Ontogenetic shifts in feeding habits of orangemouth weakfish (Cynoscion xanthulus): From estuarine benthic feeder to marine nektivore top predator
Abstract
Orangemouth weakfish is a highly important commercial and game fish species in the Tropical Eastern Pacific. However, studies assessing changes in its trophic ecology relevant to size, habitat (estuarine or marine), sex, and season are non-existent. We assessed its feeding habits and trophic position (TP) according to the aforementioned factors. Its dietary breadth was composed of 33 types of prey. The feeding habits and isotopic values (δ15N and δ13C) registered differences between sizes and habitat, indicating ontogenetic changes in habitat as well as TP, as small organisms (TP 3.1) inhabited the estuarine area, feeding on benthic and demersal organisms, whilst large individuals (TP 4.1) were found in the marine environment, preying on nektonic fauna. No differences were found according to sex or season. The isotopic niche showed that the different sizes of this species are generalist consumers with a clear separation between them and between habitats, as estuarine juvenile and preadult organisms have a larger isotopic niche than adult marine organisms, being an opportunist tertiary estuarine consumers during their early stages, and a top marine predator during the adult phase.
1 INTRODUCTION
Knowledge of the different resources consumed by fish species, along with associated spatiotemporal variations of prey consumption, allows for the comprehension of resource use in an ecosystem, especially when the species are of commercial importance. This information permits a better understanding of the effect of natural and/or anthropogenic alterations in the exploited species (Koen-Alonso, 2009), and is also the basis for research on the possible effects of climate change on the displacement and abundance of estuarine fish, which in terms allows achieving sustainable use of fishery resources (Araújo et al., 2018).
However, there is currently a lack of information regarding trophic interactions of fish species from coastal and estuarine areas of the Tropical Eastern Pacific (Barletta & Lima, 2019; Bisi et al., 2012; López-Vila et al., 2019). Specifically, in the Gulf of California, information on the trophic ecology and ontogenetic niche shift of orangemouth weakfish (Cynoscion xanthulus), a species with economic importance, is missing. This species is native to the coastal ecosystems of the Gulf of California and the Mexican Pacific, and is one of the most abundant species with an important role in local fisheries, due to its large size, abundance, and quality of meat (Robertson & Allen, 2006). The fishery for this species is in 17th place in terms of weight and economic income of the 55 fisheries registered in Mexico (http://www.conapesca.sagarpa.gob.mx).
In general, there is little information on the biological aspects of this species, which mostly comes from its introduction to an artificial hypersaline body of water; the Salton Sea in California (Caskey et al., 2007; Riedel et al., 2002; Riedel & Costa-Pierce, 2002). From these studies it is known that adult orangemouth weakfish can grow up to 65 cm, have an optimal salinity range of 33–37 g/1, spawn during the summer with a sex ratio close to 1:1., are top carnivores, and principally inhabited the open water areas of the sea, whilst the juveniles remained close to the shore.
There is only one study of this species from its native distribution; undertaken in one of the systems studied in this work from 1977 to 1978 (Díaz-González & Soto, 1988). In that study, only estuarine fish were analysed, and the principal prey items were estuarine fish, hydrozoa, and crustaceans, specifically penaeid shrimps. No attempt was made to differentiate the orangemouth weakfish into different size classes or to compare the feeding habits with specimens from the marine area adjacent to the system.
However, for other similar species of the Sciaenidae family, several authors have observed ontogenetic shifts in diet, because they increase the efficiency and capacity to capture larger prey as they grow (Blasina et al., 2015, 2016; Dos Santos et al., 2018; García, 2007; Sardiña & Cazorla, 2005; Sardiña & Lopez Cazorla, 2005), as well as habitat shifts from juvenile and subadults inhabiting estuarine systems, to adults inhabiting the coastal area outside the system (Ferreira et al., 2016, 2018). Consequently, larger individuals usually occupy higher trophic levels as they increase the range of prey size consumed by adding larger prey to their diets (Scharf et al., 2000), which has also higher mobility, as they change from benthic to nektivore predators (Ferreira et al., 2018).
Based on these results, it seems that this species exhibits ontogenetic variations in its diet, but also, that the adult organisms have a marine affinity as opposed to juveniles that seem to stay in estuarine and coastal areas (Caskey et al., 2007; Ferreira et al., 2016, 2018; Riedel & Costa-Pierce, 2002). If this is the case, new information would be available for a proper management strategy for this species, as the juveniles would be estuarine organisms looking for the refuge and feeding resources that these sites offer (Braverman, 2011), whilst adult fish would move to marine environments.
With this in mind, we evaluated the length structure and intra-specific variation in the trophic ecology of the orangemouth weakfish in coastal and marine ecosystems of the Gulf of California, with the working hypothesis that this species exhibits ontogenetic changes in habitat and feeding habits as they grow, with juvenile fish inhabiting the estuarine area and feeding principally on estuarine fish and macroinvertebrates, and adults inhabiting the marine area and preying predominantly on nekton. These changes will be reflected in the trophic levels and isotopic niches, that is, the isotopic space (δ15N & δ13C).
