Reproductive resilience of ice-dependent Antarctic silverfish in a rapidly changing system along the Western Antarctic Peninsula
Abstract
The Western Antarctic Peninsula (wAP) is globally one of the systems most heavily impacted by climate change, notably steep declines in sea ice extent. In forage species, reproductive resilience to change is particularly important because population fluctuations are rapidly communicated through the system via trophic interactions. The reproductive traits of the ice-dependent forage species Antarctic silverfish (Pleuragramma antarctica) from different areas along the wAP and at the tip of the Antarctic Peninsula were investigated through macroscopic and histological analyses of gonads, with the aim to assess its reproductive potential and to test for spatial differences in fecundity and spawning season. Fish samples were collected in late summer off Charcot Island, in Marguerite Bay and off Joinville Island; no fish were caught in the central wAP. Samples from Charcot Island and Marguerite Bay consisted of adults in developing gonad stage, whereas those from Joinville consisted almost exclusively of juveniles. Mean GSI was relatively low (2–3%) and similar in both sexes, as specimens were still far from being actively reproducing. Developing females exhibited two discrete, though partially overlapping modes of oocytes of different size, with vitellogenic oocytes measuring 0.5–1.0 mm. Absolute and relative fecundity ranged between 3000 and 12,000 eggs per female and between 80 and 190 eggs·g−1, with a strong relationship between absolute fecundity and body size. These results were consistent with a single population at Charcot Island and Marguerite Bay and indicated substantial reproductive potential, which may mitigate population isolation and reductions in habitat availability but cannot ultimately offset catastrophic loss of spawning habitat linked to sea-ice retreat.
Introduction
Studies examining climate-induced effects on the vital rates of individual populations are rare, yet recent evidence of strong interactions between life history connectivity and circulation (e.g. Hanchet et al. 2008; Ashford et al. 2010, 2012) suggest that variation in population processes is closely linked to ocean structure. As a result, geographic distributions of fish species experiencing rapid change in the marine environment are likely to reflect population boundaries and connectivity. However, abundances will also depend on the reproductive resilience of individual populations and their capacity to offset increases in mortality. Estimating fecundity, and understanding the reproductive potential over the life history, is therefore a critical part of assessing how individual populations will respond to systemic change.
In the case of forage species, reproductive resilience is particularly important because variations in abundance and distribution are rapidly communicated upward through the system via trophic interactions. The Antarctic silverfish (Pleuragramma antarctica) is a widely distributed, high latitude species found between the continental margin and the shelf break around the Antarctic (Dewitt et al. 1990). It is overwhelmingly the most abundant pelagic fish on the continental shelves of the Ross Sea and Weddell Sea, representing more than 90% the total ichthyoplankton (Hubold 1985; Guglielmo et al. 1998). Considered a high-Antarctic species (sensu Kock 1992), it is also found in the seasonal pack-ice zone, such as the shelf waters of the Western Antarctic Peninsula (wAP) (Lancraft et al. 2004; Donnelly & Torres 2008), and plays a fundamental role in the Antarctic food web as an important food resource for high-level predators such as seals, penguins and whales, as well as for other notothenioid fishes. It is characterized by a considerable feeding plasticity, taking advantage of biomass peaks of either small (copepods) or large (krill) zooplankton throughout its life span (Hubold & Ekau 1990).
Along the wAP, the continental shelf hosts a biologically rich community, in which silverfish and Antarctic krill (Euphausia superba) sustain a number of higher trophic levels in the local food web (Lancraft et al. 2004; Friedlander et al. 2011). The wAP shelf region has been the subject of an extensive field programmes (such as the Palmer Station Long-Term Ecological Research and the Southern Ocean Global Ecosystem Dynamics), aimed at understanding the dominant physical and biological processes (Smith et al. 1995; Hofmann et al. 2004 and references therein). Globally, the wAP is currently one of the most heavily impacted regions by climate change, with the fastest rate of warming water and declining sea ice around Antarctica (Clarke et al. 2007). Changes in the maximum extent of sea ice in winter and timing of its advance and retreat potentially influence fish populations living in both coastal and oceanic systems; moreover, the most critical changes are expected to be those that affect the most vulnerable periods in the life history, such as the early life stages (Moline et al. 2008).
