Adaptation of diet in a changed environment: Increased consumption of lobster krill Munida gregaria (Fabricius, 1793) by Argentine hake
Abstract
A well-replicated decadal-term (2005–2014) stomach content data set was analysed in order to infer inter-annual fluctuations in the diet of the Argentine hake, Merluccius hubbsi, an opportunistic predator in the San Jorge Gulf (SJG) ecosystem in the Southwest Atlantic. Ten research cruises were carried out each year during January from 2005 to 2014. A total of 18,461 specimens of Me. hubbsi was analysed, of which 6,777 (36.71%) contained food in their stomachs. The diet of Me. hubbsi changed markedly from 2011 onwards, with much greater consumption of the lobster krill Munida gregaria compared to the years before 2011. The frequency of occurrence (%F) of Mu. gregaria in the stomach contents of Argentine hake increased from the year 2009 onwards, most noticeably since 2011, and mostly over the southern region of the SJG. The main predators of Mu. gregaria in the SJG are two species of teleost fish (pink cusk eel, Genypterus blacodes, Argentine seabass, Acanthistius brasilianus) and three Rajidae skates (Zearaja chilensis, Psammobatis spp. and Sympterygia bonapartii), which exhibited decreased catches in the years analysed. The increased consumption of Mu. gregaria by Me. hubbsi, coupled with decreased trends in abundance of the main predators of the lobster krill during the last decade, indicate that top-down trophic dynamic control occurs in the SJG ecosystem.
1 INTRODUCTION
Conditions change in marine environments at a wide range of scales, from minutes to thousands of years, and bring pronounced changes in communities of organisms (Valiela, 1984). The modification of one or several components of marine ecosystems can have strong effects on higher or lower trophic levels, depending on whether the food web is controlled by resources or predators by bottom-up or top-down controls, (Cury, Shannon, & Shin, 2003). These systems can also be controlled by a number of key species in the middle through wasp-waist control, which is most probable in upwelling ecosystems (Cury et al., 2000). It is difficult to identify concretely how such changes affect marine communities, but opportunistic predators take advantage of a source of food outside their usual diet, changing their preferred prey type in relation to local availability, moving towards the most abundant resource (Gerking, 1994). Changes in the diet of opportunistic predators can provide valuable insights on ecosystem dynamics (Link & Ford, 2006), physical variability (Tam, Purca, Duarte, Blaskovic, & Espinoza, 2006) and large structural changes of marine communities (Becker & Beissinger, 2006). However, an opportunistic species may appear to have a specialized diet as it feeds exclusively on an abundant prey species (Mahe, Amara, Bryckaert, Kacher, & Brylinski, 2007), but an opportunistic pattern may be recognized over a broad temporal scale (Gerking, 1994).
The subfamily Merlucciinae (family Merlucciidae) comprises one genus, Merluccius, with 13 currently recognized species worldwide (Cohen, Inada, Iwamoto, & Scialabba, 1990; Nelson, Grande, & Wilson, 2016), of which most are opportunistic, generalist feeders exhibiting broad diets (Buckley & Livingston, 1997; Du Buit, 1996; Garrison & Link, 2000; Payne, Rose, & Leslie, 1987; Tam et al., 2006). Hake fisheries are present in the Atlantic (both sides and including the Mediterranean Sea and Black Sea), Southwestern Indian and Eastern Pacific (from British Columbia to the tip of South America) Oceans, and in New Zealand (Cohen et al., 1990; Nelson et al., 2016), and are economically the most important fishery in all of these localities. The Argentine hake, Merluccius hubbsi, is a demersal species, widely distributed across the Southwestern Atlantic Ocean from Brazil (21°30′S) to Southern Patagonia (55°S) (Cousseau & Perrota, 2013). There are two main stocks of Me. hubbsi in Argentina, the Northern stock (NS, 34°S–41°S) and the Patagonian stock (PS, 41°S–55°S) (Bezzi et al., 2004). Each stock has different historical exploitation patterns and is managed independently (Aubone et al., 2004).
