Sources and threats of chronic tissue loss on coral reefs in the Lakshadweep Islands, Indian Ocean
Abstract
Tissue degradation and resulting mortality are major threats to coral reefs around the world. Information on interactions of the major environmental factors that mediate tissue loss and mortality in coral reefs is of great importance. It is essential to understand the prevailing reef health conditions and to develop appropriate management actions. In the present study, a series of benthic surveys conducted in the Lakshadweep Islands revealed the interaction of major biological factors in causing tissue loss and mortality. Hierarchical regression analysis revealed interactions of various environmental scenarios. Tissue loss was prevalent in islands with high cover of massive corals (m = 20.91) and low in islands dominated by branching corals (m = 0.61). Hierarchical regression analysis revealed black band disease (β = .59; p < .001) and algal interactions (β = .48; p < .001) to be major factors responsible for coral mortality caused by tissue loss in the region.
1 INTRODUCTION
Multiple stressors mostly act synergistically and contribute to the large scale reduction of coral cover across the globe. Diseases or physical damage followed by partial mortality are common phenomena in reef-building corals. Partial mortality of reefs involves the removal or retreat of surface living tissue parts including tissue loss, lesions or tissue necrosis (Jacobson, 2012). The incidence and intensity of necrotic tissue patches depend on several factors like diseases, predation and sediment scouring (Vanwoesik, 1998). Tissue necrosis causes damage on the coral surface that eventually heals, but there is evidence from Caribbean reefs that colonies with intense tissue necrosis shift to an algal-dominated state before healing is complete (Hughes, 1994; Knowlton, 1992, 2004). Such events in major reef areas such as the Caribbean reefs demand an assessment of tissue loss patterns and influence of environmental stressors in coral reef destruction.
Coral bleaching is a prime threat to tropical corals that involves rapid loss of symbiotic zooxanthellae leaving whitened coral skeletal structures. Bleaching is normally a non-lethal response to varying water temperatures (Gates, 1990) but it becomes lethal when it is severe, prolonged and followed by secondary stresses like sedimentation, macroalgal overgrowth and diseases (Brown, 1997). Multiple stressors act individually or synergistically resulting in small- to large-scale tissue degradation and mortality. The sediment and nutrient loads from terrestrial run off are growing concerns in regions with coral reefs. Major effects of sedimentation include smothering, abrasion and shading of adult corals at greater depths (Fabricius, Wild, Wolanski, & Abele, 2003; Fabricius & Wolanski, 2000; West & Van Woesik, 2001). Varying pH and anoxic conditions caused by high sedimentation also result in reduced coral cover, altered species composition, cessation of calcification and recruitment pattern in buried corals (Fabricius, 2005 and Rogers, 1990). Scouring of surface tissue from sediments and silts may also be lethal (Nowlis, Roberts, Smith, & Siirila, 1997 and Rogers, 1990). Increased sedimentation also promotes nutrient enrichment, supporting macroalgal overgrowth.
Macroalgae compete with coral reefs for nutrients, light and space (Miller, 1998). Abrasion of the live coral tissue by macroalgae results in tissue degradation. Increased abrasion of live tissue accompanied by high allelopathic activity of macroalgae on live coral tissue catalyses the tissue degradation process (Rasher, Stout, Engel, Kubanek, & Hay, 2011; Rasher and Hay, 2014). Under adverse environmental conditions, it is difficult for coral colonies to regain their stability and macroalgae overgrowth on those coral colonies, results in phase shift from coral to macroalgae dominant ecosystems (McManus, Menez, Kesner-Reyes, Vergara, & Ablan, 2000).
An increase in the prevalence and spread of infectious diseases has also prompted a recent decrease in coral cover (Bruno et al., 2007; Rosenberg & Ben-Haim, 2002). Poor local environmental conditions may result in high prevalence of coral diseases (Williams, Aeby, Cowie, & Davy, 2010). Black band disease (BBD), which results in tissue loss leading to either partial or complete death of the colony, is a major threat to reef communities (Antonius, 1981; Kuta & Richardson, 1996). Studies have shown that tissue lesions from BBD are non-regenerative and that coral skeleton exposed by this disease remains bare of coral recruits for many years (Edmunds, 2000; Kuta & Richardson, 1997). It is therefore important to assess the regional transmission of BBD and resulting coral mortality.