Silverfish need sea ice for spawning, egg deposition and feeding grounds for newly hatched larvae (Vacchi et al. 2004). The occurrence of dense spawning aggregations has been suggested by the observation of thousands of individuals under land-fast sea ice along the Antarctic Peninsula between June and October (Daniels & Lipps 1982), and eggs of P. antarctica were consistently found floating in the platelet ice underneath thick sea ice in Terra Nova Bay in the Ross Sea until larval hatching in mid-November (Vacchi et al. 2004).
Mitochondrial DNA sequencing indicated a weak population structure around the Antarctic Continent, characterized by a small but highly significant differentiation between samples along with a lack of association between clades and geographical locations (Zane et al. 2006; La Mesa & Eastman 2012). More recent data based on stable isotope and trace element analyses performed on otoliths revealed strong heterogeneity between the northern tip of the Antarctic Peninsula (AP) near Joinville Island and the southern wAP in Marguerite Bay and near Charcot Island, suggesting that P. antarctica are distributed in independent, discrete populations (Ferguson et al. 2011; Ferguson 2012). This is consistent with the different water masses characterizing the northern tip of the AP and the southern wAP, respectively from the Weddell Sea and Bellingshausen Sea origin (Loeb et al. 1993). Nevertheless, the otolith chemistry, when coupled with particle simulations, suggested a single population in the southern wAP, with transport by the Antarctic Peninsula Coastal Current (APCC) (Moffat et al. 2008) from Marguerite Bay to Charcot Island during the first 2 years of life (Ferguson et al. 2011). As a result, spatial segregation and the southward reduction in sea ice extension along the wAP might increase the risk of extinction of local populations through mortality of ice-dependent early life stages (Torres et al. 2010).
However, potential future impacts of climate change on silverfish biology also depend on their resilience to the risk of extinction in the case of climate-linked increases in mortality and changes in habitat availability. Key information on fecundity and reproductive biology are critical to assess the reproductive potential of individual populations over their life history. In particular, the estimate of length or age at first sexual maturity with respect to the maximum size or life span and the relationship between absolute fecundity and fish size give more insights on the actual reproductive potential of the species and, hence, on its capacity of resilience as the potential for recovery from environmental disturbance.
Despite this, available data on the reproductive biology of Pleuragramma antarctica are rather scarce and fragmentary, geographically restricted (East Antarctica and Weddell Sea), and often based on the macroscopic appearance of gonads (La Mesa & Eastman 2012). Therefore, in the present study we examined the reproductive traits of P. antarctica collected in different locations along the wAP and at the tip of the AP through histological and macroscopic analyses of gonads, aiming (i) to geographically extend the knowledge of the reproductive biology of this species based on a more accurate methodological approach, (ii) to test whether populations from different areas are characterized by differences in fecundity or spawning cycle and (iii) to assess the reproductive potential of the species in the face of a rapidly changing system along the wAP.
Material and Methods
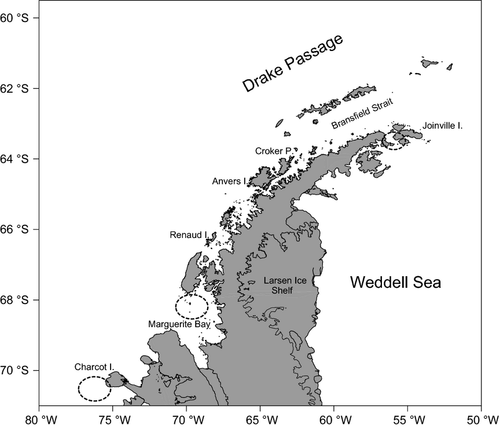
A multiple opening/closing net and environmental sensing system (MOCNESS) was used on board the R/V Nathaniel B. Palmer to sample areas along the wAP off Charcot Island, in Marguerite Bay, south of Renaud Island, off Anvers Island and in Croker Passage, as well as in Antarctic Sound between Joinville Island and the tip of Antarctic Peninsula from late March to early May 2010 (Fig. 1). The sampling system consisted of six 10-m2 nets (MOC-10) with a mesh size of 3 mm, each of them deployed at a specific depth interval (0–500, 500–300, 300–200, 200–100, 100–50, 50–0 m). Each trawl sequence lasted approximately 2 h. Overall, 48 tows were undertaken, of which 18 were off Charcot Island, 11 in Marguerite Bay and four in each of the other areas (only one in the Croker Passage).