2 MATERIALS AND METHODS
2.1 Area of study and sample collection
Orangemouth weakfish individuals were collected in the coastal zone of the south-eastern Gulf of California from August 2015 to October 2017, at bi-monthly intervals (Figure 1) at the estuarine systems of Teacapan and Huizache-Caimanero, both inside the systems and along the coastal areas outside the system at depths up to 10 m. The organisms were captured with gillnets with a mesh size of 3.5 inches in the coastal area and the channels of the estuarine systems, with a fishing gear operating time of 20 min. A cast net with a mesh size of 0.6 inches was used to capture individuals in shallow areas inside the estuarine systems. In the laboratory, total length (TL) (±0.1 cm) and weight (Ohaus digital scale: 0.1–2000 g ± 0.05) were recorded for all specimens. Organisms were preserved on ice after capture and were frozen at −20°C upon arrival at the laboratory.

2.2 Sample processing
In the laboratory, specimens were dissected and sex was determined macroscopically. Stomachs were removed and used to analyse the prey composition through stomach content analysis (SCA), and dorsal white muscle tissue from each specimen was collected to determine their recently consumed and assimilated food using stable isotope analysis (SIA).
SIA has aided in reconstructing species diets, estimating trophic positions (TPs), elucidating resource acquisition and allocation patterns, characterising feeding niches, and constructing food webs (Newsome et al., 2010). Furthermore, SIA can reveal ontogenetic shifts in consumer diet, movement patterns between habitats, species migration, and connectivity, whilst contributing to our understanding of fish population dynamics (Hobson & Wassenaar, 2018; Jara-Marini et al., 2009). δ15N exhibits a considerable degree of enrichment per trophic level, allowing, with appropriate isotopic baselines and trophic fractionation, for the estimation of the trophic positioning of an organism (Post, 2002), whilst δ13C outlines the origins of organic matter (Wissel & Fry, 2005).
When SIA and SCA are used together, it is possible to also know the taxonomic and size composition of diets and to clarify predator–prey interactions in complex systems where species have diverse consumption patterns that are difficult to identify from SIA alone (Layman et al., 2007). Its combined use improves our understanding of the feeding ecologies and functional roles of fish species and helps to clarify food web structures (Parkyn et al., 2001).
2.3 Feeding habits (stomach content analysis)
(x) = density estimation of the variable x
n = number of observations
h = bandwidth
Xi = length of the i-th fish specimen
K(•) = a smooth, symmetric kernel function integrating into one
The optimal bandwidth h selected was Sheather–Jones, because, with multimodal data, it dramatically outperforms Silverman's rule of thumb (Sheather & Jones, 1991). The resulting density distributions were rescaled to a smoothed frequency scale and decomposed into their Gaussian components. Each component's means and the standard deviation (corresponding to dominant modes or cohorts) were then obtained. The KDE procedure was performed in RStudio Version 1.2.1335.
Stomach contents were identified under a stereoscopic microscope. Prey items were typically identified to the family level unless partial digestion impeded this; they were then identified to the lowest taxonomic level possible. If items were too digested to be counted but were still recognisable as belonging to a large taxonomic group, they were described as “remains” of this category and were weighed together. If prey items were not whole or nearly whole, individual numbers were based on the identification of countable parts, such as claws and legs for crustaceans, otoliths for fishes, and beaks for cephalopods, taking into account the size and shape to determine that they came from the same individual/species. Upon identification, prey items were counted and weighed (g), and when possible, a sample of muscle tissue from each prey item was collected to determine their trophic level using SIA.
Dietary similarities among sex, size (small and large), habitat (estuarine and marine), and season, based on the seasons proposed by Amezcua et al. (2019), were tested using a permutational MANOVA (PERMANOVA) and a principal coordinates analysis (PCO; Anderson et al., 2008) to test the Ha that the feeding habits of this species changed ontogenetically and between estuarine areas and marine environments, and possibly between sex and climatic seasons. To do this a matrix was constructed that included orangemouth weakfish specimens in the columns, prey items as rows, and PSIRI as values. The data were square root transformed to reduce the effect of very abundant prey on the analysis whilst retaining the quantitative nature of the data and transformed to a resemblance matrix using Bray Curtis similarity as a resemblance measure.
2.4 Stable isotopes analysis
To obtain isotopic data on the orangemouth weakfish and its prey, muscle aliquots of these species were placed in vials with Teflon lids and dried for 24 h in a dry freezer at a temperature and pressure of −45°C and 24 to 27 × 10−3 mbar, respectively. Samples were powdered in an agate mortar, and 1-mg sub-samples were weighed and stored in tin capsules (8 × 5 mm). The δ13C and δ15N values of carbon and nitrogen isotope composition were determined by the Stable Isotope Laboratory at the University of California at Davis, USA using an Isotope Ratio Mass Spectrometer (IRMS, 20–20 mass spectrometer, PDZEuropa, Scientific Sandbach, UK) with a 0.2‰ precision for both elements. Stable isotope values (δ) were calculated using the equation: δ (%) = (Rsample/Rstandard − 1) × 1000, where R = 15 N/14 N or 13C/12C. The Rstandard is relative to international standards, the Air and V-PDB (Vienna PeeDee Belemnite) for N and C, respectively.
