Current Wild Population Status of Protected Mother-Of-Pearl Oyster Pinctada mazatlanica in Mexican Pacific Reefs
Funding: Long-term SCUBA diving monitoring has been possible through the support of the International Community Foundation, the David and Lucile Packard Foundation, the Walton Family Foundation, the WWW Foundation, National Geographic Pristine Seas, and the Leona M. and Harry B. Helmsley Charitable Trust. A.B.-G. obtained a PhD grant from SECIHTI (2021–2025, CVU 782874) and funding for this study throughout CONANP PNZMAES (PROREST/CC/736/2021), SIP-IPN 20240731, 20250136. J.G.-G., E.G.R., L.H.-S., and C.S. are SNI fellows, and J.G.-G. is also an EDI-IPN and COFAA-IPN fellow.
ABSTRACT
The mother-of-pearl oyster Pinctada mazatlanica (Hanley, 1856) obtained full protection from the Mexican government after the fishery collapse in 1939. P. mazatlanica was listed in 1994 as a threatened species in the “Special Protection” category. However, no quantitative assessment of the state of the population has been done so far. Our study is the most comprehensive summer interannual monitoring program conducted so far in the Eastern Pacific using SCUBA diving censuses conducted in 314 sampling sites located along the Mexican Pacific between 1998 and 2021. We propose the hypothesis that although P. mazatlanica had full protection with the NOM-059, global warming reported in northwest Mexico has caused a decrease in the population abundance along the Pacific coast, which may render the protection effort useless. However, we demonstrate that P. mazatlanica was the numerically dominant macro–mollusk and occupied the 18th ranked abundance place compared with the entire epibenthic macroinvertebrate fauna that included 241 species at rocky reefs of the Mexican Pacific, particularly abundant along the peninsular coast of the Gulf of California. Population frequency size distribution of P. mazatlanica dorsoventral length showed positive population growth and latitudinally similar dorsoventral length range (2–30 cm, mode 14 cm when protandry takes place) along the peninsular coast of the Gulf of California, indicating a stable population in time and space. We observed high abundances in the central Gulf of California (Baja Peninsula), mainly from Loreto to La Paz. We conclude that P. mazatlanica is a stable and healthy population along the rocky reefs of the peninsular coast of the Gulf of California even during prolonged anomalous warm events in 2013–2016. Therefore, the present protection status should be modified accordingly.
1 Introduction
The mother-of-pearl oyster Pinctada mazatlanica (Hanley, 1856) (Bivalvia: Margaritidae) is distributed exclusively in shallow rocky sea bottoms (< 30 m) of the Eastern Tropical–Subtropical Pacific. Its biogeographic distribution ranges from the west coast of the Baja California peninsula (Magdalena Bay) including the Gulf of California, Mexico, to Northern Peru. It also includes the Revillagigedo and Galapagos Islands (Keen 1971; Ulate et al. 2016). Arnaud et al. (2000) inferred three significantly distinct P. mazatlanica population groups based on genetic diversity: Northern Mexico, Southern Mexico, and Panama. Pinctada mazatlanica is a culturally and economically highly valued bivalve species that naturally forms pearls by chance or by artificially inserting a tissue graft from a donor mollusk.
Mexican populations of P. mazatlanica were listed as a species at risk under the Special Protection (Pr) category of NOM-059-ECOL-1994 in 1994, which remains in effect (NOM-059-ECOL-1994 1994; NOM-059-SEMARNAT-2019 2019). P. mazatlanica was also included in 2014 in the Agreement of Priority Species for Conservation (Diario Oficial de la Federación 2014). However, P. mazatlanica is not included in CITES and IUCN international lists of protected species. P. mazatlanica is a culturally relevant species in Mesoamerican, Andean (Peru), and Mexican civilizations, with high conservation priority in Mexico (Mazón-Suastegui 1987; Saucedo and Monteforte 1997; Saucedo et al. 2001, 2004, 2009; Monteforte 2006; Velázquez et al. 2007; Wright, Holguin-Quiñones, et al. 2009; Correa-Trigoso 2016; Ainis et al. 2019). However, no studies have determined the present population status in the Mexican Pacific. The investigation of the population status and distribution range of P. mazatlanica is relevant to evaluate the progress and efficiency of the conservation efforts conducted by the Mexican government. Some studies on the population density of P. mazatlanica have been done in the Eastern Pacific Ocean, including the population of Costa Rica (Solano-López et al. 1995), (Cipriani et al. 2008) and Mexico (carried out in the southwest Gulf of California) (Monteforte 2006; Wright, Holguín-Quiñones and Arreguín-Sánchez 2009; Bervera-León et al. 2022). Unfortunately, these studies do not provide integrative information to evaluate and justify present conservation measures because they are outdated, conducted in a small region, or have a short duration that prevents inferring the effect of climatic change on regional populations. Consequently, there is a pressing need to reassess the efficacy of conservation efforts for P. mazatlanica and identify areas of improvement or population decrease.
