Comparative Metabarcoding Analysis of Bacterial Communities and Functional Profiles in the Seaweed and Mangrove Blue Carbon Ecosystems of Goa, India
Funding: This research was supported by the Council of Scientific and Industrial Research (CSIR), India, funded project ‘Carbon Capture, Storage and Utilisation in the Mangrove Habitat’ initiative, under grant HCP0048 and institutional funding from the grant OLP2005.
ABSTRACT
Seaweed and mangrove ecosystems, as integral components of the blue carbon habitat, play pivotal roles in global carbon sequestration and coastal protection. This study explores the bacterial communities and their functional profiles from the coastal habitats of Goa, emphasising their critical roles in the blue carbon ecosystems. The bacterial diversity based on the metabarcoding analysis of the V3–V4 region of the 16S rRNA gene was assessed from the seaweed habitats at Dona Paula and the mangrove ecosystem at Chorao, Goa. Proteobacteria, Cyanobacteria and Actinobacteria dominated the seaweed ecosystems. In contrast, mangrove ecosystems had a more complex microbiota, including Firmicutes and Planctomycetes, which thrive in anaerobic conditions. A comparative reanalysis of taxonomic and functional profiles from the study locations and seven additional locations from different seaweed and mangrove ecosystems of Goa reported in previous studies was also carried out to understand the temporal changes from 2017 to 2024. The results showed a significant presence of Firmicutes at selected locations, with an increased abundance of pathogenic taxa such as Bacillus, Clostridium and Shewanella. These locations, Anjuna, Hawaii and Bogmolo in the seaweed and Ribandar, Panaji and Campal in the mangrove habitats of Goa, were situated near urban regions and influenced by anthropogenic activities, including tourism and urban runoff. Analysis of the bacterial functional profiles also showed an increased representation of the genes associated with xenobiotic biodegradation pathways in these locations. These findings emphasise the urgent need for effective conservation strategies to protect these vital ecosystems against the rising threats of anthropogenic pressures, pollution and climate change.
1 Introduction
Blue carbon ecosystems, which include mangroves, seagrasses, seaweed beds and salt marshes, are among the most productive yet extremely vulnerable habitats. The significance of these coastal marine ecosystems extends beyond their rich biodiversity, offering many ecosystem services crucial to the environment and human well-being (Hori et al. 2019; Macreadie et al. 2021). These ecosystems are highly efficient in capturing and storing carbon, termed ‘blue carbon’, far more effectively than terrestrial forests (Mcleod et al. 2011). They act as robust carbon sinks, sequestering large amounts of carbon as recalcitrant carbon in their belowground sediments for millennia (Macreadie et al. 2021). This characteristic makes the blue carbon ecosystems crucial in reducing greenhouse gas emissions, providing a natural solution to climate change mitigation. Additionally, blue carbon ecosystems play vital roles in coastal protection, providing a buffer against storm surges and coastal erosion while serving as nursery grounds for a diverse array of avian, benthic and microbial species, thus supporting biodiversity and fisheries (Alongi 2018a, 2018b; Hori et al. 2019).
Microbial communities associated with blue carbon ecosystems are pivotal in driving biogeochemical processes, including organic matter decomposition, nutrient cycling and carbon sequestration. Microorganisms, through their metabolic activities, maintain the health and functionality of blue carbon ecosystems (Ren, Liu, et al. 2022; Ren, Jensen, et al. 2022). They contribute to the breakdown of complex organic materials, enabling the recycling of nutrients and supporting the growth of vegetative cover, which is fundamental for carbon storage (He et al. 2022). An intricate balance within these microbial communities is essential for sustaining ecosystem health, productivity and resilience against environmental stressors. Despite their environmental and socio-economic importance, blue carbon ecosystems are facing unprecedented threats due to pollution and climate change. An increase in pollution, particularly from anthropogenic activities, agricultural runoff and industrial waste, disrupts the delicate nutrient balance and imposes stress on both plant and microbial life associated with these ecosystems (Lu et al. 2018; Muruganandam et al. 2023). Climate change is causing sea-level rise, increased storm frequency and ocean acidification, all of which can alter the microbial community structure and functionality of these ecosystems (Malhi et al. 2020; Williamson and Guinder 2021; Muruganandam et al. 2023).
This study focuses on two critical blue carbon ecosystems, seaweed and mangroves, each harbouring unique biodiversity. Seaweed ecosystems, composed of diverse macroalgae species, are found from intertidal zones to depths where light penetrates. These ecosystems play a crucial role in carbon sequestration and support marine biodiversity (Jagtap and Meena 2022; Fujita et al. 2023). Seaweed beds provide a protected habitat and breeding ground for a variety of marine species (Bhuyan et al. 2021; Cotas et al. 2023). The microbial communities associated with seaweed have distinct functional profiles, contributing to nutrient cycling and overall ecosystem health (Sun et al. 2018). These microbes are involved in nitrogen fixation and organic matter decomposition, which are essential for the productivity and sustainability of these ecosystems (Aires et al. 2019; Menaa et al. 2020). Earlier studies from India have reported various biotechnological applications of bacteria associated with seaweeds as important sources of enzymes and bioactive compounds for healthcare applications (Singh and Reddy 2016; Jagtap and Manohar 2021; Jagtap et al. 2022). Similarly, mangrove ecosystems, present in the dynamic intertidal zones of the tropical and subtropical regions, are characterised by their dense tangles of prop roots, known as pneumatophores. These structures stabilise coastlines and provide a sheltered nursery for numerous marine species. Mangroves are highly efficient in carbon storage, both in their above-ground biomass and the organic sediments below ground (Alongi 2012; Inoue 2019). The microbial communities in mangrove soils specialise in decomposing leaf litter, crustacean carcasses and other organic materials, contributing to nutrient dynamics and carbon cycling in these ecosystems (Palit et al. 2022; Fernandes et al. 2022; Yin et al. 2023). These microbes play a vital role in the carbon sequestration ability of mangroves. However, they are sensitive to environmental changes, making mangroves vulnerable to pollution (Ghose et al. 2024), deforestation and climate change impacts and increased storm frequency (Alongi 2018a, 2018b; Palit et al. 2022).