2.5 Trophic position
Based on the obtained isotopic values we estimated the TP of the different size classes of the orangemouth weakfish in R using the Bayesian package tRophicPosition (Quezada-Romegialli et al., 2018), employing the “twoBaselinesFull” model, because it recognises the heterogeneity of ecosystems, by including two different baselines, for example, pelagic and benthic. The tRophicPosition model includes isotopic variation in the baseline indicator, the consumer, and the trophic discrimination to provide a robust estimation of consumer TP at the population level. The model was run with 2000 iterations and 2 chains (dnorm(4, SD ± 0.1)), and the trophic level of baselines (lambda) of 1 was chosen. We included isotopic carbon and nitrogen data of phytoplankton and detritus (estuarine and marine) to provide pelagic and benthic baselines, respectively. We use the trophic enrichment factor (TEF) for fish proposed by Hoen et al. (2014) based on data on diet isotope ratios (termed the “Diet-Dependent Discrimination Factor”, DDDF) of 0.75 ± 0.10‰ for δ15N and δ13C of 0.75 ± 0.11.
2.6 Trophic spectrum
The bayesian method SIBER (Stable Isotope Bayesian Ellipses in R) was used to define the trophic amplitude among the different factors (season, habitat, sex, and size class) of the orangemouth weakfish as a measure of their isotopic resource use area at the population level. This method is based on the two-dimensional isotopic space of δ13C and δ15N, and assessed using Bayesian analysis of standard ellipses; unlike the Euclidean methods, this analysis can incorporate uncertainties such as sampling biases and small sample sizes into niche metrics (Layman et al., 2007). We used Monte Carlo simulations to correct the bivariate ellipses (δ13C and δ15N) surrounding the data points in the 95% confidence interval for the distributions of both stable isotopes (Jackson et al., 2011). The results for the orangemouth weakfish were plotted in δ13C versus δ15N diagrams to analyse differences in diet and TP between the different factors using a standard ellipse area (SEAc), which in this case is the small sample size corrected version.
Additionally, two-dimensional (δ13C and δ15N) probabilistic (95%) regions for each of the groups identified from Ward's clustering were developed using a Bayesian framework in the package “nicheROVER” (Swanson et al., 2015). This package uses a probabilistic method to calculate niche regions and pairwise niche overlap using multidimensional niche indicator data. The niche region is defined as the joint probability density function of the multidimensional niche indicators at a user-defined probability alpha (in this study 95%). Uncertainty is accounted for in a Bayesian framework, and the method can be extended to three or more indicator dimensions. It provides directional estimates of niche overlap, accounts for species-specific distributions in multivariate niche space, and produces unique and consistent bivariate projections of the multivariate niche region (Swanson et al., 2015).
The δ15N and δ13C values were used to estimate the contribution of each food item in the orangemouth weakfish diet. Bayesian isotopic mixing models were generated with R software, using the Stable Isotope Mixing Models in R “SIMMR” (Parnell & Inger, 2019). SIMMR package is an upgrade to the SIAR package and is designed to solve mixing equations for stable isotopic data within a Bayesian framework. This new version contains a more sophisticated mixing model, a simpler user interface, and more advanced plotting features. It requires a minimum of three input objects: the consumers or mixtures, the source means, and the source standard deviations. Optionally, you can also add correction data (also called TEFs) represented again as means and standard deviations, and concentration dependence values. This model estimates the probability distribution of the contribution of the prey to a mixture and also evaluates the uncertainty associated with the isotopic values of the prey and predator (Parnell & Inger, 2019).
The results of SIMMR analysis are reported as percentage distributions ranging from 0% to 99%, where the minimum and maximum values are used to determine the importance of prey in the diet (Madigan et al., 2012). To increase the discriminatory power of the isotope-mixing model, it has been recommended the use of up to six discriminated sources for C and N isotopes (Phillips et al., 2014). Therefore, we used up to six main prey items for each size class of the orangemouth weakfish to capture at least 90% of the diet to determine their relative contributions to their diet.
3 RESULTS
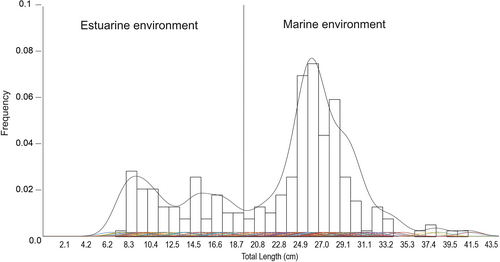
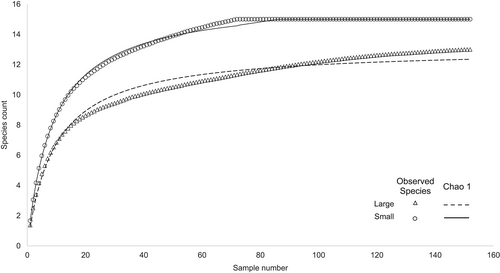
A total of 224 organisms of orangemouth weakfish were collected, with a range of sizes from 6.6 to 39.5 cm TL. The KDE procedure showed three cohorts for the TL of orangemouth weakfish: <12.5 cm (mean 8.47 cm ±1.1 SD, N = 38) corresponding to juveniles, 12.5 to 20.8 cm (mean 14.6 cm ±3.7 SD, N = 39) corresponding likely to preadults, and >20.8 cm (mean 25.7 cm ±2.1 SD, N = 147) corresponding likely to adults. Considering that the largest estuarine individual caught was 19.6 cm, the upper limit was lowered to 19.7 cm corresponding to the maximum size for the fish caught in the estuarine areas, and only two sizes were considered for the analyses, small for individuals with sizes up to 19.7 cm and caught exclusively in the estuarine area (N = 72) and large for individuals larger than 19.7 cm and caught in the marine area (N = 152; Figure 2). The Chao1 model of the richness of prey items for both orangemouth weakfish sizes indicated that the number of stomachs analysed was sufficient to describe its diet in the studied area (Figure 3).
