Distribution of the plankton assemblages during the winter and summer along the southern coast of the Kerkennah Islands (Tunisia, Eastern Mediterranean Sea)
Abstract
We studied the distribution of microphytoplankton, ciliates and mesozooplankton in relation to environmental factors in the coastal waters around the Kerkennah Islands. Diatom species were more abundant in winter, representing 74% of the total microphytoplankton abundance, whereas dinoflagellate abundance was higher in summer (58%). Naked ciliates dominated during the winter (73%) and loricate ciliates dominated during the summer (67%). Copepods were the most abundant mesozooplankton group present during the entire period of study, comprising 93%–98% of the total mesozooplankton community. The results indicate that (i) species distribution differed significantly between winter and summer, (ii) environmental factors such as temperature, salinity and nutrients influenced plankton assemblages; and (iii) the highest abundance of several pollution indicator species was due to direct exposure to the polluted coast of Sfax and the effect of tidal asymmetries generating nutrient-rich inputs from the city.
1 INTRODUCTION
Plankton represent the base of the marine food web and thus play an important role in fisheries (Drira et al., 2017). Primary productivity and plankton growth are directly related to the physico-chemical factors of seawater (Khwaja et al., 2014). Plankton communities are known to respond rapidly to fluctuations in environmental parameters, especially in coastal areas where the combination of land and marine influences drives strong spatio-temporal variability (Siokou-Frangou, 1996). Phytoplankton represent a major part of the eukaryotic primary production in marine ecosystems and play a significant role in the carbon cycling and energy flow in marine planktonic communities (Drira et al., 2008). Phytoplankton are typically classified as autotrophs, heterotrophs or mixotrophic species; in dinoflagellates, chloroplast pigments reveal the nutritional mode (Ben Brahim, Feki, Feki-Sahnoun, Mahfoudi, & Hamza, 2015a). Zooplankton are an essential component of the marine ecosystem. Among zooplankton, copepods are the most abundant group (Kiørboe, 2011). They are the principal grazers in the pelagic marine food web, representing the main pathway for transferring matter and energy from primary producers to the higher trophic levels (Ben Ltaief et al., 2017). The diversity of copepods reflects the physical and chemical properties of the water, as well as the availability of their prey, mainly phytoplankton and ciliates (Richardson, 2008). Ciliates also constitute an important zooplankton group (Hannachi et al., 2009). Marine planktonic ciliates are a major and diverse group of microzooplankton and they are divided into loricate ciliates and naked ciliates. Much attention has been given to their role as consumers of pico- and nanoplankton, as well as nutrient regenerators and as an important food source for metazooplankton and fish larvae (Elloumi, Drira, Guermazi, Hamza, & Ayadi, 2015). Therefore, investigation of the relationship between these different components of the food chain can highlight the state of the environmental conditions at a studied site.
Several studies have been conducted around the Sfax coast focusing on the spatial and seasonal variation of ultraphytoplankton and prokaryotes (Rekik, Denis, Barani, Maalej, & Ayadi, 2014), microphytoplankton (Rekik et al., 2012; Rekik, Ben, Ayadi, & Elloumi, 2016a; Rekik, Denis, Maalej, & Ayadi, 2015a; Rekik, Maalej, Ayadi, & Aleya, 2013a), ciliates (Rekik, Ben, Ayadi, & Elloumi, 2016b; Rekik, Denis, Aleya, Maalej, & Ayadi, 2013b; Rekik, Denis, Maalej, & Ayadi, 2015b; Rekik, Elloumi, Charri, & Ayadi, 2015c) and zooplankton (Rekik et al., 2012,2013a). The first investigation focused on the Kerkennah Islands concerned the distribution of the epifauna and epiflora on Posidonia oceanica leaves under the influence of regional water circulation (Ben Brahim et al., 2013) and seasonal fluctuation of heterotrophic dinoflagellates (Ben Brahim et al., 2015a) and diatoms (Ben Brahim, Feki-Sahnoun, Feki, Mahfoudi, & Hamza, 2015b). No specific studies have been conducted in the Kerkennah Islands on the spatial distribution and composition of ciliates and mesozooplankton communities. The objectives of the present study were: (i) to study the spatial and seasonal distribution of plankton community structure in relation to environmental factors; (ii) to examine the effect of industrial pollution on the water quality and the biological features. To the best of our knowledge, there have been no previous comprehensive field studies that have included all of the same parameters found in our study.
2 MATERIAL AND METHODS
2.1 Study site
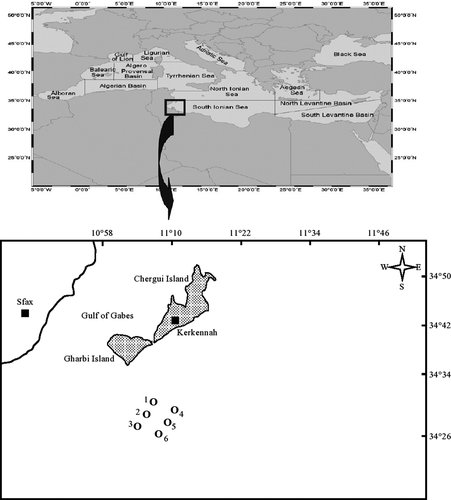
The Kerkennah Islands (34°42′N, 11°11′E; Figure 1) are composed of 10 little islands with very low landforms and a soft and fragile lithology (Ben Brahim et al., 2015b,2015a,2013). The Kerkennah Archipelago includes principally two islands: Gharbi in the west and Chergui in the east, with about 14,000 inhabitants (Trabelsi, Saad, Masmoudi, & Alfaro, 2015). The lowest areas are occupied by sabkhas, which are sterile and salty (Trabelsi & Masmoudi, 2011). The climate changes, the sea-level rise and the important variation in the local production mode and life style caused changes in the land use (Ben Brahim et al., 2015a). The Kerkennah Islands have a littoral zone subject to active water circulation (Ben Ismail et al., 2012). This water circulation is mainly affected by semi-diurnal tides that cause strong and reversing currents (Hattour, Sammari, & Ben, 2010). Tide flow around the islands is asymmetric, with relatively stronger ebb than flood tides, combined with a northward current that is occasionally strong (Ben Brahim et al., 2013), and promotes the net import of nutrients and sediments from the southeastern and northern sectors. Nutrient input originating in Sfax is rapidly flushed into the waters of the Kerkennah Islands, generating organic loads around the islands' shores and exceeding their respective concentrations by several fold (Rekik et al., 2013a).
2.2 Field sampling
To examine abiotic variables and planktonic communities, water samples were taken in winter (January 2009) and summer (July 2009) along the south of the Kerkennah Islands at six different stations (Table 1, Figure 1). The sampling stations were chosen as part of the international project Société d'Etude de Réalisation d'Aménagement et d'Hydrauliques (“SERAH”). All stations were sampled on the same day. At each station, samples for physico-chemical analyses and for microphytoplankton and ciliate identification were collected with a Van Dorn type bottle lowered at 1 m from the surface. Mesozooplankton were collected using a cylindro-conical net (30 cm aperture, 100 cm high, 100 μm mesh size). Mesozooplankton hauls were vertical. The volume of water filtered was about 1 m3. Samples for nutrient analyses (120 ml) were preserved immediately upon collection (–20°C, in the dark); those for microphytoplankton and ciliate enumeration (1 L) were preserved with Lugol (4%) iodine solution (acid Lugol solution). Mesozooplankton samples were rapidly preserved in 2% buffered formaldehyde solution after collection and were stained with rose Bengal to identify internal tissues of the different mesozooplankton species so as to facilitate dissection of copepods. Plankton samples were stored in the dark at low temperature (4°C) until analysis.
