests (16.3% of studies), seed removal experiments (2.3% of studies) and camera traps (2.3% of studies) (Table S2).
Most studies that addressed seasonality (n = 22 studies; Table S2) spanned both rainy and dry seasons, whereas just a small proportion focused exclusively on the dry season (n = 1 study; Table S2). As a result, the reviewed data mostly capture the full seasonal variability of C. thous fruit consumption patterns.
3.2 Frugivory
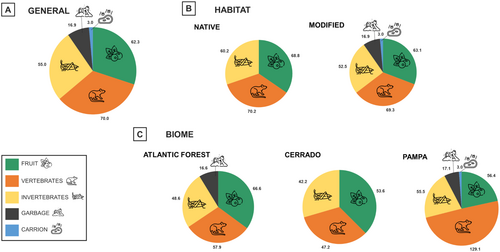
We recorded the consumption of 128 fruiting plant species in 81 genera and 43 families (Table 1). At least 18 species consumed by C. thous are exotic (cultivated). Overall, the average FO of fruits was 62.3% (±33.1; n = 29 studies), ranging from 9% to 120%. The highest frequency (120%) was recorded in a strictly protected conservation area, whereas the lowest frequency (9%) was observed in restinga (coastal tropical moist forests) and mangrove areas (Table S2). Two studies reported a total absence of fruits in the C. thous diet, specifically in a Brazilian Pampa biological reserve (length: 12 months) and in a Colombian Paramo (length: 4 days) (Table S2). Compared with other dietary items, fruits represented a substantial portion of C. thous diet overall (FO = 62.3% ± 33.1; Figure 2A). This pattern remained consistent across different habitats (native: FO = 68.8% ± 63.2, n = 13 studies; modified: FO = 63.1% ± 26.9, n = 15 studies; Figure 2B) and biomes (Atlantic Forest: 66.6% ± 37.6, n = 13 studies; Cerrado: 53.6% ± 29.8, n = 7 studies; Pampa: 56.4% ± 43.6, n = 4 studies; Figure 2C), highlighting the species' tendency to frugivory regardless of environmental context. The average PO of fruits was (34.6% ± 23.4%; n = 7 studies), from 9.6% to 75.01%. Fruit was the main item in the C. thous diet in 10 of the 29 studies (35.7%; Table S2). The mean number of fruit species consumed per study was 6.8 (±4.9).
| Family | Plant species | Fruit width (mm) | Seed width (mm) | Number of studies retrieved |
|---|---|---|---|---|
| Anacardiaceae | Anacardium humile | 26.8 | 14.3 | 1 |
| Anacardium occidentale | 36.6 | 19.0 | 2 | |
| Anacardium sp. | 31.2b | 17.4b | 1 | |
| Annona crassiflora | 129.2 | 7.5 | 2 | |
| Annona dioica | 75.1 | 8.3 | 1 | |
| Annona cornifolia | 37.6 | 4.7 | 1 | |
| Annona monticola | 95.0 | 6.0 | 1 | |
| Annona spp. | 78.4b | 10.1b | 3 | |
| Schinus terebinthifolius | 4.5 | 3.3 | 2 | |
| Mangifera indica a | 78.6 | 50.0 | 2 | |
| Spondias tuberosa | 28.6 | 13.0 | 2 | |
| Annonaceae | Duguetia furfuracea | 57.5 | 9.0 | 1 |
| Apocynaceae | Hancornia speciosa | 34.7 | 6.8 | 3 |
| Araliaceae | Didymopanax macrocarpa | 9.9 | 2.2 | 1 |
| Arecaceae | Acrocomia aculeata | 40.7 | 23.2 | 1 |
| Allagoptera arenaria | 11.5 | 10 | 2 | |
| Astrocaryum sp. | 29.5 | 20.1 | 1 | |
| Butia capitata | 21.1 | 10 | 1 | |
| Butia odorata | 29.8 | 13.9 | 1 | |
| Cocos nucifera a | 175 | 125 | 1 | |
| Copernicia alba | 16.4 | 14.2 | 1 | |
| Syagrus romanzoffiana | 21 | 15.7c | 16 | |
| Syagrus sp. | 20.8b | 16.5b | 1 | |
| Bromeliaceae | Ananas comosus a | 150 | 2 | 1 |
| Bromelia balansae | 29.2 | 6.0 | 3 | |
| Bromelia antiacantha | 29.1 | 6.6 | 2 | |
| Bromelia laciniosa | 15.0 | 4.0 | 1 | |