This study aims to gain insights into the health and functionality of the seaweed and mangrove ecosystems based on the microbial communities and their functional profiles. Progress in high-throughput next-generation sequencing technologies offers an invaluable tool to investigate bacterial diversity, overcoming the constraints of conventional culture-based methods. Metabarcoding analysis using Illumina technology provides detailed insights into the bacterial taxonomic diversity and functional profiles, which shed light on their metabolic capabilities and ecological contributions in the blue carbon ecosystems. In this study, the bacterial diversity based on the metabarcoding analysis of the V3–V4 region of the 16S rRNA gene was assessed from the seaweed habitats at Dona Paula and the mangrove ecosystem at Chorao, Goa. A comparative reanalysis of bacterial taxonomic and functional profiles from the study locations, from different seaweed and mangrove ecosystems along the Goa coast reported in previous studies, was also carried out to understand the temporal changes from 2017 to 2024. This study provides a comprehensive framework for assessing how microbial communities in blue carbon ecosystems respond to environmental changes and anthropogenic pressures by integrating metabarcoding analysis with previously reported datasets. These insights enhance our understanding of ecological interactions and temporal variations, supporting effective conservation and sustainable management strategies for coastal ecosystems.
2 Methodology
2.1 Study Region
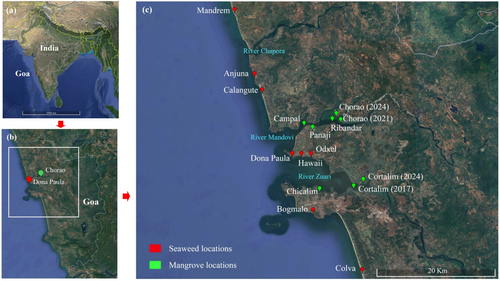
The study region, along the Mandovi-Zuari estuarine complex, harbouring the capital city of Panjim, is one of the pivotal waterways of Goa. It serves as a crucial part in providing various ecosystem services, such as coastal recreational activities, fishing and transport jetties, movement of iron ore on barges, mining activities, growing aquaculture sector and anchoring of popular floating casinos that draw a multitude of visitors to the coastal state (Figure 1). Tourism is a major contributor to the economy of Goa and plays a crucial role in its development. The estuaries serve as a leading tourist destination, attracting local and foreign tourists throughout the year (Nagvenkar and Ramaiah 2009; Khandeparker et al. 2017). A recent study shows that the annual number of tourists visiting Goa has increased significantly in recent years (Gore et al. 2021). Alongside tourism, Goa has experienced rapid urbanisation, with coastal development and smart city initiatives peaking in recent years. This has increased the vulnerability of coastal ecosystems, making Goa the state with the highest urbanisation rate in India (Sharma and Khan 2023). Large-scale tourism and coafstal developments have significantly influenced the ecological balance, energy resources and the socio-economic status of the local population, particularly affecting mangrove forests in Goa (Das et al. 2025). The surge in pollution from sewage and mining discharge is disrupting the delicate ecological balance of riverine systems and adversely affecting the surrounding blue carbon ecosystems in estuarine environments (Haldar and Nazareth 2018; Ghose et al. 2024). Earlier studies have underscored a rapid increase in urban development, which has resulted in a considerable discharge, with an estimated 7000 m3 of pollutants entering these estuarine regions every day (Nanajkar and Ingole 2010; Toraskar et al. 2022). Once considered undisturbed and pristine, coastal seaweed and mangrove ecosystems along these estuarine environments are now facing potential risks from escalating levels of pollution (Haldar and Nazareth 2018; Fernandes et al. 2022; Ghose et al. 2024; Rekadwad et al. 2024). However, there are limited reports on the microbial health in these blue carbon ecosystems along this region, under the surge in coastal development and pollution. These ecosystems are of immense ecological and commercial value and hence, it is extremely important to protect them from an environmental and industrial perspective.
2.2 Sample Collection
For this study, environmental samples including water and sediment were collected during the post-monsoon season in November 2021 from the seaweed habitat at Dona Paula and mangrove ecosystems at Chorao (near the jetty region) of Goa, India (Figure 1). Physicochemical parameters in the water samples from both seaweed and mangrove locations were assessed using standard protocols (USEPA 2013). On-field measurements of temperature, salinity, pH and Eh were conducted using a multiprobe device (Thermo Fisher Scientific, USA). DO concentrations (mg L−1) were determined using Winkler's titration method (Carpenter 1965). Biological oxygen demand (BOD) was assessed following a 5-day incubation period at 27°C, employing the same titration method. Indicators of bacterial pollution, including total coliforms (TC) and faecal coliforms (FC), were quantified using the most probable number (MPN) method, adhering to the standard guidelines of the Central Pollution Control Board (CPCB 2019). Samples for metabarcoding analysis were collected during the low tide phase to ensure consistency. At each site, sediment samples were collected in sterile 50 mL microcentrifuge tubes (Tarsons, India), in triplicate, using a spatula, while maintaining aseptic conditions to prevent contamination. To preserve the integrity of the samples, they were immediately flash-frozen upon collection and transported to the laboratory under cold conditions within 1 h. Upon arrival at the laboratory, the samples were stored at −80°C until DNA extraction was performed. All necessary precautions were taken to avoid sample contamination during storage and handling (Fernandes et al. 2022).
2.3 Taxonomic and Functional Profiling of the Bacterial Communities Based on Metabarcoding Analysis
Metagenomic DNA of the sediment samples from seaweed and mangrove locations was extracted using the FastDNA SPIN kit (MP Biomedical, USA) following the manufacturer's protocol. To ensure consistency and robustness in further analysis, extracted DNA from environmental samples collected in triplicate from each ecosystem type (seaweed and mangrove) was pooled together. This pooled DNA from each ecosystem type was then treated as representative environmental DNA for subsequent analyses. The integrity and purity of the extracted DNA were assessed based on the agarose gel electrophoresis method. The quality and quantity of the DNA samples were further evaluated using a Nanodrop spectrophotometer ND-1000 (Nanodrop Technologies, USA), with the A260/280 ratio indicating DNA quality suitable for sequencing procedures. DNA samples with a concentration of approximately 50 ng μL−1 were used for library preparation using the TruSeq DNA PCR-Free Library Prep Kit (Illumina, USA). The sequencing targeted the V3–V4 region of the 16S rRNA gene, using primer pairs 341F (5′-CCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTATCTAAT-3′). For the Illumina sequencing process, the adapter sequences used were Index 1 Read (5′ CAAGCAGAAGACGGCATACGAGAT[i7] GTCTCGTGGGCTCGG) and Index 2 Read (5′ AATGATACGGCGACCACCGAGATCTACAC[i5] TCGTCGGCAGCGTC) (Qu et al. 2018; Bora et al. 2022). Sequencing was performed at the National Institute of Biomedical Genomics, in West Bengal, India, using the NovaSeq 6000 system (v1.5, Illumina, USA). The microbial community structure and functional potential were analysed using the EZBioCloud microbiome taxonomic profiling pipeline, optimised for Illumina MiSeq paired-end reads (Yoon et al. 2017). Raw sequences were quality filtered by removing reads with lengths < 100 bp or > 2000 bp, an average Q value < 25 and non-16S sequences identified via a Hidden Markov Model (HMM)-based search. Paired-end reads were merged using overlapping regions, and identical sequences were dereplicated to reduce computational redundancy. To ensure comparability across datasets, normalisation of reads from all samples to a depth of 100,000 reads was carried out. Bias correction strategies included the removal of low-quality and non-target sequences, rarefaction to account for sequencing depth variability and dereplication to minimise overrepresentation of dominant sequences. All downstream diversity and taxonomic analyses were conducted on these rarefied datasets. Taxonomic classification was performed using the VSEARCH tool (Rognes et al. 2016) against the PKSSU4.0 database, applying a 97% similarity threshold for species-level identification, focusing on the V3–V4 hypervariable region. Sequences not meeting the 97% similarity threshold were clustered into operational taxonomic units (OTUs) using UCLUST (Edgar 2010) with a 97% cut-off, and singletons were excluded. Bacterial alpha diversity indices were calculated in R Studio (RStudio Team 2024) using the Phyloseq package (McMurdie and Holmes 2013). Functional profiles were predicted using PICRUSt2 based on KEGG orthology (Douglas et al. 2018). The predicted functional profiles with gene profiles were further grouped into KEGG pathways, following PICRUSt protocols.