| Stations | Latitude (N) | Longitude (E) | Station depth (m) |
|---|---|---|---|
| 1 | 34º30,070′ | 11º06,116′ | 22 |
| 2 | 34º29,070′ | 11º06,973′ | 47 |
| 3 | 34º27,905′ | 11º04,406′ | 47 |
| 4 | 34º29,731′ | 11º10,630′ | 45 |
| 5 | 34º28,082′ | 11º09,944′ | 45 |
| 6 | 34º26,674′ | 11º08,223′ | 26 |
2.3 Physical and chemical factors
Temperature, salinity and pH were measured immediately after sampling using a multiparameter kit (Multi 340 i/SET). The concentration of suspended matter was determined by measuring the dry weight of the residue after filtration of 500 ml seawater with a Whatman glass fiber filter membrane filter. Nutrient (nitrite, nitrate, ammonium, orthophosphate, silicate, total nitrogen and total phosphate) concentrations were analysed with a Bran + Luebbe type 3 analyser, and concentrations were determined colorimetrically according to Grasshof (1983). Percentages of dissolved inorganic nitrogen were calculated from [(NO3− + NO2− + NH4+)/T-N] × 100, where T-N = total nitrogen. Percentages of dissolved inorganic phosphate were calculated from [PO43−/T-P] × 100, where T-P = total phosphate.
2.4 Microphytoplankton, ciliate and mesozooplankton enumeration
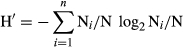
where N = total number of individuals over all species in the sample; Ni/N = frequency of species i in the sample; and n = total number of species in the sample.
2.5 Statistical analysis
The data recorded in this study were subjected to normalized principal component analyses (PCAs; Doledec & Chessel, 1989). A PCA was performed on each seasonal set of hydrological (temperature, salinity, pH, suspended matter, nutrients) and biological (abundances of microphytoplankton, ciliates and mesozooplankton groups) variables at the six stations. Simple log(x + 1) transformation was applied to the data in order to correctly stabilize he variance (Frontier, 1973). Means and SDs are reported when appropriate. The potential relationships between variables were tested with Pearson's correlation coefficients. One-way analysis of variance using XLSTAT software followed by a post hoc comparison using Tukey's test was applied to identify significant differences between winter and summer.
3 RESULTS
3.1 Physical and chemical parameters
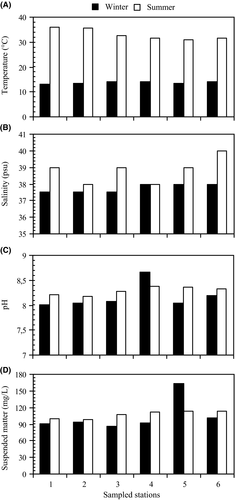
The range (min.–max.) and mean values of physico-chemical variables recorded at the six sampled points during the two-season study are summarized in Table 2 and Figures 2 and 3. The average water temperature ranged between 13 and 36°C (Table 2) with the lowest temperature detected in winter and the highest in summer at station 1. At each station, temperature showed increasing values from winter to summer. The mean temperature of the winter cruise (13.67 ± 0.41°C) was significantly different from that of the summer cruise (33.00 ± 2.19°C) (Table 2). Salinity varied from 37.50 in winter at stations 1, 2 and 3 to 40 in summer at station 6. Salinity values were low during winter (Figure 2B). The pH values ranged from 8.01 (winter, station 1) to 8.66 (winter, station 4) (Figure 2C). The mean pH values showed a non-significant difference between the two seasons. Concentrations of suspended matter varied between 85.77 (station 3) and 162.88 mg/L (station 5) during winter. Suspended matter concentration in winter was lower than that in summer, except at station 5, which had the highest value (162.88 mg/L; Figure 2D).
| Variables | Winter | Summer | F-values | p | ||||
|---|---|---|---|---|---|---|---|---|
| Minimum | Maximum | Mean ± SD | Minimum | Maximum | Mean ± SD | |||
| Physical variables | ||||||||
| Temperature (°C) | 13.00 | 14.00 | 13.67 ± 0.41 | 31.00 | 36.00 | 33.00 ± 2.19 | 512.53 | 1.53 × 10-8*** |
| Salinity | 37.50 | 38.00 | 37.50 ± 0.27 | 38.00 | 40.00 | 38.83 ± 0.75 | 6.45 | 0.03 * |
| pH | 8.01 | 8.66 | 8.17 ± 0.25 | 8.18 | 8.37 | 8.29 ± 0.08 | 0.66 | 0.44 |
| Suspended matter (mg/L) | 85.77 | 162.88 | 104.55 ± 29.03 | 98.00 | 113.88 | 107.40 ± 6.93 | 0.01 | 0.92 |
| Chemical variables | ||||||||
| NO3− (µM) | 1.96 | 2.43 | 2.22 ± 0.19 | 2.21 | 3.64 | 3.13 ± 0.50 | 10.97 | 0.01 ** |
| NO2− (µM) | 0.14 | 0.20 | 0.17 ± 0.03 | 0.31 | 0.69 | 0.46 ± 0.14 | 29.23 | 0.06 × 10-2 *** |
| NH4+ (µM) | 2.08 | 3.68 | 2.64 ± 0.65 | 0.95 | 1.99 | 1.44 ± 0.37 | 13.18 | 0.01 ** |
| Total N (µM) | 16.85 | 19.85 | 18.79 ± 1.27 | 13.90 | 18.41 | 15.47 ± 1.92 | 7.12 | 0.03 * |
| PO43− (µM) | 1.39 | 2.60 | 1.83 ± 0.48 | 0.16 | 0.32 | 0.21 ± 0.06 | 61.86 | 4.93 × 10–5 *** |
| Total P (µM) | 4.00 | 11.11 | 6.05 ± 2.59 | 1.20 | 2.61 | 2.06 ± 0.54 | 12.33 | 0.01 ** |
| N/P ratio | 1.95 | 4.03 | 2.92 ± 0.84 | 16.97 | 32.88 | 24.87 ± 6.24 | 53.42 | 8.32 × 10–5 *** |
| Si(OH)4 (µM) | 11.05 | 40.09 | 22.76 ± 11.09 | 1.52 | 5.27 | 2.96 ± 1.45 | 20.85 | 0.01 *** |
| Biological variables | ||||||||
| Microphytoplankton (×102 cells/L) | 19.00 | 107.00 | 48.50 ± 32.72 | 5.00 | 33.00 | 15.33 ± 9.83 | 4.91 | 0.06 |
| Diatoms (×102 cells/L) | 10.00 | 90.00 | 35.83 ± 29.18 | 3.00 | 9.00 | 5.17 ± 2.64 | 9.84 | 0.01* |
| Dinoflagellates (×102 cells/L) | 6.00 | 20.00 | 11.17 ± 5.27 | 1.00 | 22.00 | 8.83 ± 7.73 | 0.03 | 0.86 |
| Cyanobacteria (×102 cells/L) | 0.00 | 5.00 | 1.00 ± 2.00 | 0.00 | 3.00 | 1.17 ± 1.17 | 0.03 | 0.87 |
| Euglenophyceae (×102 cells/L) | 0.00 | 1.00 | 0.17 ± 0.41 | – | – | – | – | – |
| Dictyochophyceae (×102 cells/L) | 0.00 | 1.00 | 0.33 ± 0.52 | 0.00 | 1.00 | 0.17 ± 0.41 | 1.00 | 0.35 |
| Ciliates (×102 cells/L) | 2.00 | 53.00 | 12.33 ± 19.97 | 2.00 | 29.00 | 11.50 ± 9.46 | 0.04 | 0.84 |
| Loricate ciliates (×102 cells/L) | 2.00 | 5.00 | 3.33 ± 1.21 | 1.00 | 17.00 | 7.67 ± 5.32 | 2.87 | 0.13 |
| Naked ciliates (×102 cells/L) | 0.00 | 51.00 | 9.00 ± 20.59 | 1.00 | 12.00 | 3.83 ± 4.31 | 0.44 | 0.53 |
| Mesozooplankton (×102 ind./m3) | 61.96 | 156.26 | 102.14 ± 32.38 | 5.44 | 10.84 | 8.11 ± 2.32 | 34.69 | 3 × 10–4 *** |
| Copepods (×102 ind./m3) | 56.31 | 144.32 | 95.41 ± 29.86 | 5.19 | 10.70 | 7.92 ± 2.33 | 34.51 | 3 × 10–4 *** |
| Copepod nauplii (× 102 ind./m3) | 26.32 | 106.33 | 55.25 ± 28.66 | 0.00 | 2.14 | 0.74 ± 0.85 | 14.49 | 5 × 10–3 *** |
| Cyclopoids (×102 ind./m3) | 11.27 | 35.18 | 19.10 ± 8.46 | 0.00 | 0.58 | 0.14 ± 0.24 | 21.11 | 10–3 ** |
| Calanoids (×102 ind./m3) | 6.11 | 14.68 | 10.12 ± 3.66 | 3.77 | 10.55 | 7.01 ± 2.55 | 2.22 | 0.17 |
| Harpacticoids (×102 ind./m3) | 5.33 | 8.94 | 6.88 ± 1.47 | – | – | – | – | – |
| Poecilostomatoids (×102 ind./m3) | 2.51 | 6.37 | 4.06 ± 1.31 | 0.00 | 0.25 | 0.04 ± 0.10 | 36.56 | 3 × 10–4 *** |
| Other mesozooplankton (×102 ind./m3) | 3.12 | 11.94 | 6.73 ± 3.11 | 0.00 | 0.34 | 0.19 ± 0.15 | 31.99 | 4 × 10–4 *** |
- In the last column, the asterisks indicate different levels of significance in the ANOVA (*p < .05; **p < .01; ***p < .001).
