Environmental factors driving the distribution of the tropical coral Pavona varians: Predictions under a climate change scenario
Abstract
Climate change causes shifts in the geographical distribution boundaries of many organisms. Modelling techniques predict the distribution of species by relating climatic and physical factors with species' presence records, including potential extinction areas and new potential areas of colonization, under predicted climatic scenarios. In this study, we initially explored which environmental variables most influenced the distribution of Pavona varians, a hermatypic coral from the equatorial Indian and the Pacific Ocean, which is categorized as ‘Least Concern’ by the UICN. The most influential variables were the minimum and maximum sea surface temperature, the diffuse water attenuation and the cloud cover. These variables were used to predict habitat suitability of P. varians under a current and a future (A1B IPPC) scenario using MaxEnt. Despite P. varians is an opportunistic species, with a well-known resistance to environmental stress, the model predicted a massive decline in the suitability of its habitat in all areas by the year 2100. The information obtained by this study can be used to support the conservation decision making process to improve the preservation of the species.
1 INTRODUCTION
Climate change, particularly global warming, is impacting on the geographical distributions of living organisms of planet Earth (Noce, Caporaso, & Santini, 2019). Some species may reach new areas under favourable conditions (Parmesan et al., 1999), e.g., towards the poles or higher altitudes (González Elizondo et al., 2003; Walther et al., 2002), but others may disappear from those areas under environmental stressful conditions (Hughes, 2000; Thomas, Franco, & Hill, 2006). Distributional shifts towards suitable areas are not possible in all species, since some of them have limited plasticity in their physiological tolerance, or restricted dispersion, driving local extinctions. The disappearance of habitat-forming and foundation species is particularly worrying, as it has been shown to cause the decline and collapse of entire communities (Wernberg, Russell, Thomsen, & Connell, 2014), favouring the increase of opportunistic and invasive species (González Elizondo et al., 2003; Hoffmann & Sgró, 2011) with negative impacts on biodiversity (Ellison et al., 2005). The early detection of these potential changes, including the environmental factors that most affect species’ distributions, as well as the most affected areas, becomes a significant advantage when making conservation decisions (Guisan et al., 2013).
In the oceans, the most important effects of climate change are rising seawater temperatures, acidification resulting from increased CO2 absorption, sea level rise, and changes in the global system of ocean currents (Gattuso, Hoegh-Guldberg, & Pörtner, 2014). The potential impacts of these environmental changes on organisms depend on whether they can tolerate and adapt to these effects. The distribution of marine ectotherms is closely linked to their thermal limits (Sunday, Bates, & Dulvy, 2012), so seawater temperature changes can trigger distributional shifts. Temperatures above the organism's tolerance threshold can also compromise their survival. Additionally, organisms subjected to temperature increases, near their sub-lethal thresholds, can also become more sensitive when faced with other of the aforementioned stress factors, such as acidification in corals (Prada et al., 2017).
Coral reefs are one of the most vulnerable and threatened marine ecosystems in the world (Heron, Maynard, Hooidonk, & Eakin, 2016; Hughes et al., 2017, 2018). More than half of the world's reefs are at risk of degradation, and the vast majority has already disappeared (Burke, Reytar, Spalding, & Perry, 2011; Castro & Huber, 1997; Hughes & Kerry, 2017). Due to global warming, tropical reef-forming species are currently shifting towards higher latitudes, at an approximate rate up to 10 km per year (Takao et al., 2015). However, other factors besides temperature also limit the distribution of tropical shallow corals at high latitudes, such as lowered pH, which generates a decay of the carbonate ions necessary for the formation of coral skeletons, or low light intensities that may concurrently limit the photosynthesis of the symbionts (Couce, Ridgwell, & Hendy, 2012; Guan, Hohn, & Merico, 2015; Hoegh-Guldberg, 2012; Kleypas, McManus, & Meñez, 1999). In addition, local disturbances, such as eutrophication, sedimentation or overfishing, can also negatively affect the health of corals (Carpenter et al., 2008). The worldwide degradation and loss of coral reefs entails the loss of their associated ecosystem services, because, as foundation organisms, they provide shelter and food for marine fauna, coastal protection against wave erosion, as well as resources for the livelihood of about half a billion people (Edwards & Gomez, 2007; Jones, McCormick, Srinivasan, & Eagle, 2004). The identification of areas where corals can be most affected by these environmental changes is key for adequate conservation plans. Within this context, only few studies have aimed to predict changes, under climate change scenarios, in the distribution of corals (Chefaoui, Casado-Amezúa, & Templado, 2017; Guinan, Brown, Dolan, & Grehan, 2009; Tittensor et al., 2009; Woodby, Carlile, & Hulbert, 2009), including tropical corals (Couce, Ridgwell, & Hendy, 2013; Franklin, Jokiel, & Donahue, 2013; Freeman, Kleypas, & Miller, 2013; Rodriguez, Garcia, Carreño, & Martínez, 2019; Rodriguez, Martínez, & Tuya, 2019).
