Impact of the 2023 ENSO Event on the Benthic Community of the San Luciano Shipwreck Reef
Funding: This work was carried out with our own resources and with the infrastructure of the Faculty of Marine Sciences of the University of Colima.
ABSTRACT
Shipwrecks and artificial reefs play a critical role in restoring marine biodiversity in degraded ecosystems. This study assesses the ecological impacts of the 2023 ENSO event and chronic anthropogenic pressures on the San Luciano Shipwreck Reef (central Mexican Pacific), based on comparative monitoring during 2017–2018 and 2023. Using standardized indicators from the Global Coral Reef Monitoring Network and the biological condition gradient (BCG), we evaluated benthic communities, structural features, and physiological stress markers. Biodiversity was highest at the wreck's ends, where structural complexity increased due to metal degradation. In 2023, over 90% of coral colonies showed bleaching and mortality, accompanied by bioeroder proliferation and invasive species such as Carijoa riisei. Coral cover declined by over 85%, and biomarkers such as the Chl a/PC ratio and zooxanthellae density confirmed elevated stress levels. These changes placed the reef at level V of the BCG. The deterioration observed is strongly linked to the combined influence of ENSO-related anomalies and persistent port-related impacts, such as nutrient loading, turbidity, and invasive species transport. Findings underscore the vulnerability of artificial reefs to cumulative stressors and support the inclusion of shipwrecks as valuable sentinel sites in reef monitoring and conservation planning.
1 Introduction
Artificial reefs (ARs), including both intentionally designed structures and unintentional ones such as shipwrecks, have emerged as important tools for ecological restoration in degraded marine environments. These structures can enhance biodiversity, support fishery resources, and provide ecosystem services while also relieving pressure on natural reefs (Pitcher and Seaman 2000; Paxton et al. 2019; Higgins et al. 2022). However, ARs and shipwrecks differ substantially in terms of their origin, ecological roles, and associated challenges.
It is estimated that around 3 million shipwrecks worldwide function as opportunistic ARs. These habitats often attract diverse benthic and fish communities due to their structural complexity, but they also pose risks such as contaminant release, invasive species colonization, and trophic imbalance (Simon et al. 2013; Consoli et al. 2015; Karamitrou et al. 2023). Among invasive species, Carijoa riisei stands out due to its ability to colonize artificial substrates and disrupt benthic assemblages (Galván-Villa et al. 2023).
Climatic events such as the El Niño–Southern Oscillation (ENSO) can exacerbate these impacts. Elevated sea surface temperatures and altered nutrient dynamics during ENSO events often trigger coral bleaching and mortality. In the case of artificial structures, ENSO can also accelerate metal corrosion, increase fouling, and reduce habitat suitability for marine organisms (Hughes et al. 2018; McClanahan et al. 2020; Walker and Schlacher 2014). While the effects of ENSO on natural reefs are well-documented, its influence on ARs, particularly shipwrecks, remains poorly understood.
The San Luciano shipwreck, located in Santiago Bay on the central Pacific coast of Mexico, exemplifies the dual role of shipwrecks as both ecological assets potential sources of environmental stress. Due to its proximity to the Port of Manzanillo, the San Luciano shipwreck is subject to a range of anthropogenic pressures. These include, among others, pollution from hydrocarbons and heavy metals, the occurrence of imposex in gastropods, and the spread of invasive species (Ahumada-Martínez et al. 2018a, 2018b; Liñán-Cabello et al. 2020; Galván-Villa et al. 2023).
Recent studies have highlighted the nuanced roles of shipwrecks and ARs in marine ecosystems. For example, shipwrecks contribute to habitat heterogeneity but may differ markedly from natural reefs in trophic structure and community composition (Simon et al. 2013; Walker and Schlacher 2014). Conversely, biomimetic designs in ARs, such as concrete modules, have demonstrated greater ecological integration compared to shipwrecks (Vivier et al. 2021; Paxton et al. 2023). Further exploration of these dynamics is essential to optimize conservation strategies and mitigate ecological risks associated with artificial structures.
This study directly assesses the ecological impacts of the 2023 ENSO event on the benthic community structure and function of the San Luciano shipwreck reef, within the broader context of chronic anthropogenic stressors. By analyzing biochemical indicators and benthic community responses, we aim to contribute to the growing body of knowledge on artificial reef conservation and offer insights for the effective management of these systems within marine protected areas.
To our knowledge, this is the first application of the biological condition gradient (BCG) model to assess ecological condition on a shipwreck along the central Pacific coast of Mexico, contributing new insights into the utility of this framework in artificial reef environments.
2 Materials and Methods
2.1 Study Site
The San Luciano Shipwreck Reef is situated in the northwestern region of Santiago Bay, Colima, in the Central Pacific of Mexico (Figure 1). The Shipwreck is located approximately 11 km from the Port of Manzanillo and 250 m from La Boquita Beach, a popular tourist destination (Hernández-López et al. 2020). In 1965, the shipwreck occurred following a severe collision with a submerged rocky massif 38 miles from the Port of Manzanillo, grounding the vessel at an approximate depth of 9 m.
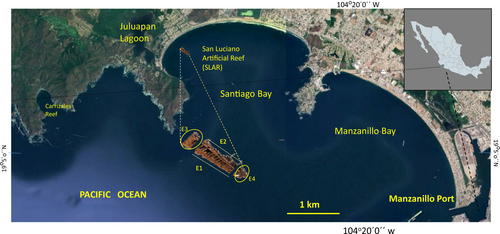
Originally, the ship had a capacity of 5000 tons, with tanks holding up to 25,000 barrels of oil. It could reach speeds of 14–15 miles (≈22–24 km/h), and its principal dimensions were 100.75 m in length, 12.23 m in beam, and 8.23 m in depth (Wrecksite.eu, n.d.). However, no historical records exist regarding the magnitude of a potential spill, its ecological impact, or the measures taken in response. Since then, the structural integrity of the shipwreck has significantly deteriorated due to dynamic forces, corrosion, and bioerosion, with estimated losses of 60% to 75% of its original physical structure. The most severe degradation is observed at the extremities of the longitudinal axis (Figure 1). Despite this deterioration, the San Luciano shipwreck continues to provide key ecosystem services as an artificial reef within Santiago Bay. It offers structural habitat for diverse benthic and fish communities, facilitates ecological connectivity with nearby natural reefs, potentially contributes to localized coastal protection by altering wave dynamics, and serves as a platform for scientific research and environmental education.