2.4 Comparative Reanalysis of Bacterial Metabarcoding Datasets to Understand the Temporal Changes in the Seaweed and Mangrove Ecosystem of Goa
A comprehensive literature review was conducted to retrieve studies on bacterial communities in seaweed and mangrove regions of Goa using NGS methods on the Illumina platform. Keywords such as ‘seaweed’, ‘mangrove’, ‘coastal’, ‘estuary’, ‘bacteria’, ‘NGS’, ‘Illumina’, ‘metabarcoding’ and ‘Goa’ were used to search for relevant studies from Google Scholar and the NCBI database. All accessible and relevant bacterial metabarcoding datasets were considered for the comparative analysis, based on their methodological consistency and the sequencing platform used (Table S1). Comparative analysis based on the available datasets could be examined to determine the temporal variations based on the bacterial metabarcoding data from seaweed and mangrove regions of Goa collected from 2017, 2021 (including the study site) and 2024 (Table S1). For this, the metabarcoding datasets from the study site at the seaweed habitats of Dona Paula (2021) were carried out with 7 other locations along the Goa coast, which included Mandrem, Calangute, Odxel and Colva (2017), Anjuna, Hawaii and Bogmolo (2024). Similarly, reanalysis of the metabarcoding datasets from the study locations at mangrove regions near the Chorao jetty region (2021) was compared with the Cortalim and Ribandar (2017); Campal and Panaji (2021) within the high-density urban limits near the state capital and 2024 metabarcoding dataset from Chorao, in the national sanctuary area and Cortalim (Table S1). The comparative analysis of the bacterial taxonomic, community structure and functional profiles was deciphered on the EZBioCloud platform. To ensure consistency across datasets, sequences were trimmed and normalisation was performed using rarefaction to equal sequencing depth and cumulative sum scaling to account for compositional biases. Functional profiles were predicted using PICRUSt2 based on KEGG orthology. The workflow was implemented using EZBioCloud, ensuring a standardised and reproducible analysis across all datasets (Yoon et al. 2017).
2.5 Statistical Analysis
Differential abundance analysis was performed to determine the significant differences in the bacterial taxonomic abundance and functional pathways identified from the seaweed and mangrove ecosystems. Statistical comparisons were conducted using Welch's t-test with a significance threshold of p < 0.05, corrected for multiple testing using the Benjamini–Hochberg method in STAMP (Parks and Beiko 2010). Correspondence analysis (CA) was also carried out using the PAST v4.17 software (Hammer and Harper 2006) to characterise the distribution of dominant bacterial genera from the sampling stations and along with the other comparative locations for each of the blue carbon habitats.
3 Results
3.1 Physicochemical Parameters in the Seaweed and Mangrove Ecosystem
The physicochemical parameters in the study locations recorded a mean temperature of 27°C ± 1.4°C at Dona Paula seaweed beds, located in the intertidal regions, which experience wide variations. The salinity was observed to be 33 ± 1.4 PSU and the pH was slightly alkaline, averaging at 7.8 ± 0.1. The redox potential (Eh) was measured at −84.1 ± 6.1 mV, suggesting a reducing environment (Table 1). The DO levels in the seaweed ecosystem were 7.7 ± 4.8 mg L−1 and BOD was recorded at 3.6 ± 2.1 mg L−1. Microbiological analysis of pollution indicator bacterial counts showed TC levels at 1109 ± 1543 MPN 100 mL−1 and FC levels at 559 ± 765 MPN 100 mL−1. Analysis of the physicochemical parameters at Chorao jetty region, in the mangrove ecosystems, showed that the mean temperature was about 28°C ± 0.4°C. The salinity was notably lower, with an average of 25 ± 11.3 PSU and the pH was measured at 7.5 ± 0.5 (Table 1). The redox potential (Eh) in the mangrove ecosystem was −63 ± 19.6 mV. DO levels were documented at 6.9 ± 1.8 mg L−1 and the BOD was lower compared to the seaweed ecosystem, at 2.4 ± 2.1 mg L−1. The mangrove study location exhibited a higher pollution indicator bacterial load, with TC at 8650 ± 10,394 MPN 100 mL−1 and FC at 1095 ± 856 MPN 100 mL−1 (Table 1) compared to the seaweed study location. However, the TC and FC levels were much higher compared to the permissible limits of 500 and 100 MPN 100 mL−1, respectively, at both the study locations.