We evaluated P. mazatlanica population density in the context of the entire macroinvertebrate community structure along the Mexican Pacific, including the Gulf of California and the oceanic islands of Revillagigedo and Islas Marías Archipelagos from 1998 to 2021. This is a novel research approach because ecological inferences are made considering the entire macroinvertebrate community with which target protected species interact in time and space. The Mexican Pacific coast has been under a long-term warming period during the last two decades that may negatively impact phytoplankton biomass (Hakspiel-Segura et al. 2022; Robles-Tamayo et al. 2022; López-Martínez et al. 2023). The tropicalization of the macroinvertebrate benthic community structure has been observed along the southeast coast of the Gulf of California during the last two decades (Favoretto et al. 2022). The increase in ocean temperatures, changes in weather patterns, and increases in storm activity may all affect the future success of pearl farms, impacting the expression of genes related to biomineralization, somatic growth, and reproduction of Pinctada margaritifera (Le Moullac et al. 2016). We propose the hypothesis that although P. mazatlanica had full protection with the NOM-059, global warming reported in northwest Mexico has caused a decrease in the population along the Pacific coast, which may render the protection effort useless. The goals of our study were: (1) to evaluate the population status in terms of regional population density of P. mazatlanica in the Mexican Pacific during 1998–2021 and contrast its relative frequency of appearance and abundance within the entire epibenthic macroinvertebrate community; and (2) to evaluate its temporal fluctuations of abundance, biomass, frequency structure of sizes, and population growth rates in the Mexican Pacific, infer its population status during the last two decades, and evaluate the results of conservation efforts. Protection of a species at risk can be expensive and requires a large amount of legal and logistical effort. Therefore, monitoring species abundance and distribution requires information to reiterate or change protection status and divert protection efforts to other species with higher risk priorities.
2 Materials and Methods
2.1 Satellite Telemetry
Five satellite environmental variables were obtained to infer the P. mazatlanica population response to climatic change along the Mexican Pacific during 1998–2021. They were daily sea surface temperature (SST, °C) obtained from the Multi-scale Ultra-high Resolution (MUR) of the Group for High-Resolution SST (GHRSST). The physiological rates (body growth, reproduction), energy metabolism, and population mortality of Pinctada species are significantly influenced by sea water temperature, with an optimal thermal window of metabolic activities between 18°C and 25°C (Saucedo et al. 2001, 2004, 2009; Le Moullac et al. 2016; Muhammad et al. 2020). Beyond this range, particularly above 30°C, metabolic efficiency may begin to decline, reflecting increased energy demands that exceed energy acquisition (Le Moullac et al. 2016). Other four environmental variables obtained from the Copernicus Marine Environment Monitoring Service (CMEMS): sea surface Chlorophyll-a concentration (Chl-a, mg Chl-a m−3), sea surface current components (u and v), mixing layer depth (MLD, m), and sea surface salinity (Sal) (details shown in Supporting Information S1). Sea surface Chl-a concentration is a proxy of phytoplankton biomass (Hakspiel-Segura et al. 2022) available for shallow benthic filter-feeding fauna, considering that P. mazatlanica is a filter-feeding mollusk mostly preying on phytoplankton (Saucedo et al. 2009). However, temperature, more than diet, exerts a stronger influence on the growth and condition of the specimens (Saucedo et al. 2009). Variations in salinity can lead to differences in larval survival and growth rates of Pinctada fucata (Muhammad et al. 2020). Cold and deep mixing layer depth near the coast can indicate upwelling conditions where phytoplankton increase their concentrations. We analyzed sea current speed as a variable related to the availability of suitable habitats for settlement, as it transports larvae by advection far away from the progenitors in regions known to predominantly promote dispersion or concentration of plankton (Marinone 2012). All environmental variables were averaged for all summers (July–September) recorded between 1998 and 2021 to infer the latitudinal environmental gradients that the epibenthic macroinvertebrate community (including P. mazatlanica) inhabits in the Mexican Pacific.
2.2 Data Collection During Visual SCUBA Surveys
Summer interannual epibenthic macroinvertebrate SCUBA diving censuses were conducted in 314 rocky reef sites (considered suitable habitats for P. mazatlanica) distributed along the Mexican Pacific coast, including the Gulf of California, using ProMARES survey method (Sánchez-Rodríguez et al. 2014). The largest and longest sampling effort was done along the southwest coast of the Baja California peninsula between Loreto and Cabo San Lucas, sampled every summer (July–September) between 1998 and 2021 (Figure 1; Table 1). Rocky reef sites located in the Gulf of California northward of Loreto, and in the Mexican Pacific south of Cabo San Lucas, were sampled opportunistically in different years (Table 1). Each survey site consisted of eight standard 30 m linear transects parallel to the coast, including four transects at 5 m deep (shallow transect) and four transects at 20 m deep (deep transect). In a few sites, only the 5 m depth transect was done due to a lack of suitable rocky habitat in deeper waters. All macroinvertebrates were counted and identified in situ to the most precise taxonomic level. The maximum length of each macroinvertebrate was measured with a PVC ruler graduated at 2 cm intervals. P. mazatlanica individuals were measured by the maximum dorsoventral length of the valve. The abundance and measurements of body length data per macroinvertebrate species were recorded on submersible sheets and transcribed into a spreadsheet file. The rest of the studied areas (Alto Golfo, Santa Rosalía, San Basilio, the oceanic islands of National Park of Revillagigedo, National Park of Islas Marías, and central and southern Mexican Pacific Bahía Banderas, Ixtapa–Zihuatanejo, and Huatulco) were surveyed using the same ProMARES method at opportunistic monitoring expeditions between July and December (Figure 1A,B). A matrix species density (ind./m2) of all epibenthic macroinvertebrates recorded in the entire study area was created to infer with which species P. mazatlanica cohabits and the environmental conditions containing the highest or lowest densities in rocky reefs of the Mexican Pacific.