| Cactaceae | Cereus fernambucensis | 5.3 | 2.0 | 1 |
| Pilosocereus arrabidae | 4.2 | 2.0 | 1 | |
| Xiquexique gounellei | 48.1 | 1.8 | 2 | |
| Pilosocereus pachycladus | 50.5 | 1.6 | 1 | |
| Pilosocereus catingicola | 42.2 | 1.0 | 1 | |
| Harrisia bonplandii | 45.0 | 2.5 | 1 | |
| Cannabaceae | Celtis chichape | 8.0 | 4.0 | 1 |
| Caricaceae | Carica papaya a | 115.0 | 6.0 | 1 |
| Celastraceae | Salacia crassifolia | 32.4 | 12.4 | 1 |
| Chrysobalanaceae | Parinari obtusifolia | 25.0 | 15.0 | 2 |
| Cucurbitaceae | Melancium sp. | 90.0 | 6.0 | 1 |
| Cucurbita sp. | 43.0b | 8.8b | 1 | |
| Citrullus lanatus a | 187.7 | 10.6 | 1 | |
| Cucumis sp. | 10.0b | 8.0b | 1 | |
| Ebenaceae | Diospyros kaki a | 52.5 | 7.0 | 1 |
| Diospyros lasiocalyx | 35.6 | 8.7 | 2 | |
| Diospyros inconstans | 21.5 | 8.3 | 1 | |
| Diospyros sp. | 34.3b | 8.3b | 1 | |
| Erythroxylaceae | Erythroxylum ovalifolium | 5.0 | 3.5 | 1 |
| Erythroxylum deciduum | 5.0 | 4.2 | 1 | |
| Euphorbiaceae | Croton floribundus | 10.0 | 1.3 | 1 |
| Fabaceae | Hymenaea stigonocarpa | 42.5 | 18.9 | 1 |
| Vachellia aroma | 20.0 | 4.0 | 1 | |
| Prosopis sp. 1 | 10.0 | 5.0 | 1 | |
| Phaseolus sp.a | 10.0 | 3.0 | 1 | |
| Goodeniaceae | Scaevola plumieri | 12.0 | 10.0 | 1 |
| Humiriaceae | Humiria balsamifera | 9.6 | 6.3 | 1 |
| Lamiaceae | Vitex cymosa | 14.5 | 9.0 | 1 |
| Vitex megapotamica | 21.7 | 8.9 | 1 | |
| Vitex montevidensis | 15.2 | 5.5 | 1 | |
| Lauraceae | Persea americana a | 95.0 | 60.0 | 4 |
| Ocotea notata | 6 | 5.0 | 1 | |
| Nectandra grandiflora | 12.3 | 10.0 | 1 | |
| Nectandra oppositifolia | 12.0 | 10.0 | 1 | |
| Nectandra sp. | 12.9b | 8.6b | 1 | |
| Malpighiaceae | Byrsonima cydoniifolia | 11.3 | 6.1 | 1 |
| Byrsonima crassifolia | 9.1 | 4.5 | 2 | |
| Malvaceae | Guazuma ulmifolia | 19.4 | 1.0 | 2 |
| Melastomataceae | Mouriri elliptica | 25.7 | 10.1 | 1 |
| Miconia hyemalis | 5.1 | 1.3 | 1 | |
| Miconia spp. | 5.2b | 1.0b | 2 | |
| Bellucia grossularioides | 30.5 | 0.7 | 1 | |
| Meliaceae | Guarea sp. | 14.5b | 9.2b | 1 |
| Metteniusaceae | Emmotum nitens | 24.0 | 12.8 | 1 |
| Moraceae | Artocarpus heterophyllus a | 295.8 | 17.4c | 2 |
| Ficus spp. | 15.6b | 1.1b | 10 | |
| Ficus crocata | 21.2 | 1.0 | 1 | |
| Ficus cestrifolia | 8.2 | 0.8 | 2 | |
| Maclura tinctoria | 20.0 | 1.5 | 1 | |
| Musaceae | Musa paradisiaca a | 30.0 | 4.0 | 1 |
| Musa acuminata a | 24.0 | 5.5 | 2 | |
| Musa balbisiana a | 35.0 | 7.5 | 1 | |
| Myrtaceae | Campomanesia aurea | 8.4 | 4.0 | 1 |
| Campomanesia xanthocarpa | 18.7 | 4.8 | 2 | |
| Campomanesia sp. | 20.7b | 5.5b | 1 | |
| Neomithranthes obscura | 16.0 | 14.0 | 1 | |
| Eugenia spp. | 17.2b | 10.7b | 3 | |
| Eugenia astringens | 12.1 | 9.1 | 2 | |
| Eugenia involucrata | 19.7 | 7.6 | 1 | |
| Eugenia pyriformis | 19.4 | 10.1 | 1 | |
| Eugenia uniflora | 23.0 | 9.2 | 1 | |
| Myrcia neuwiedeana | 17.6b | 12.7b | 1 | |
| Psidium cattleianum | 26.9 | 2.9 | 2 | |
| Psidium guineense | 24.4 | 1.3 | 1 | |
| Psidium guajava a | 52.8 | 2.9 | 7 | |
| Psidium spp. | 29.0b | 3.3b | 4 | |
| Plinia cauliflora | 27.8 | 7.8 | 3 | |
| Syzygium cumini a | 21.9 | 11.4 | 4 | |
| Passifloraceae | Passiflora mucrunata | 27.0 | 2.4 | 1 |