- ind., individuals; N/P, dissolved inorganic nitrogen/dissolved inorganic phosphate.
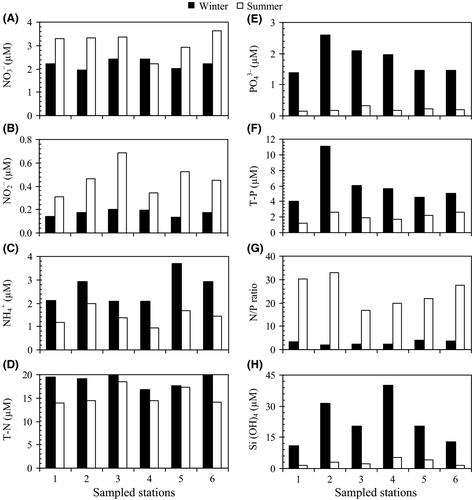
Nutrients varied significantly between winter and summer, but not among sampling stations during a given season (Table 2). Most of them were lower in summer and showed the highest values in winter, with the exception of NO3− and NO2− (Figure 3A, B). NO3− concentration varied between 1.96 and 3.64 µM in the study area (Table 2), with the highest concentration observed at station 6 in summer, whereas the highest concentration (2.43 μM) during winter was observed at station 3 (Figure 3A). The average values of NO3− concentration in winter and summer were significantly different from each other at 2.22 ± 0.19 and 3.13 ± 0.50 µM, respectively. NO2− concentration was very low in both winter and summer, varying between 0.17 ± 0.03 µM in winter and 0.46 ± 0.14 µM in summer (Table 2 and Figure 3B). NH4+ concentration varied between 0.95 µM in summer at station 4 and 3.68 µM in winter at station 5. Total nitrogen (T-N) values were about 15.47 ± 1.92 μM in summer and 18.79 ± 1.27 μM in winter. Nitrogen appeared mainly in its dissolved organic form [67.50% (summer) – 73.22% (winter)] with the dissolved inorganic form (DIN; NO3− + NO2− + NH4+) representing only 32.50% and 26.78% of the total during summer and winter, respectively. T-N and NH4+ concentrations were higher in winter than in summer, whereas NO3− and NO2− concentrations showed the opposite trend (Table 2 and Figure 3A–D). PO43− concentration was low (0.21 ± 0.06 µM) during summer, but reached its maximum value (1.83 ± 0.48 µM) during winter (Table 2). Total phosphate (T-P) concentrations varied between 1.20 μM (summer) and 11.11 μM (winter). T-P concentration values in winter were about three times higher than those in summer (Table 2). Orthophosphate concentrations accounted for 10.23% (summer) and 30.21% (winter) of T-P. The N/P ratio [dissolved inorganic nitrogen (NO2− + NO3− + NH4+) to dissolved inorganic phosphate (DIP; PO34−) ratio], ranged from 1.95 (winter) to 32.88 (summer) at station 2. N/P ratios in coastal waters in summer 2010 were about 24.87. The average N/P ratio (2.92) was less than the Redfield ratio (16) in winter, suggesting a potential N limitation. The N/P values of the winter cruise were significantly different from those of the summer cruise. The Si(OH)4 concentrations were higher during winter (22.76 ± 11.09 μM) than summer (2.96 ± 1.45 µM) (Table 2). The largest variation in silicate concentration (11.05–40.09 µM) was observed in winter (Figure 3H).
3.2 Microphytoplankton community structure and spatial distribution
A summary of the microphytoplankton species observed during the entire study period is given in Table 3A. During the investigated period, microphytoplankton abundance ranged from 5.00 × 102 cells/L (summer, station 4) to 107.00 × 102 cells/L (winter, station 1) (Table 2, Figure 4A). Diatom abundance ranged from 3.00 × 102 to 90.00 × 102 cells/L, showing a remarkable increase in winter (on average, 35.83 ± 29.18 × 102 cells/L). Diatoms were essentially represented by Navicula sp. (maximum abundance = 25.00 × 102 cells/L), Pinnularia sp. (maximum abundance = 17 × 102 cells/L), Pleurosigma sp. (maximum abundance = 11.00 × 102 cells/L) and Coscinodiscus sp. (maximal abundance = 103 cells/L) at station 1. Diatom abundance varied significantly between winter and summer, and among sampling sites (F = 6.65, df = 6, p < .05, Table 2). Dinoflagellates were numerically the second most important group and showed an important species richness. The highest (22.00 × 102 cells/L) dinoflagellate abundance was observed at station 2 and the lowest (102 cells/L) at station 4 during the summer. Dinoflagellate mean abundance was higher in winter (11.17 ± 5.27 cells/L; Table 2). Dinoflagellate diversity varied significantly with respect to season, decreasing in summer (22 species) and exhibiting a remarkable increase in winter (34 species) (Table 3A). Other microphytoplankton species were represented by Cyanobacteria (Anabaena sp., Oscillatoria sp. and Spirulina sp.), Dictyochophyceae (Dictyocha sp.) and Euglenophyceae (Euglena sp.) (Table 3A).