3.1 Feeding habits (stomach content analysis)
The dietary spectrum of orangemouth weakfish was composed of 19 prey items (Table 1) with the majority being teleosts (8 taxa), followed by crustaceans (7 taxa), and molluscs (4 taxa). Small size individuals preyed mainly on shrimps which were their main important prey item in terms of PSIRI, followed by Portunidae crabs and Stomatopoda. Large individuals preyed mainly on fish, principally small pelagics (Clupeidae and Engraulidae), and squids (Table 1).
| Small (<197 mm) N = 72 | Large (>197 mm) N = 152 | |
|---|---|---|
| Prey taxa | %PSIRI | %PSIRI |
| MOLLUSCA | ||
| Class Bivalvia | ||
| Unidentified bivalve | <0.01 | - |
| Family Mytilidae | 10.83 | - |
| Class Cephalopoda | ||
| Lolliguncula panamensis | <0.01 | 24.9 |
| Family Onychoteuthidae | - | 2.43 |
| CRUSTACEA | ||
| Class Malacostraca | ||
| Squilla mantoidea | 24.68 | - |
| Order Decapoda | - | - |
| Penaeus vannamei | 43.16 | - |
| Infraorder Brachyura | - | - |
| Unidentified crab | 25.17 | - |
| Family Aethridae | 21.05 | - |
| Family Hippidae | 8.3 | - |
| Family Xanthidae | 0.5 | - |
| Callinectes arcuatus | 37.87 | - |
| OSTEICHTHYES | ||
| Subclass Teleostei | ||
| Unidentified fish | 1.53 | 57.58 |
| Opisthonema libertate | 0 | 15.39 |
| Family Engraulidae | - | 10.62 |
| Family Eleotridae | 9.25 | - |
| Chloroscombrus orqueta | - | 11.17 |
| Family Gerridae | - | 5.72 |
| Family Haemulidae | - | 8.86 |
| Family Sciaenidae | <0.1 | 5.3 |
PERMANOVA showed statistical differences according to size (Pseudo-F1,222 = 31.53, p < 0.01) and habitat (Pseudo-F1,222 = 36.54, p < 0.01). However, no differences were found according to sex (Pseudo-F1,222 = 0.63, p > 0.05) or season (Pseudo-F1,222 = 1.39, p > 0.05). Pairwise comparison tests for the factor's size/habitat show that the feeding habits of adult-size orangemouth weakfish caught in marine environments were statistically different from the feeding habits of juvenile and preadult-size orangemouth weakfish caught in estuarine systems.
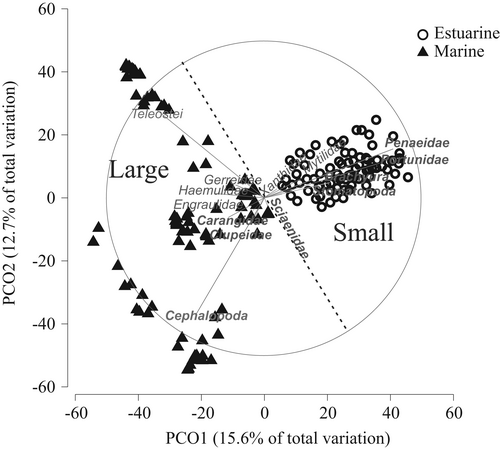
The differences in feeding habits according to size and habitat can be graphically seen in the PCO (Figure 4). Adults of orangemouth weakfish preyed on nekton exclusively (both fish and squids). There is a group of large individuals preying predominantly on cephalopods, however, these groups cannot be explained by the factors a priori established. Juvenile and preadult individuals preyed on benthic and demersal organisms, mainly crabs, bivalves, and sleeper gobies (Eleotridae), although other items were also present such as swimming crabs, stomatopods, shrimps, and weakfish as well. The prey ingestion varied according to these two factors, as the juvenile and preadult estuarine individuals showed lower biomass of prey ingested than the adults inhabiting the marine environment (Figure 5).