2.2 Fieldwork
2.2.1 Sampling Design and Field Survey Protocols
Four monitoring campaigns were conducted during two periods: 2017–2018 and 2023–2024. In both periods, sampling took place between May and November to capture seasonal variability from the late dry to the peak rainy season. The San Luciano Shipwreck Reef was divided into four monitoring stations: two on either side of the shipwreck (E1 and E2) and two at its longitudinal extremities (E3 and E4) (Figure 1). Fifty-meter-long video transects were conducted at these sites using SCUBA equipment and underwater cameras (HERO6 & HERO11). One transect was established per station and consistently repositioned at the same location during each campaign, within a depth range of 4 to 7 m. Although physical markers were not left in place between campaigns, transects were relocated using GPS coordinates and visual site references to ensure spatial consistency over time. During each survey, transects were marked with PVC measuring tapes fixed temporarily to the seabed, and observations were made within 1 meter on either side of the transect line, at an average height of one meter above the bottom, covering an area of 100 m2 per transect (Hill and Wilkinson 2004).
Each monitoring campaign covered a total area of 400 square meters (m2) for benthic species. Fish were filmed from the center of the transect, with the camera positioned at an average depth of 1.0 m (m) in the water column, oriented forward at an angle ranging from 0.79 to 1.57 rad (rad), covering a length of 50 m on each side and a width of five meters (García-Charton et al. 2000). A combination of in situ observations and post-dive video analysis was used. While general habitat and environmental conditions were recorded during the dive, species identification and quantitative metrics (e.g., abundance, coverage) were derived from video transect analysis performed in the laboratory.
2.2.2 BCG Framework
Biotic-environmental monitoring protocols followed the criteria proposed by the Global Coral Reef Monitoring Network (GCRMN, Hill and Wilkinson 2004). These methodologies are widely recognized and accepted due to their standardized approach, allowing for comparability across studies. The use of the BCG model, supplemented with detailed indicators, allows for a nuanced understanding of ecosystem health by capturing both spatial and temporal variability. Each of the seven components of the BCG model—scleractinian corals, spatial heterogeneity, macroinvertebrates, algae, octocorals, sponges, and the physical environment—serves a distinct ecological function. For example, coral health metrics indicate the structural integrity and reproductive viability of reef-building species, while spatial heterogeneity reflects habitat complexity, which supports biodiversity. This approach minimizes redundancy by providing a holistic assessment of ecosystem health, where metrics are not evaluated in isolation but integrated to characterize overall condition. The BCG ranges from Level I, representing natural structure and function, to Level VI, where both are severely degraded, with progressive structural and functional changes across intermediate levels. Observations are contextualized across seasons and sampling periods to detect localized and temporal shifts in ecosystem dynamics. A narrative description of the assemblage components analyzed in this study, conducted in the Mexican Central Pacific (MCP), is presented in Table 1. This classification is based on variables originally defined for the Caribbean by Santavy et al. (2022). Each set or element was analyzed considering the species present and their ecological equivalents in the Pacific. Additional indicators were incorporated into the scleractinian coral assemblage, including coral health (assessed using the CoralWatch scale, where Level 0 indicates severe bleaching, Level 6 represents high zooxanthellae density, and levels 1–5 reflect intermediate conditions), as well as live and dead coral coverage, bioeroder proportions, mortality rates, and bioerosion ratios. Tables 2–5 cite the sources and references for the techniques used to assess various parameters and field observations, most of which follow GCRMN protocols. By linking specific observations to broader ecological processes, this methodology enhances targeted conservation strategies and supports adaptive management of the San Luciano Shipwreck Reef.
| Assemblage or Element | Description |
|---|---|
| BCG Level 1 Natural or native condition | |
| Scleractinian Corals |
> 60% live cover of coral in fore reef habitat. High majority (> 98%) of scleractinians colonies healthy, no signs of disease or stress (discolorations, bleaching; large injuries). Low prevalence of bleached colonies may be present temporarily (Summer –Fall) High species diversity (50%–70% max. taxa for region). Large Reef-Building Coral species (LRBC) are abundant with large and medium colony sizes, healthy colonies, and a high proportion of the total live coral cover (the EW list is: Pocillopora spp., Pavona spp., Porites spp., Psammocora spp., Presence of several colonies of “rare” species such as Fungia vaughani, Pocillopora eydouxi, Pocillopora inflata, Tubastraea tagusensis, Porites evermanni, Pavona duerdeni, Pavona varians, Psammocora profundacella, and Paracyathus humilis). Most colonies within each species population are much larger than the minimum reproductive size, indicating continuous sexual and sustainable reproductive output. Abundance of smaller colonies and juveniles indicates successful settlement and survivorship during the early larval stages with high mortality stages. Presence of recruits (< 4 cm) and abundant juvenile colony sizes (≥ 4 cm and < 10 cm) |
| Spatial Heterogeneity | Complex physical structure (3D high rugosity framework) of aragonite branching, columnar, massive domes, and plates of mostly live corals. Provides habitat, refuge, and resources to a high diversity of organisms (e.g., invertebrates, fish, sea turtles, etc.) |