| Piperaceae | Piper aduncum | 6.0 | 0.5 | 2 |
| Piper lindbergii | 6.0 | 1.2 | 1 | |
| Piper gaudichaudianum | 6.0 | 0.8 | 1 | |
| Piper sp. | 5.8b | 1.3b | 1 | |
| Podocarpaceae | Podocarpus lambertii | 4.7 | 3.9 | 1 |
| Polygonaceae | Coccoloba declinata | 10.0 | 8.0 | 1 |
| Primulaceae | Myrsine sp. | 4.0 | 3.4 | 1 |
| Rhamnaceae | Hovenia dulcis a | 6.5 | 6.0 | 10 |
| Sarcomphalus mistol | 13.0 | 6.0 | 1 | |
| Sarcomphalus joazeiro | 17.5 | 7.0 | 2 | |
| Rosaceae | Fragaria vesca a | 15.0 | 2.5 | 1 |
| Rubiaceae | Genipa americana | 64.6 | 5.4 | 2 |
| Tocoyena bullata | 35.0 | 10.0 | 1 | |
| Tocoyena formosa | 36.7 | 4.2 | 1 | |
| Psychotria carthaginensis | 6.0 | 4.6 | 1 | |
| Amaioua guianensis | 10.1 | 3.1 | 1 | |
| Rutaceae | Citrus sp.a | 77.3b | 6.4b | 1 |
| Sapindaceae | Alophyllus edulis | 7.3 | 5.0 | 1 |
| Sapotaceae | Pradosia brevipes | 32.5 | 15.5 | 1 |
| Sideroxylon obtusifolium | 14.1 | 8.2 | 2 | |
| Pouteria sp. | 38.1b | 13.8b | 1 | |
| Smilacaceae | Smilax rufescens | 7.5 | 5.5 | 1 |
| Solanaceae | Solanum lycocarpum | 86.0 | 3.0 | 6 |
| Solanum americanum | 5.3 | 1.3 | 3 | |
| Solanum granulosoleprosum | 12.0 | 1.8 | 2 | |
| Solanum spp. | 20.8b | 2.3b | 8 | |
| Solanum thomasiifolium | 12.5 | 2.0 | 1 | |
| Physalis angulata | 13.0 | 2.5 | 1 | |
| Physalis pubescens | 7.5 | 1.5 | 2 | |
| Urticaceae | Cecropia pachystachya | 18.1 | 1.1 | 2 |
| Cecropia spp. | 16.5b | 1.5b | 2 | |
| Vitaceae | Vitis vinífera a | 24.0 | 4.3 | 3 |
Myrtaceae had the greatest species richness in the C. thous diet (25 spp. or 14.7% of the total number of species recorded), followed by Solanaceae (14 spp., 8.2%), Moraceae (14 spp., 8.2%), Anacardiaceae and Arecaceae (13 spp. each, 7.7%). At the species level, Syagrus romanzoffiana (Arecaceae) fruits were the most frequently consumed (16 studies, 43.2%), followed by Hovenia dulcis (Rhamnaceae—cultivated species; 10 studies, 27.0%), Psidium guajava (Myrtaceae—cultivated species; 7 studies, 18.9%) and Solanum lycocarpum (Solanaceae; 6 studies, 16.2%). The remaining fruit species were reported less consistently, with 85 of them being found in only a single study (Table 1).
3.3 Seed Dispersal Performance
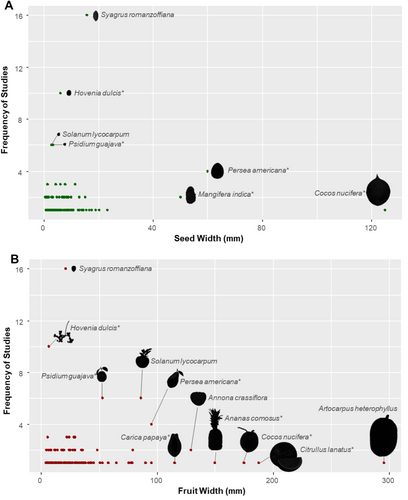
The size of consumed fruits ranged considerably, from the small fruits of Myrsine sp. (4.0 mm width) to large ones like Artocarpus heterophyllus (295.8 mm width) (Figure 3A, Table 1). The mean fruit diameter was 30.8 ± 37.4 mm. Likewise, seed size from consumed fruits also varied greatly, from the small seeds of Piper aduncum (0.5 mm width) to the large seeds of Cocos nucifera (125.0 mm width) (Figure 3B, Table 1). The mean seed size was 7.6 ± 12.1 mm. However, the largest seeds defecated whole were of S. romanzoffiana (15.7 mm) and A. heterophyllus (17.4 mm) (Table 1). We also emphasise that fruits with particularly large seeds (e.g., C. nucifera, Persea americana, Mangifera indica) likely only had their pulp or endosperm consumed by C. thous (see Macdonald and Courtenay 1996; Rocha et al. 2004). For C. nucifera, its fruits likely need to be broken before (e.g., through gravity) C. thous can utilise them, as seen in the dispersal of other large-seeded palms (Brewer 2001).