3.2 Stable isotopes analysis
The isotopic values of δ13C and δ15N in the orangemouth weakfish muscle tissues showed δ13C values between −18.9‰ and −16.2‰, and δ15N values ranging between 14.4‰ and 17.6‰. Results of the GLM for mean δ13C values showed statistical differences in habitat (t = 5.68, p < .05), but no statistical differences were found according to the other factors (tsize = 0.06, tseason = 0.17, tsex = 1.11, p > .05). In marine environments, the mean value (−16.2‰ ±0.8) was significantly higher than in the estuarine environment (−18.9‰ ±1.9; Table 2).
| Category | Group | δ13C (‰) | |||
|---|---|---|---|---|---|
| Mean | SD | t | p | ||
| Habitat | Estuarine | −18.9 | 1.9 | 5.68 | <.05 |
| Marine | −16.2 | 0.8 | |||
| Size | Small | −17.7 | 1.8 | 0.06 | >.05 |
| Large | −16.4 | 1.8 | |||
| Season | Rainy | −16.6 | 1.6 | 0.17 | >.05 |
| Dry | −17.4 | 1.9 | |||
| Sex | Male | −16.8 | 1.8 | 1.11 | >.05 |
| Female | −18.2 | 2.5 | |||
| δ 15 N(‰) | |||||
| Habitat | Estuarine | 15.1 | 4.1 | 3.188 | <.05 |
| Marine | 17.6 | 2.1 | |||
| Size | Small | 15.1 | 2.3 | 2.365 | <.05 |
| Large | 17.6 | 2.1 | |||
| Season | Rainy | 15.9 | 3.1 | 1.643 | >.05 |
| Dry | 16.4 | 2.7 | |||
| Sex | Male | 17.3 | 2.3 | 1.96 | >.05 |
| Female | 16.9 | 2.5 | |||
On the other hand, mean values of δ15N differed statistically between habitat (t = 3.19, p < .05) and size (t = 2.37, p < .05), but no differences were found according to season or sex (tseason = 1.65, tsex = 1.96, p > .05). Adult organisms inhabiting marine environments showed an isotopic value of 17.6‰ (±2.1), whilst estuarine organisms (juveniles and preadults) showed an isotopic value of 15.1‰ (±4.1; Table 2).
3.3 Trophic position
The TPs estimated with the Bayesian package tRophicPosition are in agreement with the previous results. For season and sex, the trophic levels were similar, however in terms of size the values were 4.0 ± 0.3 for juveniles and preadults (<197 mm), and 4.7 ± 0.3 (>197 mm) for adults. This indicates that it is a species that changes ontogenetically from a secondary consumer as a juvenile, to a top predator as an adult in the SE Gulf of California in benthic and pelagic communities. Also, the trophic level in the marine environment was higher (4.7 ± 0.3) than in the estuarine environment (4.2 ± 0.4; Table 4). The TP value of its main prey species as determined by PSIRI varied from 2.6 (Opisthonema libertate) to 3.6 (Chloroscombus orqueta; Table 4).
3.4 Trophic spectrum
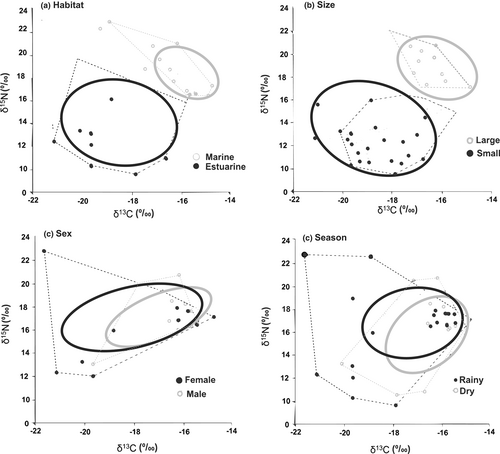
The standard ellipse areas obtained with SIBER when analysing the isotopic niche of the orangemouth weakfish indicated that clear differences existed according to habitat and size. In estuarine environments, where small individuals inhabit, the isotopic niche was wider than in the marine environment, where large individuals are found (SEAc estuarine 26.2‰, SEAc marine 3‰), and both were separated from each other (Figure 5a) indicating a different and more specialised diet in marine environments. The probability of overlap concerning the marine and estuarine habitat, according to nicheROVER, was 8.5% and 8.6% respectively. In terms of size, the isotopic niches showed high variation between small (estuarine) and large (marine) orangemouth weakfish (SEAc = 21.5‰ and 6.1‰, respectively; Figure 5b), and the probability of niche superposition between both of them was 9.7%. Also, the diet of small estuarine individuals was wider than that of large individuals, as the results from the isotopic niche of different size individuals showed.
In terms of sex and seasons regardless of size and habitat, the isotopic niches were very similar. For seasons (SEAc = 6.2‰ and 16.8‰ for rainy and dry, respectively) the probability of overlap between them was higher than 80% according to the niche region they occupy, indicating that in both periods the exploited prey isotope fields were similar (Figure 5c). For sexes, although females have a bigger niche compared to males (SEAc = 20.2‰ and SEAc = 11.4‰, respectively), the projected superimposition of their niches was 59.8% and 91.5% for males and females, respectively (Figure 5d), indicating a similar diet.