There are several modelling approaches for predicting the impacts of climate change on the geographic distribution of species (Guisan & Thuiller, 2005; Guisan & Zimmermann, 2000), but the most popular technique is the Species Distribution Model (SDM) (Elith, Kearney, & Phillips, 2010). This modelling correlates the presence records of a species with environmental conditions to produce maps of projected habitat suitability, allowing extrapolations under future climate scenarios (Elith et al., 2010). Predictive modelling is a useful tool for supporting conservation decision making (Guisan et al., 2013; Liu, White, Newell, & Griffioen, 2013). Modelling is especially relevant for species inhabiting habitats difficult to sample, as subtidal marine species, e.g., kelp forests (Franco et al., 2017; Martínez, Viejo, Carreño, & Aranda, 2012; Méléder, Populus, Guillaumont, Perrot, & Mouquet, 2010) and corals (Couce et al., 2013; Franklin et al., 2013; Freeman et al., 2013; Riul et al., 2013; Rodriguez, Garcia, et al., 2019). One of the most common methods to relate the distribution of species with climate and other physical factors is the Maximum Entropy Model (MaxEnt) (Phillips, Anderson, & Schapire, 2006). Compared to other statistical approaches, MaxEnt offers high predictive performance when species-absence data are unavailable (Jueterbock et al., 2013), as commonly occurs in the marine environment.
In this study, we focus on a scleractinian tropical coral species, Pavona varians, whose geographic distribution extends over the tropics (excepting the Atlantic), being abundant across the eastern Pacific, as well as in islands from Costa Rica, Colombia and the Clipperton Atoll (Glynn & Ault, 2000; Glynn, Veron, & Wellington, 1996; Guzmán & Cortés, 1992; Guzmán & Cortés, 2001). Due to its strictly tropical distribution, it can be considered a potential species whose centre of distribution will likely decline, if the effects of climate change are maintained according to the IPCC projections (Couce et al., 2013). However, this species is a stress-tolerant coral (Darling, Alvarez-Filip, Oliver, Mcclanahan, & Côté, 2012), which can cope with environmental stress and habitat loss, appearing in many regions where most tropical corals are declining (Wilkinson, 2006); the species is categorized by the UICN as of “Least Concern”. It is a broadcast spawner with a hermaphrodite mode of reproduction, showing increased spawning at high temperatures (Glynn, Colley, Ting, Maté, & Guzmán, 2000). These traits lead us to question if the habitat suitable for this species would decline by the negative effects of climate change, as generally predicted for coral reefs (Couce et al., 2013) or, alternatively, the available habitat would be maintained, withstanding conditions of an intermediate IPCC scenario. This study has a dual aim: understanding the relative importance of environmental variables driving the current distribution of P. varians and predicting potential habitat shifts under a future intermediate climate scenario. Additionally, the results obtained can be used to support the conservation decision making process to improve the preservation of the species.
