La Niña-related coral death triggers biodiversity loss of associated communities in the Galápagos
Abstract
During a cold La Niña period (August 2007–January 2008) in the central Galápagos archipelago, 70% of Pocillopora branching corals were severely bleached across three long-term monitoring sites, affording an opportunity to examine its impact on the persistence of these corals and their associated community of fish and mobile macroinvertebrates. Using a time series empirical approach, we tagged and tracked the fate of 96 coral heads and their associates. When surveyed in July 2008, recovered live and dead corals that were previously severely bleached supported similar levels of species richness (randomized observed and estimated Chao 1). By contrast, richness on the surviving live corals remained fairly stable, while Chao 1 estimated richness on dead corals underwent a nearly 50% increase between July and January 2009, thereafter declining to 50% of originally surveyed richness by February 2010. This nonlinear change in species richness was largely due to an influx and decline in opportunistic generalists including pencil urchin bioeroders, gastropod snails, and hermit crabs that colonized dead corals and fed on sessile invertebrates and algae that had initially recruited to dead and undefended coral substrate. Thus, dead corals retained high overall species richness until live corals had recovered, after which richness declined as dead corals eroded and disintegrated (July 2011). Live corals attracted a less speciose but stable assemblage of mutualistic xanthid crabs and fishes that increased in abundance over time with the recovery and growth of live coral tissue. Overall, coral status (live/dead), planar area and maximum branch length predicted the number of species associated with each colony. The delayed diversity loss of associated species following La Niña disturbance to a foundation species represents a local extinction debt of 32–49-month duration. A better understanding of the scale of extinction debt in foundational marine ecosystems is needed to quantify the breadth of impacts of climate oscillations on biodiversity and ecosystem functioning.
1 INTRODUCTION
Habitat-forming foundation species such as trees, grasses, salt marshes, mangroves, kelp, seagrasses and corals have a disproportionately large influence on other species through provisioning of shelter or food (Dayton & Hessler, 1972), by enhancing associated species diversity (Witman, 1985) and by serving multiple ecosystem functions (Angelini et al., 2015; Ellison et al., 2005). Although foundation species and mutualisms can serve to buffer the effects of disturbances on natural communities (Altieri et al., 2007; Ellison et al., 2005; Witman, 1987), anthropogenic impacts are reducing their abundance and distribution of foundation species (Osland et al., 2013) and decreasing the diversity of associated flora and fauna (Byrnes et al., 2011; Sorte et al., 2017), thereby threatening community resilience and functioning (Chapin et al., 1997; De Boeck et al., 2018; Duffy et al., 2015). Yet, many aspects of whole-community changes associated with disturbance-driven losses of foundation species remain poorly understood, especially related to the timing, pattern, and magnitude of associated species loss (Stella, Munday, et al., 2011; Thomson et al., 2015). Importantly, changes in species abundance or diversity of communities associated with foundation species may not occur immediately after a disturbance, resulting in extinction debt, or a significant time delay prior to the disappearance or local extinction of a species from a particular habitat patch (Kuussaari et al., 2009; Tilman et al., 1994; Watts et al., 2020). Problematically, assessing post-disturbance community-wide biodiversity loss before extinction debt has been paid could lead to an underestimation of the number and types of associated species vulnerable to local extinction (Hanski & Ovaskainen, 2002; Watts et al., 2020).
Extinction debt occurs across diverse taxa associated with a range of foundation species, with variable consequences within and across ecosystems. Indeed, the probability and timing of local extinction differs across taxonomic groups, life history traits, and in the type of relationship between the associated species (i.e., habitat specialists, generalists) and the foundation species (Hylander & Ehrlén, 2013; Kuussaari et al., 2009; Watts et al., 2020). The time to species loss after disturbance also depends on the size of the focal habitat created by the foundation species and on the intensity of disturbance (Hylander & Ehrlén, 2013). However, as compared with plant-dominated terrestrial ecosystems, these and other aspects of extinction debt remain poorly understood for marine ecosystems (Kuussaari et al., 2009). Simulations in coral reefs (for communities of 40 coral species) suggest that extinction debt of associated invertebrates and fish was up to seven times higher relative to that of terrestrial forests (Tilman et al., 1994) for the same level of disturbance (Stone et al., 1996), potentially due to the high diversity of associated species of invertebrates and fish (Canizales-Flores et al., 2021; Idjadi & Edmunds, 2006; Stella, Munday, et al., 2011). Concerningly, given dramatic declines in coral reefs due to climate-change related ocean warming, acidification, and disease (Glynn et al., 2017; Hoegh-Guldberg & Bruno, 2010; Pandolfi et al., 2011; Wellington et al., 2001), extinction debt of coral-associated species could lead to underestimates of the pace and extent of marine biodiversity loss (Kuussaari et al., 2009).
Severe climate events can serve as natural field experiments for examining the effects of climate change-related disturbances on marine foundation species and associated species diversity (Byrnes et al., 2011; Sorte et al., 2017), which may also be used to better understand extinction debt in marine ecosystems. The El Niño Southern Oscillation (ENSO) is a global climate event characterized by anomalously warm (El Niño) or cold (La Niña) Pacific ocean temperatures (Holmgren et al., 2001; Trathan et al., 2007), which have increased in intensity and duration with climate change (Cai et al., 2015), contributing to declines in foundational kelps, seagrasses, and corals (Campbell et al., 2011; Dayton et al., 1992; Hughes et al., 2017). These climate-driven declines in foundation species serve as natural experiments to investigate extinction debt, as well as to assess the links among environmental stress, foundation species and diversity change.
The Galápagos Archipelago is a place of unique marine biodiversity that has been repeatedly subjected to extreme ENSO events, systematically reducing coral cover (Glynn et al., 2018) and likely threatening coral- and reef-associated species (Edgar et al., 2010). Sustained high temperatures during the ENSO warming phases of 1982–1983 and 1997–1998 resulted in widespread loss of foundational scleractinian corals (dominated by Pavona, Porites, and Pocillopora genera) across the archipelago, with mean mortality rates of 95%–99% and 26% (10%–75%) of individuals, respectively (Glynn et al., 2001; Glynn & Wellington, 1983). Heavy bioerosion of dead corals eliminated much of the reef matrix, replacing patchy coral reefs, which previously occupied 20%–100% mean coverage across sites and islands in the mid-1970's (Glynn & Wellington, 1983), with scattered coral heads occupying <5% cover in the 1980's (Romero-Torres et al., 2020). Additional ENSO-related cold and warm phases (1997–1998 El Niño, 2007 La Niña, 2010 El Niño) resulted in further stress-related coral bleaching and death (Glynn et al., 2017, 2018). These ENSO events have strongly shaped coral populations, which despite repeated stress, have regenerated and rebounded to 20%–30% mean total cover in 2014, representing slight declines in the Eastern Tropical Pacific and Galápagos subregion since 1970 (Romero-Torres et al., 2020). However, the stress responses and fate of coral populations has substantially differed across sites and islands across the archipelago (Fong et al., 2017) and among coral species (Glynn, 1994; Glynn et al., 2001), with recent surveys (2010–2019) highlighting distinct coral species composition and size structure across islands (Riegl et al., 2019).