Fish were measured to the nearest mm (total length, TL and standard length, SL) and sexed through direct examination of gonads. Gonad stage of maturity was macroscopically assessed according to a five-point scale for notothenioids (Kock & Kellermann 1991). Individual fish weight (TW) was estimated by applying the length–weight relationship for the species reported elsewhere (Kock et al. 1985). Whenever possible, gonads were excised, weighed to the nearest 0.01 g (GW) and the gonadosomatic index (GSI) calculated as percentage of gonad weight to total weight of fish.
Generally, the number of oocytes and size frequency distribution do not differ between left and right ovaries (Hunter et al. 1985); however, to ensure no bias, fecundity was estimated from a single randomly selected ovary from each female. Using the gravimetric method (Murua et al. 2003), the number of most advanced oocytes in a weighed subsample was counted and related to the weight of the entire ovary. A subsample of each ovary, representing 3–20% of the total gonad weight, was weighed and immersed in commercial sodium hypochlorite (10% in filtered seawater, Choy 1985) for 3 min to facilitate the disintegration of ovarian lamellae, and then immersed in filtered seawater in a Petri dish. Oocytes were spaced and photographed by a Leica DFC 420 videocamera to count and to measure the size of oocytes using imaq vision builder 6 software (National Instrument Corporation, Austin, TX, USA).
The number of oocytes taken from different ovary portions (anterior, median and posterior) was compared in six specimens to check any variation across the ovary. As no statistically significant differences among portions were observed (Friedman test for dependent data, χ2 = 1.33, P = 0.513, df = 2), fecundity was estimated from a randomly selected ovary portion from all other females.
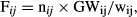
where n is the number of vitellogenic oocytes in the subsample, w is the weight of the subsample and GW is the gonad weight. Relative fecundity (Frel) was calculated as the number of vitellogenic oocytes per gram of fish body weight (TW).
The relationship between fish size (TL, mm) and absolute fecundity (F) was investigated using linear regression analysis. Data for both fish size and absolute fecundity were loge-transformed to fulfil assumptions. Normality and homogeneity of variances were tested using the Shapiro–Wilk test and the F-ratio test, respectively. No relationship was found between Frel or size of vitellogenic oocytes and fish size. Because few adult specimens were available from Joinville Island, comparisons were made exclusively between Charcot Island and Marguerite Bay. As a result, differences in absolute fecundity between geographic areas were tested using the analysis of covariance (ANCOVA, test for homogeneity of regression slopes), with female size as covariate and areas as fixed factors. All statistical analyses were performed using statistica 10 (StatSoft, Inc., Tulsa, OK, USA) software.
Histological analyses were carried out on both sexes to evaluate the stage of gonad development and to validate the macroscopical stage attribution. Particular care was taken to record the presence of postovulatory follicles (POF, indicating the occurrence of recent spawning event) and atretic oocytes. Gonad samples were dehydrated through increasing concentrations of ethanol solution, embedded in paraplast, cut in a series of transverse sections (7 μm) and mounted on slides. Sections were stained with Harrys' Haematoxylin and Eosin (Pearse 1985) and examined with a Leica DM LB light microscope at 100–630× magnification. Based on histological appearance and cell structure (West 1990), ovarian follicles were classified in the following developmental stages: I – chromatin nucleolar (immature); II – perinucleolar (immature); III – yolk vesicle or cortical alveoli formation (early maturation, endogenous vitellogenesis); IV – vitellogenic (late maturation, exogenous vitellogenesis); V – ripe (mature); VI – postovulatory follicles (post-reproductive). Each individual was staged considering the most advanced histological stage observed in ovarian sections, as notothenioids typically exhibit group synchronous ovaries (sensu Wallace & Selman 1990). Care was taken in comparing the size of oocytes at different stages of maturity between macroscopic and histological procedures, owing to the considerable shrinkage of oocytes observed after dehydration and embedding in paraplast. Based on the presence of different types of gametocytes in the seminiferous lobules (Billard 1986), male testes were classified in the following developmental stages: I –spermatogonia and spermatogonial mitoses (immature); II – first meiotic division (early maturation), spermatocytes I; III – second meiotic division (advanced maturation), spermatocytes II and spermatids; IV – spermatozoa cysts (mature); V – residual spermatozoa in collapsed lobules (post-reproductive).