2 MATERIAL AND METHODS
2.1 Distributional records
Scientific bibliography and three different databases were used to obtain presence records, including: the World Register of Marine Species (WoRMS), the Global Biodiversity Information Facility (GBIF), and the European Marine Observation Data Network (EMODnet). These databases containe georeferenced information of species' records (last accessed, December 2014); when coordinates were not available, a description of the name of location was provided. In these cases, locations were searched with Google Maps and Mapcarta to find the exact points in which P. varians was registered. Duplicated records in distances <0.1° were discarded, resulting in 68 reliable coordinates out of 350 presences of P. varians around the Indian and Pacific oceans.
2.2 Environmental variables
A total of 12 environmental variables, at a raster resolution of 0.08° (~9.2 km2), were downloaded from Bio-ORACLE (Table S1), a database of global predictors for marine species modelling (Tyberghein et al., 2012; http://www.oracle.ugent.be/, downloaded on March 2015). The layers were up-scaled to match the resolution of records (0.1°), using bilinear interpolation (De Natale, Desoli, Giusto, & Vernazza, 1993). Environmental variables were typified using several metrics, particularly the maximum and minimum, as they are directly linked to an organism' tolerance thresholds, computed from different time periods between 1997 and 2010 (Tyberghein et al., 2012). For further details on how the variables were computed from satellite sensors, see Tyberghein et al. (2012). We here included: Sea Surface Temperature (SST), the concentration of Nitrate and Phosphate, Chlorophyll a (Chla), Dissolved Oxygen (DissOx), ocean acidity (pH), Salinity, the percentage of Cloud cover (Cloud), the Available Photosynthetic Radiation (PAR), and the Diffuse attenuation at 490 mm (m−1) of the water column (Da).
A GIS project, implemented in ArcGIS 10.1 (ESRI), was used to manage all spatial information. Environmental variables were restricted to depths <100 m to exclude unsuitable seabed areas. To avoid autocorrelation between variables, a Spearman correlation matrix (Sokal & Rohlf, 1969) was obtained. When a pair of predictors were highly correlated, rs > |.85|, only the variable more related to coral biogeography was selected for subsequent analysis. The variables finally chosen for modelling were: maximum and minimum SST (SSTmax and SSTmin, respectively), Nitrate, Phosphate, Salinity, pH, maximum Cloud, PAR and Da (Cloudmax, Parmax and Damax, respectively). Multicollinearity through Variance Inflation Factor (VIF) was explored; all variables had VIF values <5 (Chatterjee & Hadi, 2015).
2.3 Species Distribution Modelling
We used the Maximum Entropy Modelling (MaxEnt, v. 3.3.3k, Phillips et al., 2006; Phillips & Dudík, 2008) to explore the importance of variables defining habitat suitability of the species. MaxEnt relates the presence records of the species with the environmental variables of a map to predict the environmental suitability of the species (Phillips et al., 2006). MaxEnt generates background data, which is useful in global marine studies where absences are unavailable, as occurred in this study. These background points define the available environment by including locations where the species is known to occur. After exploring the model with different settings (Moreno-Amat et al., 2015), we chose those which better fitted the response curves of habitat suitability to environmental predictors. To make this possible, we graphically explored the range of environmental conditions related to the presence records, by constructing histograms for each variable. Then, we chose MaxEnt features that matched better with the shape of the records versus environmental histograms. For example, if curve shapes are detected by the histograms, a quadratic feature would be advisable. Equivalently, if a descendent linear threshold is detected, “hinge” would be the best option to choose. Therefore, our model was constructed with linear, quadratic and hinge setting functions which are recommendable to detect physiological tolerance thresholds (Merow, Smith, & Silander, 2013; Moreno-Amat et al., 2015). The model was implemented with automatic regularization and β-multipliers (since models previously tested with high values oversimplified the response of some variables), and a maximum number of iterations of 1,000.