Owing to Argentine hake's commercial importance in the South Atlantic Ocean, its high abundance and the fact that it is an important predator in the Patagonian shelf waters (Laptikhovsky & Fetisov, 1999), many studies have examined its diet composition (Angelescu & Cousseau, 1969; Angelescu, Gneri, & Nani, 1958; Angelescu & Prenski, 1987; Belleggia, Figueroa, Irusta, & Bremec, 2014; Cordo, 1981; Ocampo Reinaldo, González, & Romero, 2011; Ruiz & Fondacaro, 1997; Sánchez, 2009; Sánchez & García De La Rosa, 1999; Sánchez & Prenski, 1996; Temperoni, Viñas, & Buratti, 2013). Although the feeding habits of Argentine hake have been studied in detail, there is no work analysing long-term variations in diet composition. In this context, this work analysed the stomach contents of Argentine hake collected between 2005 and 2014 in the San Jorge Gulf (SJG) region in the Southwestern Atlantic, aiming to find evidence of temporal variation in the diet. Based on this time series, the specific goals of our study were: (i) to provide a quantitative analysis of the diet composition of Me. hubbsi in the SJG in the Southwestern Atlantic, (ii) to examine temporal variability in the diet of Me. hubbsi, (iii) to identify which prey was the major contributor to the observed temporal differences in diet, and (iv) to explore the abundances of the known predators of the prey identified in (iii), to infer which type of trophic dynamics applies (bottom-up or top-down control).
2 MATERIAL AND METHODS
2.1 Study area
The study was focused on an area located between 45°S–47°S and 63.55°W–67.28°W in the Southwest Atlantic Ocean, from a depth of 60–109 m. This region includes the SJG, a major coastal embayment in the region (Acha, Mianzan, Guerrero, Favero, & Bava, 2004). The SJG is influenced in the north by a tidal mixing front during spring and summer, with stratified offshore waters and a vertically coastal mixed body of water (Acha et al., 2004). The tidal friction acts from the bottom upwards, providing efficient recirculation of nutrients and high primary production to the productive layer of water (Rydberg, 2009). The south region is influenced by a Patagonian cold estuarine front, a diluted water mass that flows from the northern extreme of the Drake Passage (55°S) 800 km northward, reaching the southern limit of the SJG and meeting the tidal mixing front here located (Acha et al., 2004). These fronts make the SJG region of utmost ecological importance for several species, including the Argentine hake, Merluccius hubbsi, for feeding and spawning (Acha et al., 2004).
2.2 Data sources
Our study was focused on repeated and systematic scientific surveys conducted during Januaries 2005–2014 by the Instituto Nacional de Investigación y Desarrollo Pesquero (INIDEP). Argentine hake stomachs were collected during bottom trawl surveys following a stratified random design in order to assess the biomass and spawn abundance of this species. Fishing was conducted during daylight hours (07.00–19.00 hr) at 3–4 knots for 30 min in each sampling site, and employed an Engel bottom trawl net (200 mm mesh in the wings, 103 mm in the cod ends, 4 m vertical opening and 15 m horizontal aperture). A total of 125 trawls was analysed, from which catches were identified to species level and weighted. Skates were grouped as Rajidae due to the inaccuracy of species identification during the first surveys of the decadal series. At each trawl location, the Merluccius hubbsi specimens were measured to the nearest cm and sexed. Finally, stomachs were excised and each prey item was identified to the lowest taxonomic level and classified as present (1) or absent (0). This decadal (2005–2014) stomach content data set of Me. hubbsi allowed us to calculate the percentage of frequency of occurrence of each prey (%F, the total number of stomachs containing a given prey expressed as percentage of the total number of stomachs excised that contained food) for each year, giving a robust and interpretable measure for quantifying diet composition (Baker, Buckland, & Sheaves, 2014).