| Macroinvertebrates | Large, healthy, and abundant colonies of hydrocorals. Absence of invasive species such as Carijoa riisei, moderate cover of zoanthids & densities of the black sea urchin Diadema spp., presence of other urchin species and echinoderms (sea stars, sea cucumbers, etc.). Abundant and diverse crustacean populations (spider crabs, stone crabs, lobsters, snapping shrimp, etc.), colorful polychaetes, and mollusks |
| Algae | Crustose coralline algae (CCA), abundant with low to moderate cover and wide distribution. Low abundance (cover) and diversity of macroalgae and turf algae. Populations grazed by the abundant herbivorous fish and sea urchins |
| Octocorals | Large and abundance colonies, high species diversity of sea fans and branching colonies provides structural complexity. Low to moderate abundance of encrusting species |
| Sponges | Low-to-moderate abundance and high species diversity of medium to large healthy barrel, branching, tube, and massive sponges. Low to moderate cover of crustose, endolithic species (e.g., genera Thoosa, Cliona) |
| Physical Environment | Favorable environmental conditions over time—low variability in temperature, salinity, pH, good water circulation, high water transparency and low sedimentation |
| BCG Level 2 Minimally disturbed | |
| Scleractinian Corals |
> 45% live cover of coral in fore reef habitat. Minimum recent mortality in LRBC include the following species: Psammocora stellata, Pocillopora verrucosa, Porites lobata, Porites evermanni, Pocillopora capitata, Pocillopora damicornis, Pavona gigantea, Pavona varians, and Porites panamensis Normal frequency distribution of colony sizes within each species size range to include large, medium, and juvenile colonies (≥ 4 cm), and presence of recruits (≤ 4 cm). A certain predominance of species that are mostly tolerant to stress is observed, for example, Pavona gigantea, Pocillopora capitata, Porites panamensis, Pocillopora damicornis, Pavona varians, and Tubastrea coccinea. Very low or background levels of disease, tissue and skeletal anomalies, and bleaching Pocillopora, Porites |
| Spatial Heterogeneity | High rugosity resulting from large living coral colonies, producing spatial and topographical complexity |
| Macroinvertebrates | Diadema spp. abundant; reef macroinvertebrates (e.g., lobsters, crabs) common and abundant. Low levels of invertebrate coral predators. |
| Algae Minimal | Algae: Minimal fleshy, filamentous, and cyanobacterial algae present. Crustose coralline algae are present, with some turf algae |
| Sponges | Phototrophic sponges dominate. Low frequency of clionid boring sponges |
| Physical Environment | Mostly high clarity, low particulates |
| BCG Level 3 Good | |
| Scleractinian Corals |
> 25% live cover of coral in fore reef habitat Higher percentage of tissue loss with signs of recent mortality especially on large reef-building genera (Pocillopora spp., Porites spp., Pavona spp.) but is still overall lower than Level BCG 1–2. Frequency distribution of colony sizes within each species size range starting to become skewed to include fewer medium and small colonies (≥ 4 cm) and lower number of recruits than expected (≤ 4 cm) Species composition and diversity: sensitive, rare species present in appropriate habitat low-to-moderate levels of disease and bleaching Pocillopor colonies are still dominant (within respective reef geomorphological zones) |
| Spatial Heterogeneity | Moderate-to-high rugosity or reef structure resulting from large living reef-forming and dead coral colonies, producing spatial complexity (or topographical heterogeneity) |
| Macroinvertebrates | Diadema present, reef macroinvertebrates (e.g., lobsters, octopus, conch) are present. |
| Algae | Minimal presence of fleshy, filamentous, and cyanobacterial algal cover Crustose coralline and tuft algae present |
| Sponges | Phototrophic sponges present, low cover and abundance of Clionid boring sponges |
| Physical Environment | Moderate clarity and particle charge that does not allow seeing the bottom of the sunken ship |
| BCG Level 4 Fair | |
| Scleractinian Corals | > 15% live cover of coral in fore reef habitat Moderate amount of recent mortality on reef-building genera (Pocillopora spp., Porites spp., and Pavona spp.) Colony size distribution: large colonies may be absent, primarily medium and small colonies Species composition and diversity: sensitive species may be absent (Pocillopora inflate, Pocillopora meandrina, Pavona duerdeni), more tolerant species present (Pocillopora capitata; Pavona gigantean, Porites panamensis; Pavona varians). Moderate levels of disease and potential bleaching on corals |
| Spatial Heterogeneity | Rugosity due to old, mostly dead coral structure |
| MacroInvertebrate | Tubastraea spp. and Palythoa may be present, sea fans and branching gorgonians are present with disease |
| Algae | Moderate to high amount of fleshy, filamentous, and cyanobacterial algal cover |
| Sponges | Moderate cover and abundance of Clionid boring sponges |
| Physical Environment | Water quality and clarity may be poor |
| BCG Level 5 Poorly degraded | |
| Scleractinian Corals |
> 5% live cover of coral in fore reef habitat Higher mortality of individual colonies is evident, or remnant colonies or reef structure bioeroded, low amount of tissue remains on colonies |
| Spatial Heterogeneity Macro-Invertebrates |
Low rugosity, that which is present may be dead coral Invasive corals such as Palythoa, Tubastraea spp., Carijoa riisei, are predominant with more gorgonians replacing coral colonies |
| Algae | Coral cover replaced by fleshy, filamentous, and cyanobacterial algae |
| Sponges | Highest presence of Clionid boring sponges Non-phototrophic sponges predominant |
| Physical Environment | Water quality and clarity mostly poor |
| BCG Level 6 Very Poor | |
| Does not meet rules for BCG Level 5 | |
| Assemblage or element | 2017–2018 | 2023 | ||||||
|---|---|---|---|---|---|---|---|---|
| E1 n = 4 | E2 n = 4 | E3 n = 4 | E4 n = 4 | E1 n = 9 | E2 n = 8 | E3 n = 8 | E4 n = 8 | |
| Scleractinian coral | ||||||||
| Proportion live cover of coral in fore reef habitat (%)1 |
3 (2–3) VI |
3 (2–3) VI |
6 (3–7) V |
7 (3–7) V |
2 (0–3) VI |
1 (1–1) VI |
1 (2–2) VI |
1 (1–3) VI |
| Proportion of healthy colonies (%)2 |
85 (83–85) II |
88 (77–89) II |
91 (88–97) I |
94 (91–97) I |
5 (0–14) VI |
3 (0–4) VI |
7 (1–9) V |
7 (1–7) V |