We aimed to develop a parsimonious reduced model, discarding the variables with limited contribution for projecting the current and future habitat suitability of P. varians. Models with few variables are preferred for explicative and predictive purposes (Austin, 2002; Elith et al., 2010; Merow et al., 2014; Moreno-Amat et al., 2015). MaxEnt shows the percent contribution of each variable to the model, i.e., the amount of variation provided by each variable. A permutation importance score is provided for each variable to test its contribution. In addition, a coefficient of importance, calculated through a Jackknife randomization, estimates the predictive power lost every time a variable is left out from the model, while its contribution is assessed when it is the only variable considered (Phillips et al., 2006). The variables with a percent contribution and permutation importance <10, and those of least importance according to Jackknife coefficients, were discarded when making projections. Additionally, we compared and related the environmental histograms and the environmental variable maps with the habitat suitability map projected by MaxEnt under current conditions.
2.4 Projections and validation
The reduced model, including only the most important variables selected by MaxEnt (see results), was used to predict the habitat suitability of P. varians under current and future conditions. The layers containing future environmental conditions of maximum and minimum sea surface temperature were downloaded from Bio-ORACLE, specifically for the A1B scenario for the year 2100 (Nakicenovic et al., 2000). This scenario considers an intermediate level of pollution, with a global CO2 emission of 750 ppm from energy and industry (IPCC, 2000), and temperatures between 1.7 and 4.4°C higher relative to nowadays (Meehl et al., 2007). These layers were rescaled and constrained to the coastline, following the aforementioned protocol, and then incorporated in MaxEnt to create a projected map of habitat suitability for 2100. As environmental projected layers were only available for SSTmin and SSTmax, we selected current conditions for the other variables. Finally, projections from the current and future climatic scenarios were compared to detect potential habitat expansions and/or contractions.
To quantify the performance of the models, we used the value of the area under the curve (AUC), operated by the Receiver Operating Characteristic curve (ROC) (Phillips et al., 2006). An internal partition of the data was applied, in which 70% of the data were randomly selected for training and 30% for testing; this procedure was repeated 10 times. Models with mean AUC values greater than 0.85 indicate good discrimination between suitable and unsuitable areas (Araújo, Pearson, Thuiller, & Erhard, 2005; Swets, 1988).
3 RESULTS
3.1 Presence records
We gathered 68 reliable sites of current presence of P. varians, distributed across the equatorial zones of the Pacific and Indian Ocean (Figure 1a), which corresponded to previously described areas (Glynn & Ault, 2000; Glynn et al., 1996; Guzmán & Cortés, 1992, 2001; Hoeksema, Rogers, & Quibilan, 2014). The species is distributed across Pacific archipelagos and atolls, such as Papua New Guinea, Guam, Northern Mariana Islands, Kiribati and Marshall Islands (Micronesia), Fiji, Cook Islands, French Polynesia, Hawaii and Pitcairn Islands. Closer to the American continent, it is found in Galapagos (Ecuador), Isla Uva (Panamá) and Isla Gorgona (Colombia). In the Indian, it appears in Seychelles, Maldives and Coco Islands. Between the Indian and Pacific Ocean, P. varians is extended from Singapore to South of Japan (Okinawa), Papua New Guinea, northern Australia and New Caledonia; the largest amount of presences is found in Indonesia. The northern latitudinal boundary is found on Midway Island (Hawaii, 28°13′N); the southern latitudinal boundary is found on Henderson Island (Pitcairn Islands, 24°22′S), both in the Pacific.
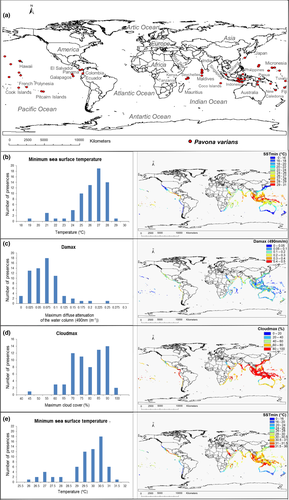
3.2 Selection of predictive variables
The full model was trained with the nine variables previously selected. After running this model with MaxEnt, a set of variables that significantly contributed to explain the geographic distribution of the species, according to a % contribution, or permutation importance >10 (Table 1a), were selected for a final predictive model (Table 1b). SSTmin had the greatest explanatory power, followed by Damax, Cloudmax and SSTmax. The five remaining variables (Parmax, Nitrate, Phosphate, pH and Salinity), whose contribution was minor, were, therefore, discarded. These results were congruent with the Jackknife's test (Figure S1), showing that Damax was the variable that contributed with the highest gain (0.22), while SSTmin was the variable that most decreased the predictive power of the model when omitted.