According to SIMMR the prey species that contributed most to the isotopic composition for both sizes of orangemouth weakfish were ix prey species (Table 3 and Figure 6). In small organisms, the main contributors were a crab (Callinectes arcuatus, 11.1%), the white shrimp (Penaeus vannamei, 16.8%), and the stomatopod (Squilla mantoidea, 1.1%). For large organisms, the three main prey items were all nektonic species: the Pacific thread herring (Opisthonema libertate), the Pacific bumper (Chloroscombrus orqueta), and the squid (Lolliguncula panamensis), all with similar percentages (18.6%, 18.5%, and 18.3% respectively).
| Season | Rain | Dry | ||
|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | |
| O. libertate | 0.24 | 0.02–0.67 | 0.22 | 0.02–0.58 |
| C. orqueta | 0.14 | 0.01–0.46 | 0.20 | 0.02–0.52 |
| L. vannamei | 0.20 | 0.02–0.64 | 0.22 | 0.02–0.67 |
| C. arcuatus | 0.21 | 0.02–0.66 | 0.19 | 0.02–0.60 |
| L. panamensis | 0.16 | 0.01–0.51 | 0.12 | 0.01–0.34 |
| S. mantoidea | 0.05 | 0.01–0.11 | 0.05 | 0.01–0.10 |
| Habitat | Estuarine | Marine | ||
| Mean | 95% CI | Mean | 95% CI | |
| O. libertate | 0 | N/A | 0.55 | 0.01–0.30 |
| C. orqueta | 0 | N/A | 0.16 | 0.01–0.50 |
| L. vannamei | 0.33 | 0–42.5 | 0 | N/A |
| C. arcuatus | 0.14 | 0–34.1 | 0 | N/A |
| L. panamensis | 0 | N/A | 0.10 | 0.02–0.62 |
| S. mantoidea | 0.17 | 0–31.7 | 0 | N/A |
| Sex | Females | Males | ||
| Mean | 95% CI | Mean | 95% CI | |
| O. libertate | 0.16 | 0.02–0.65 | 0.14 | 0.01–0.47 |
| C. orqueta | 0.23 | 0.02–0.50 | 0.22 | 0.02–0.64 |
| L. vannamei | 0.21 | 0.02–0.62 | 0.20 | 0.02–0.62 |
| C. arcuatus | 0.19 | 0.02–0.61 | 0.20 | 0.02–0.63 |
| L. panamensis | 0.14 | 0.02–0.45 | 0.19 | 0.02–0.59 |
| S. mantoidea | 0.08 | 0.01–0.17 | 0.06 | 0.01–0.17 |
| Size | Small | Large | ||
| Mean | 95% CI | Mean | 95% CI | |
| O. libertate | 00 | N/A | 0.20 | 0.02–0.63 |
| C. orqueta | 00 | N/A | 0.29 | 0.03–0.64 |
| L. vannamei | 0.46 | 0.07–0.81 | 0.0 | N/A |
| C. arcuatus | 0.13 | 0.01–0.47 | 0.0 | N/A |
| L. panamensis | 0.00 | N/A | 0.19 | 0.02–0.60 |
| S. mantoidea | 0.10 | 0.02–0.31 | 0.01 | N/A |
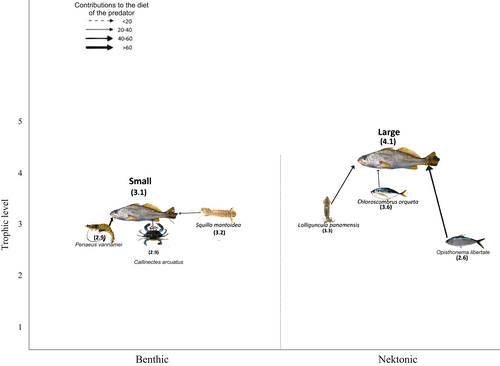
| Category | Group | TA | SEAC | PT | SD (±) |
|---|---|---|---|---|---|
| Season | Rainy | 57.5 | 16.8 | 4.1 | 0.33 |
| Dry | 32 | 16.2 | 4.4 | 0.25 | |
| Habitat | Estuarine | 53.1 | 26.2 | 4.3 | 0.36 |
| Marine | 9.9 | 3 | 4.7 | 0.22 | |
| Sex | Male | 33.2 | 20.2 | 4.4 | 0.32 |
| Female | 16.8 | 11.4 | 4.5 | 0.29 | |
| Size | Small | 24.1 | 10.5 | 4.0 | 0.26 |
| Large | 33.9 | 11.3 | 4.7 | 0.28 |
4 DISCUSSION
4.1 Feeding habits (stomach content analysis)
Studies on trophic interactions are very important from a biological point of view as they lead to the understanding of relationships within and between prey and predator species. This is of special value when the objects of study are species of commercial interest (Wootton, 1992), as is the case of the orangemouth weakfish. Our results show that the dietary spectrum of the orangemouth weakfish in this region is similar to what was previously reported, but in the present study, differences were found in feeding habits according to size and habitat.
No previous studies were found related to the length-at-maturity for this species, therefore we cannot know with certainty the exact size at which the individuals of this species change from subadults to adults. However, based on our observations from the length-frequency analysis, and previous works on the similar species Cynoscion acoupa (Ferreira et al., 2018), we consider that the small organism group included juveniles and preadults inhabiting the estuarine systems, and the large organism group included the adults inhabiting the marine environment. Our results suggest that this species shows a shift from a secondary estuarine benthic predator as a juvenile and preadult, to a top nektonic marine predator as an adult, according to the observed δ15N values from the different size classes of the studied predator, and to the fact that no small organisms were found in the marine area.