| Proportion of cover of scleractinians colonies healthy (%)1 |
92 (89–95) I |
92 (89–95) I |
90 (87–92) I |
92 (89–93) I |
5 (0–17) VI |
7 (9–16) VI |
8 (2–10) VI |
9 (0–12) VI |
| Prevalence of bleached coral (%)3 |
5 (0–11) I |
8 (7–9) II |
7 (4–8) I |
2 (1–4) I |
92 (90–95) VI |
89 (81–94) V |
97 (94–98) VI |
92 (87–91) VI |
| Coral diversity4 |
4 (4) V |
4 (3–4) V |
5 (3–6) V |
5 (4–6) 5 |
1 (0–2) VI |
1 (0–3) VI |
1 (1–4) VI |
1 (0–4) VI |
| Presence of several colonies of “rare” species1 |
0 VI |
0 VI |
0 VI |
0 VI |
0 VI |
0 VI |
0 VI |
0 VI |
| Relationship: size of coral colonies respect minimum size1 |
1:1 (0.9:1–2:1) III |
1:1 (09:1–0.95:1) III |
2:1 (2:1–2:1) III |
2:1 (2:1–2:3) III |
1:1 (2:1–3:1) V |
1:1 (1:1–4:1) V |
1:1 (0.9:1–1.1:1) V |
1:1 (1.2:1–1:9) V |
| Abundance of smaller colonies and juveniles (col m2)1 |
5 (3–9) II |
7 (2–12) I |
7 (2–9) II |
8 (7–10) I |
0 (0–2) II |
0.95 (0–3) II |
0.92 (0–0.1.1) I |
1.2 (0.2–1.3) I |
| *Normal frequency distribution of colony sizes within each species1 |
1:3:5:8 IV |
1:3:6:8 IV |
1:2.4:4:8 IV |
1:2:5:9 IV |
1:1:2:2 VI |
1:1:2.2:3 VI |
1:1.5: 2: 2.3 VI |
1:2:3:4 VI |
| Assemblage or Element | 2017–2018 | 2023 | ||||||
|---|---|---|---|---|---|---|---|---|
| E1 n = 4 | E2 n = 4 | E3 n = 4 | E4 n = 4 | E1 n = 9 | E2 n = 8 | E3 n = 8 | E4 n = 8 | |
| Spatial heterogeneity | ||||||||
| Complex physical structure (3D high rugosity framework)1 |
0.28 (n = 3) (0.18–0.3) III |
0.18 (n = 3) (0.12–0.3) III |
0.48 (n = 3) (0.2–0.4) II |
0.50 (n = 3) (0.29–0.5) II |
0.29 (n = 3) (0.19–0.31) III |
0.19 (n = 3) (0.29–0.43) III |
0.48 (n = 3) (0.41–0.64) II |
0.5 (n = 3) (0.49–0.63) II |
| Presence of charismatic megafauna Average number of sightings(reports/year)2 |
7 3 IV |
7 3 IV |
7 3 IV |
8 3 IV |
6 3 III |
6 3 III |
5 2 III |
4 3 III |
| Macroinvertebrates | ||||||||
| Abundant colonies of hydrocorals3 |
5 (1–8) III |
3 (1–7) II |
3 (1–8) II |
4 (0–10) II |
9 (7–13) III |
4 (1–7) II |
6.6 (2–14) II |
6 (0–9) II |
| Abundances of colonial anemones (specimens m−2)3 |
5 (1–8) III |
3 (1–7) II |
3 (1–8) II |
4 (0–10) II |
9 (7–13) III |
4 (1–7) II |
6.6 (2–14) II |
6 (0–9) II |
| Densities of urchins (specimens m−2)4 |
1.2 (0.1–0.9) II |
1.3 (0.2–1.0) II |
3 (0.5–9) II |
2.5 (0–4) III |
3.2 (0.2–5) IV |
4.3 (0.1–3) IV |
4.2 (0–3) IV |
4.9 (0–4) IV |
|
Presence of other echinoderms (specimens m−2)3 |
3.8 (2.1–4.1) II |
3.1 (1.8–5.1) II |
2.3 (1.1–3.1) II |
1.8 (0.8–3.1) III |
3.4 (2.7–3.7) III |
3.5 (2.6–3.7) III |
4.4 (2.9–5.7) II |
2.4 (1.7–3.8) III |
| Crustacean populations (crabs, lobsters, etc.), (specimens m−2)3 |
0.8 (0.05–1.2) III |
0.75 (0.3–1.2) III |
2.3 (1.1–2.7) III |
2.2 (0.9–1.8) III |
1.0 (0.7–0.1.4) III |
0.5 (0.4–0.7) III |
2.7 (0.43–2.8) III |
2.25 (0.8–3.7) III |
| Colorful polychaetes, and mollusks (specimens m−2)3 |
6 (4–19) II |
8 (3–8) III |
12 (12–18) III |
14 (6–19) III |
7 (9–14) III |
5 (6–11) III |
18 (13–18) IV |
21 (7–14) IV |
| Assemblage or Element | 2017–2018 | 2023 | ||||||
|---|---|---|---|---|---|---|---|---|
| E1 n = 4 | E2 n = 4 | E3 n = 4 | E4 n = 4 | E1n = 9 | E2 n = 8 | E3 n = 8 | E4 n = 8 | |
| Algae | ||||||||
| Abundance of crustose coralline algae (g m−2)1 |
7 (2–14) II |
5 (2–8) II |
15 (3–18) II |
12 (10–29) I |
16 (8–23) III |
19 (9–25) IV |
31 (15–23) IV |
35 (15–47) IV |
| Abundance and diversity of macroalgae and turf algae (g m−2)1 |
1.8 (0.8–1.5) I |
3.2 (2.2–4.2) II |
5.3 (3.2–6.3) II |
7.3 (4.2–8.8) II |
2.3 (1.6–4.5) III |
4.0 (1.2–6.0) III |
6.3 (4.6–7.9) IV |
7.8 (5.6–9.1) IV |
| Herbivorous fish contribution percentage (%)2 |
11 (2–8) III |
12 (8–14) II |
10 (3–12) II |
16 (5–18) II |
5 (5–13) III |
7 (5–16) III |
7 (6–13) III |
9 (4–11) IV |
| Octocorals | ||||||||
| Abundance and diversity (colonies m−2)3 |
1.1 (0.6–1.4) III |
1.5 (0.3–1.9) II |
1.9 (0.6–2.0) III |
1.9 (0.8–2.2) III |
0.7 (0.5–1.6) III |
0.7 (0.9–2.4) II |
0.6 (0.9–2.3) II |
0.5 (0.7–1.4) III |
| Abundance of encrusting species (colonies m−2)3 |
18 (9–21) II |
12 (6–18) II |
24 (19–27) I |
26 (18–29) I |
22 (9–31) IV |
14 (10–23) IV |
16 (14–19) III |
19 (10–31) III |
| Sponges | ||||||||
| Abundance, species diversity (specimens m−2) |
0.24 (0.11–0.7) II |
0.51 (0.22–0.7) II |
0.7 (0.4–1.2) II |
0.5 (0.25–1.1) II |
1.1 (0.75–2.8) III |
1.3 (0.85–1.7) III |
1.5 (0.37–0.19) IV |
1.4 (0.81.84) IV |
| Benthic biodiversity index5 |
3.5 (2.15–3.7) II |
3.2 (2.7–4.2) II |
4 (2.2–3.8) II |
5.1 (4.7–6.1) I |
2 (1.9–3.6) III |
2.5 (1.5–3.3) IV |
2.9 (01–3.5) III |
3.1 (2.4–4.2) III |
| Assemblage or Element | 2017–2018 | 2023 | ||||||
|---|---|---|---|---|---|---|---|---|
| E1 n = 4 | E2 n = 4 | E3 n = 4 | E4 n = 4 | E1 n = 9 | E2 n = 8 | E3 n = 8 | E4 n = 8 | |
| Physiological environmental nutrients | ||||||||
|
NO2- + NO3 μM PO4 μM SiO2 μM1 |
4.7a 1.5a 26.2a III |
4.8a 1.4a 23.3a II |
5.3a 1.36a 25.7a II |
5.6a 1.4a 22.7a II |
5.3b 1.7b 26.4b III |
4.6b 1.5b 29.4b II |
4.8b 1.4b 24.8b II |
4.7b 1.3b 23.6b II |
| Water transparency & sedimentationc | II | III | II | III | II | III | II | III |
| Surface temperature | 28.1 (27.4–28.5) | 28.3 (0.11–0.7) | 28.2 (0.11–0.7) | 28.0 (0.11–0.7) | 29.8 (0.11–0.7) | 3.0 (0.11–0.7) | 30.1 (0.11–0.7) | 30.1 (0.11–0.7) |
| Circulation patternsc | II | II | II | I | II | I | II | I |
- Note: 1Grasshoff (1983).
- a Average value of measurements taken between July and November 2017.
- b Average value of seven monthly measurements taken between March and September 2023.
- c These variables were not included in the PCA analysis.
2.2.3 Structural Assessment and Biochemical Analysis
Structural complexity was determined with a 10-m-long chain that was laid out following the outline of the bottom along the transects. Subsequently, the total distance was measured in a straight line from the beginning to the end of the chain (Rogers et al. 2001), and surface coverage of uncolonized substrate (bare or corroded metallic substrate) was recorded. During each sampling period, small samples of the coral Pavona gigantea were collected for biochemical analysis, as this species was the most abundant and representative across all four stations. Biochemical diagnostics included the analysis of total carotenoids, total chlorophyll, and mycosporine-like amino acids (MAAs).