| (A) Microphytoplankton taxa (cells/L) | Winter | Summer | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 1 | 2 | 3 | 4 | 5 | 6 | |
| Cyanophyceae | ||||||||||||
| Anabaena sp. (Bornet & Flahault, 1886) | 100 | 100 | 100 | 100 | 100 | 300 | ||||||
| Oscillatoria sp. (Gomont, 1822) | 400 | |||||||||||
| Spirulina sp. (Gomont, 1892) | 100 | |||||||||||
| Bacillariophyceae | ||||||||||||
| Amphiprora paludosa (Smith, 1853) | 100 | 100 | 100 | |||||||||
| Amphora marina (Smith, 1857) | 400 | 100 | 100 | 100 | ||||||||
| Bacteriastrum sp. (Shadbolt, 1854) | 100 | |||||||||||
| Chaetoceros sp. (Ehrenberg, 1844) | 200 | |||||||||||
| Cocconeis sp. (Ehrenberg, 1836) | 100 | |||||||||||
| Coscinodiscus sp. (Ehrenberg, 1839) | 1000 | 200 | 300 | 200 | 100 | 100 | 200 | |||||
| Grammatophora marina (Kützing, 1844) | 100 | 100 | 300 | |||||||||
| Guinardia delicatula (Hasle & Syvertsen, 1996) | 400 | 300 | 100 | |||||||||
| Guinardia striata (Hasle & Syvertsen, 1996) | 200 | 700 | 200 | 100 | 200 | 100 | ||||||
| Gyrosigma sp. (Hassal, 1845) | 100 | |||||||||||
| Hemiaulus hauckii (Van Heurck, 1882) | 100 | 100 | 100 | |||||||||
| Hemiaulus sp. (Heiberg, 1863) | 100 | |||||||||||
| Leptocylindrus danicus (Cleve, 1889) | 200 | |||||||||||
| Licmophora gracilis (Ehrenberg, 1838) | 100 | |||||||||||
| Melosira sp. (Agardh, 1827) | 100 | |||||||||||
| Navicula sp. (Bory de St Vincent, 1822) | 2500 | 1000 | 500 | 1200 | 300 | 100 | 100 | 200 | 500 | |||
| Nitzschia longissima (Ralf, 1861) | 600 | 100 | 500 | 100 | ||||||||
| Nitzschia sp. (Hassall, 1845) | 100 | 100 | 100 | 100 | 100 | |||||||
| Odontella sp. (Grunow, 1884) | 100 | |||||||||||
| Pinnularia sp. (Ehrenberg, 1843) | 1700 | 100 | 400 | 100 | 700 | 100 | 300 | 100 | ||||
| Plagiotropis lepidoptera (Kuntze, 1898) | 100 | |||||||||||
| Pleurosigma simonsenii (Hasle, 1990) | 1100 | 600 | 400 | 100 | 100 | 100 | ||||||
| Pseudo-nitzschia sp. (Perag & Perag, 1900) | 300 | |||||||||||
| Rhabdonema adriaticum (Kützing, 1844) | 100 | |||||||||||
| Rhizosolenia alata (Van Heurck, 1882) | 100 | |||||||||||
| Rhizosolenia sp. (Brightwell, 1858) | 100 | 200 | 100 | |||||||||
| Rhizosolenia styliformis (Brightwell, 1858) | 200 | 200 | 200 | 200 | ||||||||
| Skeletonema costatum (Cleve, 1873) | 100 | |||||||||||
| Striatella sp. (Agardh, 1832) | 200 | 100 | ||||||||||
| Striatella unipunctata (Agardh, 1832) | 400 | |||||||||||
| Tabellaria sp. (Kützing, 1844) | 100 | |||||||||||
| Thalassionema nitzschioides (Mereschkowsky, 1902) | 100 | 100 | ||||||||||
| Thalassiosira subtilis (Gran, 1900) | 100 | 200 | ||||||||||
| Dinophyceae | ||||||||||||
| Alexandrium minutum (Halim, 1960) | 300 | |||||||||||
| Alexandrium sp. (Balech, 1985) | 100 | 100 | ||||||||||
| Blepharocysta sp. (Ehrenberg, 1873) | 100 | |||||||||||
| Tripos carriensis (Gomez, 2013) | 100 | |||||||||||
| Tripos furca (Gomez, 2013) | 600 | |||||||||||
| Tripos lineatus (Gomez, 2013) | 100 | |||||||||||
| Tripos pentagonus (Gomez, 2013) | 400 | 100 | ||||||||||
| Tripos sp. (Gomez, 2013) | 100 | 100 | ||||||||||
| Coolia monotis (Meunier, 1919) | 100 | |||||||||||
| Dinophysis rotundata (Claparède & Lachmann, 1859) | 100 | 100 | ||||||||||
| Dinophysis sp. (Bütschli, 1885) | 200 | |||||||||||
| Gonyaulax digitale (Kofoid, 1911) | 100 | 100 | ||||||||||
| Gonyaulax polygramma (Stein, 1883) | 100 | 100 | ||||||||||
| Gonyaulax spinifera (Diesing, 1866) | 100 | 100 | 100 | |||||||||
| Gymnodinium aureolum (Hulburt, 1957) | 100 | |||||||||||
| Gymnodinium sp. (Stein, 1878) | 200 | 200 | ||||||||||
| Gyrodinium sp. (Freudenthal & Lee, 1963) | 300 | 100 | 300 | |||||||||
| Gyrodinium spirale (Kofoid & Swezy, 1921) | 100 | |||||||||||
| Karenia selliformis (Haywood et al., 2004) | 100 | |||||||||||
| Karlodinium sp. (Larsen, 2000) | 100 | |||||||||||
| Karlodinium veneficum (Larsen, 2000) | 100 | |||||||||||
| Noctiluca scintillans (Kofoid & Swezy, 1921) | 100 | |||||||||||
| Noctiluca sp. (Macartney, 1810) | 100 | |||||||||||
| Ostreopsis sp. (Schmidt, 1901) | 200 | 100 | ||||||||||
| Oxyrrhis marina (Dujardin, 1841) | 100 | |||||||||||
| Peridinium sp. (Ehrenberg, 1830) | 100 | 200 | 100 | |||||||||
| Podolampas palmipes (Stein, 1883) | 100 | |||||||||||
| Polykrikos sp. (Bütschli, 1873) | 300 | 500 | 400 | 100 | 200 | 100 | 300 | |||||
| Prorocentrum gracile (Schütt, 1895) | 200 | |||||||||||
| Prorocentrum lima (Stein, 1878) | 100 | |||||||||||
| Prorocentrum micans (Ehrenberg, 1834) | 100 | 300 | ||||||||||
| Prorocentrum rathymum (Sherley & Schmidt, 1979) | 200 | 100 | ||||||||||
| Prorocentrum sp. (Ehrenberg, 1834) | 100 | 100 | ||||||||||
| Protoceratium sp. (Meunier, 1910) | 100 | |||||||||||
| Protoperidinium depressum (Balech, 1974) | 200 | |||||||||||
| Protoperidinium diabolum (Balech, 1974) | 100 | |||||||||||
| Protoperidinium divergens (Balech, 1974) | 100 | 100 | 100 | |||||||||
| Protoperidinium ovatum (Pouchet, 1883) | 100 | 100 | 100 | 100 | ||||||||
| Protoperidinium quinquecorne (Balech, 1974) | 100 | |||||||||||
| Protoperidinium sp. (Balech, 1974) | 100 | 300 | 100 | |||||||||
| Scrippsiella subsalsa (Steidinger & Balech, 1977) | 100 | |||||||||||
| Scrippsiella trochoidea (Stein, 1883) | 100 | 100 | 400 | |||||||||
| Dictyochophyceae | ||||||||||||
| Dictyocha sp. (Ehrenberg, 1841) | 100 | 100 | 100 | |||||||||
| Euglenophyceae | ||||||||||||
| Euglena acusformis (Schiller, 1925) | 100 | |||||||||||
| Thecofilosea | ||||||||||||
| Ebria sp. (Borgert, 1891) | 200 | |||||||||||
| Hermesinium sp. (Rhodes & Gibson, 1981) | 100 | 100 | 100 | |||||||||
| (B) Ciliate taxa (cells/L) | Winter | Summer | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 1 | 2 | 3 | 4 | 5 | 6 | |
| Loricate ciliates | ||||||||||||