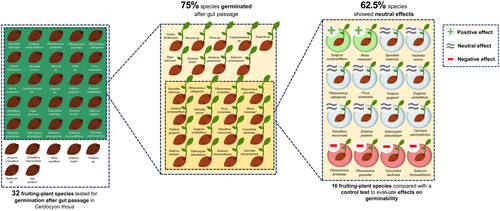
Seeds ingested by C. thous are commonly defecated intact and viable (Table 2; Figure 4; Figure S2). Of the 32 fruiting plant species tested for germination after gut passage, 24 species germinated (75%; Figure 5). From those compared with a control test (n = 16 spp.), effects on germinability were positive in two species (12.5%), negative in four species (25%) and neutral in 10 (62.5%; Figure 5). We could not evaluate the effect on germination for nine species since they had no control comparison (Table 2; Figure 5).
| Plant species | Effects on germinability | Effect quality | Germination percentage (%) | Seed viability after gut passage (undamaged seeds) | |
|---|---|---|---|---|---|
| Anacardiaceae | Annona crassiflora | Unobserved1 | — | — | Yes1 |
| Spondias tuberosa | Observed8 | Neutral8 | ≈16.68 | Yes8 | |
| Araliaceae | Didymopanax macrocarpa | Unobserved1 | — | — | Yes1 |
| Arecaceae | Syagrus romanzoffiana |
Unobserved1,3 Observed2 |
—Positive2 | —16.72 | Yes1,2,3 |
| Cactaceae | Pilosocereus arrabidae | Observed7 | Negative7 | 0.07 | Yes7 |
| Xiquexique gounellei | Observed8 | Negative8 | 22.88 | Yes8 | |
| Pilosocereus catingicola | Observed8 | Neutral8 | 4.08 | Yes8 | |
| Euphorbiaceae | Croton floribundus | Observed3 | No comparison with control test3 | 30.03 | Yes3 |
| Fabaceae | Vachellia aroma | Observed4 | Neutral4 | ≈32.04 | Yes4 |
| Melastomataceae | Miconia sp. | Observed1 | No comparison with control test1 | 90.91 | Not mentioned1 |
| Moraceae | Ficus sp. | Observed1 | No comparison with control test1 | 52.01 | Not mentioned1 |
| Ficus crocata | Observed7 | Neutral7 | 0.07 | Yes7 | |
| Musaceae | Musa balbisiana a | Observed2 | Positive2 | 23.32 | Yes2 |
| Myrtaceae | Campomanesia sp. | Observed1 | No comparison with control test1 | 95.01 | Not mentioned1 |
| Eugenia sp. | Observed1 | No comparison with control test1 | 100.01 | Not mentioned1 | |
| Plinia cauliflora | Unobserved1 | — | — | Yes1 | |
| Psidium sp. | Unobserved1 | — | — | Yes1 | |
| Psidium guajava a | Observed2 | Neutral2 | 93.82 | Yes2 | |
| Eugenia astringens | Observed5,7 | Neutral5,7 |
56.05 28.07 |
Yes5,7 | |
| Passifloraceae | Passiflora mucrunata | Observed7 | Neutral7 | 0.07 | Yes7 |
| Piperaceae | Piper aduncum | Observed2 | No comparison with control test2 | 95.32 | Yes2 |
| Polygonaceae | Coccoloba declinata | Observed7 | Negative7 | 0.07 | Yes7 |
| Rhamnaceae | Hovenia dulcis a | Observed1,3 | No comparison with control test1,3 |
49.11 56.23 |
Not mentioned1 Yes3 |
| Sarcomphalus mistol | Unobserved4 | — | — | Yes4 | |
| Sarcomphalus joazeiro | Observed8 | Neutral8 | 6.48 | Yes8 | |
| Rubiaceae | Amaioua guianensis | Observed1 | No comparison with control test1 | 100.01 | Not mentioned1 |
| Sapotaceae | Sideroxylon obtusifolium | Observed8 | Neutral8 | 41.68 | Yes8 |
| Solanaceae | Solanum americanum | Observed1 | No comparison with control test1 | 50.01 | Not mentioned1 |
| Solanum thomasiifolium | Observed6 | Negative6 | 53.06 | Not mentioned6 | |
| Solanum sp. | Unobserved1 | — | — | Yes1 | |
| Urticaceae | Cecropia pachystachya | Observed2 | Neutral2 | 76.02 | Yes2 |
| Vitaceae | Vitis vinifera a | Unobserved1 | — | — | Yes1 |
- Note: ‘Effect Quality’ refers to the effects of seed ingestion on germination rates when compared to control seeds, which can be ‘positive’ (germination rate of ingested seeds significantly higher than control's), ‘neutral’ (no significant differences between ingested and control seeds) or ‘negative’ (germination rate of ingested seeds significantly lower than control's). 1Motta-Junior et al. (1994), 2Cheida (2002), 3Rocha et al. (2004), 4Varela and Bucher (2006), 5Cazetta and Galetti (2009), 6Vasconcellos-Neto et al. (2009), 7Raíces and Bergallo (2010), 8Henriques e Souza et al. (2021).
- a Exotic/Cultivated species.
For captive C. thous individuals, the average seed passage time was 7.7 h, measured for three sizes of artificial seeds and wild seeds from the fleshy-fruited species Sarcomphalus mistol, Vachellia aroma, Celtis tala and S. romanzoffiana (Table S2). C. thous defecates in all types of habitats but displays a general trend for open habitats, like shrub grasslands and wooded savannas (up to 94% of scat samples [n = 39] in Rio Pratudão Ranch, Central Brazil, Table S2). Scats are mostly found in unfavourable sites for germination, including trails, dirty roads and forest edges (n = 6 studies, Figure 4; RBC, pers. obs.). The canid can transport seeds distances of 8.9 to 10.9 km (n = 2 studies; Figure 4).