During transect surveys, complementary searches for coral species were conducted through roving dives (free diving outside the transects) to complete the inventory of scleractinian corals present in the study area. Opportunistic encounters with other fauna, such as large fish and reptiles, were also recorded.
2.2.4 Laboratory Work
Species identification from images and videos was conducted using taxonomic guides (Cortés and Guzmán 1998; Ketchum and Reyes-Bonilla 2001; Allen et al. 2003; Bastida-Zavala et al. 2014; Chávez-Comparán 2018). Species quantification was performed through a detailed digital analysis of underwater videos and photographs. This approach allowed for the identification of organisms and the recording of relevant data, including their size and density. Images were analyzed frame-by-frame using ImageJ, ensuring accurate identification and quantification of both individual and colonial organisms. Dead and live corals, the proportion of bioeroders, the mortality rate, and the bioerosion ratio were calculated using the equations proposed by English et al. (1997) and Zamani (2020), based on frame-by-frame quantification. These metrics allowed for a standardized assessment of bioerosion intensity across sampling stations.
2.2.5 Laboratory Work
To assess carotenoid pigment concentration, coral fragments were preserved in 100% methanol at 4.0°C. After 24 h, the fragments were homogenized, sonicated for 15 s, and centrifuged at 1500 × g for 5 min at 4°C. The resulting extracts were immediately used for absorbance measurements by scanning wavelengths from 300 to 750 nm at 1 nm intervals. Pigment content was determined using the extinction coefficients for β-carotene (145.0 L·g−1·cm−1) and peridinin (132.5 L g−1·cm−1) carotenoids (Jeffrey and Haxo 1968), and for diadinoxanthin (223.0 L·g−1·cm−1) and dinoxanthin (135.0 L·g−1·cm−1) xanthophylls (Vernon 1960). Total chlorophyll concentration (Chl a) was calculated using equations from Jeffrey and Humphrey (1975), with turbidity correction as recommended.
MAA concentrations were determined following Shick et al. (1992), using the peak absorbance at 325 nm with a Biotek Epoch Microplate Spectrophotometer, and the molar extinction coefficient (36,200) provided by Bandaranayake (1998).
The density of endosymbiont dinoflagellates was quantified from formaldehyde-preserved fragments following a modified protocol by Zamoum and Furla (2012). Coral tissue was removed from the skeleton by incubation in 10 mL of 4 M NaOH at 65.0°C for 1 h. The homogenate was kept overnight at 37.5°C, and Symbiodiniaceae cell density was quantified from a 10-μL aliquot using a Neubauer hemocytometer (n = 8 replicates).
2.2.6 Statistical Analysis
Principal component analysis (PCA) was performed to assess the distribution and association patterns of various indicators related to the reef's structural and functional characteristics. These indicators included live coral, coral health, coral cover, bleaching, coral diversity, rare corals, small coral colonies, coral rugosity, charismatic fauna, and hydrocorals, as well as anemones, urchins, other echinoderms, crustaceans, mollusks, algae, macroalgae, and herbivorous fishes. Additional variables encompassed octocorals, fouling abundance, sponges, benthic diversity, and environmental factors such as nutrient levels, chlorophyll a sedimentation, carotenoids, Chla/PC ratio, MAAs, zooxanthellae density, phosphorus, silicates, TSS, and turbidity.
Before conducting the PCA, 35 variables were standardized to ensure comparability across different scales. Each variable was standardized to a mean of 0 and a standard deviation of 1. This preprocessing step was essential to prevent variables with larger magnitudes from exerting disproportionate influence on the analysis. Additionally, three variables were excluded due to collinearity or low contribution to the overall data structure.
To evaluate the effects of temporal and spatial variation on coral biochemical parameters, we applied a two-way ANOVA (Model I), treating both sampling period (2017–2018 vs. 2023) and monitoring station (E1–E4) as fixed, crossed factors. This model allowed us to assess both main effects and their interaction. Prior to the analysis, data normality and variance homogeneity were verified using Shapiro–Wilk and Levene's tests, respectively. When significant differences were found, post hoc comparisons were performed using Tukey's Honest Significant Difference (HSD) test to identify differences among stations. All analyses were conducted using InfoStat v8, with a significance threshold set at α = 0.05.
3 Results
3.1 Impacts of the 2023 ENSO Event on Coral Health
The results obtained from the 2017 to 2018 and 2023 monitoring periods at the San Luciano Shipwreck Reef reveal significant changes in biodiversity, coral health, and habitat structure. Color thresholds, used as indicators of coral bleaching and health based on the CoralWatch scale, ranged from D4 to D0, with D0 being predominant in species such as P. gigantea and Pocillopora spp. (Table 2, Figure S1). Bleached colonies were often observed detached from their substrates (Figure 2a), indicating high coral mortality rates.

3.2 Reduction in Coral Cover
Live coral cover experienced a dramatic decline, from 368 m2 in 2017–2018 to 38.8 m2 in 2023. This impact was particularly severe at stations E2, E3, and E4, where the Gradient Biological Condition indicated levels V–VI (Table 2).
3.3 Changes in Biodiversity and Community Structure
In 2017–2018, 148 species were recorded, comprising 96 benthic and 52 fish species. By 2023, total richness decreased to 135 species, including 103 benthic and 32 fish species, reflecting a reduction in community complexity. Fish assemblages showed persistence of dominant species such as Diodon holocanthus, Arothron meleagris, and Melichthys niger, particularly at stations E3 and E4. No new dominant species emerged post-ENSO, and although detailed abundance data were not available to perform dissimilarity analyses, the observed trend suggests a general loss of diversity rather than a shift in species composition. Benthic communities were dominated by macroinvertebrates (hydrocorals, anemones, echinoderms, crustaceans, polychaetes, and mollusks) and algae (crustose coralline and macroalgae), with herbivorous fish contributing notably. In 2023, densities of Anthopleura spp. and echinoderms such as Ophiothela mirabilis, Diadema mexicanum, and Echinometra vanbrunti increased, alongside macroalgae, turf algae, and sponges (Chelonaplysilla violacea and Haliclona caerulea). These dynamics were inversely correlated with the decline in coral cover (Figure 5). Additionally, bioerosion activity by Lithophaga spp. and boring sponges (Cliona spp.) intensified, particularly at stations E3 and E4.
3.4 Dominance of Invasive Species
In 2023, the invasive species C. riisei was documented at station E2, covering areas up to 15.2 m2 along one side of the shipwreck, corresponding to an absolute percent cover of 15% at that station. Smaller colonies were also observed in low-light zones (Figure 2b).