ENSO-related warming events have had particularly detrimental effects on Pocillopora corals (family Pocilloporidae), which are the dominant habitat-forming stony corals at shallow depths, and the only branching corals in the central Galápagos Archipelago (Glynn, 1976). Although patchy in their distribution and abundance on Galápagos reefs, Pocillopora corals (hereafter also termed Pocilloporids, as per Glynn & Wellington, 1983), have been historically dominant at shallow depths, providing significant and unique habitat and food for obligate guard crabs (Canizales-Flores et al., 2021; Gotelli et al., 1985) and a diverse assemblage of facultative and opportunistic invertebrates and fishes (Abele, 1976; Glynn, 1984; Hickman, 1999; Holbrook et al., 2008). Many of these coral-associated species were negatively affected by ENSO-related declines in Pocillopora coral health and cover (Edgar et al., 2010; Glynn et al., 1985), as well as potentially by ENSO-related temperature anomalies (Glynn et al., 2018; Grove, 1985; Ruttenberg, 2000). Considering the significant impact of coral bleaching and/or death on these associated organisms (Booth & Beretta, 2002; Glynn, 1985; Glynn et al., 1985, 2014; Leray et al., 2012; Stella, Munday, et al., 2011), as well as the overall influence on reef ecosystems (Figueroa-Pico et al., 2021; Olivier et al., 2022), in comparison to the relatively weaker effects of temperature anomalies on the organisms themselves (Mora & Ospina, 2001, 2002; Urban, 1994), it is plausible that the primary impacts are linked to alterations in Pocilloporid habitat. However, the assessment of ENSO events' impacts on branching coral-associated community diversity and diversity-function relationships remains unexplored, as does the evidence regarding a potential whole-community extinction debt in the Galápagos.
In this study, we aimed to investigate the impacts of a coral bleaching event in the Galápagos Islands caused by a cold-water anomaly during the 2007–2008 La Niña phenomenon on Pocilloporid corals and their associated communities of mobile macroinvertebrates and fishes. Our objective was to assess the indirect effects of the 2007–2008 La Niña event on the composition and diversity of coral-associated invertebrates and fishes by comparing the communities on corals that died as a result of the bleaching event with those on live, recovered corals. We employed a time series approach also used in prior studies to examine potential extinction debt (Kuussaari et al., 2009; Ridding et al., 2021), thereby collecting data post-bleaching disturbance in July 2008, January 2009, July 2009, and February 2010.
We addressed the following questions: (1) How did a La Niña-related cold-water anomaly and associated coral bleaching alter the availability and quality of Pocilloporid habitats? (2) Did coral bleaching reduce associated species richness?, and (3) if so, was there a time lag in associated species loss and diversity changes (extinction debt) in the communities inhabiting corals? (4) Post-bleaching, did the community structure of associated invertebrates and fishes (species abundance, composition) differ between dead versus live (recovered) Pocilloporids? Finally, (5) what attributes of the live and dead foundational Pocilloporid habitats predicted the species richness of the associated community? Here we use the term extinction to refer to local extinction, which is the disappearance of a species from a habitat patch (Kuussaari et al., 2009; Watts et al., 2020), and not as a reference to regional or global species' extinctions.
2 METHODS
The extent of coral bleaching was first observed in January 2008, and the general fate of the Pocillopora corals was tracked over a 49-month period, starting in July 2008 and ending in July 2011 (Figure S1). This period of time included two additional ENSO-related temperature events: a 2008–2009 La Niña and a 2009–2010 El Niño (NOAA National Weather Service Climate Prediction Center: https://origin.cpc.ncep.noaa.gov/products/analysis_monitoring/ensostuff/ONI_v5.php).
Subtidal temperatures were recorded at 10 min intervals during the 2008–2009 La Niña period by Onset Tidbit™ loggers placed at 6 and 15 m depth at two of the three study sites: Baltra (00 24.705 S, 090 16.482 W) and Guy Fawkes (00 29.964 S, 090 30.773 W). No temperature data is available for Rocas Beagle (00 24.797 S, 90 37.685 W), due to logger malfunctioning at this site. However, temperatures were also recorded (to the accuracy of ±0.21°C) at 15 m depth at an additional reference site, Cuatro Hermanos (00 84.787 S, 090 74.908 W), located 40 km southwest of Guy Fawkes to provide insight into the spatial scale of the cold-water event. Bleaching of Pocillopora corals during cold water events has been recorded at temperature thresholds of 19°C in the Gulf of California (Paz-García et al., 2012), while temperatures of 18.5–19°C stressed P. verrucosa in lab experiments (Rodríguez-Troncoso et al., 2013). Sustained cold water temperatures well-below reported thresholds of 16°C (11 and 12°C, respectively) resulted in widespread bleaching and mortality of many scleractinian coral species along the Florida reef tract (Kemp et al., 2011; Lirman et al., 2011). We thus considered the 2007–2008 Galápagos records for the amount of time (i.e., total time duration in hours and days) that temperatures were less than or equal to one or both of two cold-water thresholds identified in the literature (16 and 18°C), which serve as conservative indicators of the low temperature stress to Pocillopora colonies (Table 1). In addition to our temperature loggers, we also obtained information regarding the cold-water plume (Figure S2) from the NOAA Coral Reef Watch Website (National Satellite and Information Service Center: https://coralreefwatch.noaa.gov/product/5km/index_5km_composite.php).