4 Discussion
4.1 The Importance of Fruits in Cerdocyon thous Diet
Our results indicate that fruit consumption represents a substantial proportion of C. thous diet. Fruits were the main food item in 10 out of 27 studies (35.7%) that evaluated the C. thous diet. Fruits were also among the most common diet items with high FO (≥ 50%). Moreover, in some instances, fruit can account for up to 56.4% of the biomass intake (Juarez and Marinho-Filho 2002; for biomass percentage, see also Bueno and Motta-Junior 2004; Rocha 2008) and up to 44.0% of the volumetric intake (Facure and Monteiro-Filho 1996; for volume percentage, see also Macdonald and Courtenay 1996; Facure et al. 2003; Bossi et al. 2019). This is noteworthy considering the variety of biomes in which this canid's diet was evaluated—for example, tropical rainforests, savannas, dry forests, grasslands—and the wide diversity of fruit-bearing plants consumed by C. thous (Table 1; Tables S1 and S2; Figure S1). Although these biomes differ in overall vegetation structure and species composition, many of them offer a year-round supply of fleshy fruits (Kuester et al. 2020; Silva et al. 2020). This broad regional availability of fruit resources likely contributes to maintaining high levels of frugivory at the population level, as C. thous individuals across different habitats consistently incorporate fruits into their diet. While an individual's fruit consumption may vary depending on local availability, the overall pattern of frugivory remains high across the species' range due to the diversity and ubiquity of fruit-bearing plants in the Neotropics (Fleming et al. 1987).
As suggested by Vieira and Port (2007), FO of fruits in C. thous diet can be related to habitat type or the energetic demands of individuals. High fruit frequencies may be related to highly productive habitats in the Neotropical regions (e.g., forests) (Haugaasen and Peres 2007; Brockerhoff et al. 2017). Low frequencies may be associated with ecosystems where plants with fleshy fruits are less common (e.g., grasslands) or with the necessity of C. thous to rely upon high-energy food (e.g., small vertebrates) in colder regions (Vieira and Port 2007). This pattern is reflected in our results, where fruit FO tends to be higher in biomes with more complex vegetation structures (Atlantic Forest, Cerrado; Figure 2C) compared to more open habitats (Pampa; Figure 2C). Ultimately, particular behaviours of local C. thous populations (e.g., hunting efficiency) and limited sample size can also explain low frequencies of fruits in the species' diet (Pedó et al. 2006; Delgado-V and Zurc 2007).
Fruit-eating by C. thous varies seasonally, with debate over the dominant fruit-consuming season. Several studies indicate that fruit consumption is higher during the rainy season (Motta-Junior et al. 1994; Facure et al. 2003; Bueno and Motta-Junior 2004; Rocha et al. 2004; Vilagran 2004), which coincides with peak fruiting periods in many tropical rainforests and woodland savannas (Mendoza et al. 2017). Conversely, other studies suggest higher fruit consumption in the dry season (Vieira and Port 2007; Amaral 2007; Araujo 2008), potentially reflecting the phenology of plant species that fruit asynchronously or persist as dry-season resources, particularly in more seasonal environments like dry forests (Cortes-Flores et al. 2019; Pereira et al. 2022). There is also evidence to the effect that fruits may be consumed equally by C. thous throughout the year, irrespective of the season (Cheida 2002; Gatti et al. 2006a; Raíces and Bergallo 2010; Bianchi et al. 2014; Porto and Rui 2019), a pattern that may be associated with vegetation physiognomies where fruit availability remains more stable across seasons, such as wetter rainforests (Mendoza et al. 2017).
The studies encompassed a broad seasonal range, covering both wet and dry seasons, with most conducted along the central-south axis of Brazil (Table S2; Figure S1). This geographic concentration ensures robust temporal representation but may introduce regional biases that limit broader inferences across the entire Neotropical range. While our data set captures seasonal variation in C. thous fruit consumption, differences in fruiting phenology among biomes suggest that general trends may not be uniform across the species' distribution. Ultimately, C. thous' fruit consumption seems to be driven by the local fruiting patterns of the species exploited (Kuester et al. 2020), which may vary seasonally and geographically (Ting et al. 2008; Miranda et al. 2023). This pattern is unsurprising since C. thous has an opportunistic feeding strategy, consuming items that are readily available (Motta-Junior et al. 1994; Novaes et al. 2011; Santiago et al. 2023).
4.2 What Is on the Menu? Fruits Consumed by Cerdocyon thous
We found that C. thous feeds on at least 128 species of fruiting plants. Many of them (n = 85 spp.) had a single record of consumption, whereas only four species were frequently consumed. As noted by Facure et al. (2003), most fruit species consumed by C. thous have a low recurrence and contribute little to the total volume ingested; some, on the other hand, dominate and are consumed in great quantities (e.g., cultivated species). This fruit consumption pattern indicates a nonuniform resource use rather than a lack of dietary diversity (Jácomo et al. 2004). Instead of consuming all available food items uniformly, C. thous likely relies more frequently on a subset of resources, including specific fruit species, while incorporating others less frequently. We can only speculate about the reasons for that, but it may have to do with the differential availability of fruit species and/or with the canid's preferences guided by the nutrient contents of fruits. Thus, C. thous seems to concentrate its fruit consumption on a few species (Gatti et al. 2006b)—particularly S. romanzoffiana, H. dulcis, P. guajava and S. lycocarpum—highlighting this species' tendency to rely disproportionately on specific, key fruit items while maintaining an overall opportunistic feeding strategy.