Results
Fish samples
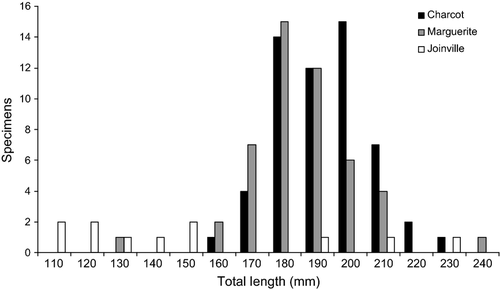
Pleuragramma antarctica were only caught off Charcot Island, in Marguerite Bay and south of Joinville Island. Off Charcot Island, gonad samples consisted of 15 males of 163–196 mm TL and 41 females of 152–225 mm TL (Fig. 2). All specimens were developing (stage 3 of macroscopic scale of gonad maturity). In Marguerite Bay, gonad samples consisted only of 48 developing females ranging from 124 to 233 mm TL (Fig. 2). Off Joinville Island, only four adult fish were collected, and these were all females ranging from 148 to 230 mm TL; two were maturing virgin (stage 2) and two were developing (stage 3). All other fishes (n = 7) were unsexed juveniles ranging from 103 to 144 mm TL, at stage 1 of gonad maturity (immature) (Fig. 2).
Macroscopic analysis
Gonadosomatic index values calculated for each sex and macroscopic stage of gonad maturity are summarized in Table 1. Mean GSI was significantly different between Charcot and Marguerite Bay (two-sample t-test, t = 3.45, df = 85, P = 0.0008). Mean GSI of developing males collected off Charcot was slightly higher than that of females (Table 1). The macroscopic analysis of ovaries in developing females revealed the presence of two discrete although partially overlapping modes of oocytes of different size (Fig. 3), confirming the group synchronous oocyte development in this species. Based on histological analysis (see below), the first mode consisted of smaller previtellogenic oocytes and the second of relatively larger vitellogenic oocytes at yolk vesicle or yolk granule stages. The two modes were separated by a threshold size of approximately 400 μm (Fig. 3). Consistent with GSI, mean and maximum size of vitellogenic oocytes were both significantly different between the two sampling areas (two-sample t-test = 4.10, df = 22, P = 0.0004; t = 4.16, df = 22, P = 0.0004, respectively), with samples from Marguerite Bay possessing slightly larger oocytes than samples from Charcot (Table 2). The two developing females from Joinville exhibited oocytes within the size range of samples from the other areas (Table 2).
| Site | Sex | n | Maturity stage | GSI (%) range | GSI (%) mean ± SE |
|---|---|---|---|---|---|
| Charcot | ♂ | 15 | 3 | 1.09–4.00 | 2.87 ± 0.20 |
| Charcot | ♀ | 41 | 3 | 1.48–3.46 | 2.25 ± 0.06 |
| Marguerite | ♀ | 46 | 3 | 1.54–4.35 | 2.62 ± 0.08 |
| Joinville | ♀ | 2 | 3 | 1.29–5.25 | 3.27 ± 1.98 |
| Joinville | ♀ | 1 | 2 | 0.05 | – |
| Site | n | Mean size (mm) range | Mean size (mm) ± SE | Max. size (mm) range | Max. size (mm) ± SE |
|---|---|---|---|---|---|
| Charcot | 12 | 0.46–0.56 | 0.52 ± 0.01 | 0.61–0.81 | 0.68 ± 0.01 |
| Marguerite | 12 | 0.54–0.70 | 0.59 ± 0.02 | 0.68–1.00 | 0.81 ± 0.02 |
| Joinville | 2 | 0.48–0.69 | 0.58 ± 0.10 | 0.57–0.99 | 0.78 ± 0.21 |