| A | |||||
|---|---|---|---|---|---|
| Site | Depth (m) | Mean | Min | Max | Median |
| Baltra | 6 | 20.9 | 16.3 | 24.7 | 21.2 |
| 15 | 20.4 | 15.3 | 24.7 | 20.7 | |
| Guy Fawkes | 6 | 20.2 | 15.8 | 23.6 | 20.3 |
| 15 | 19.6 | 13.9 | 23.4 | 19.8 | |
| Cuatro Hermanos | 15 | 18.9 | 14.0 | 22.5 | 19.0 |
| B | |||||
|---|---|---|---|---|---|
| Site | Depth (m) | Hours ≤16°C | Days ≤16°C | Hours ≤18°C | Days ≤18°C |
| Baltra | 6 | NA | NA | 151.5 | 6.3 |
| 15 | 14.1 | 0.6 | 451.8 | 18.8 | |
| Guy Fawkes | 6 | 0.7 | NA | 237.3 | 9.8 |
| 15 | 54.6 | 2.3 | 825.3 | 34.3 | |
| Cuatro Hermanos | 15 | 127.3 | 5.3 | 1428.1 | 59.5 |
In January 2008, as part of our bi-annual biodiversity monitoring surveys, we observed that the branching (Pocilloporidae) and massive (Poritidae, Pavonidae) corals across our survey sites in the central Galápagos Archipelago were severely bleached. Following our regular survey design (150 m2 surveys) we recorded densities of Pocillopora corals at depths of 6–11 m. In July 2008, when we returned to monitor corals at three of these survey sites (Baltra, Guy Fawkes, and Rocas Beagle) we observed that the corals had recovered from severe bleaching (≥50% live tissue, with the majority of individuals with 100% live tissue) or died (<50% live tissue, with the majority of individuals with 0% live tissue) and were colonized by sessile invertebrates and algae. Based on these observations, we haphazardly selected and tagged dead and live coral colonies with numbered plastic labels at 4–15 m depth across areas of approximately 600 m2 at these sites. Tagged corals consisted of five known morphospecies/morphotypes, including Pocillopora verrucosa (n = 18 live, 18 = dead), Pocillopora capitata (n = 7 live, n = 15 dead), Pocillopora damicornis (n = 4 live, n = 9 dead), Pocillopora eydouxi (n = 1 live, n = 7 dead), and Pocillopora meandrina (n = 0 live, n = 4 dead). Due to the challenges associated with identifying the species of many dead corals, as their calcium carbonate structure had deteriorated or was obscured by invertebrate colonization, a significant number of them (n = 24 dead, n = 1 live) lacked a recognizable morphospecies/morphotype. Given these limitations, and also due to the imbalanced design of our surveys, we opted for aggregating individual colonies at the Genus level (Pocillopora). In addition, it is important to note that all these morphotypes represent only two distinct species (Type 1 and 3) based on molecular analyses conducted by (Pinzón & LaJeunesse, 2011). In total, we tracked 96 discrete Pocillopora coral heads across all time-series surveys at the three sites (Baltra n = 29, Guy Fawkes n = 31, and Rocas Beagle n = 36).
We surveyed the fish and mobile macroinvertebrates (1.0 cm total body length or larger) associated with the tagged corals in July 2008, January 2009, July 2009, and February 2010. A diver slowly approached each tagged coral and first (at a short distance) identified and counted all visible fishes sheltering within, swimming in and out of, or hovering over the coral head. We only counted fishes within 10 cm of coral branches, with the exception of juvenile damselfish, which were included in fish counts but were often slightly further from coral heads. The diver then approached the coral more closely and identified all cryptic fishes (e.g., blennies and gobies) and mobile macroinvertebrates (e.g., crustaceans, echinoderms, and gastropods). One observer conducted all the in situ counts to maintain the consistency of methodology and species identifications. Gastropods were identified with the assistance of local experts at the Charles Darwin Research Station. We did not census mobile invertebrates smaller than 1.0 cm total body length because it was hard for the diver to visually identify organisms <1 cm with the naked eye (the same size cut-off was used by [González-Gómez et al., 2018]), nor did we survey sessile invertebrates or algae that recruited onto the dead corals, because our focus was on the mobile associated macrofauna. Diver-based visual methods of surveying coral-associated fishes and invertebrates are the most common technique for sampling reef fish (Kulbicki et al., 2010) and the most efficient method for sampling visible coral-associated taxa (Knowlton et al., 2010), especially for macro-invertebrates of at least 1.0 cm in size (González-Gómez et al., 2018). Although visual methods may underestimate reef-associated richness relative to destructive methods (Beisiegel et al., 2017; Willis, 2001), these methods were necessary both for repeated sampling of coral-associated species on individual coral heads (Beisiegel et al., 2017), and for ethical considerations given the ecological importance, sensitivity, and increasing rarity of these habitats (Gotelli et al., 1985) in the Galápagos Marine Reserve.
The planar area of each tagged coral head (which is known to scale with coral surface area and volume [House et al., 2018]) was measured as the 2D surface area from a digitized overhead photograph of the colony. Photographs were taken at a fixed distance from the substrate by using a rigid rectangular frame placed on the substrate, following methods outlined in (Edmunds & Elahi, 2007). Photographs were taken on slide film with a quadrapod camera framer (Witman, 1985) designed to hold a Nikonos camera, a 15 mm lens corrected for barrel distortion and two strobes, with photographs taken perpendicular to the substrate to obtain a standard overhead view in a 0.25 m2 photo quadrat. Images were later analyzed using Image J to obtain the total planar area of each live or dead coral, following similar methods to those outlined in (Hoogenboom et al., 2017). We also surveyed the maximum branch length per coral head. To minimize disturbance to the fish and potential displacement from corals caused by photography equipment and additional SCUBA divers, surveys of the associated communities were conducted separately from photographing the coral planar area and during a different dive. Additionally, to prevent diurnal differences in the composition of associated communities from confounding the results, the surveys were conducted at midday. This timing helped to isolate the variation in associated composition or abundance over time rather than being influenced by natural diurnal shifts. To avoid recounting fish that might have moved between adjacent coral habitats, the surveys were conducted on a single day at each time point, allowing for a more accurate assessment of the associated communities' composition. During the initial bleaching survey in January 2008, and on the four subsequent sampling events (July 2008–February 2010), we recorded the status of tagged corals as live if they had at least 50% cover of live tissue. For dead corals, we recorded if they had eroded, broken apart into rubble, or completely disappeared. A final survey was conducted in July 2011 at all three sites by JD. Witman to only determine the persistence of the tagged, dead corals (note that none of the live corals were surveyed at this last time point).
2.1 Statistical analyses
We compared the richness of coral-associated macrofauna on live and dead corals over time, first of all surveyed macrofauna, as well as for fishes and mobile macroinvertebrates, separately. We calculated randomized observed species richness (‘observed species richness’) of the community associated with coral heads by constructing randomized Species Accumulation Curves (SACs), calculating the mean SAC and its standard deviation from random permutations of the data, or subsampling without replacement (Gotelli & Colwell, 2001). We also calculated Chao 1 estimated species richness (‘Chao 1 estimated richness’) for the entire community, fishes and mobile invertebrates, of coral heads using the Chao 1 estimator, a non-parametric species estimator for abundance data (Chao et al., 2009), including 95% confidence intervals based on the actual Chao estimator (Colwell & Elsensohn, 2014). Statistical differences were deemed to be significant when the 95% confidence intervals of the means of the different groups of comparisons did not overlap (Ramsey & Schafer, 2012).