| Parameters | Seaweed | Mangrove |
|---|---|---|
| Temperature (°C) | 27 ± 1.4 | 28 ± 0.4 |
| Salinity (PSU) | 33 ± 1.4 | 25 ± 11.3 |
| pH | 7.8 ± 0.1 | 7.5 ± 0.5 |
| Eh | −84.1 ± 6.1 | −63 ± 19.6 |
| DO (mg L−1) | 7.7 ± 4.8 | 6.9 ± 1.8 |
| BOD (mg L−1) | 3.6 ± 2.1 | 2.4 ± 2.1 |
| TC (MPN per 100 mL) | 1109 ± 1543 | 8650 ± 10,394 |
| FC (MPN per 100 mL) | 559 ± 765 | 1095 ± 856 |
3.2 Metabarcoding Analysis of Bacterial Diversity and Taxonomic Composition in the Seaweed and Mangrove Ecosystems
To assess the bacterial diversity in the seaweed and mangrove ecosystems, metabarcoding data were analysed for richness and evenness using various diversity indices. About 106 reads were taxonomically annotated, which resulted in 66,224 valid bacterial read sequences from the seaweed sampling location, Dona Paula and 57,587 reads from the mangrove Chorao jetty location (Table S2). Rarefaction curves approached saturation at the 97% similarity threshold, indicating sufficient sequencing depth for comprehensive microbial community analysis (Figure S1). Taxonomic analysis of the bacterial sequences resulted in an OTU abundance of 1424 from the seaweed ecosystem at Dona Paula and a marginally higher OTU number of 2747 from the mangrove habitat at the Chorao jetty location. The diversity indices evaluated based on the OTUs showed a Shannon index of 5.03, a Simpson index of 0.02, and ACE and Chao1 richness estimators were 1908 and 1892, respectively. Diversity indices were slightly higher at the mangrove location of Chorao, with a Shannon index of 5.32 and a Simpson index of 0.03, which indicates evenness. The ACE and Chao1 values were 3681 and 3775, respectively, which were higher in the mangrove sampling station compared to the seaweed location (Table 2).
| Study location | Valid reads | OTUs | Diversity indices | |||
|---|---|---|---|---|---|---|
| Shannon | Simpson | ACE | CHAO | |||
| Seaweed ecosystem | ||||||
| Odxel-2017 | 76,804 | 6556 | 7.61 | 0.001 | 6940 | 6736 |
| Calangute-2017 | 71,231 | 6409 | 7.40 | 0.002 | 6817 | 6571 |
| Colva-2017 | 74,102 | 4616 | 6.28 | 0.01 | 4793 | 4685 |
| Mandrem-2017 | 76,639 | 5113 | 6.87 | 0.005 | 5395 | 5236 |
| Dona Paula-2021a | 66,224 | 1424 | 5.03 | 0.02 | 1908 | 1892 |
| Anjuna-2024 | 68,005 | 493 | 3.17 | 0.10 | 509 | 497 |
| Bogmalo-2024 | 59,826 | 1175 | 3.40 | 0.09 | 1354 | 1279 |
| Hawaii-2024 | 59,174 | 1802 | 3.82 | 0.13 | 1856 | 1821 |
| Mangrove ecosystem | ||||||
| Chicalim-2017 | 78,618 | 5332 | 6.95 | 0.004 | 5684 | 5471 |
| Cortalim-2017 | 94,102 | 3630 | 5.83 | 0.01 | 4562 | 4641 |
| Ribandar-2017 | 95,029 | 3514 | 5.29 | 0.02 | 5029 | 4918 |
| Campal-2021 | 57,587 | 5998 | 6.03 | 0.02 | 8896 | 9047 |
| Panaji-2021 | 90,511 | 5835 | 5.67 | 0.04 | 8873 | 8830 |
| Chorao-2021a | 88,980 | 2747 | 5.32 | 0.03 | 3681 | 3775 |
| Chorao-2024 | 84,475 | 7079 | 7.49 | 0.002 | 9670 | 9604 |
| Cortalim-2024 | 85,424 | 5093 | 6.67 | 0.004 | 7297 | 7212 |
- a Present study.
Taxonomic metabarcoding analysis showed that Proteobacteria (36.20%) was identified as the dominant phylum (p < 0.001) in the seaweed ecosystem, followed by significant proportions of Cyanobacteria (19.50%) and Planctomycetes (16.20%) (Figure 2). Bacteroidetes (12.20%), Actinobacteria (8.60%) and Verrucomicrobia (5.50%) were also significantly dominant (p < 0.001). In contrast, the mangrove ecosystem was characterised by a significant dominance of Firmicutes (p < 0.001), which constituted about 61.60% of the bacterial population (Figure 2a). Other phyla such as Proteobacteria, Actinobacteria and Planctomycetes were also present but in markedly reduced abundance in the mangrove location compared to the seaweed station. Minor abundance of phyla such as Chloroflexi, Acidobacteria and unassigned bacterial taxa were detected in both the sampling locations; however, they were significantly dominant in the mangrove habitat at the Chorao location (Figure 2). Notably, the mangrove samples showed a relatively higher abundance of unassigned bacterial phyla, BRC1, Gemmatimonadetes and taxa such as GU454958 that were absent or present in very low abundance from the seaweed samples. Additionally, the presence of Saccharibacteria_TM7, Armatimonadetes, Tenericutes, Latescibacteria_WS3 and Chlorobi exclusively in mangroves underscores the distinct microbial niches within these two blue carbon ecosystems (Figure 2a).
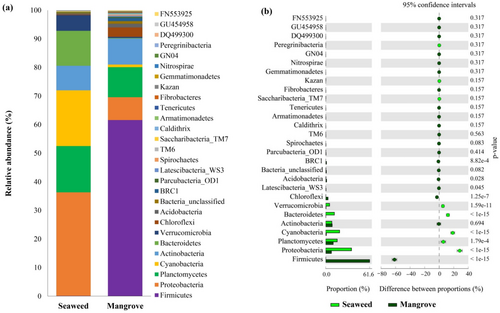
Bacterial communities at a class level were also estimated from both study locations. In the seaweed sample, Alphaproteobacteria were the most abundant class, representing 25.19% of the community, indicative of its versatile roles in marine environments, from symbiosis to nutrient cycling (Figure S2). This was closely followed by the Chroobacteria (19.02%) and Acidimicrobiia (14.89%). Flavobacteria, Verrucomicrobiae and Planctomycetia also had a notable presence (~10%). The unique microbial classes such as Sphingobacteriia, Cytophagia, Oligoflexia and Deinococci were also detected in the seaweed habitat (Figure S2). Conversely, the mangrove ecosystem was dominated by classes within the Firmicutes phylum, with Clostridia and Bacilli accounting for 45.16% and 35.87% of the microbial community, respectively. Several classes were exclusively identified in the mangrove ecosystem, such as Actinobacteria_unclassified (3.78%), Negativicutes (3.45%) and others like Erysipelotrichi, Vampirovibrio_unclassified and Hormogoneae (Figure S2).