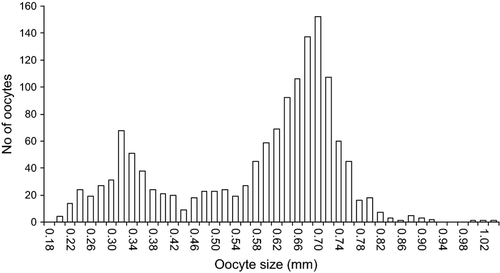
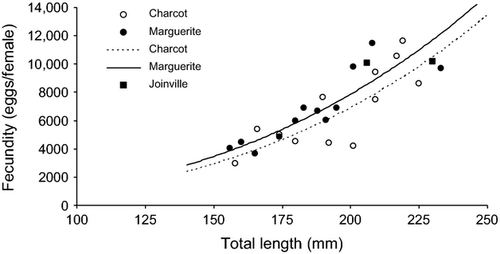
Absolute and relative fecundity of developing females were estimated from the number of vitellogenic oocytes (Table 3). No significant differences in relative fecundity were found between females sampled off Charcot and those in Marguerite Bay (two-sample t-test; t = 0.11, df = 22, P = 0.91). Females sampled off Joinville showed absolute and relative fecundity within the values observed in the other areas (Table 3, Fig. 4). The relationships between absolute fecundity (F) and fish size (TL, mm) from Charcot and Marguerite Bay followed the exponential equations
| Charcot | F = 0.000915 TL 2.99 | (n = 12, r2 = 0.61) |
| Marguerite Bay | F = 0.002181 TL 2.85 | (n = 12, r2 = 0.80) |
| Site | N | F (eggs per female) range | F (eggs per female) mean ± SE | Frel (eggs per g) range | Frel (eggs per g) mean ± SE |
|---|---|---|---|---|---|
| Charcot | 12 | 2953–11,613 | 6818 ± 1968 | 78–193 | 136 ± 39 |
| Marguerite | 12 | 3637–11,492 | 6700 ± 1934 | 108–189 | 157 ± 45 |
| Joinville | 2 | 10,043–10,174 | 10,109 ± 7148 | 119–171 | 145 ± 103 |
Comparing the regression slopes of the above equations with female size as covariate and areas as fixed factors, absolute fecundity was positively related to fish size in both areas, with no significant difference between them (ANCOVA, Table 4).
| Parameter | Degree of freedom | Wald statistic | P |
|---|---|---|---|
| Site | 1 | 0.098 | 0.753 |
| loge TL | 1 | 59.73* | 0.00000 |
| Site * loge TL | 1 | 0.078 | 0.780 |
- *indicates statistically significant value.
Histological analysis
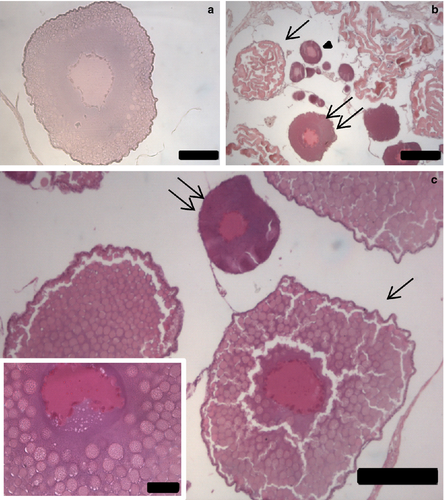
From a histological point of view, the different stages of development were very similar in the samples from the different areas, so that they are described regardless of the geographical origin. The analyses of ovaries in females (n = 18) revealed the presence of oocytes at different development stages, such as previtellogenic, cortical alveoli and vitellogenic. A single small maturing female showed small ovaries filled exclusively with previtellogenic oocytes of two different sizes (62 ± 14 and 142 ± 15 μm; histological stage II). The developing females (17) had relatively larger ovaries. Most of them (13) exhibited mainly oocytes at late cortical alveoli stage (367 ± 73 μm, histological stage III, Fig. 5a), whereas ovaries of all other females (n = 4) consisted of oocytes in exogenous vitellogenesis, completely filled with yolk granules (418 ± 32 μm, histological stage IV; Fig. 5c) and a few oocytes at an early cortical alveoli stage (143 ± 38 μm). Only two developing females showed oocytes under resorption through atretic processes (430 ± 51 μm, from histological sections; Fig. 5b). No post-ovulatory follicles were recorded.