We also compared the composition of coral-associated macrofauna on live and dead corals over time by performing a principal components analysis (PCA) using raw abundances of coral-associated macrofauna as the response variables and treating each individual coral head in each survey as an individual sample. The PCA algorithm used unweighted singular value decomposition on values fitted with unweighted linear regression of chi-transformed data (Legendre & Legendre, 2012). We then calculated Euclidean dissimilarities in composition using multiscale bootstrapped resampling.
Finally, we assessed the attributes that predicted associated richness by constructing linear mixed effects models of associated richness per coral head, first for all macrofauna, and then for fishes and mobile macroinvertebrates, separately. Coral vital status (live/dead), planar area, maximum branch length, time (series of sampling events), and the interaction between coral status and planar area, vital status and branch length, and vital status and time were treated as fixed effects, and individual coral heads and sites were included in the model as random intercepts. In the analysis, several factors were considered as fixed effects, including the vital status of the corals (live/dead), planar area, maximum branch length, and time (represented by a series of sampling events). The interactions between coral status and planar area, vital status and branch length, and vital status and time were also included as fixed effects in the model. To account for the potential variation between individual coral heads and sites, random intercepts were incorporated into the model. By treating these factors as fixed effects and including random intercepts, we aimed to capture the influence of specific variables while accounting for the inherent variability across individual coral heads and sites.
All statistical analyses were conducted using R software (version 3.6.2, R Core Team, 2021), including various packages. We ran mixed effects models using the lme4 package (Bates et al., 2015), enabling us to account for random effects within the data. To examine species accumulation curves and conduct principal components analysis, we utilized the vegan package (Oksanen et al., 2020). Additionally, we calculated Chao 1 total estimated richness using the SpadeR package (Chao & Chiu, 2016).
3 RESULTS
3.1 Temperature
Minimum temperatures at two of the study sites ranged from 13.9°C at Guy Fawkes (at 15 m) to 16.3°C (at 6 m) depth at Baltra, and were similarly low (14°C) at the Cuatro Hermanos reference site (Table 1A, Figure 1). Temperatures were at or below the threshold of 18°C for Pocillopora bleaching for 6.3 to 34.3 days at Baltra (6 m) and Guy Fawkes (15 m), respectively (Table 1B). At Guy Fawkes, the subtidal environments experienced the colder bleaching threshold of 16°C or lower for less than 1 h at 6 m, but this time period increased to 54.6 h at 15 m depth. Temperatures were colder at the Cuatro Hermanos reference site, where they were 18°C or lower for 59.5 days and 16°C or lower for 5.3 days (Table 1B).
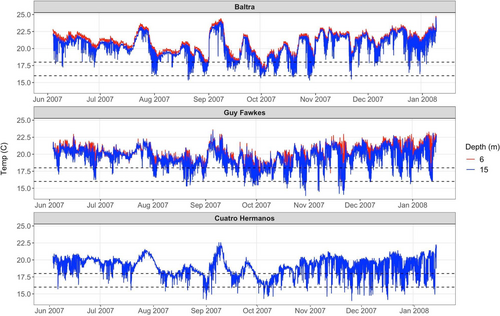
Satellite images of minimum sea surface temperatures (SST) showed temperatures of 15–20°C in August–October 2007 (Figure S2), in general matching the ranges of our logger data (Table 1). They also depicted a cold-water plume moving northwest along the South American Coast and deflecting offshore to reach our study area in the central Galápagos. We recorded substantial temperature declines at 6 and 15 m depth during this same time period (Figure 1). By January 2008, the minimum SST had increased to 20–25°C (Figure S2).
3.2 Effects of ENSO-related cold-water anomalies on Pocillopora corals
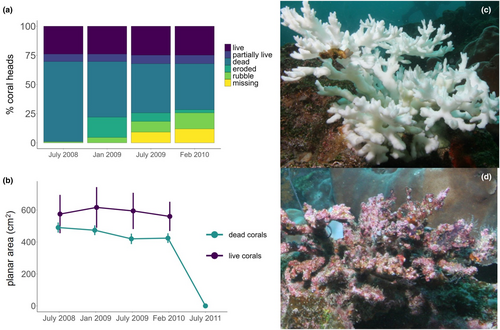
In January 2008, we observed that Pocilloporids occurred at average densities of 0.04/m2 (0.017 SD), 0.10/m2 (0.08 SD) and 0.04/m2 (0.04 SD) at depths of 6–11 m at Baltra, Rocas Beagle and Guy Fawkes, respectively (n = 3, 150 m2 plots per site), with dense aggregations of up to 3.7 Pocillopora heads per m2 at 5 m depth at Rocas Beagle (anecdotal observations, J. Witman). At this time, we also observed that all Pocillopora corals (as well as massive corals of the Genus Pavona and Porites) were severely bleached. Although we did not record the extent of bleaching using color cards, visual observations of corals photographed during this survey suggest that they exhibited the lowest level of saturation and highest level of brightness (Figure 2c), reflecting severely low symbiont density and chlorophyll ⍺ content (Siebeck et al., 2006). After the 2007–2008 La Niña bleaching, we found that a minority (24%) of bleached Pocilloporids corals recovered their pigmentation and survived, while most died and eroded (Figure 2). When surveyed 6–13 months post-bleaching in July 2008 (Figure 2a), we found that colonies that were previously severely bleached, only 24% (n = 26) had fully recovered their color (highest level of saturation and lowest level of brightness), 6% had partially recovered live tissue, but experienced at least 25%–75% tissue loss, and 69% (n = 75) of all bleached coral colonies (as in Figure 2c) had died and were colonized by mobile and sessile invertebrates, fish and algae (as in Figure 2d; also see Figure S3 for overhead photographs of live and dead individuals of each morphospecies, and Table S1 for additional information including planar area, live planar area, branch length, and water depth of surveyed corals). Subsequent monitoring between July 2008–February 2010 revealed that 3% (n = 3) of corals had visibly eroded, 14% (n = 15) had detached from the substrate and broken into coral rubble, and 12% (n = 13) had completely disappeared, with their fragments likely washed away by currents (Figure 2a). Over this survey period, live corals (560–616 cm2) had not lost planar area, whereas dead corals (419–474 cm2) had lost an average of 13% planar area and were on average 24% smaller than live corals (Figure 2b). All Pocillopora corals that had initially bleached and died at the beginning of the study in January 2008 had disintegrated by July 2011 (Figure 2b). Over the survey period, the live corals did not bleach or deteriorate subsequent to the 2007–2008 La Niña (J. Witman and O. Rhoades, pers. obs.). For subsequent analysis, we separated these corals into two basic categories: live (≥50% live tissue, with the majority of individuals with 100% live tissue) and dead (<50% tissue, with the majority of individuals with 0% live tissue).