3.5 Shifts in Community Composition
3.5.1 Biochemical Indicators and Coral Stress
Pigment concentrations varied across monitoring periods (Figure 3). In 2017–2018, chlorophyll a (Chl a) levels were consistently higher across all stations compared to 2023, with peak concentrations at station E3 during both periods. Carotenoid levels exhibited a similar trend, with higher concentrations in 2017–2018 and a sharp decline in 2023. Conversely, the Chl a/PC ratio increased significantly at all stations in 2023, with the largest rise at station E3.
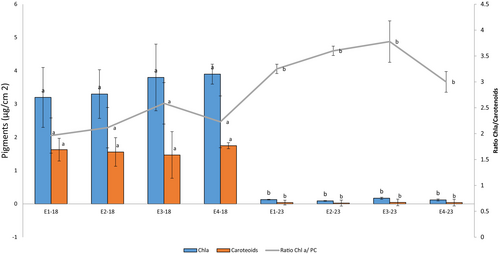
Statistical analysis revealed significant main effects of both period (F(1,24) = 9.87, p = 0.004) and station (F(3,24) = 4.65, p = 0.01), with no significant interaction between factors. Post hoc comparisons (Tukey HSD) identified significant differences between stations E3 and E4 relative to E1 in 2023 (p < 0.05). These findings align with the observed elevations in the Chl a/PC ratio, indicating heightened physiological stress in the structurally degraded extremities of the shipwreck.
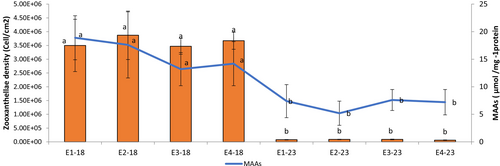
Figure 4 shows that both MAA concentrations and zooxanthellae densities were significantly higher in 2017–2018 compared to 2023. ANOVA results indicated significant effects of period (MAAs: F(1,24) = 12.45, p = 0.002; zooxanthellae: F(1,24) = 10.62, p = 0.003) and station (MAAs: F(3,24) = 5.71, p = 0.004; zooxanthellae: F(3,24) = 4.33, p = 0.01), with no interaction effects. Post hoc tests showed lower 2023 values at E1 and E2 (MAAs: p < 0.05), consistent with higher bleaching stress in these frontal zones. Zooxanthellae densities dropped from ~5.2 × 106 to ~2.1 × 106 cells cm−2 at E1 and from ~5.0 × 106 to ~2.3 × 106 at E2.
3.5.2 Physiological Environmental Nutrients
With regard to the environmental conditions, the most significant change was observed in sea surface temperature during 2023. Moderate to high levels of nitrogen, phosphorus, and potassium compounds were also recorded during both monitoring periods, corresponding to levels II to III on the BCG. Similar levels were identified for water transparency and sedimentation (Table 5).
3.5.3 Associations Among Coral Monitoring Variables Via PCA
The PCA results (Figure 5) reveal significant temporal separation between stations sampled in 2017–2018 and those in 2023, indicating a substantial shift in shipwreck conditions. In 2017–2018, stations exhibited a homogeneous profile with variables such as coral health, live coral cover, octocorals, and biotic indicators, including Chl a and zooxanthellae density. By contrast, in 2023, the stations displayed greater variability, with significant contributions from stress-related biomarkers, including surface temperature, bleaching, eutrophication, sponges, and the Chl a/Carotenoids ratio. Stations E3 and E4, as well as E1 and E2, demonstrated clustering in both periods, suggesting associations with specific variables. In 2017–2018, E3 and E4 clustered with higher values of live coral cover, chlorophyll a, coral rugosity, and zooxanthellae density—indicators of healthier reef conditions. Conversely, in 2023, E1 and E2 were associated with elevated Chl a/PC ratios, bleaching levels, and bioeroder abundance, reflecting increased physiological and structural stress.
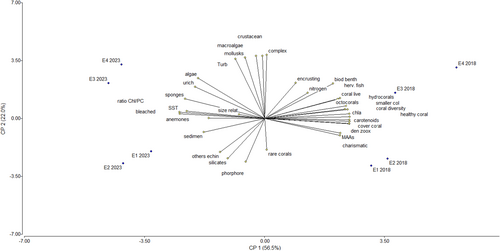
The proportion of the variation explained by these two components was 77.2%. The classification variables, eigenvalues, and eigenvectors from the PCA are provided in Figure S2.
In addition, a complementary PCA was performed to separately examine the structure of biological and environmental variables. The resulting ordinations, included in Figure S3, highlight temporal and spatial variation in biological assemblages (Figure S3a) and environmental conditions (Figure S3b) across the sampling stations.
4 Discussion
For centuries, human-made structures submerged in the ocean have provided enduring evidence of anthropogenic influence on the marine environment (Higgins et al. 2022). Shipwrecks, in particular, have been recognized for contributing to marine biodiversity by offering structural habitats, fostering biological productivity, potentially enhancing biodiversity, and promoting sustainable fishing. However, their structural components can also disrupt natural habitats by acting as conduits for the spread of invasive species and potentially contributing to marine pollution, especially when compared to more sensitive marine ecosystems (Simkanin et al. 2012; Hermosillo-Nuñez et al. 2016; Bhagat and Chauhan 2019).
4.1 Reef Structure Attributes
The tropical Eastern Pacific is reported to have relatively low richness and abundance of scleractinian corals, with 67 species identified in the region (Glynn and Morales 1997; Reyes-Bonilla 2002). Thirteen species of zooxanthellate corals have been documented along the Colima coast, particularly at the Carrizales reef, located just 7.5 km from San Luciano Shipwreck Reef, which is generally considered a well-preserved reef community (Reyes-Bonilla et al. 2013; Hernández-Zulueta et al. 2017). In our study, four species of scleractinian corals were identified during both sampling periods, with P. gigantea being the most dominant, followed by Pocillopora spp. and Tubastraea coccinea. This dominance influenced the “Proportion of Live Cover” indicator, which ranged between levels 5 and 6. The lower diversity and coral coverage on ARs reefs, compared to neighboring natural reefs, have been noted by other researchers (Burt et al. 2009; Perkol-Finkel et al. 2006). However, factors such as the metallic composition, spatial orientation, and structural rugosity of shipwrecks like the San Luciano Shipwreck Reef may play a crucial role in providing adequate light and suitable substrates for coral settlement and development.