Parrot fish corallivory has also resulted in chronic partial mortality (Bruckner & Bruckner, 1998; Rotjan & Lewis, 2005; Sanchez, Gil, Chasqui, & Alvarado, 2004) as evident from the Virginia Islands, USA (Bythell, Gladfelter, & Bythell, 1993), and Curaco (Meesters, Pauchli, & Bak, 1997). Parrotfish predation produces characteristic conspicuous grazing scars (Rotjan & Lewis, 2005) that heal under low predation pressure. Moderate predation can cause partial to complete mortality that compromises coral growth and reproduction (Van Veghel & Bak, 1994). Spatial competitors such as macroalgae and sponges can colonize on the tissue eroded area when the tissue regeneration process is incomplete or too slow, resulting in partial to complete coral mortality.
It is evident that coral reefs are increasingly threatened across the globe. Detailed knowledge of the regional occurrence of stresses and coral mortality is therefore essential for the management of coral reefs.
Apart from some reports on coral reef bleaching, diseases and recovery patterns (Arthur, 2000, 2005; Pillai & Jasmine, 1989; Ravindran & Raghukumar, 2006), few studies have detailed environmental stresses, including tissue loss and resulting mortality, in the Lakshadweep archipelago. Based on the assemblages of dominant coral genera, there are three distinct coral communities in the lagoons of Lakshadweep (Pillai, 1986):
- Porites community (massive community): the Porites community dominated by Porites lutea and Porites solida forms an inner lagoon community. Favia, Favites, Platygyra and Goniastrea mix with the Porites community, forming the massive coral cover.
- Acropora community (branching community): the Acropora community consists of various species of Acropora in the lagoon.
- Heliopora community: fungiids and faviids are common in the Heliopora community.
Understanding the interaction of factors contributing to the tissue loss pattern and mortality in these assemblages may allow for more effective conservation strategies of coral reefs of the Lakshadweep archipelago. The present study was intended to understand the responses of Lakshadweep corals to different tissue loss scenarios and to assess the hierarchy of these stresses in causing coral mortality.
2 MATERIAL AND METHODS
Lakshadweep is an archipelago of coral atolls scattered in the Arabian Sea at about 200–400 km from the Kerala coast, India, between 8°–12°N latitude and 71°–74°E longitude. The reefs in the islands are flat, rarely rising more than 2 m and consist of fine coral sand and boulders compacted into sandstone. Most atolls have northeast and southwest orientation with the islands lying on the eastern rim, and a mostly submerged reef on the western rim enclosing the lagoon (Pillai 1986). The Lakshadweep group of islands comprises 36 islands, of which 11 are inhabited (Kavaratti, Agatti, Amini, Kadmath, Chetlath, Andrott, Kalpeni, Kiltan, Bitra, Bangharam and Minicoy). The average depth of the lagoons varies from 0.5 to 8 m (Pillai 1971). The climate is typically tropical and is affected by the warm waters of the Indian Ocean. The monsoon cycle and reversal of water currents have a major influence in this island group. This region is of special importance to oceanographers, policy makers and to the nation of India at large because of its impressive biodiversity.
Two series of surveys were conducted perpendicular to the shore within a depth ranging from 0.5 to 8 m along seven islands (Kavaratti [KV], Agatti [Ag], Thinnakaran [Th], Kalpeni [Kp], Minicoy [My], Chethlath [Ch] and Bangaram [Ba]) of the Lakshadweep archipelago (8°–12°N latitude and 71°–74°E longitude) during March–April 2011 and November–December 2011. Line intercept transects 30 m long were surveyed at all islands to understand the environmental conditions and tissue loss pattern. Benthic conditions were photographed using a Canon D10 underwater camera. Five transects geo-tagged using the Garmin GPS system were laid at each island to represent all major benthic features in all surveyed islands. Transects for continuous monitoring were selected based on the availability of coral cover and prevailing tissue loss conditions.
Coral colonies were identified to the genus level and classified as massive and branching in order to understand the prevalence of tissue loss and mortality at the community level. Coral communities (branching or massive) that covered more than 50% of the total cover in the surveyed transects were considered dominant coral forms. Tissue loss prevalence and interacting biological parameters such as BBD, algal contact and predation scars were recorded from 30 × 2 m belt transects (English, Wilkinson, & Baker, 1997).