| Region | No. of sites | Total No. of transects | Years of expeditions | Total abundance (No. ind) | Mean abundance (ind/m2) | Standard error Sx | Mean biomass (g/m2) | Standard error Sx |
|---|---|---|---|---|---|---|---|---|
| Alto Golfo | 23 | 306 | 2009, 2016 | 6 | 0.0006 | 0.0046 | 0.0165 | 0.1366 |
| Santa Rosalia | 10 | 128 | 2009, 2010, 2016 | 45 | 0.0122 | 0.0347 | 0.2610 | 0.8062 |
| San Basilio | 20 | 85 | 2019 | 40 | 0.0156 | 0.0372 | 0.8014 | 2.5084 |
| Loreto | 36 | 1827 | 1998–2019 | 2956 | 0.0545 | 0.1260 | 1.5345 | 4.0431 |
| Corredor | 37 | 989 | 2000–2019 | 1803 | 0.0608 | 0.0991 | 1.8817 | 3.5844 |
| La Paz | 56 | 1021 | 1998–2021 | 1922 | 0.0624 | 0.1105 | 1.6471 | 3.2343 |
| La Ventana | 16 | 349 | 2009–2018 | 789 | 0.0762 | 0.1372 | 2.3139 | 4.5340 |
| Cabo Pulmo | 20 | 915 | 2009–2020 | 1114 | 0.0403 | 0.0818 | 1.1372 | 2.4404 |
| Los Cabos | 15 | 511 | 2009–2021 | 76 | 0.0051 | 0.0207 | 0.1462 | 1.1846 |
| Revillagigedo | 29 | 309 | 2006 | 1 | 0.0001 | 0.0018 | 0.0046 | 0.0823 |
| Islas Marías | 24 | 268 | 2010, 2018 | 211 | 0.0274 | 0.0462 | 0.7536 | 1.8880 |
| Bahía Banderas | 7 | 40 | 2013 | 8 | 0.0089 | 0.0226 | 0.2222 | 0.6329 |
| Ixtapa-Zihuatanejo | 15 | 80 | 2017 | 19 | 0.0079 | 0.0226 | 0.0965 | 0.2827 |
| Huatulco | 7 | 38 | 2016 | 12 | 0.0127 | 0.0358 | 0.8201 | 4.3529 |
- Note: The gray rows indicate the latitudinal regions where P. mazatlanica was most abundant in the Mexican Pacific.
2.3 Pinctada mazatlanica Relative Dominance in the Macrobenthic Invertebrate Community
An Olmstead–Tukey analysis was conducted to infer the ecological position in terms of the frequency of appearance and abundance of P. mazatlanica within the community of macromollusk and within the entire community of macroinvertebrates surveyed (ProMARES survey 1998–2021) in the rocky reefs of the Mexican Pacific.
2.4 Latitudinal Population Size Structure in the Gulf of California
We compared the size-frequency distribution of P. mazatlanica between 1998 and 2021 in seven regions located along the peninsular coast of the Gulf of California (Loreto, Corredor, Bahía de La Paz, La Ventana, Cabo Pulmo, Los Cabos, and Islas Marías) to infer the existence of a latitudinal cline or regional bias toward small individuals, which may indicate that regional populations might be subjected to illegal extraction. A second latitudinal population size–frequency distribution of P. mazatlanica was conducted per year to track the interannual trend of mean population growth rates using the software FiSAT II version 1.2.2 (https://www.fao.org/fishery/en/topic/16072/en). Sex assignment of P. mazatlanica was inferred with data from Saucedo and Monteforte (1997) (details in Supporting Information S1).
2.5 Abundance and Biomass Estimation
Standardized abundance of P. mazatlanica (ind./m2) was estimated from the total number of individuals per transect divided by the area surveyed (30 m2). The regional average abundance was calculated with the standardized abundance of all the transects recorded at each region divided by the total number of transects conducted between 1998 and 2021 along the peninsular coast of the Gulf of California. The standardized regional abundance of P. mazatlanica was compared among latitudinal regions as a function of time (years) to infer interannual variability of population abundance. The biomass of P. mazatlanica was estimated using the length–weight power regression model (W = 0.0005418DVL2.7301) reported by Wright, Holguin-Quiñones, et al. (2009), where W is body weight (g) and DVL is dorsoventral length (cm). The product of individual weights (g) and abundance was used to estimate the P. mazatlanica mean population biomass per transect (gr/m2), which was later used to estimate mean regional biomass.
2.6 Ecological Ordination
A biodiversity matrix was constructed with the 241 macroinvertebrate species registered (columns) and 314 sampling sites (rows, average abundance of the transect replicas per site) distributed along 14 ecological regions surveyed in the Mexican Pacific recorded between 1998 and 2021. A hierarchical two-way cluster analysis (CA) was conducted using the Bray Curtis link method and Flexible Beta distance (selected value −0.250) and matrix coding of relative abundance per species to detect spatial community structure similarities among macroinvertebrate distributions of each sampling site and the abundance of each macroinvertebrate species recorded in the study area. The CA was calculated with the PC-ORD software (ver. 6; http://home.centurytel.net/~mjm/pcordwin.htm) (McCune et al. 2002, Supporting Information S1).
A canonical correspondence analysis (CCA) was conducted to infer with which species typically cohabit P. mazatlanica along the Pacific coast of Mexico and under which environmental conditions P. mazatlanica reaches its highest population abundances (Supporting Information S1). The macroinvertebrate abundance of each species was Log (x + 1) transformed to decrease variance and linearize variables. The environmental variables were relativized per column to standardize the scale of each variable considering their distinct original range of variability (McCune et al. 2002). The CCA used two matrices to perform ecological ordination of the macrobenthic community structure. The species matrix included 241 macroinvertebrate species (columns) and 314 sampling sites (rows, average abundance of the transect replicas per site per sampling day). The environmental matrix included averages of five environmental variables recorded during summer (July–September) each year (columns) and 314 sampling sites (rows). These environmental variables were sea surface temperature (°C), sea surface Chl-a concentration (mg/m3), the thickness of the mixed layer (m), sea surface salinity (kg/L), and sea surface current speed calculated from their u and v components of the nearby pixels located in the 314 sampling sites (rows) (explained in section 2.2). The CCA was calculated with the PC-ORD software (ver. 6).