| Acanthostomella norvegica (Kofoid & Campbell, 1929) | 100 | 200 | ||||||||||
| Cymatocylis sp. (Campbell, 1929) | 100 | |||||||||||
| Codonellopsis pusilla (Jörgensen, 1924) | 100 | |||||||||||
| Dictyocysta elegans (Ehrenberg, 1854) | 100 | |||||||||||
| Eutintinnus fraknoii (Daday, 1887) | 300 | |||||||||||
| Eutintinnus medius (Kofoid & Campbell, 1929) | 100 | 100 | 300 | 400 | ||||||||
| Eutintinnus sp. (Kofoid & Campbell, 1939) | 100 | 100 | ||||||||||
| Favella ehrenbergi (Claparède & Lachmann, 1858) | 300 | 100 | ||||||||||
| Helicostomella subulata (Ehrenberg, 1833) | 100 | 400 | ||||||||||
| Parundella sp. (Jörgensen, 1924) | 100 | |||||||||||
| Petalotricha ampulla (Fol, 1881) | 200 | |||||||||||
| Rhabdonella amor (Cleve, 1900) | 300 | 100 | ||||||||||
| Rhabdonella spiralis (Fol, 1881) | 200 | |||||||||||
| Salpingella subconica (Kofoid & Campbell, 1929) | 100 | 200 | 100 | |||||||||
| Tintinnidium balechi (Barra de Cao, 1981) | 200 | 100 | 200 | |||||||||
| Tintinnopsis aperta (Brandt, 1906) | 100 | 100 | ||||||||||
| Tintinnopsis beroidea (Stein, 1867) | 100 | 200 | 100 | 100 | 100 | |||||||
| Tintinnopsis lobiancoi (Daday, 1887) | 100 | |||||||||||
| Tintinnopsis radix (Stein, 1867) | 100 | 100 | ||||||||||
| Tintinnopsis sp. (Stein, 1867) | 100 | 300 | 100 | |||||||||
| Undella sp. (Meunier, 1910) | 100 | |||||||||||
| Naked ciliates | ||||||||||||
| Euplote charon (Müller, 1786) | 100 | 200 | ||||||||||
| Euplote sp. (Müller, 1786) | 100 | 300 | ||||||||||
| Holosticha sp. (Dragesco, 1970) | 100 | 100 | ||||||||||
| Leegardiella sol (Lynn & Montagnes, 1988) | 300 | 100 | 300 | |||||||||
| Lohmanniella oviformis (Leegaard, 1915) | 100 | 100 | ||||||||||
| Strobilidium sp. (Schewiakoff, 1892) | 500 | 300 | ||||||||||
| Strombidium capitatum (Leegaard, 1915) | 100 | |||||||||||
| Strombidium sp. (Claparède & Lachmann, 1859) | 200 | 200 | 100 | |||||||||
| Uronema marinum (Dujardin, 1841) | 4500 | |||||||||||
| (C) Mesozooplankton taxa (individuals/m3) | Winter | Summer | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 1 | 2 | 3 | 4 | 5 | 6 | |
| Nauplii of copepods | 5571 | 3215 | 2632 | 4755 | 6341 | 10633 | 145 | 25 | 25 | 34 | 214 | |
| Cyclopoids | ||||||||||||
| Oithona nana (Giesbrecht, 1892) | 1216 | 1846 | 1013 | 2890 | 1165 | 1152 | 58 | |||||
| Oithona similis (Claus, 1866) | 274 | 49 | 18 | 34 | 85 | 288 | 25 | |||||
| Oithona helgolandica (Claus, 1863) | 9 | 147 | ||||||||||
| Oithona plumifera (Baird, 1843) | 192 | 66 | 48 | 447 | 94 | 174 | ||||||
| Oithona linearis (Giesbrecht, 1891) | 72 | 58 | 48 | 44 | 30 | |||||||
| calanoids | ||||||||||||
| Acartia clausi (Giesbrecht, 1889) | 130 | 41 | 41 | 22 | 36 | |||||||
| Acartia discaudata (Giesbrecht, 1881) | 6 | |||||||||||
| Acartia italica (Steuer, 1910) | 58 | |||||||||||
| Paracartia latisetosa (Kriczaguin, 1873) | 29 | 25 | ||||||||||
| Acartia sp. (Giesbrecht, 1889) | 12 | 9 | 51 | 12 | 25 | |||||||
| Calanus helgolandicus (Claus, 1863) | 67 | |||||||||||
| Centropages kroyeri (Giesbrescht, 1892) | 29 | 25 | 200 | 256 | 201 | |||||||
| Centropages typicus (Krøyer, 1849) | 29 | 74 | 250 | 342 | 302 | 179 | ||||||
| Centropages hamatus (Lilljeborg, 1853) | 49 | 125 | 371 | 34 | ||||||||
| Eucalanus attenuatus (Dana,1848) | 251 | 291 | 93 | 302 | 92 | 576 | ||||||
| Eucalanus monachus (Giesbrecht, 1888) | 114 | 29 | 33 | 36 | 12 | |||||||
| Labidocera sp. (Al-Yamani & Prusova, 2003) | 25 | 29 | ||||||||||
| Paracalanus parvus (Claus, 1863) | 358 | 891 | 481 | 618 | 347 | 156 | 232 | 221 | 100 | 57 | 167 | 641 |
| Paracalanus aculeatus (Giesbrecht, 1888) | 276 | 108 | ||||||||||
| Paracalanus sp. (Al-Yamani & Prusova, 2003) | 18 | |||||||||||
| Temora longicornis (Muller, 1792) | 25 | |||||||||||
| Temora stylifera (Dana, 1849) | 12 | |||||||||||
| Temora sp. (Baird, 1856) | 143 | 33 | 169 | 139 | 114 | 54 | ||||||
| Harpacticoids | ||||||||||||
| Clytemnestra scutellata (Dana, 1852) | 17 | |||||||||||
| Euterpina acutifrons (Dana, 1852) | 382 | 411 | 400 | 674 | 397 | 402 | ||||||
| Microsetella norvegica (Boeck, 1864) | 170 | 41 | 195 | 71 | 90 | |||||||
| Microsetella rosea (Dana, 1848) | 286 | 242 | 174 | 25 | 65 | 84 | ||||||
| Corycaeus clausi (Dahl, 1894) | 120 | 48 | 173 | 51 | 78 | 25 | ||||||
| Corycaeus speciosus (Dana, 1849) | 36 | 12 | 42 | 0 | 12 | |||||||
| Oncaea conifera (Giesbrecht, 1891) | 203 | 251 | 348 | 57 | 228 | 439 | ||||||
| Oncaea mediterranea (Claus, 1863) | 72 | 18 | 90 | 51 | 102 | |||||||
| Conaea rapax (Giesbrecht, 1891) | 6 | |||||||||||
| Other mesozooplankton | 312 | 463 | 565 | 832 | 674 | 1194 | 25 | 25 | 29 | 34 | ||
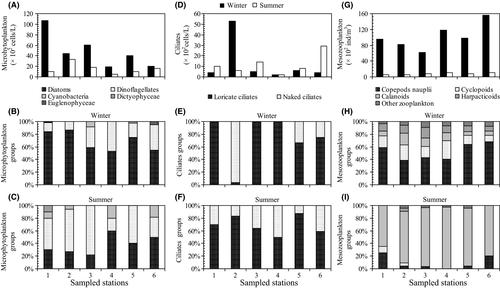
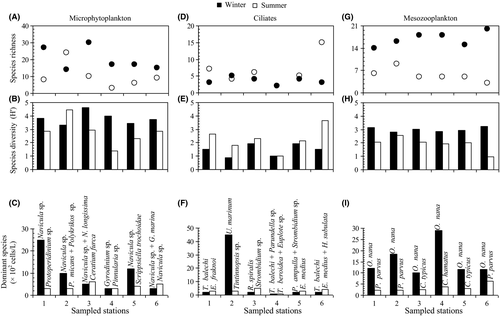
In the present study, 82 phytoplankton taxa were observed, of which 36 were identified to species level. Dinoflagellates were the richest group with 44 taxa, followed by diatoms with 33 taxa. The genus Protoperidinium (six taxa) was the most diverse among dinoflagellates and the genus Rhizosolenia (three taxa) among diatoms. Other groups such as Dictyochophyceae and Euglenophyceae were represented by only one species each. The microphytoplankton species richness varied from three taxa at station 4 in summer to 30 taxa at station 3 in winter (Figure 5A). The species diversity [Shannon index (H′)] of the microphytoplankton community was lower in summer than in winter. This was particularly clear at station 4 where microphytoplankton were represented by only three taxa (Pinnularia sp., Anabeana sp. and Gyrodinium spirale; H′ = 1.37 bits/cell, Figure 5B) during summer. H′ reached its maximum (H′ = 4.61 bits/cell, 30 species, station 3) during winter. Similar values were also recorded at stations 2 (H′ = 4.45 bits/cell, summer) and 4 (H′ = 4.00 bits/cell, winter) (Figure 5B).