2.3 Statistical analyses
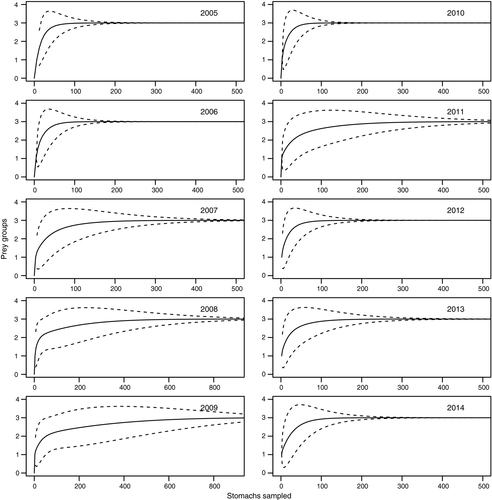
The differences in size frequency distributions among years were evaluated by pairwise Kolmogorov–Smirnov tests (Crawley, 2005). In order to assess whether the number of stomachs analysed was sufficient to accurately describe the diet of each year considered (Cortés, 1997; Ferry & Cailliet, 1996), the stomachs sampled were randomized 100 times, and an accumulation prey curve was constructed as a function of stomach number. The cumulative prey curves with their confidence intervals (95%) were fitted and the sample sizes were considered sufficient to describe diets when curves reached an asymptote.
The stomachs containing food were grouped and averaged by sampling site. The average number of each prey by sampling site was used to build a Bray–Curtis dissimilarity matrix (Krebs, 1989; Zuur, Ieno, & Smith, 2007). An analysis of similarities (ANOSIM) was performed to evaluate Argentine hake diet differences among years, and pairwise comparisons were considered significant if pairwise R > Global R and p < .05 (Clarke, 1993). In addition, the degree of separation among years increases as the R value increases (Clarke, 1993). Similarity percentages (SIMPER) analyses were used to assess the contribution of each prey to the diet differences observed in the ANOSIM, and also to find the most erratic prey (Clarke, 1993; Zuur et al., 2007). Following SIMPER, the most erratic prey was defined as the prey item that contributed most to the dissimilarity among years in the diet of Argentine hake. Hereafter, the frequency of occurrence of the most erratic prey was calculated by sample site (%Ft), as the number of stomachs of Argentine hake in which the erratic prey was found, expressed as the percentage of stomachs with food, in each sampling site. The %Ft calculated were interpolated using the ordinary Kriging interpolation function to create a grid for each year and plotted.
Finally, the main predators of the most erratic prey were identified following previous studies focused on the SJG ecosystem (Sánchez & Prenski, 1996). The catches of the species considered predators of the erratic prey in the scientific surveys were analysed by year using the loess procedure, which fits smooth curves using non-parametric techniques to the relationship between variables (Crawley, 2005). The yearly averaged catch of the main known predators of the most erratic prey expressed in kilograms was used as the dependent variable to explore temporal variability in relative abundance of these species, and to identify an ecosystem control mechanism (bottom-up or top-down control) (Richardson & Schoeman, 2004). Statistical analyses were performed in the open R language using R 3.2.2 with the libraries MASS, stasts and vegan (R Core Team, 2014).
3 RESULTS
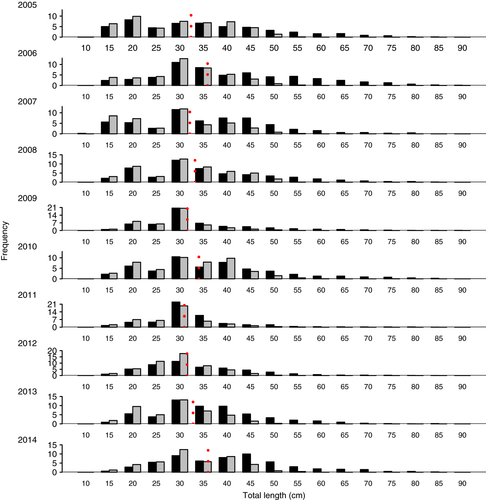
A total of 18,461 specimens of the Argentine hake Merluccius hubbsi was analysed, of which 6,777 (36.71%) contained food in their stomachs. The cumulative prey curves indicated that the number of stomachs analysed in each year was adequate to study the diet of this species (Figure 1). Size frequency distributions of Me. hubbsi (Figure 2) were generally significantly different among years (Kolmogorov–Smirnov test, p < .05), with the exception of the years 2009 and 2011 (D = 0.046, p = .14), which did not differ. The mean total length did not show any differences when 2005 was compared with 2007 or 2013, 2006 with 2014, 2007 with 2009, 2012 or 2013, 2008 with 2013, and 2009 with 2012 (Student's t test, p > .05; Figure 2). The variances of the total length did not show differences for the comparisons of 2005 with 2006, 2007 with 2010, 2008 with 2010 and 2014, and 2009 with 2013 (variance F test, p > .05; Figure 2).