The main environmental variables that influence invertebrate abundance and distribution were identified using the CCA ordination technique. Then, we used the multi-response permutation procedure (MRPP) to test five null hypotheses contrasting that the epibenthic community of macroinvertebrates was not significantly distinct among regions categorized into the following five categorical variables: (1) 14 latitudinal regions (Alto Golfo, Santa Rosalia, San Basilio, Loreto, Corredor, La Paz, La Ventana, Cabo Pulmo, Los Cabos, Revillagigedo, Islas Marías, Bahía Banderas, Ixtapa–Zihuatanejo, and Huatulco), (2) sea surface water masses defined with temperature and salinity range criteria described in Portela et al. (2016) (California Current water CCW, Gulf of California water GCW, Tropical surface water TSW and Transitional water TW), (3) three biogeographic regions inferred from resident shore fishes in the Tropical Eastern Pacific: Cortez province, oceanic islands, and Panamic provinces (Robertson and Cramer 2009); (4) cross-shelf habitats distinguishing Coast, Continental islands (Isla Angel de la Guarda, Isla Coronado, Isla Carmen, Isla Danzante, Isla Monserrat, Isla Catalana, Isla San Marcos, Isla Santa Cruz, Isla San José, Isla Espíritu Santo, Isla Cerralvo, and Islas Marías), and Oceanic Island (Islas Revillagigedo), and (5) a classification of sampling site combining sea water masses of the Gulf of California described in detail in Ulate et al. (2016) and biogeographic patterns of shore fishes Robertson and Cramer (2009) dividing regions as North, Central, South of the Gulf of California, Island (Revillagigedo), and Panamic region. The MRPP was calculated using PC-ORD v.6 following the criteria of McCune et al. (2002) (Supporting Information S1).
Indicator species analysis (ISA) was used to test the fidelity of macroinvertebrate species (expressed in standardized abundance) present per sampling site using each of the same five null hypotheses (Ho). The ISA method measures the average abundance estimated in all SCUBA visual transects done per sampling site defined by each null hypothesis. ISA produces indicator values for each species in each environmental station group based on their relative abundance, their frequency of appearance, and an indicator that combines relative abundance and relative frequency of appearance. The highest indicator value for each species is tested for statistical significance using a Monte Carlo randomization technique (Dufrene and Legendre 1997). Although ISA was calculated for all macroinvertebrate species, we report only ISA values of P. mazatlanica to test the null and alternative hypotheses.
3 Results
The 1998–2021 epibenthic macroinvertebrate community included 241 species recorded in the 314 rocky reef sites located along the Mexican Pacific, including the peninsular coast of the Gulf of California. A total of 6866 SCUBA diving censuses were conducted in the study area. P. mazatlanica was recorded in 38% of the total sampling sites (2622 positive transects) and distributed in virtually all the study areas (recorded in 233 out of the 314 sites, 73% of the sampling sites, n = 9002 individuals; Figure 1A,B). P. mazatlanica was observed on Socorro Island but absent in the other three Revillagigedo islands (Clarion, Roca Partida, and San Benedicto; Figure 1, Table 1).
Pinctada mazatlanica was rarely observed in the Alto Golfo but more frequently observed in the transition of the subtropical and temperate communities in the Gulf of California (Figure 1; Table 1). It was more frequent and abundant in Santa Rosalía. It was the numerically dominant macromollusk species between Bahía de Loreto National Park and Cabo Pulmo National Park (26°34°–23°27 N). P. mazatlanica was also rarely observed in the southern tropical regions of Mexico, except at Islas Marías, where it was also a numerically dominant species but in lower abundance than those observed along the peninsular coast of the Gulf of California. However, Islas Marías, Bahía Banderas, Ixtapa–Zihuatanejo, and Huatulco regions had, so far, low sampling efforts (Figure 1A,B). The highest P. mazatlanica abundances were recorded along the peninsular coast of the Gulf of California, which we consider the present core of the P. mazatlanica population in Mexico (Figure 1; Table 1).
3.1 Macroinvertebrate Dominance
Pinctada mazatlanica occupied the 18th position in the macroinvertebrate community. The community was typically dominated in abundance by the hexacorals Porites panamensis Verrill, 1866, and Pocillopora grandis Dana, 1846, the sea fan Muricea austera Verrill, 1869, the sea urchin Eucidaris thouarsii (L. Agassiz and Desor, 1846), and the ascidian Rhopalaea birkelandi Tokioka, 1971 (Figure 2; Table S1). P. mazatlanica was the most frequent and abundant macromollusk in the rocky reefs of the Mexican Pacific coast (Figure 2; Table S2), which is an unusually high relative abundance for a species with protected status in Mexico.