Similar to our findings, where substrate rugosity, along with mollusks and crustaceans, were the variables contributing most to the construction of principal component 2 in the PCA analysis (Figure 5)—Jimenez et al. (2016) reported comparable observations. In their study of scleractinian coral cover on two shipwrecks in the Levantine Sea, Cyprus, with an age range of 40 to 70 years, they identified only four species of scleractinian corals, compared to 13 species on surrounding natural reefs. They concluded that factors such as location, depth, orientation, material, and structural rugosity were critical in determining the complexity of encrusting communities. In the specific case of the San Luciano Shipwreck Reef, additional physical factors may have influenced biological colonization. Notably, coastal modifications implemented since 2003—particularly the opening of a channel connecting the Juluapan Lagoon—have been linked to significant reductions in coral cover at the nearby La Boquita coral patch, just 400 m from the shipwreck (Liñán-Cabello and Michel-Morfín 2018). Furthermore, the San Luciano Shipwreck Reef is located only 250 m from a heavily frequented beach, which experiences high visitor pressure and alterations in oceanographic circulation patterns, characterized by two distinct hydrodynamic regimes (Galicia-Pérez et al. 2008).
Asner et al. (2022) reported a positive correlation between shipwreck length and both coral species abundance and genus-level richness. The San Luciano ship, originally 100.75 m-long (Wrecksite.eu, n.d.), currently exhibits significant structural collapse and oxidative degradation at both longitudinal ends. As a result, only 70 m of the structure remain intact, with predominant walls showing localized points of failure. Based on this observation and the aforementioned factors, we infer that the shipwreck under study may be insufficient in size to provide adequate substrate for the diversification and settlement of additional coral species.
4.2 Functional Variables
In terms of substrate rugosity, stations E4 and E3 exhibited the highest values during both sampling periods compared to E1 and E2. These areas corresponded to regions with greater structural heterogeneity, primarily due to the dispersion of metal fragments from the shipwreck, which consequently enhanced light availability. This pattern was reflected in the PCA, which revealed strong associations between substrate complexity and variables such as live coral, herbivorous fish, hydrocorals, octocorals, and overall benthic biodiversity, as indicated by their high loadings on the same principal component. This finding is consistent with Asner et al. (2022), who identified light availability as a key factor in their study of the ecology of 29 sunken ships, noting its influence on primary productivity and, consequently, coral formation and coverage.
In general, during both sampling seasons at E3 and E4, there is a higher prevalence of grass-like algae, Corallina spp., and turf algae, which could be attributed to the increased light availability indicated by turbidity measurement. Eutrophication, previously reported by Liñán-Cabello et al. (2016) and associated with the proximity of the Juluapan Lagoon's outlet to San Luciano Shipwreck Reef (280 m), may also influence the presence of different algal groups. However, the presence of Corallina sp. in the region has been associated with a 60% coral mortality rate during restoration processes (Muñiz-Anguiano et al. 2017), and its abundance increased at all stations during the 2023 period. This increase was linked with other encrusting groups such as mollusks, sponges, grass-type algae, urchins, and polychaetes. Among the mollusks, lithophagous bivalves of the genera Jenneira, Lithophaga, and Leiosolenus were identified, previously recognized as active bioeroders in the Mexican Pacific (Cantera and Contreras 1988; Landa-Jaime et al. 2013). The genera Thoosa, Cliona, and Siphondictyon were primarily observed during the 2023 campaigns, and the bioerosion action of Cliona vermifera in the Central Mexican Pacific is well-documented for its ability to fragment coral colonies and interfere with their growth and health (Carballo et al. 2008). Nonetheless, no significant evidence of erosion has been detected on the coral reef mounds at the studied stations.
Observations of charismatic fauna showed an association with stations E3 and E4, with few variations compared to 2023. The greater topographical heterogeneity and surface complexity of the shipwreck in these areas were primarily associated with habitat use by moray eels for living and/or reproduction. Additionally, the presence of sea turtles and the American crocodile (Crocodylus acutus) has been documented, with the latter being particularly noticeable during the rainy season. Notably, this species is native to the Juluapan Lagoon, located 280 m from the entrance (Noyola 2016).
Among the fish species observed during both sampling periods across all stations, D. holocanthus, A. meleagris, and M. niger consistently exhibited high abundance, with the highest numbers recorded at stations E3 and E4 during the 2023 period. These species share a similar trophic profile, primarily feeding on sea urchins, algae, and sponges, although they are also known to consume corals from the Pocillopora genus (Glynn et al. 1972; Guzmán and López 1991). Additionally, other species such as Stegastes acapulcoensis, Thalassoma lucasanum, and Microspathodon dorsalis were consistently identified during both periods. Notably, S. acapulcoensis and M. dorsalis exhibit flexible feeding habits, functioning as facultative herbivores that primarily cultivate and consume algae within their territories. However, they can opportunistically incorporate small invertebrates, sea urchins, sponges, and even coral into their diet when necessary (Glynn et al. 1972; Guzmán and López 1991). This trophic plasticity likely contributes to their persistence across both sampling periods. Similarly, no significant signs of fish-induced bioerosion were observed. According to Glynn (1988), during the 1983 ENSO event in the Gulf of Chiriquí, Panama, there was a progressive increase in the density of Diadema sp. from 7 to 18 individuals per meter. In this study, urchin densities increased from 1.2–2.5 individuals per meter in 2017–2018 to 3.4–4.9 individuals per meter during the 2023 season, values lower than those reported by Glynn, possibly due to the grazing activity of D. holocanthus, A. meleagris, and M. niger.
The 2023 ENSO event had a profound and community-wide impact on the benthic assemblage associated with the San Luciano shipwreck. This was evidenced by a sharp reduction in live coral cover (> 85%), increased bleaching prevalence (CoralWatch D0 dominant), and a marked shift in community structure, including proliferation of turf algae, bioeroding sponges (Cliona spp.), and echinoids (Diadema mexicanum). The BCG declined from level II to V across most stations, and pigment-based biomarkers confirmed heightened physiological stress. These changes point to a system-wide collapse in structural and functional integrity driven primarily by thermal anomalies and nutrient shifts associated with ENSO conditions.
Colonies of octocorals, including Leptogorgia alba, Leptogorgia rigida, and Leptogorgia cuspidata, were predominantly found at depths of 6 to 9 m. These findings align with those previously reported by Abeytia et al. (2013), who observed a high density of these same species within a depth range of 5 to 10 m along the southern Pacific coast of Mexico. This suggests an inherent ability of these species to anchor themselves to metallic substrates, likely as a means of protection against wave action, a factor identified as a primary contributor to the mortality of gorgonians (Yoshioka and Yoshioka 1991).
4.3 Anthropogenic Influence
It is important to highlight that San Luciano Shipwreck Reef is located 11 km from the entrance to the commercial port of Manzanillo, Mexico's most significant port. Evidence of port-related contamination is apparent, as the first case of disseminated neoplasms was reported in benthic organisms, specifically in the foot and gonad of the gastropod P. columellaris (formerly known as Plicopurpura pansa), in the Port of Manzanillo (Ahumada-Martínez et al. 2018a, 2018b).
Liñán-Cabello et al. (2020) later reported an 18% incidence of imposex in the gastropod P. columellaris within the rocky intertidal zone of Bahía Carrizales, located 7 km northwest of the study site, with another occurrence documented 11 km to the southwest. Given the study site's intermediate position between these two affected areas, it is highly likely that similar environmental pressures contribute to imposex within this region.