3.3 Functional Profiles of Bacterial Communities in the Seaweed and Mangrove Ecosystem
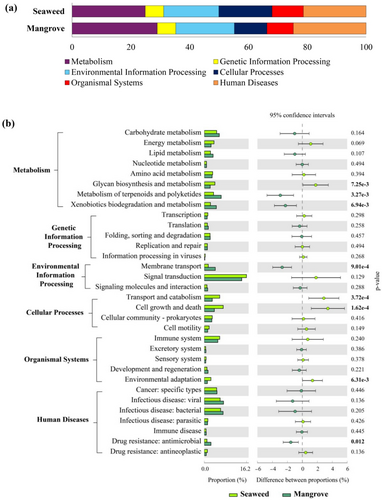
The functional diversity of the seaweed and mangrove bacterial communities from the Dona Paula and Chorao sampling stations was analysed based on the KEGG pathway analysis. The analysis revealed that in the seaweed and mangrove location, major pathways related to metabolism contributed about 24.87% and 28.98%, respectively (Figure 3a). Significant representation was also found in pathways related to human diseases at the seaweed location (21.32%) and was marginally higher (24.76%) in the mangrove sampling station. The gene sequences of pathways associated with environmental information processing, cellular processes (18.10%) and organismal systems (10.66%) and genetic information processing pathways (6.26%) were identified from the seaweed location (Figure 3a). A similar trend of gene sequences within the environmental information processing pathways, cellular processes, organismal systems and genetic information processing pathways was observed in the mangrove Chorao jetty station (Figure 3a). A detailed analysis of KEGG pathways delineated a range of metabolic functions with varying levels of activity in the seaweed and mangrove sampling station (Figure 3b). In the seaweed Dona Paula location, the abundance of genes associated with glycan biosynthesis and metabolism, signal transduction, transport and catabolism, cell growth and death and environmental adaptation pathways showed a significant increase as compared to the Chorao mangrove location (p < 0.001). In contrast, the mangrove location showed a significant increase in the abundance of genes associated with the metabolism of terpenoids and polyketides, xenobiotic biodegradation and metabolism, membrane transport and drug resistance: antimicrobial pathways (p < 0.001) (Figure 3b).
3.4 Comparative Reanalysis of Metabarcoding Bacterial Datasets to Understand the Temporal Changes in the Seaweed and Mangrove Ecosystem of Goa
Temporal changes in the bacterial diversity and its functional profiles were studied at the different seaweed and mangrove locations along the Goa coast based on a reanalysis of published metabarcoding datasets from 2017, 2021 (including the present study) and 2024 (Table S1). In 2017, seaweed bacterial communities had the highest OTUs (4616–6556), with Odxel-2017 showing the highest richness (6556 OTUs). By 2021, OTUs had dropped to 1424 at Dona Paula. In 2024, the decline was even more pronounced, with OTUs ranging from 493 (Anjuna) to 1802 (Hawaii). Shannon diversity indices were highest in 2017 (6.28–7.61) and 5.03 in 2021 (Dona Paula) and dropped to 3.17–3.82 in 2024 and a similar trend was observed in the Simpson index (Table 2). Additionally, richness estimators (ACE and CHAO) also followed the same declining trend, with the highest values in 2017 and the lowest reported in 2024. The sequencing depth and total valid reads reported from the seven datasets of bacterial diversity from different seaweed locations along the Goa coast remained relatively stable from 2017 to 2024. Despite stable sequencing depth, OTUs declined significantly over time, indicating a loss of microbial richness (Table S2). The taxonomic composition of seaweed-associated bacterial communities exhibited substantial shifts between 2017, 2021 and 2024, with changes in dominant phyla and fluctuations in their relative abundance (Figure 4). Proteobacteria, Acidobacteria, Planctomycetes and Actinobacteria were the dominant phyla reported from 2017, 2021 and 2024 from seaweed locations along the Goa coast.
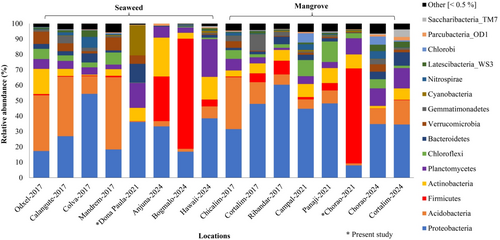
Comparative reanalysis of the microbial diversity in the mangrove locations along the coastal regions of Goa showed distinct temporal and spatial variations. All datasets from 2017, 2021 and 2024 yielded sufficient sequencing depth, ranging from 57,587 to 95,029 reads, relatively higher than those obtained from seaweed habitats (Figure S1 and Table S2). Alpha diversity analysis showed an increasing trend in OTU richness from 2017 to 2024. A similar trend was observed based on Shannon diversity, which was moderate in 2017 (5.29–6.95), remained relatively stable in 2021 (5.32–6.03); however, it increased in 2024 (6.67–7.49). The Simpson index, richness estimators (ACE and CHAO) also followed a similar pattern, increasing in 2024 (Table 2). The composition of mangrove bacterial communities also exhibited substantial shifts between 2017 and 2024, with variations in dominant taxa and overall relative abundance patterns. Proteobacteria, Actinobacteria, Acidobacteria, Planctomycetes and Chloroflexi were the most abundant phyla from the 2017, 2021 and 2024 metabarcoding datasets. The significant (p < 0.001) presence of pathogenic phylum Firmicutes was reported in 2017 and 2021 (Figure S3), which could be due to the sampling location within the urban areas and jetty locations.
Differential abundance analysis revealed distinct taxonomic shifts in bacterial communities across seaweed and mangrove habitats over the period analysed. In 2017, phyla such as Acidobacteria, Chloroflexi and Verrucomicrobia were significantly enriched in seaweed samples, while Proteobacteria, Firmicutes and Gemmatimonadetes showed higher relative abundance in mangrove samples (Figure S3a). In 2021, the microbial composition had notably changed; seaweed samples exhibited higher levels of Cyanobacteria, Bacteroidetes, Planctomycetes and Verrucomicrobia, whereas Firmicutes, Chloroflexi and Acidobacteria were more dominant in mangrove locations (Figure S3b). In the 2024 dataset, seaweed-associated communities were predominantly composed of Firmicutes and Actinobacteria, while Proteobacteria, Acidobacteria and Bacteroidetes were more abundant in mangrove habitats. These compositional differences were supported by differential abundance testing (p < 0.05), indicating a strong influence of habitat type and temporal variation on microbial community structure (Figure S3c). At the genus level, distinct community signatures were also observed between seaweed and mangrove habitats across the dataset (Figure S4). In 2017, seaweed samples were enriched with genera such as Woeseia, Gaiella, Acidibacter, PAC001869_g and AC001990_g, while mangrove samples exhibited a higher relative abundance of Halomonas, Marinobacter, Methylophaga and Pseudomonas. The 2021 dataset showed a shift in dominant seaweed-associated genera to Pleurocapsa, Mariniblastus, Ponticoccus and unclassified Rhodobacteraceae, whereas Pseudomonas, Bacillus, Shewanella and DQ811856_g were more prominent in mangrove samples. However, in 2024, Vibrio, Bacillus, Exiguobacterium and Planococcus dominated seaweed communities, while mangrove sites featured increased representation of Blastocatella, Acidibacter, DQ811856_g, PAC002137_g, Woeseia and unclassified Planctomycetaceae (Figure S4). These genus-level distinctions were consistent with the differential abundance trends observed at higher taxonomic ranks and were statistically significant (p < 0.05), underscoring the ecological divergence (Figures S3 and S4).