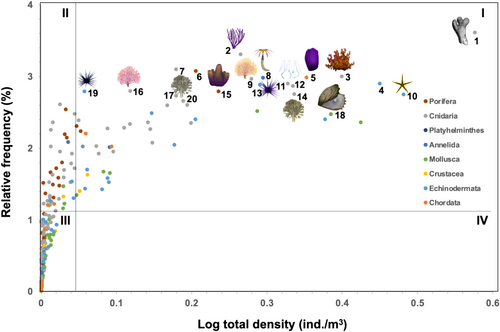
3.2 Population Size Structure
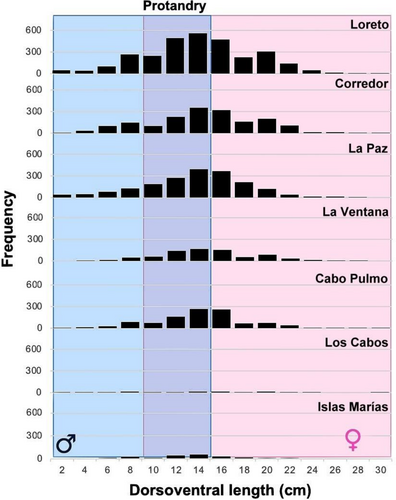
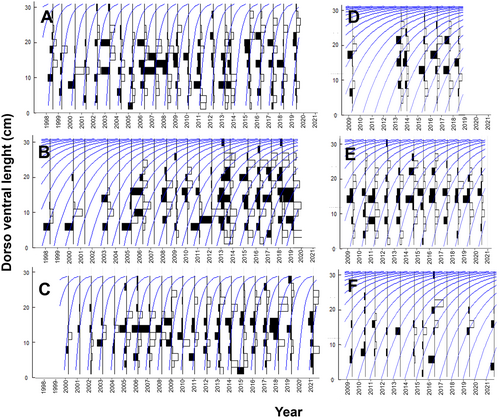
The dorsoventral length (DVL) frequency distribution of P. mazatlanica measured during 1998–2021 along the peninsular coast of the Gulf of California, where sampling effort was consistent through the entire time series, showed individuals between 2 cm and 30 cm DVL (Table S3). Overall, the Loreto, Corredor, and Bahía de La Paz regions showed the most complete population distribution range between 2 cm and 30 cm. P. mazatlanica had low abundance and missed large individuals (> 26 cm DVL) in the Los Cabos region even though it also had a high-frequency sampling effort. Islas Marías, with limited temporal data (November 2010 and June 2018), only had a few records of P. mazatlanica individuals between 6 cm and 24 cm DVL (Figure 3). The frequency of small individuals (males) was low regardless of the latitudinal region. The highest frequency in the distribution range was observed for individuals between 10 cm and 15 cm DVL, when P. mazatlanica likely changes sex to females (protandry), which occurs with a mode of 14 cm DVL in most of the latitudinal regions (Figure 3). Females decrease their abundance as they increase in size (probably driven by mortality), particularly for specimens > 24 cm DVL (Figure 3). No evident latitudinal cline was observed in the size-frequency distribution of P. mazatlanica, which indicates a stable population structure along the peninsular coast of the Gulf of California and high resilience to climatic changes. P. mazatlanica has an estimated lifespan of 4–5 years based on evidence of the size-frequency distribution and growth rates estimated through FiSAT II software (Figure 4A–F, Supporting Information S1). However, most P. mazatlanica individuals observed in our study were < 3 years old (Figure 3).
3.3 Population Abundance and Biomass of P. mazatlanica
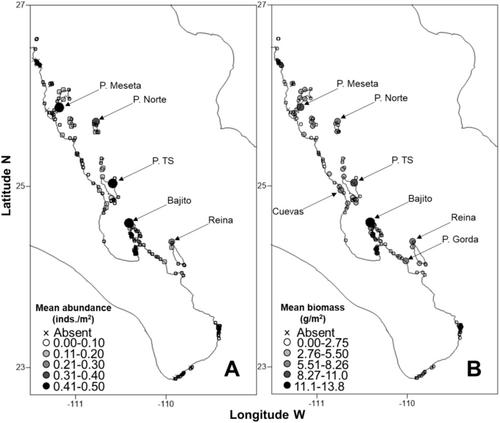
The mean abundance of P. mazatlanica per latitudinal region along the Mexican Pacific coast had the highest abundances (0.04–0.08 ind/m2) and biomass (1.14–2.31 g/m2) between Loreto and Cabo Pulmo regions (Table 1). The highest mean abundances (0.23–0.5 ind/m2) and biomass (5.9–13.77 g/m2) by sampling sites were distributed from Loreto to La Ventana (peninsular coast of the Gulf of California; Figure S2 and Figure 5A,B). Pinctada mazatlanica population's biomass had a similar distribution pattern to abundance because no significant latitudinal difference in the population size–frequency distribution of the DVL was observed (Figure S3).
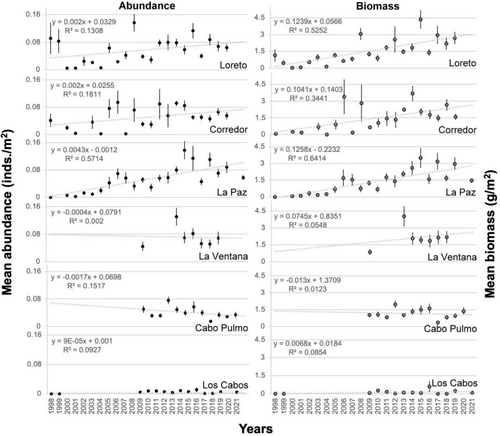
3.4 Time Series of Population Abundance and Biomass
Our hypothesis argues that if P. mazatlanica has been fully protected from 1994 to the present, population growth must be positive from 1998 to 2021. However, due to anomalous warming conditions in this period, we expected a decrease in the average population density and biomass. The annual average abundance (and standard error) of P. mazatlanica distributed between Loreto and La Paz showed that population densities increased (significant positive slope; Figure 6). However, no significant population abundance change throughout time (slope not significantly different from zero) was detected at La Ventana (Figure 6). Contrary to our initial expectations, P. mazatlanica population decreased (negative slope) at the no-take Cabo Pulmo National Park, fully protected since 1995 to the present (Figure 6). The slightly negative but stable trend of P. mazatlanica abundance in Cabo Pulmo National Park was associated with minimum abundance in 2017 of 0.05 ind./m2, just after the strong El Niño 2015–2016 event (Figure 6).