Imposex, characterized by the masculinization of female gastropods through the development of male sexual traits, severely disrupts reproductive function and poses a significant threat to population stability. This phenomenon has been strongly linked to port activities, shipping traffic, and organotin pollution, all of which are prevalent stressors in marine coastal ecosystems (Shim et al. 2000; Iannacone et al. 2023; Skazina et al. 2023). The spatial proximity to known imposex-affected zones highlights the need for targeted monitoring and assessment to evaluate the potential extent and impact of contamination in this area.
Processes associated with eutrophication, driven by runoff into the bays of Manzanillo and pollution from chemical compounds and hydrocarbon spills, previously reported by Olivos-Ortiz et al. (2008), Liñán-Cabello et al. (2016), and Bejarano-Ramirez et al. (2017), could intensify from July to January, when a cyclonic circulation pattern occurs in the area (Galicia-Pérez et al. 2008), causing water masses to move from the port towards the San Luciano Shipwreck Reef area. This circulation pattern may facilitate the transport of port-derived pollutants, such as organotin compounds from antifouling ship paints—known to induce imposex—and hydrocarbons.
The influence of port activities is evident, as dense colonies of C. riisei were specifically recognized at station E2 during the 2023 monitoring (see Figure 1). This species has successfully colonized numerous habitats, displacing native species and altering the ecological balance in the habitats it invades (Goldberg and Wilkinson 2004). The gradual colonization from the port to San Luciano Shipwreck Reef has been previously reported by Galván-Villa and Ríos-Jara (2018) and Galván-Villa et al. (2023). A recent study on the impacts of climate change on the spread of C. riisei identified proximity to the port, temperature, and bathymetry as the most influential variables (Mohamed Nisin et al. 2023). Among these, the authors noted that increased temperature was most strongly correlated with the expansion of distribution, up to a threshold of 30°C. This suggests that proximity to port activities and circulation patterns may have facilitated the spread of C. riisei between 2017–2018 and 2023.
4.4 Biochemical Indicators
Regarding the physiological state of P. gigantea, the PCA analysis indicated that biochemical variables such as chlorophyll a (Chl a), coral diversity, total carotenoids (PC), the Chl a/PC ratio, and zooxanthellae density were associated with other biotic variables linked to the healthy state of San Luciano Shipwreck Reef during 2017–2018. These variables significantly contributed to the construction of the first principal component (see Figure S2). However, the concentrations of these variables recorded in SLAR during this period were lower than those reported for P. gigantea by Leyva (2018) at the Carrizales reef community, a minimally disturbed reef, with average values of 4.74 mg cm−2, 4.76 mg cm−2, 1.008, and 5 × 106 cells cm−2, respectively.
At the San Luciano Shipwreck Reef, larger and more well-defined P. gigantea mounds were observed in areas with greater afternoon solar exposure. This finding suggests that the orientation and structure of the shipwreck do not enhance light availability, contrary to previous assumptions. The lower biochemical provisioning could be directly related to the reduced density of zooxanthellae, as proposed by Warner et al. (1996) and Izumi et al. (2023), who discuss the direct relationships between pigment concentration, symbiont cell abundance, and light availability. According to Figure 3, an increase in the Chl a/PC ratio, which becomes significant at stations E3 and E4, was observed. In this context, carotenoids are recognized for their significant role in eliminating reactive oxygen species associated with coral bleaching (Ambarsari et al. 1997).
Regarding MAA concentrations during 2017–2018, this was the only parameter in the San Luciano Shipwreck Reef that exceeded the average levels of 11.7 μmol mg−1 protein reported for P. gigantea in Carrizales by Leyva (2018). These compounds are known to be activated through complex metabolic pathways with high energy demands and respond to adverse conditions, including antioxidant activity, osmotic regulation, heat stress, and nitrogen storage (Yakovleva et al. 2004; Korbee et al. 2006; Oren and Gunde-Cimerman 2007).
The high levels of this indicator observed during 2017–2018 may suggest a preexisting adaptive mechanism in P. gigantea specimens associated with the San Luciano Shipwreck Reef, potentially in response to tidal influxes, temperature variability, and salinity gradients linked to discharges from the Juluapan Lagoon. In this context, Yakovleva et al. (2004) demonstrated that scleractinian corals with elevated concentrations of MAAs, particularly mycosporine-glycine (Myc-Gly), exhibited enhanced resistance to oxidative stress induced by elevated temperatures, underscoring the critical role of MAAs in thermal tolerance.
This study represents the first documented application of the BCG model on a shipwreck in the study area, highlighting the potential of this framework to evaluate ecological condition beyond natural reef systems.
5 Conclusions
This study demonstrates that the San Luciano Shipwreck Reef experienced severe ecological degradation due to the combined effects of the 2023 ENSO event and anthropogenic stressors, including port activity and invasive species. The coral bleaching rates, reaching 85% to 94%, and the shifts in the BCG from level II in 2017–2018 to level V in 2023, underscore the significant structural and functional deterioration of the reef.
The findings highlight the critical vulnerability of ARs to extreme climate events and human-induced pressures. The incorporation of biomarkers within and around shipwreck sites offers deeper insights into the physiological impacts on corals and serves as a valuable tool for the early detection of environmental stress.
Key biomarkers, such as coral bleaching, the abundance of species like C. riisei, Cliona spp., Diadema spp., and the Chl a/PC ratio, offer essential indicators of coral health and environmental stress. Integrating these parameters into AR assessments can enhance the accuracy of reef health diagnoses, ultimately aiding conservation efforts and resilience strategies.
5.1 Global Implications
The results from San Luciano Shipwreck Reef suggest broader implications for artificial reef conservation and management strategies. Given the increasing deployment of ARs worldwide, particularly as a method to restore biodiversity in degraded marine environments, it is crucial to ensure that these structures are resilient to both natural and anthropogenic stressors. The vulnerability observed in San Luciano Shipwreck Reef reinforces the need for careful planning in reef design, considering factors such as structural complexity, substrate material, and proximity to human activities.
Future research should focus on expanding the analysis of artificial reef systems across diverse environmental and geographical contexts to better understand the generalizability of these findings. Additionally, the influence of global climate patterns, such as ENSO, on artificial reef ecosystems warrants further investigation. Long-term monitoring of ARs, coupled with advanced biochemical and ecological metrics, will be essential for developing adaptive management strategies and mitigating the impacts of climate change on marine biodiversity.
By informing the creation of resilient ARs networks and promoting the establishment of marine protected areas, this research provides critical insights for the conservation of marine ecosystems globally.
Acknowledgments
We thank the support of the various students from the Faculty of Marine Sciences at the University of Colima and the members of local cooperative societies for their assistance in conducting the fieldwork for this research.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
All data generated or analyzed during this study are included in this published article.