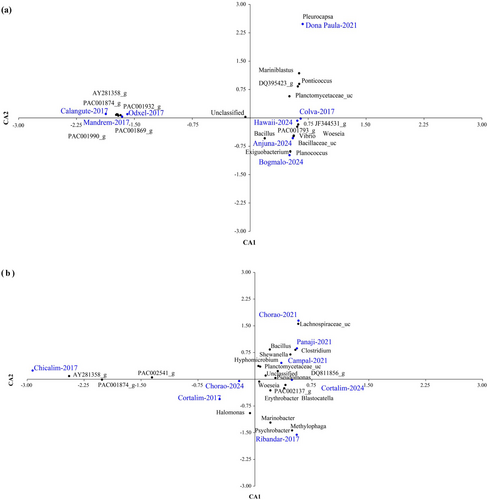
Correspondence analysis of the dominant genera (> 1%) across the sampling datasets from 2017 to 2024 showed a distinct clustering of samples within the datasets analysed, especially within the 2017 and 2024 representatives in the seaweed habitats (Figure 5a). The seaweed datasets from 2017 and 2021 reflected a habitat-specific microbial profile. In 2017, the habitat showed high uncultivated bacterial taxa, and in 2021, it was dominated by genera such as Pleurocapsa, Mariniblastus and Ponticoccus. A significant increase in microbial pollution was evident in the 2024 seaweed samples, dominated by potentially pathogenic genera such as Bacillus, Vibrio, Exiguobacterium and Planococcus, indicating a temporal shift in microbial community structure (Figure 5a). Correspondence analysis of the bacterial community from the mangrove habitats at the genus level also showed a relatively higher abundance of pathogenic genera of Firmicutes such as Bacillus, Clostridium and Shewanella from the urban regions of Campal, Panaji and Chorao (Jetty region, this study) in 2021 (Figure 5b). However, the taxonomic profiles of Chicalim, Cortalim (2017) and Chorao sanctuary region from 2024 showed a dominance of genera such as Marinobacter, Halomonas, Woeseia and Erythrobacter belonging to Proteobacteria (Figure 5b).
The metabolic potential of seaweed microbial communities analysed from the 2017 to 2024 metabarcoding dataset showed that core metabolic functions were represented in all three data sets (Figure S5). The biosynthetic pathways associated with glycan biosynthesis and biosynthesis of secondary metabolites, energy, carbohydrates, lipids and amino acids were observed to be significantly dominant in the seaweed habitats compared to the mangrove habitats from 2017 to 2024 (p < 0.001). Analysis of the functional potential of mangrove-associated microbial communities showed significant changes, especially in the representation of xenobiotic biodegradation pathways in the 2017–2024 dataset. A significant increasing trend in the disease-related pathways and antimicrobial drug resistance was also observed to increase in 2024 in the mangrove ecosystems along the Goa coast (Figure S5).
4 Discussion
Coastal blue carbon ecosystems, including mangroves and seaweed habitats, serve as critical carbon sinks while supporting diverse microbial communities that drive biogeochemical cycling. This study provides a metabarcoding analysis of the bacterial taxonomic and functional profiles at Dona Paula, seaweed habitat and Chorao, mangrove ecosystems. A systematic comparative reanalysis of data sets from 2017, 2021 and 2024 was also carried out, which provides additional insights into the temporal changes of the bacterial community and functional profile within these blue carbon ecosystems.
4.1 Physicochemical Variations in Seaweed and Mangrove Ecosystems
Analysis of physicochemical parameters and bacterial community analysis in the seaweed and mangrove ecosystems revealed detailed insights into the health of these blue carbon habitats along the Goa coast. The values of temperature, pH, Eh and DO were within the prescribed permissible limits for coastal waters and marine outfalls (Toraskar et al. 2022). However, a decrease in the water quality was observed at the seaweed habitat based on the BOD value, which was beyond the permissible 3.0 mg mL−1 limit (CPCB 2019). The observed decline in water quality at the seaweed habitat, particularly the elevated BOD levels, may be attributed to a combination of factors common along the Goa coast, including organic matter accumulation from seaweed biomass. Discharge of untreated urban wastes and recreational activity from coastal tourism, along the rocky shoreline, all add to the increased organic load and microbial proliferation in these ecosystems (Nanajkar and Ingole 2010; Toraskar et al. 2022; Cotas et al. 2023). Lower salinity values were recorded at Choarao, a characteristic feature of the estuarine mangrove habitat, which causes stratification in these regions. This often leads to decreased circulation and water exchange, increasing bacterial load (Toraskar et al. 2022). This was reflected in the mangrove study location, which exhibited a higher pollution indicator bacterial load compared to the seaweed study location. The elevated TC and FC levels, exceeding the permissible limits of 500 and 100 MPN 100 mL−1, respectively (Table 1), indicate substantial microbial contamination at both study locations. The elevated levels of pollution indicator bacteria detected with wide fluctuations in both these ecosystems suggest external influences could be due to recreational activities or direct contamination from nearby urban human settlements, disrupting the natural microbial balance (Mitra and Zaman 2016; Sharma et al. 2022; Subramanian et al. 2023). The mangrove ecosystem, situated at the land-sea interface, naturally experiences a combination of marine and freshwater influences, leading to more variable physicochemical conditions such as salinity, tidal variations and the accumulation of detritus, favouring rich and complex microbial diversity (Dinesh et al. 2016). This level of microbial pollution not only alters the microbial dynamics of blue carbon ecosystems but also threatens their ecological functions, such as carbon sequestration and severely impacts the biodiversity of these habitats (Wang and Gu 2021; Palit et al. 2022). The presence of high pollution indicator pathogenic bacterial loads in both ecosystems, despite their differing physicochemical environments, points to their declining ecosystem health with rising pollution.