In several studies, S. romanzoffiana was the most common fruit species in C. thous diet (Facure and Monteiro-Filho 1996; Rocha et al. 2004, 2008; Bossi et al. 2019), consumed nearly year-round (Facure et al. 2003; Kuester et al. 2020) and in such quantity that its faeces can be dominated by S. romanzoffiana seeds (Reitz 1974). S. romanzoffiana is a widely distributed palm tree in South America (Glassman 1987; Carvalho 2006; Lorenzi et al. 2010), commonly found in the wild and also used as an ornamental plant (Noblick 2017; Carvalho 2006; Soares 2023). This palm produces fruit year-round, with up to 800 yellow-orangish fruits per bunch (Galetti et al. 1992). These features likely explain its prevalence in C. thous diet.
C. thous consumes fruits of 18 cultivated species, two of which (H. dulcis and P. guajava) feature among the most frequently consumed. C. thous' consumption of cultivated fruits is well documented in the literature (Motta-Junior et al. 1994; Facure and Giaretta 1996; Facure and Monteiro-Filho 1996; Cheida 2002). Several studies in our literature review were performed in modified habitats, including rural, peri-urban and natural areas with different degrees of disturbance. The occurrence of C. thous in human-disturbed areas with high availability of cultivated plants (e.g., farms, orchards, crops, agricultural fields) may explain their high frequency on its diet (Facure and Monteiro-Filho 1996; Facure et al. 2003). Such is the case with the consumption of the Japanese raisin tree (H. dulcis; Rhamnaceae), one of the most important invasive species in Brazilian protected areas (Zenni and Ziller 2011; Hendges et al. 2012). H. dulcis is particularly common near human settlements and modified habitats, where C. thous is prone to consume it (Facure et al. 2003; Facure and Monteiro-Filho 1996; Rocha et al. 2008). The strong correlation between the abundance of H. dulcis and C. thous highlights the canid's role in spreading this invasive species (Lima et al. 2015). This raises concerns about C. thous spreading H. dulcis in natural habitats and protected areas (Amaral 2007), potentially interfering with native species' recolonisation due to H. dulcis' allelopathic properties (Boeni 2011). In fact, the phenomenon of canids acting as dispersers of exotic, potentially invasive plants is not uncommon, as most canids are opportunistic generalist feeders (Spennemann 2021).
4.3 Seed Dispersal by Cerdocyon thous
4.3.1 Fruit and Seed Sizes
Cerdocyon thous is capable of consuming fruits and seeds of various sizes (fruit width: 4.0–295.8 mm; seed width: 0.5–125.0 mm). As with other mesocarnivores, the range of fruits dispersed by C. thous is less strongly determined by fruit morphology than is the case in birds (Nakashima and Do Linh San 2022). Body size determines the range of fruit sizes a frugivore can consume, so large frugivores commonly disperse large seeds (Chazdon 2013; Lim et al. 2020). C. thous is the second largest canid in South America (Castelló 2018), consuming large-sized fruits. However, we emphasise that, although some fruits exhibit a considerably large diameter (e.g., Artocarpus heterophyllus, Citrullus lanatus, Annona crassiflora), C. thous does not consume them whole, but piecemeal (see Juarez and Marinho-Filho 2002; Moura and Pires 2014). For seed dispersal, more relevant than the fruit itself is the integrity of seeds during fruit consumption. Many of the large fruits C. thous eats display multiple, yet proportionally smaller seeds (e.g., Annona crassiflora, A. monticola, Solanum lycocarpum). Hence, even if C. thous consumes these large fruits piecemeal, it can swallow their small seeds without damaging them. The largest seeds C. thous can swallow whole are from S. romanzoffiana (15.7 mm width) and A. heterophyllus (17.4 mm width). Usually, large amounts of intact S. romanzoffiana seeds are seen in C. thous scats (Reitz 1974; Kuester et al. 2020; Varela and Bucher 2006; Figure S2), indicating their whole ingestion. There are also records of C. thous swallowing A. heterophyllus (jackfruit) seeds, although some are also discarded during consumption (Moura and Pires 2014). For species with extremely large seeds that cannot be swallowed, such as those of Mangifera indica (50.0 mm width; mango), C. thous probably discards seeds during pulp consumption (Cazetta and Galetti 2009). However, if C. thous carries these fruits out of the parent tree area before consumption, this behaviour could still contribute to seed dispersal, specifically through stomatochory (McConkey et al. 2024).
4.3.2 Germination and Seed Viability After Gut-Passage
At least 75% of the plant species tested still germinate successfully after being ingested by C. thous, indicating that seed germinability remains highly possible despite gut passage. Nevertheless, we mainly observed neutral effects on germination rates compared with control seeds. The results from our literature review contrast with those of Nakashima and Do Linh San (2022), who concluded that C. thous has an overall positive effect on germination rates. However, their study considered a smaller number of plant species (n = 3 spp.) compared with the data set in our review (n = 32 spp.). The findings presented here align with studies reporting a mostly neutral effect on seed germination after gut passage of other South American canids, such as maned wolves (Motta Junior and Martins 2002) and Culpeo foxes (Maldonado et al. 2018).