3.5 Ecological Ordination of Epibenthic Macroinvertebrate Community
The MRPP showed that the macroinvertebrate community structure was significantly different contrasting the five hypotheses tested (Table S4). The hypothesis of biogeographic regions based on Robertson and Cramer (2009) biogeographic criterion (based on shore fishes) showed the most distinct, but coherent latitudinal regionalization of survey sites and species assemblages. We focused the results on this tested hypothesis. Descriptions of the other four hypotheses are shown in the supporting Information S1. The two-way cluster analysis using Robertson and Cramer's (2009) criteria clustered 19 groups of macroinvertebrate species with a 25% similarity cut value. Seventeen clusters were small groups of species, including common and rare species. We focused on the cluster where P. mazatlanica was grouped, which included 40 epibenthic macroinvertebrate species (including 36 of the most numerically dominant species with a high frequency of appearance). Three out of the five tested hypotheses showed a clear latitudinal regionalization in the macroinvertebrate community. Robertson and Cramer's (2009) biogeographic regionalization criterion was significantly distinct, where Revillagigedo (Island region) mixes with several survey sites of San Basilio (Figure S4). The Panamic region from the southern part of Mexico was grouped with sampling sites between La Paz and Los Cabos, and the Cortez region clustered sampling sites from Alto Golfo to Los Cabos (Figure S4). The indicator species analysis showed that P. mazatlanica had significantly high fidelity to Cortez province based on the biogeographic criteria of Robertson and Cramer (2009) (Table S5).
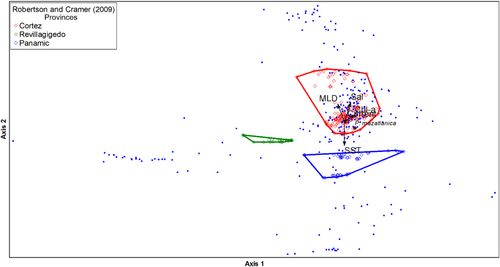
3.6 Canonical Correspondence Analysis (CCA)
The first two axes of the CCA ordination, based on Robertson and Cramer's (2009) biogeographical provinces as categorical variables, explained 7.6% of the accumulated variance in the epibenthic macroinvertebrate abundance in function of the environmental gradients (Table S6). Environmental variables for the CCA along maximized axis 2 showed a high negative correlation with sea surface temperature gradient and a positive but decreasing correlation of salinity, mixing layer depth, sea surface Chl-a concentration, and sea current speed (Table S6; Figure 7). The transects showed a clear latitudinal regionalization, showing the average multi-dimensional position of P. mazatlanica habitat based on each categorical variable considered in the five hypotheses. The most evident distinction of the macroinvertebrate community was obtained using the categorical biogeographic province criteria of Robertson and Cramer (2009), showing three statistically distinct, well-defined groups, where P. mazatlanica had a clear association with the Cortez province (Figure 7).
3.7 Linear Regression Models
Spatial linear regression models show a significant latitudinal trend of P. mazatlanica abundance in function of MLD (positive correlation) and sea surface salinity (negative correlation) (Table S8). 1998–2021 temporal linear regression models showed a marginally significant positive association of P. mazatlanica abundance in function of annual mean sea surface Chl-a concentration and significant negative correlations with mixing layer depth (positive correlation) and sea surface salinity (negative correlation) (Table S8).
4 Discussion
Pinctada mazatlanica has a broad distribution pattern in the coastal and insular habitats of the Mexican Pacific. P. mazatlanica population abundance increased between the Loreto and La Paz region and decreased between La Ventana and Cabo San Lucas during the last two decades, even though the region had prolonged anomalously warm events during the 2003–2016 period (Hakspiel-Segura et al. 2022; Ventura-Domínguez et al. 2022). A test for the impact of demographic history on the genetic diversity of P. mazatlanica specimens collected in 1997–1998 was congruent with a then-recent decline of population sizes in eight sites in Mexico and one site in Panama (Arnaud et al. 2000). We suggest that the population size increased since then, at least in the region of the peninsular coast of the Gulf of California, where the temporal sampling effort was robust. Arnaud et al. (2000) showed that genetic differentiation followed a scheme of isolation by distance, with low levels of differentiation at the scales of 10–100 km and stronger and significant genetic structure detected at a larger geographical scale distinguishing three populations: Northern Mexico, Southern Mexico, and Panama.
4.1 Dominance Within the Epibenthic Macroinvertebrate Fauna
We demonstrated that P. mazatlanica was the most abundant macromollusk inhabiting the rocky reefs of the Mexican Pacific. In a previous study that reported 32 macroinvertebrate species at Isla Espíritu Santo (Bahía de La Paz), P. mazatlanica was the 4th most dominant species, surpassed only by Tripneustes depressus A. Agassiz, 1863, Toxopneustes roseus (A. Agassiz, 1863), and Phataria unifascialis (Gray, 1840) (González-Medina et al. 2006). If we constrain the same taxonomic groups evaluated in our study, P. mazatlanica would also be in the 4th place, surpassed only by Eucidaris thouarsii (L. Agassiz and Desor, 1846), P. unifascialis, and Diadema mexicanum A. Agassiz, 1863 (Table S1). This suggests some regional variation among the most dominant macroinvertebrate species, but P. mazatlanica's relative abundance has been stable in the macroinvertebrate community structure.