3.3 Post-bleaching shifts in coral-associated species richness
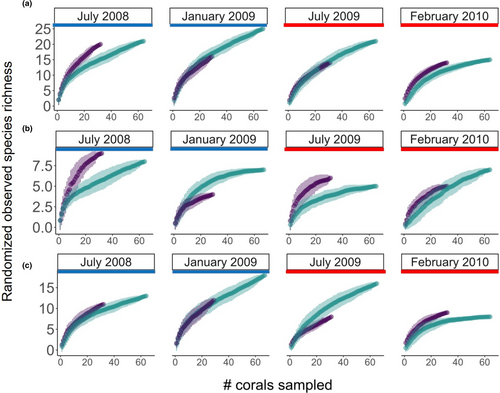
Randomized observed species richness (Figure 3) and Chao 1 estimated species richness (Figure 4) of associated species varied significantly across certain time points and/or between live and dead corals. In July 2008, which was 6–13 months post-bleaching and within the 2007–2008 La Niña period, live and dead corals supported similar observed (20–21 species) and Chao 1 estimated (40–41 species) total species richness (Figures 3a and 4a). However, observed total species richness (and observed invertebrate richness) on dead corals increased to 25 species in January 2009 relative to July 2008 (including seven fishes and 19 macroinvertebrate species), and then it declined thereafter until February 2010 (Figure 3a). By contrast, observed total species richness (and observed fish richness) on live corals was initially higher in July 2008 (and higher than on dead corals in July 2008) and comprising of 20 species (including 9 fishes and 11 macroinvertebrate species), decreased in January 2009, and (for total observed richness) remained stable through February 2010 (Figure 3a,b).
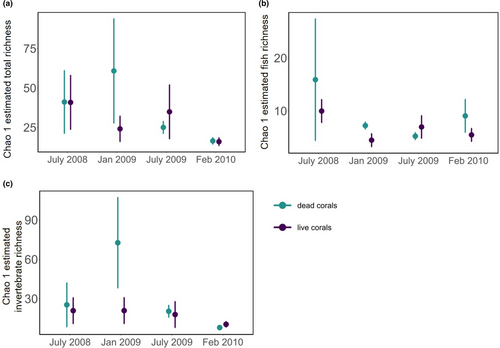
Across all survey dates, Chao 1 mean estimated total richness was 16–41 and 16–61 total species (Figure 4a), 4–10 and 5–16 fish species (Figure 4b), and 10–21 and 8–73 invertebrate species (Figure 4c) associated with live and dead corals, respectively. Similar to observed species richness, between July 2008 and January 2009, Chao 1 estimated total species richness and invertebrate species richness on dead corals underwent a nearly 50% increase, from 41 to 61 total species and from 25 to 73 invertebrate species, then declined significantly to 25 total and 20 invertebrate species in July 2009, and ultimately to 16 total and eight invertebrate species in February 2010 (Figure 4a,c). By contrast, Chao 1 estimated total and invertebrate species on live corals did not significantly differ across most time points, and fluctuated from 41 to 24 to 35 total species and from 21 to 21 to 18 invertebrate species from July 2008 to 2009, but it significantly declined to 16 total and 10 invertebrate species in February 2010 (Figure 4a,c). By contrast, Chao 1 estimated fish richness declined over time on dead corals (from 16 to 7 to 5 to 9 species) and live corals (from 10 to 4 to 7 to 5 species), and in July 2008 it was significantly higher than in any other time periods (Figure 4b).
3.4 Differences in coral-associated species on live and dead corals
Principal component analysis (PCA) of the entire community of fish and mobile macroinvertebrates associated with Pocillopora corals indicated that the overall species composition of the communities differed significantly between live and dead corals (Figure 5a, see Figure S4 for explanation of PCA species scores). Species composition also differed among certain time points, especially between the first time point (July 2008) relative to other time points for live corals and dead corals, and between the last time point (February 2010) relative to other time points for live corals (Figure 5A). Live corals were characterized by high PC1, which increased across time points, and was strongly correlated with the presence of mutualistic xanthid crabs Trapezia ferruginea and Trapezia digitalis (Figure S4a). Dead corals were characterized by lower values of PC1, which was strongly correlated with the presence of the pencil urchin Eucidaris galapagensis (Figure S4a). Relative to the latter points, earlier time points were characterized by lower PC2 values, which were strongly correlated with the presence of the pencil urchin and juveniles of the ring-tailed damselfish Stegastes beebei, as well as a speciose assemblage of opportunistic snails (Figure S4b).
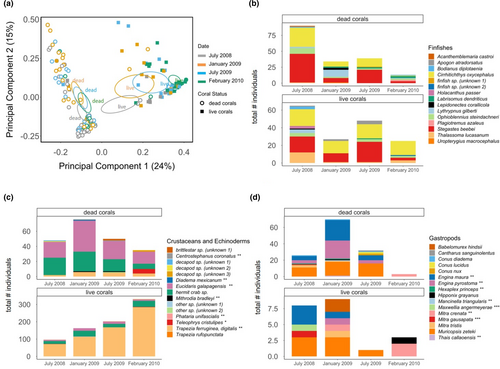
Live and dead corals exhibited strong overlap in fish assemblages (Figure 5b), but particular differences in the community of associated invertebrates (Figure 5c,d). With respect to coral-associated fishes, live and dead corals were dominated by two territorial and residential fish species: the coral hawkfish Cirrhitichthys oxycephalus and juveniles of the ring-tailed damselfish Stegastes beebei; though the abundance of these species varied across time points, and ultimately declined by 65% and 90%, respectively (particularly on dead corals) between July 2008 and February 2010 (Figure 5b). Other common species at certain time points (e.g., the large-banded fanged blenny Ophioblennius steindachneri in July 2008 and the Galápagos blue-banded goby Lythrypnus gilberti in January 2009 on dead corals and the Cortez rainbow wrasse Thalassoma lucasanum in July 2008 on live corals) also exhibited high variability in abundance across time points (Figure 5b).