Defining the quality of seed gut passage in carnivores can be controversial, as there is evidence pointing to positive (Nakashima and Do Linh San 2022), neutral (Traveset 1998), and negative (Torres et al. 2020) effects. This variation could be due to the kind of food ingested, which influences the effect of gut passage on germination (Traveset 1998). Since many carnivores are omnivorous and capable of exploiting different food resources (Ewer 1998; Stains and Lariviere 2023), it is assumed that the combination of the type and quality of items consumed can modulate the effects on germination due to impacts on gut passage time and acidity levels to which seeds are exposed in their stomach (Traveset 1998; Nakashima and Do Linh San 2022). Furthermore, experimental conditions under which tests are run (e.g., greenhouse, laboratory, field) may influence the germination percentages between ingested and uningested seeds (Traveset and Verdú 2002). Factors other than germinability need to be considered to assess effects on germination, including germination speed, synchronism, species context and prevailing environmental conditions (Henriques e Souza et al. 2021). The presumption that an increase in germination rates is necessarily positive is poorly supported since higher percentages after gut passage do not necessarily equate to an increase in plant fitness (Traveset 1998). Thus, greater scrutiny of the effects on seeds resulting from passage through C. thous gut is required, especially considering that other aspects of germination have not been fully evaluated so far.
4.3.3 Legitimacy and Seed Dispersal Effectiveness
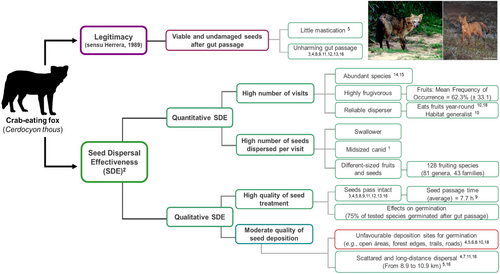
The studies reviewed here suggested that C. thous can act as a legitimate seed disperser, providing reasonably effective dispersal for many of the plant species it consumes (Figure 4). Bustamante et al. (1992) list three attributes that should be considered to assess the positive effects of frugivores upon plants: (i) legitimacy, (ii) efficiency and (iii) effectiveness. According to Herrera (1989), seed dispersal legitimacy in canids is indicated by the occurrence of undamaged seeds in their faeces. Likewise, but for a different taxon, Traveset (1994) considers as legitimate dispersers species that swallow fruits, defecating or regurgitating their seeds intact. For most plant species consumed by C. thous, seeds remain viable after gut passage (Table 2). C. thous faeces also often contain intact seeds of shrub and tree species (Rocha et al. 2004; Figure S2). Canids do not usually chew much of their food, nor do they possess specific adaptations for cellulose digestion (Juarez and Marinho-Filho 2002). These characteristics help prevent seeds from being damaged during consumption or digestion.
Efficiency and effectiveness can be seen as subcomponents within Schupp's (1993) ‘Seed Dispersal Effectiveness’ framework, as suggested by Reid (1989). A disperser's effectiveness is directly related to quantitative components—for example, the number of visits it makes to the plant, seeds removed per interaction—as well as qualitative components—for example, the quality of seed treatment during consumption and deposition sites (Schupp et al. 2010). To estimate seed dispersal effectiveness, various factors must be considered, requiring a thorough evaluation of pre- and postdissemination stages (Schupp et al. 2010). Despite a relative paucity of relevant literature, C. thous can likely be considered an effective disperser for most fruits consumed. In terms of quantitative components, we understand that C. thous has high efficiency, as it can disperse a large number of individual seeds per scat (Figure S2).
The number of seeds dispersed per interaction is affected by the disperser's handling method (Schupp 1993). Carnivores generally do not handle seeds with their paws, often swallowing them whole and undamaged (Nakashima and Do Linh San 2022). Hence, C. thous can be classified as a ‘swallower’ (sensu Corlett and Lucas 1990), which increases its likelihood of dispersing viable seeds (Schupp 1993). Additionally, a frugivore's body size limits the amount of fruit it can swallow (Jordano 2000), and C. thous, as a midsized canid, can consume several fruits per visit, depending on fruit size.
In terms of qualitative components, C. thous shows a high-quality treatment of the seeds but low-quality deposition, which would counterbalance the positive aspects of its qualitative performance. Seed treatment in the mouth and gut directly affects dispersal quality as it can harm seeds or alter their germination (Schupp 1993). Most seeds ingested by C. thous come out intact and viable in its faeces, suggesting that they are not harmed during consumption or through gut passage. Additionally, seed passage through the gut of C. thous affects seed germinability (Motta-Junior et al. 1994; Cheida 2002), germination speed (Cazetta and Galetti 2009; Paulino-Neto et al. 2016) and dormancy break (Varela and Bucher 2006; Henriques e Souza et al. 2021).