| Environmental variable | Percent contribution | Permutation importance |
|---|---|---|
| a) Full model | ||
| SSTmin | 26.1 | 42 |
| Damax | 19.5 | 12.2 |
| Cloudmax | 16.4 | 5.8 |
| SSTmax | 13.6 | 17.2 |
| Parmax | 7.6 | 3.3 |
| Nitrate | 4.8 | 3.5 |
| Phosphate | 4.5 | 4.3 |
| pH | 3.8 | 3.2 |
| Salinity | 3.7 | 8.5 |
| b) Reduced model | ||
| SSTmin | 41.5 | 56.2 |
| SSTmax | 23.3 | 25.5 |
| Damax | 19.2 | 4.8 |
| Cloudmax | 16 | 13.8 |
3.3 Habitat suitability at current conditions
According to the histogram relating coral presences to SSTmin values (Figure 1b-left); the minimum SST with occurrences of P. varians ranged between 19 and 29°C, with the highest frequency between 25 and 28°C. The latter range corresponded to the Indian and Pacific islands (e.g., Mauritius, Maldives, Philippines and Micronesia). Preference for lower SSTmin values occurred only at high latitudes in the Pacific, e.g., Hawaii and Japan (ranging from 20 to 22°C), and close to the equator in Galápagos (between 16 and 22°C), due to the cold current of Humboldt, which causes lower temperatures than those expected for this latitude. Minimum temperatures >28°C showed a marked decrease in the species' presence (Figure 1b-left), i.e., just one occurrence (Tuvalu) corresponding to 29°C and none above this temperature.
According to projections of habitat suitability under current climatic conditions, high suitability values were estimated for coasts from the middle western America (e.g., Panama and El Salvador) and for islands and peninsulas from low latitudes in the Indian (e.g., Mauritius, Maldives and Thailand) and the Pacific (e.g., Philippines and Micronesia; Figure 2a). The exception was the coasts from Ecuador and Galapagos, due to the regional effect of the cold Humboldt current (Figure 2a).
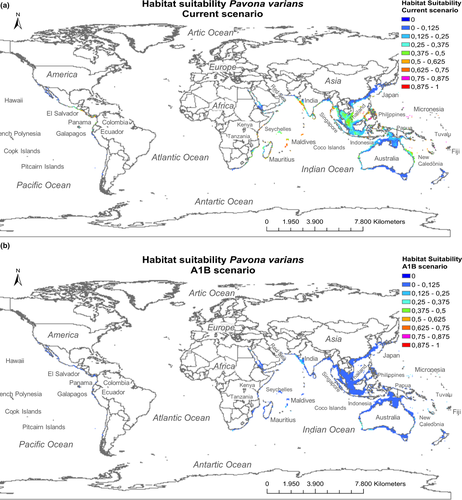
By large, SSTmin marked the latitudinal distribution limits of P. varians, being the variable with the highest importance in the SDM (Table 1b). However, habitat suitability varied within tropical regions because of the influence other variables. In terms of maximum sea surface temperatures, P. varians appeared in cells with temperatures up to 31.5°C; the most favourable temperatures ranged from 28 to 30.5°C (Figure 1e-left). These temperatures appeared in oceanic regions close to the equator, such as central America, India, Indonesia and the Philippines (“Coral Triangle”), north-eastern Australia and Micronesia. Extremely warm temperatures, >31.5°C, were not adequate for the coral, showing low suitability, e.g., in northern Australia and the Red Sea. Despite SSTmax did not limit the northern and southern latitudes in the distribution of P. varians, this variable limited the coral distribution in certain areas of high temperatures. For this reason, it was the variable with the second % of contribution in the SDM (Table 1b).