With regards to coral-associated invertebrates, dead corals hosted opportunistic and predatory macroinvertebrate species, dominated by the pencil urchin Eucidaris galapagensis, hermit crabs (Figure 5c), and a rich assemblage of 16 species of gastropods, especially two species of the Genus Engina (E. maura and E. pyrostoma) as well as Muricopsis zeteki, which increased between July 2008 and January 2009 (Figure 5d). The total (cumulative) abundance of pencil urchins surveyed collectively across dead corals increased initially by 100% (from 21 to 42 individuals) between July 2008 and January 2009, and then declined by 62% between January 2009 and February 2010 (Figure 5c). Pencil urchins were observed feeding directly on the calcium carbonate structure of dead and occasionally bleached live coral tissue (urchins are known bioeroders, grazing heavily on basal branches of Pocillopora [Glynn et al., 1979]), while gastropods and hermit crabs were frequently observed feeding on the sessile invertebrates (barnacles, sponges, ascidians, bryozoans) that had colonized dead coral skeletons (O.K. Rhoades, pers. obs.). By contrast, the cumulative abundance of coral-associated invertebrates surveyed collectively across live Pocilloporids included hundreds of mutualistic xanthid crabs (Trapezia spp. including Trapezia ferruginea and Trapezia digitalis) (Figure 5c). The cumulative abundance of mutualistic crabs on live corals increased by 300% (from 71 to 284 individuals) between July 2008 and February 2010 (Figure 5c). Gastropods were considerably (10 times) more abundant on the collective assemblage of dead corals relative to live corals (Figure 5d).
3.5 Relationships between coral habitat attributes and associated species richness
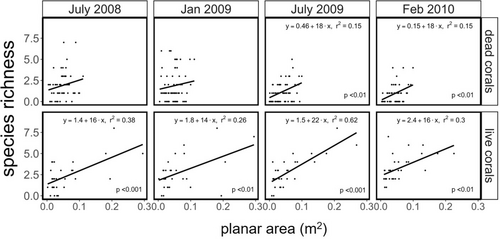
Three physical attributes of Pocilloporid habitat structure: coral status (live/dead) (p < .0001), planar area (p = .0004) and maximum coral branch length (p =< .0001), and the interaction between coral status and time (p < .0001) predicted the number of species associated with the corals (Table 2). ENSO phase (cool versus warm), the predictor associated with recurring ENSO events, was not a significant predictor of species richness, and the best fit model using AIC and BIC criteria excluded this predictor, but included time and the interaction between time and vital status (Table S2). Ordinary Least Squares (OLS) regression analysis indicated that total species richness was positively and linearly related to the planar area of the coral colony, although the significance and strength of this relationship varied between live and dead corals and across time points (Figure 6). Species-area relationships were significant for live corals across all time points (Figure 6, bottom panel), whereas they were only significant for dead corals for the two latter time points (Figure 6, top panel). Planar area also explained a greater proportion of total variation in species richness for live versus dead corals (r2 = 0.26 to 0.62 versus r2 = 0.15, respectively, Figure 6). The richness of coral-associated macrofauna increased by 14–22 species/m2 planar area for live corals across time points, and by 18 species/m2 planar area for dead corals (Figure 6). Tighter species-area relationships on live corals were driven by fishes (r2 = 0.47 to 0.58) relative to invertebrates (r2 = 0.19 to 0.29). For dead corals, richness of associated species was poorly predicted by coral planar area for both fishes (r2 = 0.08 to 0.14) and invertebrates (r2 = 0.06 to 0.14) (Figure S5a,b).
| Predictor | ML estimate | Significance |
|---|---|---|
| Coral status (live/dead) | −0.37 | p < .0001 |
| Coral planar area | 0.25 | p = .0004 |
| Coral max branch length | 0.21 | p < .0001 |
| Time series | −0.24 | p = .1042 (NS) |
| Coral status*time series | 0.36 | p < .0001 |
| Coral status*planar area | −0.11 | p = .3090 (NS) |
| Coral status*branch length | −0.05 | p = .6648 (NS) |
- Note: Continuous predictors are centered and standardized, such that the full range of a continuous predictor is 2 units (compared to 1 unit for categorical predictors).
Species richness of the associated community was also predicted by the length of live coral branches (Figure S6). The richness of coral-associated macrofauna increased in live corals by 8–25 species/m increase in coral branch length at three of four sampling events (r2 = 0.29 to 0.43) (Figure S6 bottom panel). On the other hand, branch length of dead corals showed either a weak positive (r2 = 0.21, July 2009) or weak negative (r2 = 0.17, February 2010) relationship with species richness of the associated community (Figure S6 top panel).
4 DISCUSSION
Nearly 70% of Pocillopora corals bleached and died between August 2007 and July 2008 at our three study sites in the Galápagos, which coincided with the 2007–2008 La Niña-related cold-water anomaly. This represents an biologically significant loss of live coral foundational habitat for associated fishes and mobile macroinvertebrates, especially at shallow depths where these corals historically occurred in dense patches of 20%–100% mean coverage across sites and islands in the mid-1970's (Glynn & Wellington, 1983) and more recently estimates of 30% cover in 2014 (Romero-Torres et al., 2020), and densities of 0.10/m2 (0.08 SD) and 0.04/m2 (0.04 SD) at depths of 6–11 m at our sites in 2008. Nevertheless, dead coral colonies were still able to retain the ecosystem function of hosting high species richness of associated fish and mobile macroinvertebrates for more than two years after the disturbance. However, the species composition of these assemblages (and particularly of invertebrates) significantly varied between live and dead corals; mutualistic xanthid crabs occupied live corals while opportunistic predatory pencil urchins, hermit crabs, and gastropod snails occupied dead corals. Moreover, species composition of these assemblages shifted over time on dead and live corals, likely due to changes in structural characteristics and food resources (e.g., replacement of live tissue with colonizing sessile invertebrates and algae) on foundational coral habitats, and loss or growth of live coral tissue. Furthermore, the speciose and opportunistic associated assemblage on dead corals ultimately declined in richness due to erosion and disappearance of the complex coral calcium carbonate structure, and likely led to loss of coral-associated richness after 32–49 months.
4.1 Cold water effects on corals and coral associates
Our observations suggest that an ENSO-related cold-water anomaly led to bleaching and death of Pocillopora corals. The bleaching period coincided with the arrival of a plume of cold water (15–20°C minimum SST) flowing up the west coast of South America and arriving in the central Galápagos during October 2007, which was reflected in similarly low temperatures at our study sites. During this time period, corals were exposed to temperatures at or below published cold water thresholds for coral bleaching, including 18°C for up to 34 days and 16°C or less for 2.3 days at Guy Fawkes (15 m depth). Temperatures at Baltra (15 m) were at or below the 18°C threshold for 18.8 days. As expected, temperatures decreased with depth from 6 to 15 m. Longer periods of unusually cold water occurred at the reference site Cuatro Hermanos (where corals are rare) likely related to the presence of stronger upwelling currents relative to those at Guy Fawkes or Rocas Beagle (Witman et al., 2010).