A total of 21 prey items was identified in the stomach contents of Me. hubbsi during 2005–2014: seven fishes, seven crustaceans, five cephalopods and two others (Table 1). Among fish, predation on Engraulis anchoita and cannibalism were present throughout the decade of this study (Table 1). The crustaceans Themisto gaudichaudii, Euphausia spp., Munida gregaria and Peisos petrunkevitchi, and the cephalopod Illex argentinus were also present during all years from 2005 to 2014 (Table 1). The %F of all crustaceans showed an increasing trend over time, whereas fish and cephalopods declined (Table 1).
| Prey items | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 |
|---|---|---|---|---|---|---|---|---|---|---|
| %F | %F | %F | %F | %F | %F | %F | %F | %F | %F | |
| Crustaceans | 44.13 | 64.38 | 84.16 | 77.59 | 91.42 | 70.97 | 89.95 | 80.66 | 84.94 | 89.37 |
| Themisto gaudichaudii | 6.68 | 5.68 | 31.69 | 13.79 | 22.57 | 18.16 | 6.70 | 11.00 | 9.61 | 10.54 |
| Euphausia spp. | 36.03 | 12.52 | 20.00 | 26.25 | 24.25 | 25.28 | 1.41 | 28.82 | 28.28 | 23.19 |
| Munida gregaria | 1.42 | 1.37 | 0.26 | 9.77 | 25.37 | 12.73 | 63.32 | 38.67 | 43.35 | 52.06 |
| Peisos petrunkevitchi | 0.81 | 45.60 | 37.40 | 30.46 | 22.76 | 14.98 | 19.93 | 4.17 | 3.97 | 4.12 |
| Pleoticus muelleri | – | 0.39 | – | 1.15 | 0.75 | 1.12 | 3.88 | 1.99 | 3.42 | 2.93 |
| Pterigosquilla armata armata | – | – | 0.26 | – | 0.56 | 0.37 | 0.18 | – | 0.09 | 0.09 |
| Unidentified crustaceans | – | – | – | – | – | – | 0.35 | 0.19 | – | 0.09 |
| Fish | 34.01 | 20.35 | 12.47 | 21.07 | 8.96 | 15.36 | 8.99 | 6.92 | 12.48 | 6.42 |
| Engraulis anchoita | 12.55 | 3.33 | 3.12 | 6.13 | 1.49 | 5.99 | 0.71 | 3.60 | 0.18 | 0.73 |
| Merluccius hubbsi | 12.75 | 11.55 | 6.23 | 9.96 | 4.66 | 8.05 | 6.00 | 2.65 | 11.18 | 4.22 |
| Patagonotothen ramsayi | 0.40 | 0.20 | 0.26 | – | – | – | 0.18 | – | 0.09 | – |
| Genypterus blacodes | – | – | – | – | – | – | – | – | – | 0.09 |
| Stromateus brasiliensis | 0.20 | – | – | – | – | – | – | – | – | – |
| Raneya brasiliensis | 0.00 | 0.20 | – | – | 0.19 | – | – | – | – | – |
| Unidentified fish | 8.10 | 5.28 | 2.86 | 4.79 | 2.61 | 1.31 | 2.12 | 0.66 | 1.02 | 1.37 |
| Cephalopods | 22.06 | 17.42 | 4.42 | 1.72 | 0.75 | 14.61 | 1.94 | 13.93 | 3.70 | 4.67 |