3.3 Ciliate community structure and spatial distribution
Tables 2 and 3B report the abundances of the dominant groups and species for each season. Ciliates were particularly abundant at stations 2 (winter) and 6 (summer). Ciliate density ranged from 2 × 102 to 53 × 102 cells/L (on average, 11.50 ± 9.46 × 102 cells/L in summer, 12.33 ± 19.97 × 102 cells/L in winter; Figure 4D). Loricate ciliates were observed at all stations although they were poorly represented in terms of abundance. The majority of loricate ciliates was found in winter (100% of total ciliate abundance, Figure 4E). Loricate ciliate abundance varied from 102 to 17.00 × 102 cells/L; the highest value was observed in station 6 during summer, associated with a large proliferation of Eutintinnus medius and Helicostomella subulata (Table 3B and Figure 5B). The mean naked ciliate abundance was 3.83 × 102 and 9.00 × 102 cells/L in summer and winter, respectively. Naked ciliates were observed in great abundance during winter (73% of total ciliate abundance) whereas they were poorly represented during summer (33% of total ciliate abundance, Figure 4F).
The ciliate community consisted of 30 taxa (15 taxa in winter and 21 taxa in summer) belonging to 21 genera and two groups: loricate ciliates and naked ciliates. Loricate ciliates were the most diverse ciliate group with 21 taxa (12 and 14 taxa in winter and summer, respectively). The genus Tintinnopsis was dominant among loricate ciliates (five taxa), followed by Eutintinnus (three taxa) (Figure 5F, Table 3B). Ciliate richness, considered as a whole, was higher in summer than in winter at stations 1, 3 and 6 (Figure 5D). Ciliate community diversity increased from station 2 in winter (H′ = 0.90 bits/cell, five taxa) to station 6 in summer (H′ = 3.68 bits/cell, 15 taxa). The lowest value of H′ was associated with a great proliferation of Uronema marinum (45 × 102 cells/L, station 2, winter; Figure 5E, F).
3.4 Mesozooplankton community structure and spatial distribution
A summary of the mesozooplankton species observed throughout the study period is given in Table 3C. The total mesozooplankton abundance varied from 5.44 × 102 individuals (ind.)/m3 (station 2, summer) to 156.26 × 102 ind./m3 (station 6, winter) (Figure 4G), the highest mean abundance being recorded in winter (102.14 ± 32.38 × 102 ind./m3, Table 2). Low densities of mesozooplankton were recorded during summer (8.11 ± 2.32 × 102 ind./m3, Table 2), a 12–13-fold drop compared to winter (Figure 4G). Copepods clearly dominated the mesozooplankton community during the two seasons, accounting for 93%–98% of total mesozooplankton abundances (Figure 4H, I). The largest copepod abundance values were observed at stations 4 (109.89 × 102 ind./m3) and 6 (144.32 × 102 ind./m3) in winter. During summer, copepod abundance did not exceed 10.70 × 102 ind./m3 (Table 2). High numbers of copepod nauplii were observed, ranging between 0 and 106.33 × 102 ind./m3. The mean values of copepod nauplii abundance in summer and winter were 0.74 ± 0.85 × 102 and 55.25 ± 28.66 × 102 ind./m3, respectively (Table 2). Calanoids dominated with the largest percentage (10%–86% of total mesozooplankton), followed by cyclopoids (2%–19% of total mesozooplankton). Harpacticoids varied from 0% to 7% and were exclusively present in winter (6.88 ± 1.47 × 102 ind./m3) and poecilostomatoids varied from 0.5% to 4% (Figure 4H). Other mesozooplankton presented low abundances during the entire study period (2%–7% of total mesozooplankton abundance, Figure 4H, I). The majority of other mesozooplankton (Cladocera, Polychaeta larvae, Amphipoda, Appendicularia, Euphausiacea and Neogasteropoda) was found in winter (between 3.12 × 102 and 11.94 × 102 ind./m3; Table 2). Mesozooplankton composition was significantly different (p < .001; Table 2), except for calanoids and harpacticoids, between the two seasons.
Mesozooplankton assemblages were dominated by copepods with a total of 33 species. Copepod richness was more pronounced in winter (25 species) than in summer (14 species) (Figure 5G, Table 3C). The diversity index (H′) varied between the six stations and the two seasons. Diversity was more pronounced in winter than in summer. During summer, the H′ varied between 0.98 bits/cell (station 6) and 2.57 bits/cell (station 2). It was higher in winter, varying in the range of 2.81 bits/cell (station 2) to 3.25 bits/cell (station 6) (Figure 5H). A total of 14 copepod genera were found at every station (Figure 5I, Table 3C), with Oithona nana dominating the total abundance of copepods in winter (Table 3C). Copepods consisted particularly of Paracalanus parvus (stations 1, 2 and 6), Centropages typicus (stations 3 and 5) and Centropages hamatus (station 4) in summer (Figure 5I).
3.5 Statistical analysis
For the winter data, PCA distinguished four separate groups that surrounded the F1 (40.73%) and F2 (26.62%) component axes. These explained 67.35% of the variance in the environmental parameters. The G1 was formed by salinity, suspended matter, NH4+ and N/P ratio at stations 5 and 6. The G2 formed by total nitrogen at station 1. G3 was formed by PO43− and T-P, selected at station 3. G4 was formed by abiotic parameters [temperature, pH, NO3−, NO2− and Si (OH)4; Figure 6A]. PCA showed that F1 and F2 axes explained 67.67% of the variance for biological parameters in winter (Figure 6B). Loricate ciliates showed a close link to Dictyochophyceae and dinoflagellates in G1. G2 was formed by diatoms and cyanobacteria. The spatial distribution of the copepods (calanoids, cyclopoids and harpacticoids) was linked to the abundance of naked ciliates (G3). The density of the other mesozooplankton was associated with copepod nauplii and Euglenophyceae (G4).
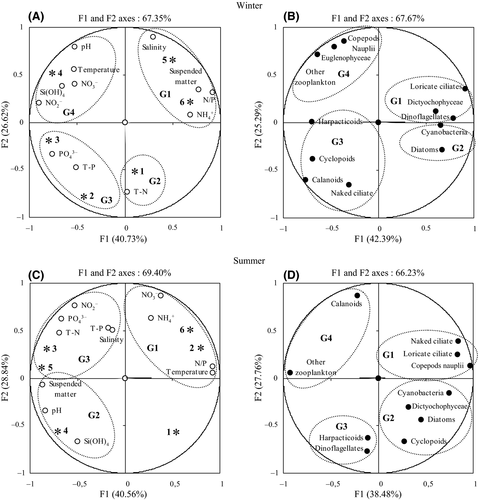
PCA selected three groups around the components of the F1 and F2 axes explaining 69.40% of the variance during the summer for environmental variables. The G1, formed by temperature, NO3−, NH4+ and N/P ratio (stations 2 and 6). The G2 formed by several physico-chemical variables [pH, suspended matter and Si (OH)4]. The last group, G3, was mainly related to salinity, NO2−, T-N, PO43− and T-P (stations 3 and 5) (Figure 6C). PCA explained 66.23% of the variance, of which 38.48% was attributed to the first axis and 27.76% to the second for the plankton community during the summer (Figure 6D). A positive relationship was shown between ciliates (loricate ciliates and naked ciliates) and copepod nauplii (G1). G2 showed close links between cyclopoids and microphytoplankton groups (cyanobacteria, diatoms and Dictyochophyceae). A negative relationship was shown between dinoflagellates and harpacticoids (G3). G4 contained calanoids and other mesozooplankton.