4.2 Variation in the Bacterial Diversity and Community Structure in the Seaweed and Mangrove Ecosystems
The bacterial diversity based on the metabarcoding analysis in the seaweed and mangrove ecosystems reveals notable differences in the microbial community composition and richness of these two distinct blue carbon habitats (Table 2). The diversity indices collectively suggested that a highly diverse bacterial community was present in the mangrove habitat at Chorao compared to the seaweed ecosystem at Dona Paula. In the seaweed ecosystem, the dominance of Proteobacteria, Cyanobacteria and Actinobacteria reflects a community engaged in nutrient cycling and primary production, crucial for marine ecosystems (Singh and Reddy 2014; Menaa et al. 2020). The presence of Bacteroidetes, Verrucomicrobia and Planctomycetes, known for their roles in organic matter decomposition and nitrogen cycling, further underscores the functional diversity within this habitat (Xie et al. 2024; Zhang et al. 2024). These findings were consistent with the global studies on seaweed-associated bacterial communities, spanning a decade, showing the dominant presence of key bacterial phyla such as Proteobacteria, Actinobacteria, Bacteroidetes, Verrucomicrobia, Planctomycetes and Cyanobacteria in these ecosystems (Burke et al. 2011; Lachnit et al. 2011; Bengtsson et al. 2012; Mancuso et al. 2016; Cleary and Huang 2020). The occurrence of minor taxa like Chloroflexi, Acidobacteria and unassigned bacterial taxa (Figure 2a) highlights these specialised niches harbouring unique bacterial taxa, which have potential functional roles in the seaweed ecosystem. Conversely, the mangrove ecosystem was characterised by a predominance of Firmicutes, suggesting an adaptation to the sediment-rich and potentially anaerobic conditions of mangroves (de Santana et al. 2021; Chithira et al. 2021; Fernandes et al. 2022). In the mangrove ecosystem, the dominance of Clostridia and Bacilli within Firmicutes, along with exclusive classes like Actinobacteria_unclassified and Negativicutes, underscores a microbial community adapted to thrive in anaerobic conditions and involved in the fermentation and breakdown of organic matter (Jeyanny et al. 2020; Chithira et al. 2021). The significant presence of class Clostridia in the studied mangrove ecosystem indicates faecal contamination (Ghose et al. 2024). This indicates that increasing pollution has a greater impact on the mangrove ecosystem, with a higher abundance of pathogenic bacterial groups than the seaweed ecosystem at the sampling stations in 2021 (Figure 2).
4.3 Functional Profiles of Bacterial Communities and Their Ecological Implications
Functional profiles of bacterial communities revealed the prominence of environmental information processing and cellular process pathways within the seaweed microbiome, suggesting a highly adaptive bacterial community. This indicates that the microbiome is well-equipped to interact with its surroundings and is proficient in preserving cellular function and integrity, reflecting a dynamic and resilient microbial ecosystem capable of responding to environmental stimuli and stresses (Egan et al. 2014; Ghaderiardakani et al. 2020; Ren, Liu, et al. 2022; Ren, Jensen, et al. 2022). Interestingly, the relatively high representation of pathways associated with human diseases could indicate the presence of potential pathogens, which points to the impact of rising pollution on these ecosystems due to the lack of proper coastal conservation and management systems (Ghaderiardakani et al. 2020; Mancuso et al. 2023). The significant presence of metabolism pathways suggests a versatile microbiome engaged in robust biochemical activities, essential for nutrient cycling and energy flow in the seaweed ecosystem (Miranda et al. 2022; Ren, Liu, et al. 2022; Ren, Jensen, et al. 2022). The moderate presence of organismal systems pathways and the lower abundance of genetic information processing pathways reflect a balanced microbial community, with less emphasis on genetic maintenance and more on environmental interaction and organismal processes (Ghaderiardakani et al. 2020). Conversely, the mangrove microbiome shows a greater emphasis on metabolic pathways, underscoring its crucial role in nutrient cycling and organic matter degradation within the mangrove ecosystem (Lin et al. 2019). The well-represented environmental information processing pathways suggest a microbiome capable of effectively responding to environmental changes (Yu et al. 2023; Zhu et al. 2022). The lower representation of cellular processes and organismal systems pathways compared to the seaweed microbiome might reflect a different ecological strategy adopted by the mangrove microbiome, focusing more on metabolic activities than on cellular or organismal interactions (Lin et al. 2019). The notably higher presence of human disease pathways within the mangrove microbiome than in the seaweed microbiome showed potential pathogenic interactions within the mangrove ecosystem (Liu et al. 2019); this showcases that increased anthropogenic activities, urbanisation and improper waste management practices are some of the leading sources of significant contamination of the mangrove environment (Sharma et al. 2022; Ghose et al. 2024).
The detailed KEGG pathway analysis further delineates these differences in the seaweed microbiome, with the notable presence of carbohydrate metabolism and glycan biosynthesis and metabolism pathways highlighting its role in primary production and organic matter processing (de Oliveira et al. 2012; Weigel et al. 2022). The substantial representation of cell growth and death pathways and environmental adaptation pathways suggests a dynamic microbial community actively engaged in growth, adaptation and environmental interaction (Ghaderiardakani et al. 2020). In contrast, the mangrove microbiome exhibits a unique functional profile with a significant abundance of pathways involved in the metabolism of terpenoids and polyketides, reflecting its capacity to process complex organic compounds typical of mangrove ecosystems (Liao et al. 2020; Mohapatra et al. 2021). The pronounced membrane transport pathways and the more abundant carbohydrate metabolism pathways in mangroves further underscore their specialised metabolic functions (Lin et al. 2019). However, the elevated xenobiotic degradation and metabolism pathways in mangroves highlight the adaptive capabilities of their microbial communities. Mangroves, polluted by urban and industrial runoff, accumulate xenobiotics in their sediments. In response, microbes evolve efficient pathways to metabolise these compounds, maintaining ecological balance and resilience.
4.4 Comparative Reanalysis of Bacterial Communities in Seaweed and Mangrove Ecosystems and Their Temporal Variations
Comparative reanalysis of the microbial diversity in the seaweed and mangrove locations along the coastal regions of Goa showed distinct variations. Despite stable sequencing depth, a significant decline in microbial diversity from 2017 to 2024 was recorded in seaweed locations. The consistent reduction in OTUs and reduced values of diversity indices indicate a loss of microbial functions, potentially affecting nutrient cycling and ecosystem stability. The sharp decline in diversity in 2024 underscores the need to investigate the possible influence of point source anthropogenic influences and their ecological impact. The dominance of major bacterial phyla such as Proteobacteria, Acidobacteria and Bacteroidetes, along with notable contributions from Actinobacteria, Planctomycetes and Cyanobacteria (Figure 4), underscores their ecological significance in seaweed microbial communities. These taxa play vital roles in nutrient cycling and organic matter degradation, essential for maintaining the ecological balance of seaweed habitats, as observed across all three analysed datasets. Urbanisation appears to influence microbial community composition, as evident from shifts observed in the 2024 data from Anjuna, Hawaii and Bogmalo. A decline in Proteobacteria, coupled with an increase in pathogenic Firmicutes such as Bacillus, Vibrio, Exiguobacterium and Planococcus (Figure 5a), suggests ecological disturbances likely driven by anthropogenic activity and environmental stressors. These changes may have broader implications for ecosystem stability, as such microbial shifts are known to affect both human and plant health (Korlević et al. 2021; Muruganandam et al. 2023).