| Illex argentinus | 21.05 | 17.03 | 4.16 | 0.77 | 0.56 | 13.67 | 1.76 | 13.84 | 3.42 | 4.67 |
| Doryteuthis gahi | 0.81 | 0.20 | – | 0.57 | 0.19 | 0.94 | – | – | – | – |
| Pulpo spp. | – | – | – | – | – | – | – | 0.09 | 0.09 | – |
| Semirossia tenera | 0.20 | 0.20 | 0.26 | 0.38 | – | – | 0.18 | – | 0.09 | – |
| Unidentified cephalopods | – | – | – | – | – | – | – | – | 0.09 | – |
| Others | 1.01 | 0.00 | 0.52 | – | – | 0.19 | – | 0.19 | 0.65 | 0.64 |
| Pleurobrachia sp. | 0.81 | – | – | – | – | – | – | – | – | – |
| Unidentified remains | 0.20 | – | 0.52 | – | – | 0.19 | – | 0.19 | 0.65 | 0.64 |
There were significant differences in the diet of Me. hubbsi among years (ANOSIM, Global R = .171, p < .001). The pairwise comparisons indicated that the greatest change in the diet occurred since 2011 (R2005–2011 = .86, p < .001; R2006–2011 = .46, p < .001; R2007–2011 = .66, p < .001; R2008–2011 = .43, p < .001; R2009–2011 = .26, p < .001; R2010–2011 = .50, p < .001). The diet did not show any differences when 2011 was compared with 2012, 2013 and 2014 (pairwise R < Global R, and p > .05). The SIMPER analysis revealed that the squat lobster, Munida gregaria, was the most erratic prey and thus contributed most to the differences observed among years [contribution to dissimilarity (C)2005–2011: 41.0%; C2006–2011: 48.9%; C2007–2011: 42.5%; C2008–2011: 46.8%; C2009–2011: 43.9%; C2010–2011: 43.9%].
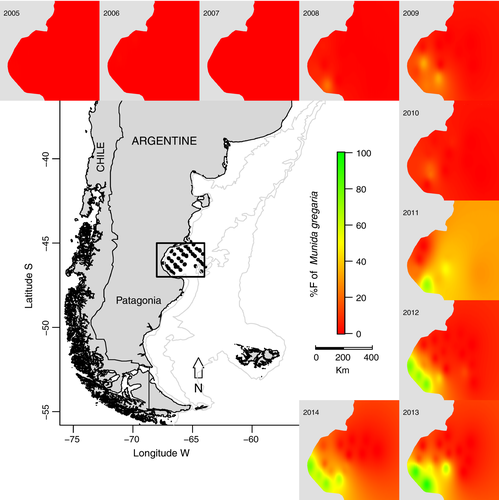
Mapping the %Ft of Mu. gregaria by year revealed that the increased consumption by Me. hubbsi started during 2008 over the southern region of the SJG, and geographically spread in 2011–2014 (Figure 3). The shift in the diet of Me. hubbsi exhibited a pronounced pattern from 2011 to 2014, mainly in the southern region of the SJG, where 100% of the stomachs contained Mu. gregaria as prey (Figure 3).
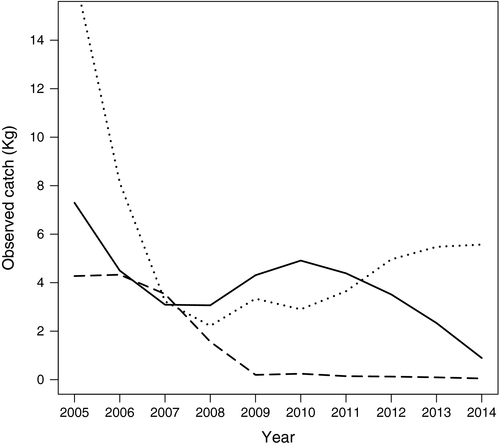
In the SJG ecosystem, the lobster krill Mu. gregaria was mainly predated by two species of teleost fish [pink cusk eel, Genypterus blacodes (Mu. gregaria was 43% of its diet), Argentine seabass, Acanthistius brasilianus (54%)] and three Rajidae skates [Zearaja chilensis (30%), Psammobatis spp. (73%) and Sympterygia bonapartii (67%)]. The catches of these main predator species decreased through time from 2005 to 2014 (Figure 4).