4 DISCUSSION
Within the ultra-oligotrophic Eastern Mediterranean Basin, the Kerkennah Islands have the peculiarity of being highly productive and rich in natural resources. Around 65% of the national fish production in Tunisia is from this area (CGP, 1996). It has become particularly important to study the coast of the Kerkennah Islands and its highly productive ecosystem owing to its unique position within the Eastern Mediterranean Basin and the subsequent decline in its biological resources due to anthropogenic pressures (Rekik et al., 2012). The present study is the first to examine the distributions of plankton assemblages during the winter and summer in a coastal area in the south of the Kerkennah Islands.
During this study, the recorded values of temperature and salinity were typical of arid to semi-arid zones (Ben Brahim et al., 2015a). The winter salinity variation may be due to an intrusion of modified Atlantic water, the circulation of which through the Gulf of Gabes was recently established (Bel Hassen et al., 2009). Salinity increased from winter to summer, a trend that can reasonably be attributed to evaporation.
Relatively high phosphate concentration compared with DIN could be a result of fast regeneration of orthophosphates (Li, Zhang, Wu, Lin, & Chen, 2001). Nitrogen, which is the most common element limiting microphytoplankton growth in most marine ecosystems (Livingston, 2001), was found at substantial concentrations during the summer along the coast of the Kerkennah Islands, while orthophosphate concentrations were low and N/P was higher than the Redfield ratio (16). Orthophosphate was still at a very high concentration with respect to the rest of the ultraoligotrophic Eastern Mediterranean basin where phosphorus is a limiting factor for plankton growth (Krom, Emeis, & Cappellen, 2010), comparable results were found in the Eastern (Aktan, 2010) or Western (Marty, Chiaverini, Pizay, & Avril, 2002) Mediterranean or both (Pujo-Pay et al., 2011). This is probably due to the shallow waters of the Kerkennah Islands, combined with the large tides favoring sediment re-suspension and so maintaining the presence of nutrients throughout the year. In addition, Saharan dust deposits are likely to be an important source of nutrients although this remains to be quantified by in situ investigations (Hamdi et al., 2015). Total phosphate concentration presented the same pattern as orthophosphate and was three to nine times more abundant. Phosphate concentration was related to the plankton groups in winter. The abundance of different plankton groups correlated positively with the high concentrations of orthophosphate (r = .77, n = 6, p < .05) during the winter.
The environmental conditions observed in the Kerkennah Islands' coastal waters appear to be highly favorable to microphytoplankton development with high temperature, non-limiting nutrients and solar energy in winter and summer (Feki et al., 2016). The dominance of taxonomic groups within the microphytoplankton changed from winter to summer. This was related to the increase in diatoms during winter (74% of total microphytoplankton), contrasting with a progressive increase of dinoflagellate species abundance during summer (58% of total microphytoplankton). This pattern illustrates the basic characteristics of microphytoplankton succession in temperate coastal waters described elsewhere (Smayda, 1980) and mainly explained by the nutrient availability during the winter and summer (Marty et al., 2002). A predominance of diatoms in winter has also been described for other areas along the coast of Sfax (Rekik et al., 2015a, 2013a) and in the Gulf of Gabes (Feki-Sahnoun, Hamza, Mahfoudi, Rebai, & Bel, 2014). Spatial variability of diatoms was revealed in the same area by Ben Brahim et al. (2015b), who found that the lowest diatom abundance was detected in winter. Winter diatom blooms may result from the replenishment of surface waters with nutrients due to winter overturning of the water column (D'Ortenzio & Ribera d'Alcalà, 2009). Diatoms are known to be opportunistic organisms (Fogg, 1991) and can thus take advantage of the newly available nutrients resulting from winter mixing. Concerning the diatom community composition, there was a substantial contribution from Navicula sp., Grammatophora marina, Nitzschia sp. and Nitzschia longissima. These diatom species were also found previously in the Kerkennah Islands (from Cercina station located in the western coast of Kerkennah during 2007, Ben Brahim et al., 2015b), on the north (Rekik et al., 2013a, 2015a) and south (Rekik et al., 2015c) coasts of Sfax and in the Gulf of Gabes (Feki-Sahnoun et al., 2014). Large diatoms (30–180 μm), known as benthic species, characterized the microphytoplankton community in the present study. Indeed, Navicula species have been known as benthic species (Welker, Sdrigotti, Covelli, & Faganeli, 2002). Navicula sp. was also detected in significant numbers by Ben Brahim et al. (2015b) it was the main species making difference between the three tidal periods for all seasons. The density of diatoms was correlated with water temperature (r = .88, n = 6, p < .05) and salinity (r = –.71, n = 6, p < .05). A similar relationship was also reported for the north coast of Sfax (Rekik et al., 2015a). High temperature may be an important factor limiting the growth of some diatom taxa (Ben Brahim et al., 2015b; Weisse, Gröschl, & Bergkemper, 2016). A previous study suggested that diatoms have a large growth range temperature; 13–25°C was found to be the optimal temperature range (Fang, Yang, Ji, Yao, & Liu, 2013). The concentrations of orthophosphate and total phosphate recorded in winter were higher than those reported during summer in the present study, resulting in the prevalence of diatoms. High dissolved P concentrations are generally accompanied by high abundances of diatom species (Ben Brahim et al., 2015b; Rekik et al., 2012). The concentrations of silicate were generally higher than 11 μM at all stations. Diatoms can continue to grow as long as the silicate concentration is above 2 μM (Egge & Aksnes, 1992). Silicic acid is known to be an important predictor for the abundance of diatoms because it is used to build their frustules (Ben Brahim et al., 2015b). Regenerated silicate appears to stimulate winter diatom production, associated with a low developing of dinoflagellate population (Rekik et al., 2012). Microphytoplankton assemblages were dominated by dinoflagellates in summer. Similar results were found by Ben Brahim et al. (2015a) at Cercina station in the Kerkennah Islands, Ben Ltaief et al. (2015) in the Gulf of Gabes, Abdennadher et al. (2012) in southern Tunisia and by Anderson et al. (2012) in other Mediterranean marine environments. High temperature and salinity are favorable for dinoflagellate growth (Feki et al., 2016), suggesting that this group is well adapted to the warm and salty waters of the present study area (Ben Brahim et al., 2015a). High abundance of dinoflagellates, in contrast to diatoms, were reported in a previous study when the water column was well stratified and characterized by a high level of inorganic nitrogen (Cermeno et al., 2008). A decrease in water turbulence was favored dinoflagellate development (Smayda, 1997). In fact, more recent work has shown that they prefer a stable water column and warm temperature (Lasternas et al., 2011). Thus, the stability of the water column during summer stratification in the present study provided an opportunity to take full advantage of this stability. The fact that nutrients had the greatest influence on dinoflagellate abundance in our study area is clear from the strong positive correlation between their abundance and NO3− (r = .63, n = 6, p < .05) and NH4+ (r = .78, n = 6, p < .05). Dinoflagellates are generally considered slow growers with a low maximum uptake capacity for dissolved inorganic nutrients (Feki et al., 2016), which makes them inferior competitors in situations where nutrients are plentiful. However, they can overcome the lack of nutrients by diversifying their trophic modes (autotrophic, mixotrophic and heterotrophic; Jeong et al., 2010). About half of dinoflagellate species in marine plankton lack chloroplasts (Sherr & Sherr, 2007).