Within the latitudes bounded by SSTmin and areas with no lethal temperatures marked by SSTmax, the coral showed a preference for areas with low diffuse attenuation, up to a maximum of 0.25 nm/m (Figure 1c-left). The lowest values of Damax (<0.1 nm/m) were found in small islands, where the occurrence of the coral was high (Figure 1c-left-right). Values of diffuse attenuation >0.25 nm/m were found in continental coasts (Figure 1c-right), where projected habitat suitability was low, with the exception of certain eastern African coasts (e.g., Kenya) and central America (e.g., Ecuador), which have Damax values between 0.1 and 0.2 and habitat suitability >0.5. Larger islands, such as those between the Indian and Pacific Oceans, also had high diffuse attenuation values, as do continents, and so their habitat suitability was also low (>0.25; Figure 2a). Damax was the third variable with the greatest capacity to explain the distribution of P. varians (Table 1b), explaining differences of habitat suitability between islands and continental coasts.
Presences of P. varians appeared under cloud covers >45% (Figure 1d-left); the highest frequencies were found under cloud covers between 70% and 90%. Much of the areas with a cloud cover >70% appeared in the tropical continental coasts and islands (Figure 1d-right), coinciding with the Intertropical Convergence Zone (ITCZ). Current habitat suitability values >0.5 were always found under a cloud cover >40% (Figure 2a). Therefore, the variable Cloudmax had an explanatory importance on the distribution of P. varians.
3.4 Model validation and future habitat suitability
The model showed an excellent performance, i.e., mean AUC value = 0.925. All the presence records appeared in areas of high suitability (Figures 1a and 2a); the response curves of the SDM (Figure S2) showed similar patterns relative to those of the histograms (indicating the environmental ranges where the species was present, Figure 1).
According to future projections a notable thermal threshold was detected within the training data, with a decline reaching habitat suitability = 0 at a temperature of 35.5°C; therefore extrapolations of temperatures >35.5°C correspond to a value of habitat suitability = 0 in the future projection. A general and acute decrease of habitat suitability of P. varians was predicted by the year 2100. All regions had habitat suitability values very low, from 0 to 0.25, indicating major unsuitability for P. varians (Figure 2b).
4 DISCUSSION
Detecting and understanding the main variables that drive the global distribution of a specific coral can guide and reduce the efforts of habitat conservation managers by focusing on the most relevant variables. SDMs can detect which are these variables, by extracting their importance in a geographic model built with remotely-sensed data. The main predictors can be considered first when managing strategic conservation plans at global scales (e.g., top-down management of large ocean Marine Protected Areas (MPAs) (Gaymer et al., 2014; Toonen et al., 2020); leaving other potential impact factors for secondary local steps. Additionally, the prediction of the species habitat suitability can also help to prioritise their most vulnerable areas (Marshall, Glegg, & Howell, 2014). Among the set of variables considered in this study, the minimum sea surface temperature was the variable that best explained the current distribution of P. varians in tropical latitudes. The results also suggested the importance of the maximum sea surface temperature, showing a thermal threshold at 31.5°C where the coral does not appear, most likely, because the seawater temperature is too high for its survival. Other variables, such as the maximum cloud coverage and the coefficient of diffuse water attenuation, were also important in determining the habitat suitable for P. varians. In summary, the best suitable areas were those with minimum sea surface temperatures from 24 to 29°C and maximum sea surface temperatures from 28 to 30.5°C. Such areas mostly occur in equatorial zones, also characterized by low diffuse water attenuation and high cloud cover, except in areas of high runoff that promoted increased light attenuation. Nutrients, pH, diffuse attenuation and salinity were not relevant factors in the SDM because their variability did not affect the species distribution, or their local effects were masked by the global effects of the other variables here selected. Thus, they should be tackled in second place, in more specific local approaches.