Comparative reanalysis of the microbial community profile along the mangrove regions of the Goa coast from 2017 to 2024 reflected an increasing trend in microbial richness and diversity in mangrove-associated communities. The increase in OTUs and diversity indices, particularly in 2024, indicates enhanced microbial colonisation and stability. This contrasts with the decline observed in seaweed-associated communities, highlighting differences in ecosystem resilience. The observed patterns suggest that mangrove environments may support more stable or recovering microbial populations, potentially due to their adaptive capacity and ecological conditions. The dominance of Firmicutes in the bacterial community structure at Ribandar (2017), the Chorao mangrove station (jetty region, this study) and the urban-influenced mangrove habitats of Campal and Panaji (2021) (Figure 4) suggests a strong influence of anthropogenic activities and environmental stressors in shaping microbial composition in these regions. However, the shift in microbial dominance over time indicates that anthropogenic pressures and environmental changes have significantly influenced microbial community composition, as evidenced by the 2017 data from the Chicalim and Cortalim mangrove stations, where Proteobacteria, Acidobacteria and Bacteroidetes (Figure 4) are known to play a central role in organic matter degradation and nutrient cycling. The significant increase in pathogenic taxa such as Bacillus, Pseudomonas and Clostridium suggests that urbanisation and pollution create favourable conditions for these bacteria at the Campal and Panaji locations, which are within the urban city limits (Ghose et al. 2024). Firmicutes thrive in high organic pollution environments, indicating a shift toward taxa that can efficiently utilise organic matter from urban runoff (Ghose et al. 2024; Chithira et al. 2021). The surge in Firmicutes and pathogenic genera underscores the health risks in mangrove ecosystems, which are linked to human-induced pollution. The 2024 data from Cortalim and Chorao, a relatively pristine mangrove sanctuary, suggests a notable reduction in pathogenic bacterial forms, indicating that minimal human disturbance and better ecological conditions may support a more stable and resilient microbial community. Additionally, the presence of lesser-known or unclassified taxa points to the potential for discovering novel functions and interactions in these environments.
The persistence of core metabolic functions across the 2017 to 2024 metabarcoding datasets suggests that fundamental microbial processes in these ecosystems have remained relatively stable despite environmental changes (Figure S5). However, the greater dominance of biosynthetic pathways associated with glycan biosynthesis, secondary metabolite production and the metabolism of energy, carbohydrates, lipids and amino acids in seaweed habitats compared to mangrove ecosystems suggests a functionally distinct microbial community. This distinction underscores the specialised metabolic adaptations of seaweed-associated bacteria, likely driven by the unique ecological conditions and nutrient availability in these environments over time. The increasing representation of xenobiotic biodegradation pathways in mangrove-associated microbial communities from 2017 to 2024 along the Goa coast (Figure S5) suggests a growing microbial response to persistent anthropogenic pollution. This trend highlights the adaptive capacity of mangrove microbiomes to degrade environmental contaminants, potentially mitigating the impact of pollutants over time. However, the concurrent rise in pathways associated with infectious diseases raises concerns about the deteriorating ecological health of these ecosystems, likely driven by urban runoff, industrial discharge and other human-induced stressors. These findings emphasise the urgent need for sustained monitoring and pollution mitigation strategies to preserve the functional integrity of mangrove habitats. Xenobiotics are often introduced through industrial effluents, agricultural runoff, coastal tourism and urban waste discharge, which serve as point sources of pollution, particularly in coastal areas (Wang and Gu 2021; Sharma et al. 2022). Mangroves, often located near estuarine zones, act as sinks for these contaminants due to sediment trapping and low water flushing rates, creating hotspots for microbial xenobiotic metabolism (Cabral et al. 2019; Kannan et al. 2023). These findings underscore the critical role of microbial communities in mitigating pollution by breaking down harmful xenobiotics. However, the persistent and increasing presence of these pathways also highlights the pressing need for better pollution management and wastewater treatment strategies to reduce the anthropogenic load on coastal ecosystems.
5 Conclusion
This study highlights the significance of the bacterial communities and their functional dynamics from the coastal habitats of Goa, emphasising their critical roles in the blue carbon ecosystems. The metabarcoding analysis reveals that seaweed habitats at Dona Paula are dominated by Proteobacteria, Cyanobacteria and Actinobacteria, reflecting their role in nutrient cycling and primary production. In contrast, the mangrove ecosystem at Chorao harbours a more complex microbial community, with Firmicutes and Planctomycetes indicative of anaerobic and pollutant-degrading microbial adaptations. The comparative reanalysis revealed characteristic temporal changes in the bacterial diversity and functional profiles from 2017 to 2024. Major changes in the bacterial diversity included a notable increase in the pathogenic genera of Firmicutes at the seaweed habitats of Anjuna, Bogmolo and Hawaii in 2024, suggesting a declining eco-health in these locations. The comparative reanalysis of mangrove habitats confirms the consistent dominance of Proteobacteria, Acidobacteria, Bacteroidetes, Actinobacteria and Planctomycetes, essential for ecosystem stability. However, urban-influenced locations such as Ribandar, Campal, Panaji and the Chorao jetty area showed a higher prevalence of pathogenic genera. These findings show that the microbial communities in blue carbon ecosystems situated near urban regions were greatly affected by an increased abundance of pathogenic groups and metabolic pathways related to human diseases and xenobiotic degradation. These salient findings from this study underscore the need for continuous monitoring and effective pollution management to preserve the health of blue carbon ecosystems.
Author Contributions
Ashutosh Shankar Parab: formal analysis, investigation, methodology, writing – original draft, writing – review and editing. Mayukhmita Ghose: formal analysis, methodology, writing – original draft. Vitasta Jad: formal analysis, literature review. Sumit Sudhir Phakatkar: methodology, writing – original draft. Aiswarya Kalathil Jayan: formal analysis, writing – original draft. Cathrine Sumathi Manohar: conceptualisation, funding acquisition, project administration, resources, writing – review and editing.
Acknowledgements
The authors are thankful to the director of the CSIR National Institute of Oceanography, Goa, for extending all the support and required facilities to accomplish this work. This is NIO contribution number 7413.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The metabarcoding data generated in this study have been deposited in the National Centre for Biotechnology Information (NCBI) Sequence Read Archive (SRA) under the Bioproject number PRJNA1065591.