Our data suggest that coral bleaching occurred during a 5–7-month window, between June 2007 (during the prior bi-annual biodiversity monitoring, when bleaching was not present) and January 2008 (when it was first observed) (Figure S1). Despite another La Niña occurring in October 2008–April 2009 and an El Niño in June 2009–April 2010, these live corals did not bleach subsequent to the 2007–2008 La Niña (J. Witman and O. Rhoades, pers. obs.), nor did these corals significantly change in abundance or live planar area across all time points (Figure 2a,b), suggesting that the 2007–2008 La Niña cold-water anomaly and bleaching triggered the initial death and deterioration of most of the coral heads. Additionally, there was not a significant change in total species richness of live coral associates over time and across time points (July 2008–Feb 2010), which suggests that after July 2008, subsequent ENSO events did not strongly influence community processes for the live corals. Indeed, coral-associated total community richness was not well-predicted by categorical or continuous independent variables associated with ENSO events, but instead by the interaction between time and coral status (live/dead), indicative of distinct successional shifts on live versus dead corals. However, we recognize that without pre-disturbance data on coral associates for the 2007–2008 La Niña event, we cannot fully attribute causation to this or other subsequent (or prior) ENSO events. Given that these corals and coral-associates have been strongly shaped by ENSO events (Glynn et al., 2018), changes in coral associates on live and dead corals over time may be partly attributable to these recurring perturbations and/or shifts from cooling to warming (2008 and 2009 La Niña, 2010 El Niño) and not solely the effects of the 2007–2008 La Niña on Pocillopora corals.
In general, cold-water coral bleaching has received less attention than warm water bleaching, although it can cause extensive coral mortality. For example, (González-Espinosa & Donner, 2020) documented cold-water coral bleaching at 14 sites in the Eastern Tropical Pacific alone from 1998–2017. One of these events was reported from the northern islands of Darwin and Wolf in the Galápagos where bleaching of three coral species (Porites lobata, Pocillopora spp. and Pavona clavus) occurred in 2007 when SST were 16°C (Glynn, 2009; Glynn et al., 2017), which was likely caused by the same La Niña event reported in this study. Given that climate models predict that the frequency of extreme La Niña events and associated cold-water anomalies will increase with climate warming (Cai et al., 2015), our study suggests that more attention should be paid to the ecological consequences of coral bleaching caused by low temperatures, especially for coral-associated species loss and local extinction debt.
4.2 Coral-associated richness
Our study of Pocillopora colonies on rocky reef ecosystems of the central Galápagos contributes to the growing number of studies that highlight the diversity of fishes and invertebrates that associate with these branching corals, which are the only Genus of branching corals in this subregion. Our surveys showed that recently recovered Pocillopora colonies supported up to 20 observed species (randomized observed species richness), and up to 41 total estimated species (Chao 1 estimated species richness), including nine known fishes and 11 known mobile macroinvertebrate species. Dead colonies of the same Genus supported an even greater diversity of up to 25 observed species and 61 total estimated species, including seven known fishes and 18 known mobile macroinvertebrate species. Even so, these are underestimates of the actual diversity of the entire community of associated species, since we only surveyed mobile macrofauna >= 1 cm and (for ethical considerations and to effectively survey individual coral heads repeatedly over time) we chose not to collect corals and exhaustively census the mobile fauna living in the interstices of the coral, or the sessile invertebrates and algae that colonized the dead Pocillopora colonies. Nevertheless, they are a repeatable assay for visually surveying the taxa associated with Pocillopora. across live and dead corals and across time points.
The biodiversity of communities associated with Pocillopora corals has been extensively studied in other tropical regions, and the range of our estimated values of species richness (20–41 species associated with live corals depending on the mode of analysis) falls within the range (though at the lower end, due to our use of non-destructive sampling) of published values of Pocillopora communities in other regions. The seminal work of (Abele & Patton, 1976) demonstrated that off the Pacific coast of Panama (close to the Galápagos study region), Pocillopora colony 3D area predicted the species richness of the decapod community associated with the corals, supporting a total of 61 species. Subsequent work has found that 36–127 species were found associated with Pocillopora corals at locations from the Red Sea to Hawaii (Britayev et al., 2017). Since Pocillopora corals are the only branching corals on Galápagos reefs that provide unique structural complexity and support obligate and facultative decapod symbionts (Glynn, 1985; Glynn et al., 1985; Leray et al., 2012; Stella, Pratchett, et al., 2011), continued declines in Pocilloporids appear to represent an important loss of foundational habitat for the shallow reef community, and especially for mobile macroinvertebrates.
4.3 Extinction debt of coral associates
Coral associates experienced biodiversity loss multiple years after disturbance to their foundational habitat, which coincides with declines in richness in other ecosystems due to losses of other foundation species. Indeed, bleached and dead Pocilloporids retained high levels of species richness for 13–19 months after the bleaching disturbance occurred; in fact, species richness of mobile macroinvertebrates considerably increased during this time period. However, this trend was reversed by a significant loss of mobile macroinvertebrate richness 19–25 months post-bleaching. During the final survey of associated species, total Chao 1 estimated species richness on dead corals was significantly lower relative to corals at earlier time points, and relative to live corals at that time. Depending on how long after our initial survey (June 2007) bleaching actually occurred, and how long before our final survey (July 2011) corals disintegrated, we estimate that it took between 32 and 49 months for the bleached Pocillopora to disintegrate and entirely disappear, driving the species richness of associated fish and macroinvertebrates down even further, possibly to zero as their habitat was lost.
4.4 Coral-associated richness on live versus dead corals
As with standing dead wood forests on land that have died due to disease or herbivorous insect attacks (Seibold et al., 2015; Stokland et al., 2012; Thorn et al., 2020), bleached and dead Pocillopora corals retained their biodiversity-enhancing function for multiple years after cold-water bleaching and death. This is both related to the habitat provided by the complex branching structure of these corals, as well as the food provided by sessile invertebrates and algae that rapidly recruited to and grew on the dead and undefended corals (Hadfield & Paul, 2001; McCook et al., 2001). In this study, both planar area and maximum branch length of Pocillopora corals significantly predicted the number of associated species, aligning with theory that habitat complexity is a leading predictor of species diversity (MacArthur & MacArthur, 1961), and that coral colony planar area and branch length are indicators of the habitable area or volume for associated fishes and invertebrates (Abele & Patton, 1976; Britayev et al., 2017). Our study further demonstrated that live tissue area was particularly important in determining the richness of associated species; planar area (and coral branch length, to a lesser extent) accounted for a much greater proportion of the total variation in species richness on live corals versus dead corals (26%–62% versus 15%). As with other recently disturbed and dead foundational habitats, live coral habitat has greater structural integrity and complexity relative to dead coral structure, such that live coral area is more representative of habitat area. Additionally, dead corals provide supplementary, ephemeral functions related to provisioning of food, which differ from the habitat and food functions provided by live corals, which attract a disproportionately rich assemblage of opportunistic coral associates.