The minimum seawater temperature marked the northern and southern limits in the distribution of P. varians across the Pacific and Indian Ocean. An exception was the southern limit in south America, near the Ecuador (equatorial region), as a result of the upwelling of cold waters associated with the Humboldt current (Humboldt, 1856). Suitable temperatures for corals usually range between 20 and 30°C (Badenas & Aurell, 1999; Nybakken, 2001), with the best range between 25 and 29°C (Kinsman, 1964). Simulation experiments to detect thermal thresholds of a battery of tropical corals (Jones, Randall, & Wilder, 1976) suggested that mean upper tolerances varies between 30 and 33°C. Specifically, for P. varians, upper limits range between 30.5 and 32.5°C. These temperatures closely coincided with those accounted by the histograms of this study, since P. varians showed presence in cells between 19°C (SSTmin) and 31.5°C (SSTmax), with high frequencies between 24 and 29°C for SSTmin. The minimum temperature limit of P. varians extracted from our data was 19°C; this limit is common for hermatypic corals, because they contain symbiotic organisms (zooxanthellae) that require warm temperatures. The cold temperature intolerance of the coral is dependent on the cold photosynthetic tolerance of the zooxanthellae; when seawater temperature declines, the photosynthetic potential of the zooxanthellae also decreases, reducing the fitness of the holobiont (Thornhill, Kemp, Bruns, Fitt, & Schmidt, 2008). A study by Suwa, Hirose, and Hidaka (2008) applied to species of the genus Pavona (P. divaricata and P. decussata) showed that low temperatures (<18°C) decreased the photosynthetic yield of their symbionts, which explains the limitation of the minimum temperature in the distribution of corals.
As most corals, P. varians requires clear waters (Castro & Huber, 1997; Ceh et al., 2013; Nybakken, 2001). Waters with a low diffuse attenuation (<0.25) is typical of zooxantheallae corals, as sunlight can penetrate easily into the water column, providing sufficient light to carry out the photosynthesis (Castro & Huber, 1997; Lesser, Slattery, & Leichter, 2009; Nybakken, 2001). Continental coasts generally have more land erosion and pollution than archipelagos, what is associated with turbid waters, via terrestrial runoff (Fabricius, 2005). This could have contributed to the low habitat suitability of these regions, as predicted by the current SDM. Only small continental coasts, e.g., Kenya, in Africa, and Ecuador, in central America, had low diffuse attenuation and, consequently, high suitability values, coinciding with areas of low human impacts (Halpern et al., 2008).
According to our study, P. varians lives in regions with a high percentage of cloud cover, ranging from 70% to 90%. Tropical regions, typically within the ITCZ, commonly have a monsoon climate, including a wet season with abundant rain, and a dry season with less rain. Although corals need sunlight, clouds can provide protection from extremely hot temperatures. In this sense, endosymbionts inside zooxanthellate corals, as occurred with P. varians, can emit dimethyl sulfide (DMS), which is released into the atmosphere, facilitating cloud condensation (Charlson, Lovelock, Andreae, & Warren, 1987; Fischer & Jones, 2012; Jones & Trevena, 2005; Leahy, Kingsford, & Steinberg, 2013; Swan, Jones, & Deschaseaux, 2012). The ability to release DMS, and produce cloud cover above reefs, is a mechanism to regulate environmental conditions, maintaining the maximum seawater surface temperature below 30°C. This mechanism protects corals from possible water temperature anomalies and buffer strong solar radiation leading to reef whitening (Kleypas, Danabasoglu, & Lough, 2008; Leahy et al., 2013; Swan et al., 2012; Thompson & van Woesik, 2009).