The number of coral colonies encountered with tissue loss (Figure 1a and b) divided by the total number of colonies observed in the transect gave tissue loss prevalence. Sediment flux in the surveyed transects was estimated by fixing sediment traps (English et al., 1997) at all surveyed sites (Figure 1c). One sediment trap fixed at each transect in all surveyed islands within the depth range of 2–3 m was retrieved every 30 days. Paired bite scars obtained from survey transects (Figure 1d) evidenced the existence of parrotfish predation (Rotjan et al., 2006) in the Lakshadweep system. Tissue loss by predation was recorded as predation scar prevalence by a ratio of coral colonies with prominent bite scars to total colonies in the transect. Prevalence of macroalgal contact (Figure 1e) and BBD (Figure 1f) were recorded as percentage prevalence. Tissue eroded coral colonies with low possibility of regeneration and the presence of macroalgae mats observed from benthic surveys were logged as the percent of corals with partial/complete mortality. Coral colonies with a low rate of tissue degradation and colonies that showed signs of regeneration in the continuous surveys were not counted as coral colonies with partial/complete mortality.

An independent samples t test was conducted with the statistical tool R to understand any statistically significant variation in tissue loss prevalence between massive and branching coral forms. A hierarchical multiple regression model was developed using R (R Core Team, 2016) to understand the effects of BBD, algal contact, predation scars and sediment flux upon large-scale tissue degradation and coral mortality. Preliminary analyses were conducted to test the assumptions of normality, linearity and homoscedasticity. In the hierarchical multiple regression model all predictor variables (causative factors) were added in the following sequence:
- Step 1: coral mortality ~ sediment flux.
- Step 2: coral mortality ~ sediment flux + predation scars.
- Step 3: coral mortality ~ sediment flux + predation scars + algl contact.
- Step 4: coral mortality ~ sediment flux + predation scars + algal contact + BBD.
3 RESULTS
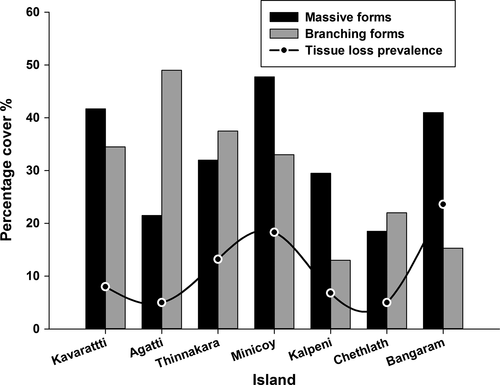
Massive coral coverage was maximum at Bangaram Island (41.7%) and lowest at Chethlath Island (18.5%). Percentage cover of branching coral forms was maximum at Agatti Island (49.8%) and lowest at Kalpeni Island (13%). Maximum tissue loss prevalence was recorded at Bangaram Island (23.6%) and minimum at Chethlath (5%) and Agatti (5%) islands (Figure 2). Benthic observations indicated that the prevalence of tissue loss tends to decrease with a reduction of massive coral cover, suggesting a greater susceptibility to tissue loss in massive corals than in branching forms. This observation was confirmed with a t test showing a statistically significant difference in prevalence of tissue loss between branching (Mean = 0.16; SD = 0.35) and massive (Mean = 20.91; SD = 7.88) coral forms (t(33) = 10; p = .0001, which is less than the alpha value 0.05).
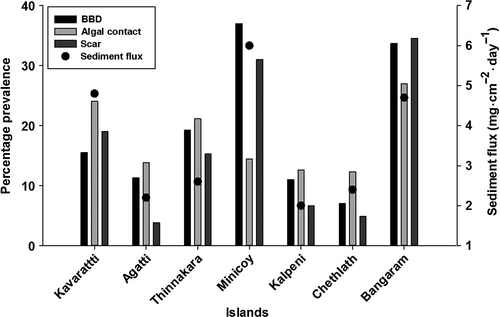
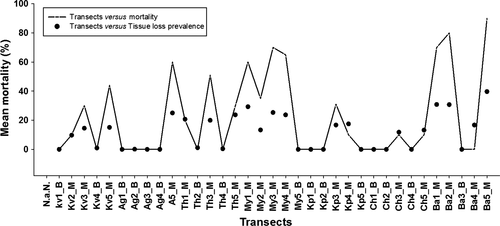
Visual documentation of environmental factors causing tissue loss and its prevalence was recorded (Figure 3). The maximum sediment flux was from Minicoy Island (6 mg·cm−2·day−1) and the minimum was from Kalpeni Island (2 mg·cm−2·day−1). Predation scars were highest at Bangaram (34.6%) and lowest at Agatti (3.8%). Bangaram Island (26.9%) was recorded as having the maximum algal contact prevalence and the minimum was at Chethlath (12.3%). BBD prevalence was highest at Minicoy (37%) and lowest at Chethlath (7%). Coral mortality was common on transects dominated by massive corals and was lower in transects dominated by branching corals. The highest recorded coral mortality of 85% was at Bangaram Island (transect Ba5_M) and mortality was found to increase with prevalence of tissue loss (Figure 4).