The macroinvertebrate community structure in rocky reefs showed a clear latitudinal regionalization in the Gulf of California, with two distinct epibenthic macroinvertebrate species assemblages. The southern Gulf of California contains oligotrophic tropical conditions that favor a high abundance of stony corals (Pocillopora hexacorals), which is a habitat where the P. mazatlanica population has low densities and biomass (Halfar et al. 2005; Ulate et al. 2016). In the central region of the Gulf of California prevail subtropical conditions, prompted by weak summer upwelling conditions driven by southeast winds (Lavín and Marinone 2003) with relatively higher phytoplankton productivity, which favors shallow water suspensivorous organisms like octocorals and hexacorals in rocky reefs where the population of P. mazatlanica attains the highest population densities recorded in present and previous studies (Ulate et al. 2016) (Figure 1A). The high frequency of appearance and abundance of P. mazatlanica between Bahía de Loreto National Park and Cabo Pulmo National Park is a result of at least three combining effects. First, this is the region with the highest ProMARES sampling effort done in our study (74% of the total linear transects) (Table 1); thus, the probability of detection is high. Second, this region has suitable rocky reef habitats with tropical–subtropical environmental characteristics and a relatively low human population (Ulate et al. 2016). Third, three national parks operate in this region (Parque Nacional Bahía de Loreto, Parque Nacional Archipiélago de Espíritu Santo, and Parque Nacional Cabo Pulmo) where partial or full protection of P. mazatlanica is more likely to be enforced efficiently and effectively.
Paradoxically P. mazatlanica population declined in Parque Nacional Cabo Pulmo (a no-take park and the most successful in Mexico) located south of the Baja California peninsula, and the population increased in the other two multi-use national parks with enforced partial protection (Figures 1 and 6). P. mazatlanica's population abundance in multi-use Cabo San Lucas National Park has been low since 1998, likely because environmental conditions here are naturally unfavorable (high temperature) and mostly due to summer storm–hurricane perturbations to the epibenthic community. For example, in 2014, during hurricane Odile (Category 4, September 10–14, 2014), breaking waves reached the top of Cabo San Lucas Arch (61 m height), clearing most of the epibenthic macroinvertebrate fauna (Carlos Sánchez, personal observations). Those perturbations cause rapid episodic changes in macroinvertebrate community structure, decreasing and reshaping local biodiversity.
4.2 Population Size Structure and Growth Curves
Previous studies reported a maximum P. mazatlanica DVL of 14 to 18.7 cm (Table S7) (Wright, Holguin-Quiñones, et al. 2009). The maximum size recorded in our study was 30 cm DVL (11 individuals) recorded between the San Basilio and Los Cabos region. Although DVL frequency distribution curves of P. mazatlanica showed a normal distribution in most of the latitudinally surveyed regions (Figure 3), this does not imply that this represents the actual population size–frequency structure. ProMARES surveys can potentially have a bias in the detection of juveniles (recruitment) of several epibenthic species, including P. mazatlanica. Censuses were carried out over the rocky habitat trying to observe conspicuous macroinvertebrates settled between crevices, but without altering the ecosystem. The pediveliger last planktonic larva stage of P. mazatlanica (15–30 d old) settle on the seafloor as juveniles between narrow crevices and under the rocks sheltered away from their predators or inhabit different habitats passed unnoticed by survey divers. A similar distribution pattern of size structure of P. mazatlanica was also observed in previous studies, even in those that used different sampling methodologies (Monteforte and Cariño 1992; Solano-López et al. 1995, 1997; Monteforte and Morales-Mulia 2000; Wright, Holguín-Quiñones and Arreguín-Sánchez 2009). P. mazatlanica showed two regionally distinct population growth patterns: populations with slow-growth curves (K < 0.50) at Corredor, La Ventana, Cabo Pulmo, and Los Cabos regions and populations with fast growth curves (K > 1.8) like those observed between Loreto and La Paz. This latitudinal variability previously reported that the fast growth curve of P. margaritifera may be related to a predation condition and/or a high phytoplankton productivity near the coast (Pouvreau et al. 2000).
4.3 Time Series of Population Abundance and Biomass
Our estimations of P. mazatlanica abundance (ind/m2) and biomass (gr/m2) cannot be extrapolated to larger areas (i.e., ha) because we do not have precise and explicit spatial information about the type of seafloor habitat in the Mexican Pacific (Aburto-Oropeza and Balart 2002; Ramírez-Zúñiga et al. 2024). Any attempt to extrapolate abundance to larger areas can easily result in overestimating the erroneous assignation of P. mazatlanica's available habitat, as has likely occurred in several previous studies (Table S7). P. mazatlanica distributes in shallow waters (< 30 m depth), covering a narrow habitat along the coast often associated with rocky cliffs on land, which are highly vulnerable to illegal catch by tourists and outlaw fishermen.
Estimated abundances reported in previous studies provide a reference to population variability through space and time. The estimated mean abundance of P. mazatlanica was 0.15 ind./m2 in Ensenada de La Paz (Villamar 1965), 0.5 ind./m2 (maximum abundance 1.2 ind./m2) in La Ventana (Monteforte and Cariño 1992), and 0.03 ind./m2 between Loreto and La Paz (1997–1999) (Wright, Holguin-Quiñones, et al. 2009). The highest abundance (0.13 ind./m2) of small individuals (< 4 cm) was in Ensenada de La Paz. González-Medina et al. (2006) surveyed a large area recording quite low population densities, perhaps because they did their censuses over several types of habitats at Bahía de La Paz. Our regional mean abundances typically ranged between 0.04 ind./m2 and 0.08 ind./m2, with the highest abundances recorded up to 1.6 ind./m2 (Table S7). Thus, after several decades, there has been no significant change in the mean magnitude of population abundance, providing reasonable doubts if the increases of population abundance recorded between Loreto and La Ventana during our study are ecologically relevant population increases for P. mazatlanica in the Gulf of California. Although P. mazatlanica is currently a protected species, illegal extraction still exists for this species (Flores-Garza et al. 2012). Climatic and latitudinal regional environmental conditions contribute to the latitudinal declines in population densities, but overall low densities and narrow DVL range occur over the southern Mexican Pacific, where ecosystem health is more compromised for all macroinvertebrate communities. This study provides observational evidence that although P. mazatlanica has been shown to have positive population growth in the Gulf of California, population growth has not been observed in all regions of its distribution ranges. Caution must be taken to modify the present protection status of this culturally iconic macromollusk species.