On the other hand, C. thous seemingly offers low-quality seed deposition. The quality of deposition is related to the probability of seeds being deposited in a viable site for their survival (Schupp 1993). Concerning deposition patterns, C. thous usually defecate in unfavourable sites for seed germination and establishment of new seedlings, such as forest edges, open areas, trails and roads (Cheida 2002; Rocha et al. 2004; Vieira and Port 2007). Such sites are hostile to plant development (Raíces and Bergallo 2010) and are characterised by abiotic stressors (e.g., excessive sunlight, low humidity, high soil compaction), which cast doubt on the effectiveness of C. thous for high-quality site deposition (Rocha et al. 2004). Such deposition pattern (favouring open areas) seems to be common among mesocarnivores (Nakashima and Do Linh San 2022), including other South American canids (Bustamante et al. 1992; Motta Junior and Martins 2002; Rodrigues 2002; Eigenheer 2018).
Although C. thous often deposits seeds in seemingly unfavourable sites for germination, the effectiveness of its seed dispersal may depend on the plant species. For example, heliophilous and pioneer plants, such as those in the genera Cecropia, Psidium, Piper and Syagrus, benefit from deposition in open sites, where conditions favour their growth (Cheida 2002). This may also apply to invasive plants such as H. dulcis (Borba 1994). Additionally, anthropogenic linear gaps (e.g., rails, trails, roads) can enhance the abundance and diversity of pioneer and exotic species (Suárez-Esteban et al. 2016). While open sites are often associated with abiotic stressors such as excessive sunlight and low humidity, it is possible that the prevalence of scats in these areas is influenced by sampling bias as opposed to the active use of these sites by dispersers (Rodrigues 2002; Suárez-Esteban et al. 2013).
Regarding movement patterns, C. thous likely succeeds in transporting seeds away from the mother plants. Like other canids, C. thous has large home ranges and moves around over great distances and across different landscapes (Nakano-Oliveira 2002; Maffei and Taber 2003; Kanda 2021). C. thous home ranges can extend up to 12.8 km2, and some individuals may roam nearly 11 km during their period of greatest daily activity, usually at night (Juarez and Marinho-Filho 2002; Maffei and Taber 2003). Consequently, C. thous is likely to disperse seeds for long distances, as evidenced by records of S. lycocarpum seeds dispersed up to 8 km (Eigenheer 2018). Additionally, the seed gut passage time is relatively long (average retention of 7.7 h; Varela and Bucher 2006) compared to other frugivores like birds and bats, whose gut retention time spans from a few minutes to more than an hour, depending on seed traits (Traveset 1998; Barnea et al. 1991; Jacomassa and Pizo 2010; Baldwin and Whitehead 2015). This retention period increases the chances of C. thous depositing consumed seeds farther from the mother plant.
5 Conclusion
Our review sheds light on important aspects of C. thous frugivory and seed dispersal. C. thous feeds frequently on fruits, including a wide range of fruits of different species, families, and sizes in the main Neotropical biomes. The evidence so far indicates that C. thous can be a legitimate and effective seed disperser for most of the plant species it consumes. Considering its widespread distribution, generalist habits, high fruit consumption and long-distance movements, C. thous can be a relevant dispersal agent for several native or exotic plant species. Its capacity to thrive in disturbed habitats may be particularly important for restoration purposes given the lack of large-sized seed dispersers in such habitats (Dirzo et al. 2014). However, its role in the dissemination of cultivated and invasive species, such as H. dulcis, raises concerns regarding its potential impact on the natural regeneration of disturbed landscapes. Further research is required to assess how C. thous frugivory may influence plant community dynamics, particularly in degraded areas.
Therefore, although we have explored many aspects of frugivory and seed dispersal by C. thous, there are still knowledge gaps that warrant further assessment. For instance, deeper explorations of fruit preferences, the number of seeds consumed per interaction, dispersal distances, seed deposition patterns, in situ germination success and seedling recruitment under natural conditions will bring significant advances to the knowledge of the seed dispersal performed by C. thous. Moreover, C. thous' role as a seed disperser should also be explored in less studied biomes, such as the Amazon or Pantanal. To the best of our knowledge, our study is the first to propose an in-depth review of aspects of frugivory and seed dispersal of a canid at a regional level in the Neotropics. We hope it may inspire future research regarding the ecology of frugivory interactions involving C. thous and other Neotropical carnivores, often neglected as seed dispersers.
Author Contributions
Rodrigo Béllo Carvalho: conceptualisation, data curation, formal analysis, methodology, visualisation, writing – original draft, review and editing. Liana Chesini Rossi: conceptualisation, writing – original draft, review and editing. Marco A. Pizo: conceptualisation, supervision, writing – review and editing.
Acknowledgements
We thank Universidade Estadual Paulista—Rio Claro and Stanford University for their institutional support of this study. We are grateful for the fruitful feedback from LECAVE, Rodolfo Dirzo, and the members of the Dirzo Lab on this manuscript. We sincerely appreciate Antônio Lucas Barreira Rodrigues for his assistance with the figure shapefiles. We also extend our gratitude to the two anonymous reviewers for their constructive input and critical evaluation, which greatly enhanced the final version of this work. We are deeply grateful to Martin Freeland for his generous help in enhancing the manuscript's readability.
Disclosure
The authors have nothing to report.
Ethics Statement
This study did not involve the use of animals or human participants. Ethical approval was not necessary since the research relied exclusively on a review of existing literature.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data used for the research described in the article are provided in the main text and Data S1 submitted with the paper.