In the present study the average ciliate abundance during winter was similar to that observed in summer, but their composition and distribution differed between winter and summer. For example, in terms of temporal scale, some dominated the samples during the winter (naked ciliates), while the other peaked in the summer (loricate ciliates). With regard to the structural composition, the ciliate community on the coast of the Kerkennah Islands was dominated by aloricate ciliates (Uronema marinum, Leegardiella sol and Strombidium sp.) during winter, which is typical of many coastal and oceanic waters, such as the Irish Sea (Edward & Burkill, 1995), the Irminger Sea (Montagnes et al., 2010), the Baltic Sea (Mironova, Telesh, & Skarlato, 2009) and the North Sea (Stelfox-Widdicombe, Archer, Burkill, & Stefels, 2004). The mixotrophic naked ciliate Uronema marinum was the most abundant species, particularly at station 2 (45 × 102 cells/L, salinity 37). Hannachi et al. (2009) reported that Uronema marinum is able to thrive not only offshore of the Gulf of Gabes (salinity 38) and along its coast in a nearshore salty station (salinity 48; Kchaou et al., 2009) but also in the man-made Sfax solar salterns (salinity 150–300; Elloumi, Guermazi, Ayadi, Bouaın, & Aleya, 2009). These ciliates are free-living and ubiquitous in the marine environment, preferentially feeding on bacteria residing in sediments, and are abundant in coastal waters (Alvarez-Pellitero et al., 2004). The adaptation of the small Uronema marinum to different ecosystems may be linked to its opportunistic feeding strategy and the availability of nanoplankton prey (Hannachi et al., 2011). The summer assemblage included Tintinnopsis sp., Eutintinnus fraknoi, Tintinnopsis beroidea, Eutintinnus medius and Helicostomella subulata. Loricate ciliates account for a large proportion of the ciliate communities in the Gulf of Gabes (Elloumi et al., 2015), the Adriatic Sea (Bojanić et al., 2005) and the Yellow Sea (Jiang et al., 2011). The genus Tintinnopsis has usually been reported to be the dominant planktonic ciliate in both temperate and tropical coastal systems (Dolan, 2006). This suggests that this genus performs better than other loricate ciliates, probably due to more flexible adaptive strategies (Reynolds, 1997). According to Hannachi et al. (2009), the quantitative dominance of loricate ciliates during their study was related to pollution from the surroundings in the Gulf of Gabes. Our survey demonstrating the prevalence of loricate ciliates confirms the results reported by Hannachi et al. (2009). The winter variations in the naked ciliate abundance were significantly related to environmental variables (PO43− and T-P). Correlation analysis demonstrated that the spatial variations in loricate ciliate abundance were significantly correlated with changes in nitrate concentrations, alone or in combination with salinity (r = .93, n = 6, p < .05). Thompson, Alder, and Boltovskoy (2001) reported that stable salinity gradients would probably favored loricate ciliate abundance. In addition, most loricate ciliate species are found in waters with salinity <35 (Lei, Xu, Ki, Pyo, & Wickham, 2009).
Mesozooplankton assemblages in the coastal area around the Kerkennah Islands were dominated by copepods that accounted for 93% and 98% of the total mesozooplankton abundance in winter and summer, respectively. The winter peak of copepods was recorded at the same time as the maximum abundance of microphytoplankton was 48.50 × 102 cells/L. Also, a high ciliate abundance was recorded in winter and may be responsible for the high abundance of copepods in this season (Drira et al., 2010). The copepod community was characterized by the high dominance of copepod nauplii, which constituted a large part of the total density of mesozooplankton (54% in winter and 9% in summer). Rekik et al. (2012) also reported that copepod nauplii were the most abundant copepods in coastal samples. During the winter cruise, small copepod species, particularly Oithona nana, were found to dominate copepod communities. Large copepods (Paracalanus parvus, Centropages typicus and Centropages hamatus) recorded very high abundances in summer. The community appeared to be diverse and largely composed of small copepods with temperate or cosmopolitan distributions during winter (Papantoniou, Danielidis, Spyropoulou, & Fragopoulu, 2015). This has also been recorded as a common feature of copepod communities in the coastal region of Sfax (Rekik et al., 2013a), in the Gulf of Gabes (Ben Ltaief et al., 2015), in the north of Tunisia (Daly Yahia, Souissi, & Daly Yahia Kefi, 2004), in the Balearic Sea (Fernandez de Puelles, Gras, & Hernandez, 2003) and in the Adriatic Sea (Vidjak, Bojanic, Kuspilic, Gladan, & Ticina, 2007). In the present study, oithonids recorded very high abundances with O. nana (15.47 ± 7.19 × 102 ind./m3) being the most abundant species in winter. Oithona nana has been recognized as the most ubiquitous copepod species in the world's oceans (Riccardi & Mariotto, 2000). It was reported to occur at very high abundances in neritic temperate seas (Rekik et al., 2012). This species exhibits a certain ability to endure environmental perturbations, relying on its low respiratory rate and omnivorous diet (Gallienne & Robins, 2001). Several studies have shown that O. nana is able to proliferate in fluctuating ecosystems (e.g., Williams & Muxagata, 2006). This copepod is known to be omnivorous (Lampitt & Gamble, 1982), opportunistic (Lam-Hoai & Rougier, 2001), capable of colonizing polluted environments (Rekik et al., 2013a) and areas subject to eutrophication (Richard & Jamet, 2001). Oithona nana's physiology may show greater flexibility with respect to environmental constraints than calanoids (Paffenhöfer, 1993). These characteristics may explain its occurrence in the present study site. Indeed, studies in the Mediterranean have shown that, although this species is known to develop in spring, it is also found in great numbers in winter (Siokou-Frangou, 1996). Oithonid abundance declined significantly during the summer, being replaced by large calanoid species.
Microphytoplankton populations showed strong spatio-temporal variations in the study area, following relatively small nutrient variations. Top-down control may account for the observed microphytoplankton abundance variations. Previous studies have reported significant activity of grazers feeding on microphytoplankton (Zervoudaki et al., 2011). Ciliates are important intermediaries between larger and microbial components of marine pelagic food webs (Turner, 2004). Ciliates represent an important microzooplankton group able to impose top-down control on microphytoplankton populations; however, ciliates are actively consumed by copepods (Löder, Meunier, Wiltshire, Boersma, & Aberle, 2011). The dominance of copepod species is probably related to their capacity to exploit a wide range of food resources including microphytoplankton and ciliates.
5 CONCLUSIONS
The results of this study indicate that the spatial variations in the microphytoplankton, ciliate and mesozooplankton communities of the coastal area around the Kerkennah Islands are related to the area's hydrographic properties. The microphytoplankton community structure shows clear spatial variations along the sampled coastal stations and is dominated by a community of large-sized diatoms in winter. The dominance of loricate ciliates is related to pollution from anthropogenic and industrial activities. Copepods are the most abundant mesozooplankton, with species such as Oithona nana, which indicates a poor and polluted environment, spreading abundantly along the coast. Because these disturbances may impact the biological communities, Tunisian authorities undertook the Taparura project, which consists of removing tons of phosphogypsum spread over more than 60 km2 (Rekik et al., 2012, 2013a). To improve our understanding of microphytoplankton, ciliate and mesozooplankton dynamics along the coast of the Kerkennah Islands, we are currently investigating the plankton community in more detail as well as the small-scale variability in three regions (west, central and east coasts of the Kerkennah Islands; Rekik, Ayadi, Elloumi, unpublished data).
ACKNOWLEDGEMENTS
This work was conducted in the Biodiversity and Aquatic Ecosystems UR/11ES72 research unit at the University of Sfax.