Hierarchical multiple regression analysis explored the influence of various possible causative factors responsible for coral mortality in the Lakshadweep region. All correlations among predictor variables were moderate to high, ranging between r = .45; p < .05 and r = .84; p < .001 (Table 1). This shows that multi-colinearity was unlikely to be a problem (Tabachnick and Fidell, 2007). All predictor variables were statistically correlated with coral mortality (r = .59; p < .001 to r = .84; p < .001). This showed that the data were correlated well with the dependent variable and hence examination through hierarchical multiple regression is reliable.
| Variables | TL | SF | PS | AC | BBD |
|---|---|---|---|---|---|
| Tissue loss prevalence (TL) | 1 | ||||
| Sediment flux (SF) | 0.67*** | 1 | |||
| Predation scars (PS) | 0.59*** | 0.51** | 1 | ||
| Algal contact (AC) | 0.78*** | 0.63*** | 0.45* | 1 | |
| Black band disease (BBD) | 0.84*** | 0.72*** | 0.54*** | 0.59*** | 1 |
| Means | 11.42 | 1.49 | 19.79 | 18.53 | 1.96 |
| SD | 11.96 | 1.41 | 22.12 | 16.57 | 2.12 |
| Range | 39.7 | 6.32 | 61 | 50 | 6.2 |
| Possible range | 0–39.7 | 0.08–6.4 | 0–61 | 0–50 | 0–6.2 |
| Cronbach's alpha | 0.52 | 0.73 | 0.69 | 0.56 | 0.71 |
- Statistical significance: *p < .05; **p < .01; ***p < .001.
In the first step of the hierarchical model, sediment flux was entered as the predictor. This model was statistically significant (F(1,33) = 26.21; p < .001) and explained 44.3% of variance in coral mortality (Table 2). Adding predation scars as the second-order variable resulted in an increase of total variance of 8% (R2 change = .08), i.e. a total of 53%. At the same time, it was evident that in the second step, the highest influence on coral mortality was from predation scars (standardized co-efficient value [β]of .34; p < .001) rather than the sediment flux (β = .49; p < .05). Introduction of algal contact with the third step explained another 17% of variance in coral mortality (R2 change = .17). This resulted in a total variance of 71% (F(3,31) = 24.69; p < .001), in which algal contact significantly explained more coral mortality (β = .55; p < .001) than predation scars (β = .25; p < .05) and sediment flux (β = .19; p < .05) combined. Adding BBD into the fourth step of the analysis explained an additional 14% of the variance in coral mortality and explained a total variance of 84.9% (F(4,30) = 42.28; p < .001). Only two of the four predictor models used in the analysis were statistically significant in explaining coral mortality, with BBD exhibiting a higher standardized co-efficient value (β = .59; p < .001) than algal contact (β = .48; p < .001). Predation scars and sediment flux were not statistically significant in explaining coral mortality in the final step of the analysis. It is hence clear from the analysis that BBD is the most important variable influencing coral mortality in the Lakshadweep system, followed by algal contact, predation scars and sediment flux.
| R | R 2 | R2 change | B | SE | β | t | |
|---|---|---|---|---|---|---|---|
| Step 1 | .66 | .44 | |||||
| Sediment flux | 5.64 | 1.1 | .66*** | 5.12 | |||
| Step 2 | .73 | .53 | .07 | ||||
| Sediment flux | 4.16 | 1.19 | .5* | 3.48 | |||
| Predation scars | 0.19 | 0.08 | .34*** | 2.44 | |||
| Step 3 | .84 | .71 | .17 | ||||
| Sediment flux | 1.63 | 1.13 | .19* | 1.44 | |||
| Predation scars | 0.13 | 0.06 | .25* | 2.15 | |||
| Algal contact | 0.39 | 0.09 | .55*** | 4.28 | |||
| Step 4 | .92 | .85 | .14 | ||||
| Sediment flux | −0.86 | 0.94 | −.1 | −0.92 | |||
| Predation scars | 0.07 | 0.05 | .13 | 1.45 | |||
| Algal contact | 0.32 | 0.07 | .45*** | 4.70 | |||
| Black band disease | 3.30 | 0.61 | .59*** | 5.36 |
- R2, amount of variance explained by predictors; R2 change, additional variance introduced in dependent variable; B, unstandardized co-efficient; SE, standard error with 95% confidence interval; β, standardized co-efficient; t, estimated co-efficient (B) divided by its own SE.