Accordingly, the richness and composition of the associated communities differed between live and dead Pocilloporid habitats, and these differences magnified over time. Though the composition and abundance of fishes (dominated by coral hawkfishes Cirrhitichthys oxycephalus and juvenile ring-tailed damselfishes Stegastes beebei) was similar on live and dead corals, abundance was highest at the first community census and then fluctuated over time on live corals, while both fish species decreased in abundance over time on dead corals. Moreover, live and dead corals hosted distinct assemblages of invertebrates, with symbionts and specialists occupying live corals, versus opportunistic generalists occupying dead corals, and each of these assemblages became more and more distinct as live corals recovered and grew, while dead corals simultaneously eroded into rubble and disappeared.
Species composition on recovered, live corals exhibited a shift over time toward total dominance by xanthid crabs (Genus Trapezia), highlighting the importance of live coral habitat for this species. Trapeziid crabs are obligate residents that feed on live coral tissue while also defending corals from attacks (Gotelli et al., 1985; Stewart et al., 2006). Trapezia crabs exhibited a marked increase in abundance on live corals between July 2008 and February 2010, possibly due to redistribution of individuals from recently dead to remaining live (recovered) corals, which has been found to occur after widespread bleaching and subsequent coral recovery (Glynn et al., 2017; Glynn & D'Croz, 1990; Gotelli et al., 1985; Stella, Munday, et al., 2011), according to colony size (Canizales-Flores et al., 2021). It is possible that the large increase in the number of Trapezia occupying the live coral habitats from July 2008 to February 2010 at the Galápagos sites reflects habitat limitation due to the reduction of live coral habitats after the bleaching-induced mortality of Pocilloporids, in addition to growth of live tissue on those colonies over time. Overall high mobility of the fish, crustacean, and echinoderm fauna associated with live and dead corals suggests that active dispersal could explain the surge of species richness in the dead coral habitats between July 2008–January 2009 and the increase in abundance of Trapezia crabs in live corals. Our results also suggest that a metapopulation perspective (Hanski & Ovaskainen, 2002) may be a useful conceptual framework for investigating post-disturbance patterns of live and dead coral habitat occupancy, at least in this system of patchily distributed coral heads in the Galápagos, and for tropical reefs of other regions where disturbances result in increasingly patchily and sparsely distributed branching coral colonies.
By contrast, bleached and dead corals exhibited rapid colonization by opportunistic and transient urchins, crabs and gastropod snails, which ultimately declined due to loss of structure. These mobile macroinvertebrates contributed to a marked, non-linear change in associated community richness; first an increase in total species richness on recently dead corals between the first two community surveys (July 2008–Jauary 2009), and thereafter a substantial decrease over the next 13 months to February 2010. These species included large numbers of the common pencil urchin bioeroder, Eucidaris galapagensis, as well as hermit crabs and a number of species of gastropod snails. These species were observed feeding on dead coral tissue and sessile invertebrates, which may have arrived via migration of juveniles and adults and/or larval recruitment onto undefended coral skeleton habitats. These species declined in abundance and richness over time as corals disappeared into rubble, serving to temporarily enhance overall coral-associated community abundance and richness until obligate specialists recovered on live corals. This pattern of ephemeral colonization by opportunistic species followed by species loss with coral habitat deterioration has also been observed in response to a coral mass mortality event on the Caribbean coast of Panama (Nelson et al., 2016).
4.5 Consequences of Pocillopora coral declines for coral specialists versus generalists
Theory predicts that extinction debt should vary depending on the degree of habitat specificity of associated species (Hylander & Ehrlén, 2013; Kuussaari et al., 2009; Watts et al., 2020), with generalists characterized as having broad habitat requirements and specialists “largely dependent on one particular habitat type” (Watts et al., 2020). Specialists with low dispersal should have relatively short duration extinction debt, while generalists with high dispersal would have the longest extinction debt, as they could utilize other habitats after a disturbance. Of the Pocillopora associated community of fishes and mobile macroinvertebrates, we have sufficient information on small-scale (within-habitat) distribution to estimate that at least six species can be considered largely dependent on the Pocilloporid habitat. These include three species of Trapezia crabs, which are known obligate Pocillopora mutualists (Glynn, 1976; Hickman & Zimmerman, 2000), the spider crab Telephyrs cristolipes described as inhabiting Pocillopora corals in Hickman and Zimmerman (2000), the corallivorous gastropod Babelomurex hindsii, and the coral hawkfish Cirrhitichthys oxycephalus, which is frequently observed in Pocillopora corals and among black corals in the Galápagos (J. Witman, pers. obs.). As in most communities of mobile species such as birds (Watts et al., 2020), insects (Hanski & Ovaskainen, 2002), and reef fishes (Kritzer & Sale, 2006), the community of fishes and macroinvertebrates associated with live and dead Pocillopora corals represents a mix of habitat and food specialists and generalists. Future research to identify habitat specialist species on Galápagos reefs is needed to understand those species most vulnerable to habitat loss and to overall biodiversity loss following future temperature stress from climate oscillations.
ACKNOWLEDGMENTS
We thank L. Dee for assistance in the field, J. Palardy for assistance with preliminary data exploration, to the boat captains (L. Cruz and W. Aguirre) and researchers (N. Tirado, H. Jager, M. Romoleroux) at the Charles Darwin Foundation, and J. Suarez at Galápagos National Park for their assistance. We thank the Galapagos National Park Directorate and the Charles Darwin Research Foundation for permission to conduct this research, which was performed under permits PC-40-13, PC-06-18, PC-02-18 and PC 74-19 from the Galápagos National Park. We also thank the Hakai Institute, the Tula Foundation, the University of British Columbia, Florida International University, the Smithsonian Institution, and the University of California, Davis for their support during the completion of this manuscript. This publication is contribution number 2523 of the Charles Darwin Foundation for the Galápagos Islands.
FUNDING INFORMATION
This work was funded by the United States National Science Foundation (Award Numbers OCE-1061475, OCE-1450214 and OCE-1623867) to J. D. Witman.
CONFLICT OF INTEREST STATEMENT
The authors declare no competing interest.
Open Research
DATA AVAILABILITY STATEMENT
Should the manuscript be accepted, the data supporting the results will be archived in an appropriate public repository (Dryad, Figshare, or Hal) and the data DOI will be included at the end of the article.