4 DISCUSSION
The diet of the Argentine hake, Merluccius hubbsi, changed during the decade of the study, with decreasing proportions of fish and cephalopods, and increasing proportions of crustaceans. The major increase among crustaceans occurred for the squat lobster, Munida gregaria. The benthic phase of this prey substantially increased its relative availability and abundance in the SJG ecosystem, from 1,779.2 to 1,483.3 kg nm−2 in 2009 and 2010, respectively, to 4,040 kg nm−2 in 2011 (Ravalli, De La Garza, & López-Greco, 2013). This shift in diet composition towards the most abundant resource confirms the extreme plasticity in the opportunistic foraging behavior of Me. hubbsi. This pattern has also been observed in other opportunistic hakes such as Merluccius gayi peruanus, which changed its diet due to the reduction in its preferred prey during El Niño events (Tam et al., 2006). Pinnegar, Trenkel, Tidd, Dawson, and Du Buit (2003) suggested that fish species exhibit some flexibility in their feeding preferences but they have a ‘portfolio’ of suitable prey types, and they respond to changes in relative abundance of prey within their particular portfolio.
The shifts observed in the feeding habits of Me. hubbsi, a generalist predator, provided interesting insights into the distribution, boom and availability of the prey Mu. gregaria. The genus Munida with 243 species (Baba et al., 2008) is the most specious genus of the family Munididae (Ahyong, Baba, Macpherson, & Poore, 2010). Munida gregaria is distributed in the Southern Hemisphere around New Zealand and Southern Australia, from 36°S to 51°S, and around Southern South America, from 41°S to 55°S in the Pacific and from 35°S to 55°S in the Atlantic (Ahyong & Poore, 2004; Baba et al., 2008; Boschi, Fischbach, & Iorio, 1992). Munida gregaria is one of the most abundant decapod species in the Southwestern Atlantic (Diez, Tapella, Romero, Madirolas, & Lovrich, 2016). It represents a direct trophic shortcut in this marine food web incorporating organic matter into the food web: it feeds at a low trophic level, upon algae, detritus and sediments, representing an important link between the particulate organic matter and many benthic and demersal top predators (Diez, Tapella, et al., 2016; Pérez-Barros, Romero, Calcagno, & Lovrich, 2010; Romero, Lovrich, Tapella, & Thatje, 2004; Vinuesa & Varisco, 2007).
Munida gregaria usually exhibits high abundances in Southern Chile and Argentina in the Drake Passage and Beagle Channel (Diez et al., 2012; Pérez-Barros, Tapella, Romero, Calcagno, & Lovrich, 2004). During the past decade scientists have started to examine the ecological role of these abundant organisms in an effort to take a more integrated ecosystem approach to fisheries management (Lovrich & Thiel, 2011). Diez, Cabreira, Madirolas, and Lovrich (2016) have more recently noted that between 2009 and 2014 pelagic swarms of Mu. gregaria increased their occurrence and abundance in both the Beagle Channel and on the Argentine Patagonian Shelf. Moreover, Mu. gregaria is actually the most important crustacean in the by-catch of the Patagonian shrimp fishery (Varisco, Vinuesa, & Góngora, 2015). Its commercial potential was recognized, and it was even included in cookbooks from Argentina (Bigongiari, 2016). In accordance with our results, an increase in Mu. gregaria swarms and benthic aggregations occurred in southern areas of the SJG (Diez, Cabreira et al., 2016; Roux & Piñero, 2006), probably driven by natural population growth in the absence of high predation-related mortality, coupled with oceanographic factors. Both aspects are discussed below.