The change in habitat is reflected in its diet, as small individuals prey on estuarine benthic and demersal fauna, and large organisms feed exclusively on nekton prey (squid and pelagic fish). This change in prey selection can be related to the development of the visual system, as well as changes in the energy requirements of the species' reproductive stages (Torres-Rojas et al., 2020). It is known that body length plays a central role in predator–prey interactions (Sheldon et al., 1977), and the different isotopic composition of the transition from juvenile to adult orangemouth weakfish might be reflective of differences in feeding strategies associated with the increase in size and the change of habitat. Several authors note that trophic levels may increase as fish grow (Cousins, 1987; Scharf et al., 2000; Warren & Lawton, 1987); this is because as fish size increases the efficiency to capture prey also increases as the senses are fully developed and they can capture larger and faster prey. This pattern was evident in our study because body length and trophic level were correlated, the larger organisms have a higher TP compared to small organisms.
As previously stated, a prior study undertaken in the region 40 years ago (Díaz-González & Soto, 1988), found a similar dietary spectrum, although in that study all the samples were collected inside the estuarine system, and as such, the number of large individuals was very scarce, no attempt was made to differentiate the orangemouth weakfish into different size classes. However, based on the results from both studies in the area, the feeding habits, size structure, and life cycle of the orangemouth weakfish may have remained similar in the area during the last 40 years.
Studies undertaken on Gulf weakfish (Cynoscion othonopterus) have found similar results (Bajeca Serrano, 2016; Encinas-Rivera, 2008), as they report that this species feed mainly on fish, however, both of these studies were undertaken in marine environments and the reported lengths of the predators correspond to the adult part of these species (Mendivil-Mendoza et al., 2018). Other authors have mentioned that the food of the weakfish depends on the habits of the prey rather than a selective preference on the part of the predator towards it (Matlock & Garcia, 1983). For example, studies conducted in the Upper Gulf of California for the Gulf weakfish mention that its main diet is the anchovy Cetengraulis mysticetus with a contribution to its diet greater than 95%, and that this has not changed for more than 20 years due to the great abundance of this prey in the area (Bajeca Serrano, 2016).
However, it seems that, although the specific diet can be directly related to prey abundance, the orangemouth weakfish exhibits an ontogenetic change in habitat use and feeding habits, a result that is supported by studies on the tropical Acoupa weakfish (C. acoupa), which exhibits the same ontogenetic changes in a very similar tropical area in Brazil (Ferreira et al., 2016). Juveniles and preadult organisms inhabit the estuarine system, because the conditions in these zones are favorable for these specific stages, which are nursery and feeding grounds for juveniles, and feeding grounds for preadults, due to the high densities of prey in estuarine zones. As they reach the adult stage, a migration occurs to the marine environment, changing from an estuarine benthic predator as juveniles, preying mainly on sessile or slow-moving small prey invertebrates, then remaining an estuarine benthic predator, but transitioning to preying on larger and more mobile organisms as preadults, to finally become a marine pelagic feeder as an adult, making this species a marine-estuarine dependent, relying on the estuary to complete its life cycle (nursery and early development; Blaber, 2007; Blaber & Blaber, 1980).
4.2 Trophic position and trophic Spectrum
The δ13C values of orangemouth weakfish varied from −18.86 in small estuarine organisms to −16.24 in large marine organisms. These differences in carbon isotopic values could indicate the change in habitat as small organisms inhabit the estuarine zone, which generally presents more negative carbon isotopic values compared to organisms of larger sizes inhabiting the marine environment that present more enriched δ13C values (Tue et al., 2012). This could also indicate that the sources of carbon differ between both habitats, as estuarine organisms have increased assimilation of autochthon δ13C primary producers in the base of their food web, whilst large marine organisms reflect marine basal sources of δ13C, which are larger values when compared to estuarine and freshwater environments (Bouillon et al., 2008). The values found in our study are similar to other estuarine benthic feeders in the case of small juvenile organisms, and large nektonic predators, as in the case of large marine orangemouth weakfish (Torres-Rojas et al., 2020).
Isotopic data of predators with a standard deviation higher than 1 indicates an isotopic variation that can be associated with feeding on prey with different isotopic values or from different trophic levels (Bearhop et al., 2004), and in most of our results SD > 1. Considering this, a broad isotopic niche (i.e., SD > 1) is indicative of heterogeneous diets, comprising a wide variety of food sources with different isotopic values; their final isotopic composition reflects the combination of the food sources they have consumed. Concerning the isotopic niche breadth of orangemouth weakfish concerning season and sex, the δ15N and δ13C values indicate no differences between specimens of this species according to these two factors. The absence of seasonal differences could be related to the fact that upper levels in the food web do not vary seasonally (Simenstad & Wissmar, 1985), and considering that most of the analysed species in this work occupy high levels of the coastal food web, this might be masking differences in lower trophic levels. However, such analysis was beyond the scope of this paper.
In comparison, the δ15N and δ13C values concerning habitat and size were significantly different. Organisms captured in estuarine systems show a higher variation in their isotopic values opposite to the organisms of the marine environment that reflect a smaller isotopic niche (nektivores). Multivariate analysis shows that organisms captured in estuarine and marine areas have different food preferences. These different feeding strategies could be responsible for the observed isotopic variability, as this may directly relate to the type of prey species consumed and assimilated. The presence of significant differences in both isotopes indicates that predators use different feeding habitats (estuarine/marine) or consume different prey species (Newsome et al., 2010). In addition to this, the organisms that reside in estuarine zones are characterised by presenting a higher isotopic variability as these environments have a large number of potential sources of organic matter due to the input of subsidies by the surrounding environment as it is high in situ production.