P. mazatlanica has a wide tropical–subtropical distribution. However, the highest densities were observed above the transition zone of tropical-subtropical waters (peninsular coast of the Gulf of California). The population of P. mazatlanica showed a notable population increase in the central region of the peninsular coast of the Gulf of California. This was despite this region experiencing prolonged anomalous warmth with anomalously low sea surface Chl-a concentration conditions (particularly during 2013–2016) due to the combined influence of several marine heatwaves in 2013–2014 and El Niño 2015–2016 event (Favoretto et al. 2022; Hakspiel-Segura et al. 2022; Ventura-Domínguez et al. 2022). The population status of P. mazatlanica is still uncertain in the southern region of the Mexican Pacific due to the low sampling effort from these regions. In this context, the observed population size increase of Pinctada mazatlanica could be attributed to biological or ecological resilience mechanisms. The high reproductive rates of the species and positive population growth rates, along with its capacity to colonize diverse benthic habitats are relevant adaptations to respond to recent anomalous warm conditions (Le Moullac et al. 2016). Alternatively, stability could indicate ecological interactions, such as decreased natural predators or increased trophic resources associated with ecosystem shifts and most importantly an illegal status of fishing commercialization. These potential causes require further investigation to fully understand the factors contributing to the persistence of this species in the Mexican Pacific coast. The increase in P. mazatlanica population abundance in our study contrasts with trends observed in other regions facing similar environmental conditions. For instance, P. mazatlanica populations in the Las Perlas Archipelago and Coiba, Panama, have not recovered since historical collapses due to extensive harvesting, showing densities significantly lower than those in La Paz, Mexico, where densities can reach up to 12,000 individuals per hectare (Cipriani et al. 2008). This suggests that local management practices and environmental resilience may differ, influencing population dynamics across regions. Our results imply that P. mazatlanica is likely recovering after decades of full law protection (at least along the west coast of the Gulf of California). The present protection status must be evaluated with the Species Risk Assessment Method (Método de Evaluación de Riesgo, MER in Spanish), which is the official legal tool to assess species protection status in Mexico. The MER of the Mexican Standard NOM-059-SEMARNAT-2010 (2010) determines the risk category of a species based on scores of four criteria and places a species as “Extinct in the wild, subject to special protection, threatened, or in danger of extinction”. However, other international conservation evaluations can also be used to determine the population risk status (CEP IUCN 2019). The results of the present study emphasize the strategic relevance of long-term monitoring surveys to understand the population dynamics of wild marine species in the context of climate change. Our entire benthic assemblage study to infer status of target protected species approach provides detailed information on the health of local populations and allows for the generation of knowledge that can be extrapolated to similar protected benthic species of other regions of the world. The establishing standardized and collaborative monitoring networks of the entire macroinvertebrate community across countries for tropical and subtropical latitudes can help to infer if those ecosystems are especially vulnerable to anomalous warming events and other environmental changes like ocean acidification and deoxygenation associated with mean increase of SST (Keeling et al. 2010). These initiatives would facilitate a more complete understanding of biological and ecological responses to climate pressures, strengthening the management and conservation of marine resources worldwide.
5 Conclusion
Our study is the first geographically extensive and prolonged (1998–2021) monitoring survey that evaluates the status of the wild population of P. mazatlanica along the Mexican Pacific. This survey helps to assess the long-term efficiency of Mexican conservation efforts for this culturally relevant species. This study provides an integrative perspective on how P. mazatlanica is one of the most abundant, numerically dominant, and widely distributed species of macromollusk in the entire rocky reef in the Mexican Pacific. We conclude that P. mazatlanica shows signs of population recovery along the peninsular coast of the Gulf of California (Loreto to La Ventana regions). The southern Gulf of California (with high sampling effort) and the Central and southern Mexican Pacific region (with low sampling effort) had low P. mazatlanica population densities. We propose that the population in the Gulf of California (but not in the southern Mexican Pacific) shows sufficient recovery to be considered out of risk of extinction.
Author Contributions
A.B.-G., J.G.-G., and C.S. conceived the ideas and designed the methodology; A.B.-G., C.S., and E.G.-R. collected the data; A.B.-G. and J.G.-G. analyzed the data. A.B.-G. and J.G.-G. led the writing of the manuscript. L.H.-S. helped with writing and editing. All authors contributed critically to the manuscript and gave final approval for publication.
Acknowledgments
We deeply thank the entire Reef Fauna Program of the Universidad Autónoma de Baja California Sur, Mexico, monitoring team (students and volunteers) that has been monitoring the rocky reefs of the Mexican Pacific over the past two decades. We particularly thank Octavio Aburto-Oropeza from Scripps Institution of Oceanography and Centro para la Biodiversidad Marina y la Conservación for his long-term leadership. Ramiro de Jesus Arcos Aguilar for the drawings.
Ethics Statement
This study has the corresponding authorizations for SCUBA Diving monitoring census of macroinvertebrates by CONANP in Bahía de Loreto National Park, Archipelago Espiritu Santo National Park, Cabo Pulmo National Park, and Revillagigedo National Park, Mexico.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
This research utilized census data collected by Programa Fauna Arrecifal (UABCS) and publicly available upon reasonable request to the corresponding authors.