The population expansion of Mu. gregaria agrees with our results, and can be explained by both oceanographic and biological factors. The former is supported by the Patagonian current, a diluted plume that occurs with an excess of rainfall in the Southeast Pacific and the continental discharge along the west coast of South America (Acha et al., 2004), which transports low salinity waters, mixed by tides and winds, 800 km northwards along the west coast of South America from the Drake Passage to the southern limit of the SJG (Acha et al., 2004). Munida gregaria can remain pelagic after metamorphosis for up to 6 months; adults are benthic but can perform vertical migrations into the water layer (Diez et al., 2012; Zeldis, 1985), which would facilitate transport from the Beagle Channel to the frontal and highly productive region of the Southern SJG ecosystem. However, further studies considering oceanographic and environmental variables are necessary in order to explain the geographic expansion of Mu. gregaria and also to understand the fluctuations in its abundance.
The decrease in the abundance of the predators of Mu. gregaria can be considered among the biological factors explaining the population expansion. The time series herein analysed include both decreases in predator abundance and the corresponding increase in Munida spp. abundance. This may indicate that the absence of predators is the determinant of prey abundance when a linear predator–prey relationship is assumed. A similar pattern was identified for the Atlantic cod (Gadus morhua) and northern shrimp (Pandalus borealis) interaction across the North Atlantic Ocean, where shrimp populations were strongly inversely related to predator abundance (Worm & Myers, 2003). Varisco et al. (2015) hypothesized that the lower abundance of Me. hubbsi could explain the demographic expansion of Mu. gregaria. However, other species exhibited decreasing trends and were the main predators of Mu. gregaria in the SJG ecosystem during the 1990s, for instance: the pink cusk eel, Genypterus blacodes, Argentine seabass, Acanthistius brasilianus and Rajidae skates (Zearaja chilensis, Psammobatis spp. and Sympterygia bonapartii) (Sánchez & Prenski, 1996).
The increased consumption of the lobster krill Munida spp. by the opportunistic Me. hubbsi combined with the decreased trends in abundance of the main predators of the lobster krill suggest a top-down trophic dynamic control in the SJG ecosystem. Bottom-up controls result in a positive correlation between predator and prey abundances, whereas top-down control should result in a negative correlation between predators and prey (Richardson & Schoeman, 2004). Similarly, in a neighboring area, a wasp-waist ecosystem control supported by the amphipod Themisto gaudichaudii was suggested (Padovani, Viñas, Sánchez, & Mianzan, 2012). The abundance and species composition in an environment are determined by a combination of competitive interactions among the species and the effect of predation on those species (Valiela, 1984). Our study suggests that the biological system was top-down controlled by several predators like the pink cusk eel, G. blacodes, the Argentine seabass A. brasilianus and Rajidae (Z. chilensis, Psammobatis spp. and S. bonapartii), which exhibited inverse correlations with their prey, the lobster krill Mu. gregaria, in the SJG ecosystem.
5 CONCLUSIONS
Our results show that the diet of the Argentine hake, Merluccius hubbsi, changed during the decade of the study in the SJG ecosystem, with increased consumption of the lobster krill Munida gregaria.
Moreover, this work exhibited decreased catches for the main known predators of Mu. gregaria (pink cusk eel, Genypterus blacodes, the Argentine seabass, Acanthistius brasilianus, and three Rajidae species), which could be one of the biological factors that contributed to the lobster krill bloom. These results suggest a top-down trophic dynamic system controlled by predators in the SJG ecosystem.
ACKNOWLEDGEMENTS
We thank the Instituto Nacional de Investigación y Desarrollo Pesquero (INIDEP) for specimens collected and the scientific team: Hugo, Luis, Nono, Lia, Claudia Datto, Buratti, Paola Betti, Emiliano, Castruchi. We also thank the librarians from INIDEP for providing very helpful documents. This work was supported by CONICET and INIDEP. The authors were supported by CONICET; INIDEP provided samples and was the workplace. The authors declare that they have no conflict of interest. All applicable international, national and/or institutional guidelines for the care and use of animals were followed.