Concerning size, significant differences were not found concerning δ13C, however, concerning δ15N the isotopic values of the organisms did show differences between sizes with lower values in organisms of small sizes compared to large sizes which registered higher values. This was also observed through the analysis of stomach contents, as small-sized organisms fed on benthic invertebrates and demersal fish, and larger organisms were exclusive nektivores, which has been reported in other species of the Scianidae family (Blasina et al., 2015; García, 2007; Sardiña & Cazorla, 2005).
The results in this study are congruent with size-based marine food webs, where the size of potential prey increases with predator size as their diet is generally constrained by their morphometric characteristics such as the size of their jaws, feeding behavior, and prey availability along with other factors (Gerking, 2014; Lundvall et al., 1999; Sheldon et al., 1977). Consequently, size-related dietary shifts may be prevalent in many fish species (Davis et al., 2012; Galvan et al., 2010). The previous results were corroborated by the SIMMR mixing model, which shows the TP of both sizes of orangemouth weakfish, as well as the relative contribution of the prey consumed by each size.
Our results highlight the importance of considering the ontogenetic variations in the analysis of trophic studies in estuarine and adjacent marine areas where there is an abundance of juvenile fish and macroinvertebrates that use these types of systems as a refuge and feeding area (Rodríguez-Graña et al., 2018). Our work only includes organisms captured in the estuarine and marine areas close to shore. As such, organisms of the maximum reported lengths were not caught, so other differences in feeding habits and TP might occur if compared with larger organisms likely captured at higher depths or in other environments. It is necessary to consider that larger organisms might not have been caught because of the selectivity of the fishing gear used. Local fishers use gillnets of a mesh size of up to 6 inches to catch larger fish. Therefore, there is the likelihood that larger fish could return to the estuarine system to spawn, or they may remain outside the marine zone permanently, however that goal was outside of the scope of this work. In this sense, and although our results show isotopic differences between estuarine and marine habitats related to ontogenetic changes in both prey selection and habitat, more information is still required to sample across all age classes to determine the extent of ontogenetic differences in diet.
However, results from this study indicate that this species has an important role in the ecological connectivity between coastal ecosystems; through the migration of juveniles from the estuarine system to subtidal waters, this species transfers carbon among nearshore systems (Bouillon & Connolly, 2009), as our results from δ13C indicate that the carbon source for small organisms is riverine. Also, these individuals prey on estuarine biota and then migrate to the marine area, and they are likely to be predated when they leave the estuarine system, thus producing the effect of a relay system that transfers energy from the estuarine to the marine ecosystem, a phenomenon known as Trophic Relay (Kneib, 1997). This sort of energy transfer from the estuarine systems to the marine environment in migrating fish is a potentially important mechanism of carbon transport in tropical ecosystems (Bouillon & Connolly, 2009).
In conclusion, our study demonstrates that the orangemouth weakfish shows an ontogenetic shift in its diet spectrum and its TP, but also shows that this species has a differential use of habitat as it grows, shifting from a secondary benthopelagic predator in estuarine systems to a top nektonic predator as an adult, thus playing an important role as a species regulator and in the ecological connectivity trough carbon transport in coastal ecosystems. Such information can be useful for the development of robust trophic models which allow for an accurate representation of the trophic flows associated with demersal fish in the Gulf of California.
AUTHOR CONTRIBUTIONS
Víctor M. Muro-Torres: Conceptualisation, Formal analysis, Funding acquisition, Investigation, Methodology, Visualisation, Writing—original draft, Writing—review and editing. Felipe Amezcua: Conceptualisation, Formal analysis, Investigation, Funding acquisition, Project administration, Methodology, Supervision, Visualisation, Writing—original draft, Writing—review and editing. Lucinda Green: Formal analysis, Investigation, Methodology, Writing—original draft, Writing—review and editing. Jorge Payan: Formal analysis, Investigation, Methodology, Writing—original draft. Eduardo F. Balart-Páez: Investigation, Visualisation, Writing—original draft, Writing—review and editing. Felipe Amezcua-Linares: Formal analysis, Investigation, Methodology, Writing—original draft.
ACKNOWLEDGEMENTS
We thank A. Garcia, E. Hernandez, and all the fishers that helped us with the sampling process. Dr. M. Soto assisted us with the SIA. The research leading to these results received funding from Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México. Victor Muro was awarded a Ph.D. grant from CONACYT.
CONFLICTS OF INTEREST
The authors have no relevant financial or non-financial interests to disclose.
CODE AVAILABILITY
Not applicable.
ADDITIONAL DECLARATIONS FOR ARTICLES IN LIFE SCIENCE JOURNALS THAT REPORT THE RESULTS OF STUDIES INVOLVING HUMANS AND/OR ANIMALS
The fishers collecting the samples had all the proper fishing permits issued by the relevant authority (https://www.gob.mx/conapesca).
CONSENT TO PARTICIPATE
All the authors have given their consent to participate in this work.
CONSENT FOR PUBLICATION
All the authors have given their consent to submit and publish this work, if accepted, in Aquatic Ecology.
Open Research
DATA AVAILABILITY STATEMENT
Data information will be available at UNINMAR (http://www.icmyl.unam.mx/uninmar/).