- Statistical significance: *p < .05; ***p < .001.
4 DISCUSSION
The higher influence of BBD upon tissue loss prevalence among Lakshadweep corals compared to the other factors tested as explained by the hierarchical multiple regression analysis corroborates the observations of Rutzler et al. (1983). They reported a rapid spreading rate and high coral tissue degradation in the massive coral Montastrea annularis with increasing BBD incidence. BBD is a widespread coral disease that affects mainly the massive framework-building corals (Barneah, Ben-Dov, Kramarsky-Winter, & Kushmaro, 2007; Frias-Lopez, Klaus, Bonheyo, & Fouke, 2004; Richardson & Carlson, 1993; Zvuloni et al., 2009). The significant influence of BBD upon tissue loss and the specific occurrence of BBD in massive coral forms explain the higher prevalence of tissue loss among massive coral forms compared with branching forms in the Lakshadweep region. Massive corals are also more susceptible to multiple stresses and large-scale tissue degradation than the branching forms (Thinesh, Mathews, & Edward, 2011). Tissue lesions and skeleton erosion caused by BBD are reportedly non-regenerative, causing partial or complete mortality in coral reefs (Viehman, Mills, Meichel, & Richardson, 2006).
Studies have shown that continuous contact of macroalgal thalli on coral tissue can result in tissue lesions from abrasion and in some cases from macroalgal allelochemicals (Morrow, Paul, Lilesand, & Chadwick, 2011). The larger surface area in massive corals, which are also susceptible to multiple stressors, might support increased macroalgal growth resulting in continuous physical contact. Such increased macroalgal coverage increases the chances of coral surface abrasion induced by water movement and intense allelopathic interactions, possibly resulting in large-scale tissue loss among massive corals.
Reef areas with a high abundance of massive corals such as M. annularis, Montastraea faveolata and Porites sp. have a higher prevalence of feeding scars, often exceeding 20% (Garzon-Ferreira & Reyes-Nivia, 2001). Parrotfish predation is specific to massive coral forms. This explains the high predation scar prevalence and induced tissue loss in massive coral dominant islands such as Minicoy and Bangaram. While, the dominance of branching corals in Agatti and Chethlath islands has resulted in reduced parrot fish predation and mediated tissue loss in these islands as branching corals are impervious to parrotfish predation. Such a reduced predation pattern might have contributed to the low tissue loss and mortality pattern recorded here for islands with branching coral dominance.
Although algal contact and parrotfish predation contribute to tissue loss in Lakshadweep corals, the hierarchical regression model shows that the influence of these factors upon coral mortality is low when compared to BBD. This could be because of the better healing ability exhibited by corals with algal abraded tissue lesions (Vanwoesik, 1998). However, a synergy of surface abrasion and allelopathy reduces tissue recovery leading to coral mortality (Morrow et al., 2011). This may explain the increased hierarchy of macroalgae over predation scars and sediment flux in terms of causing coral mortality. Tissue lesions from predation scars will generally heal unless scars are further spoiled with sediment filling and macroalgal succession. Sediment filling and macroalgal succession on predation scars results in coral mortality (Hoegh-Guldberg et al., 2007). Sediment flux exhibited an insignificant relationship with tissue loss prevalence in the Lakshadweep system and its role in coral mortality is minimal owing to the low sediment flux observed in the region.
5 CONCLUSIONS
Results from this study show that tissue loss induced by BBD, algal interactions and parrot fish predation is responsible for coral mortality in the region and that damage was more intense in massive forms than in branching forms. Reduced growth and slow recovery from tissue loss in massive corals resulted in significant colony mortality. BBD-mediated mortality should be addressed in management plans to safeguard the reef complexity of the Lakshadweep archipelago.
ACKNOWLEDGEMENTS
The authors are grateful to the Director and Dean of Centre of Advanced Study in Marine Biology and the authorities of Annamalai University for support and encouragement. The authors also thank the National Remote Sensing Centre, Hyderabad, for providing financial support, Department of Science and Technology and Department of Environment and Forest, Lakshadweep, for providing the necessary permission to conduct the coral survey. We thank the anonymous reviewers for their critical comments and suggestions for improvement. The contents and views expressed in the paper are of the individual authors and do not reflect the views and positions of their institutions.