The coral P. varians is a stress-tolerant species (Darling et al., 2012), behaving as a robust and opportunistic species under environmental stress (Wilkinson, 2006), being the first to colonize denuded areas (Guzmán & Cortés, 1992; Guzmán & Cortés, 2001). This species is expected to acclimatise better to global warming than their branched counterparts (Loya et al., 2001). For example, P. varians recruitment success was favored by positive sea surface temperature anomalies, ranging from 0.5 to 1.5°C, at Uva Island (Panamá) (Glynn et al., 2000). According to Glynn et al. (2000), densities of Pavona in the eastern Pacific declined after the strong Southern Oscillation of El Niño (ENSO) in 1982 and 1983; 10 years later, the number of colonies in Caño Island (Costa Rica) and Uva Isla (Panama) were reestablished to pre-ENSO levels. In addition, Sheppard, Harris, and Sheppard (2008) detected a marked increase in the cover of juvenile colonies of P. varians in 2006, after a high mortality event caused by a period of extreme temperatures in 1998 in Chagos. This archipelago, located in the center of the Indian Ocean, coincides with an area in which the current habitat suitability map showed high probability of favorable conditions. Initially, this body of research suggests this species may colonize new territories, while coping with climate change, as already suggested for other species, e.g., Acropora spp. (Takao et al., 2015). However, Vargas-Angel et al. (2019) recorded an 85% decline in the Pavona densities in Jarvis Island (Pacific ocean), after an El Niño event in 2016, compared to pre-bleaching levels observed in 2015. Additionally, Glynn et al. (2000) also detected a limit in recruitment when monthly maximum SST anomalies exceeded 1.6°C. Moreover, van Woesik, Irikawa, and Loya (2004) demonstrated that P. varians showed no bleaching after anomalous high temperatures in 1998, but it was less resistant after the high temperatures of 2001. The absence of P. varians under temperatures >31.5°C (e.g., northern Australia), along with the massive decline in almost all equatorial and tropical regions predicted by our future SDM, is consistent with these results. This outcome coincides with Couce et al. (2013), stressing future declines in the habitat suitable for coral reefs in tropical latitudes, due to seawater temperature increases. As a result, the initial ability of P. varians to withstand environmental stress may not be enough to persist under extremely high temperatures. The maximum thermal tolerance of the species, according to the response curves of this study, is at 31.5°C (habitat suitability <0.3), which will occur across most of the entire study region by 2100 (Figure S3), likely causing a general loss in the suitable habitat. Those regions with temperatures <31.5°C (e.g., southern Australia) would not be suitable, due to restrictive values of other relevant factors driving P. varians distribution (i.e., damax and cloudmax). Declines in habitat suitability do not necessarily mean that that the species will be extinct in the future. Rather, this suggests that the habitat will not be adequate according to the values used within the training data. Obviously, the incorporation of other sources of information, e.g., physiological and genetic adaptation of corals, dispersal and species interactions, or the effects of local anthropogenic disturbances, would improve the reliability of SDMs projections (Diniz-filho et al., 2019; Donner, 2009; Gardner & Gaston, 2019; Rodriguez, Garcia, et al., 2019; Singer, Schweiger, Kühn, & Johst, 2018). Likewise, corals' symbionts can differ among species and play an important role in corals adaptation (Baker, Starger, McClanahan, & Glynn, 2004; Rodríguez et al., 2019). Therefore, further research, considering also corals' symbionts in SDMs would improve the robustness of its predictions. We must be aware that the use of SDMs have some limitations, but these tools are still useful to generate relevant information at broad scales.
In summary, among the variables studied in this research, the main climatic variable determining the global distribution of P. varians was the minimum sea surface temperature. Other relevant variables contributing to explain the distribution of P. varians were diffuse water attenuation, cloud cover and maximum sea surface temperature. Despite being considered a stress tolerant coral, our future SDM predicts an evident decrease in the habitat suitable for this coral, coinciding with global ocean warming. This research provides relevant information to support further management decisions to conserve the species in the future decades.
ACKNOWLEDGEMENTS
Laura Rodríguez was supported by a FPU fellowship (Formación del Profesorado Universitario) from the Spanish Ministry of Education, Culture and Sports (Ref AP2012-3702). We want to thank María Ruiz and Maite Goñi for sharing their experiences with modelling, and the two anonymous reviewers for their constructive criticism.
CONFLICT OF INTEREST
On behalf of all authors, the corresponding author states that there is no conflict of interest.