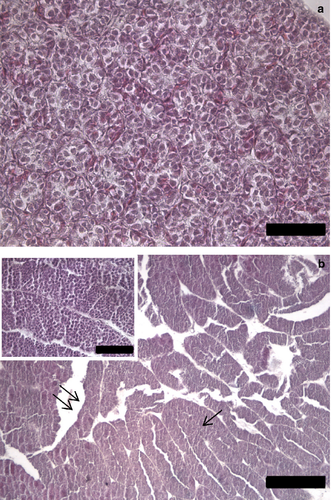
Males were in two different stages of maturity. Four immature males (staged macroscopically unsexed juveniles) presented very small and compact testes, with only cysts of spermatogonia in the lobule epithelium (histological stage I). Lobule lumina were not distinguishable (Fig. 6a). Developing males (n = 5) showed testes with spermatocytes I in the lobule epithelium, and enlarged and empty lobule lumina (histological stage II), possible signs of a previous reproduction (Fig. 6b).
Discussion
Reproductive biology of Antarctic silverfish
Examining the gonads of Antarctic silverfish collected along the wAP and at the tip of the AP, we found two discrete modes of oocytes of different size in the ovaries of developing females (Fig. 3), confirming that oocyte development is group synchronous in this species. The first mode consisted of previtellogenic oocytes and the second of vitellogenic oocytes at yolk vesicle or yolk granule stages. Mean absolute fecundity was estimated at 6800 eggs per female sampled off Charcot Island and 6700 eggs per female in Marguerite Bay. However, there was a strong relationship with fish size: those larger than 200 mm reached absolute fecundities larger than 10,000 eggs per female, compared with only 2000–6000 eggs per female in those smaller than 175 mm. In contrast, there was no relationship between fish length and size of eggs, estimated at 0.52 mm in fish sampled off Charcot Island and 0.59 mm in fish in Marguerite Bay. Mean absolute fecundity of 10,100 eggs per female off Joinville Island was consistent with the large size of the adult fish sampled there.
The knowledge of the Antarctic silverfish reproductive traits is still largely based on a few scattered data, geographically restricted to the Weddell Sea and Indian Ocean sector of the Southern Ocean (La Mesa & Eastman 2012). In this study, we were able to sample during late summer into early winter prior to ice formation, and extend the geographic limits to the wAP. Sampling from south to north of the Peninsula, we found no silverfish in historically abundant areas south of Renaud Island and off Anvers Island, and only one in Croker Passage, suggesting population fragmentation as a result of rapid change in the shelf system (Ferguson 2012). Nevertheless, we were able to capture fish from the southern wAP, in Marguerite Bay and off Charcot Island, and at the tip of the AP in Antarctic Sound that covered the range of life stages from juvenile to large adult.
Comparing the reproductive traits of Plueragramma antarctica from the wAP with other regions, the timing of gonad development and the reproductive effort represented by the gonadosomatic index (GSI) were consistent with a similar annual gonad development cycle around the Antarctic. Thus, in the Weddell Sea and in the Cosmonaut, Cooperation and Mawson Seas of Eastern Antarctica, most specimens collected in January–February were in their resting stage, with a mean GSI comprising approximately 1–2% in both sexes (Faleeva & Gerasimchuk 1990; Hubold 1992; Duhamel et al. 1993). At this stage of gonad maturity, two well defined groups of oocytes of different size were already distinguishable in females, with a threshold size of about 0.4 mm. The larger group consisted of oocytes starting the vitellogenic process (Faleeva & Gerasimchuk 1990), with a maximum size of 0.6 mm (Hubold 1992). In late summer (March–April) GSI started to increase rapidly in both sexes, attaining a mean value of 2.5–3.5% in populations from Eastern Antarctica and the Western Antarctic Peninsula. At the same time, vitellogenic oocytes showed a steady increase in size (up to about 1 mm), leading to a clear separation between vitellogenic and previtellogenic oocytes, which formed the batch to be spawned in the current season and the reserve stock, respectively (Faleeva & Gerasimchuk 1990; present data). GSI of males and females were also comparable at this stage of gonad development, and this has been tentatively considered an adaptation to pelagic spawning and egg release (Faleeva & Gerasimchuk 1990), as has been observed in the marbled rockcod, Notothenia rossii (Camus & Duhamel 1985; Kock & Kellermann 1991).
Histological analyses also showed similarities geographically, with those from the wAP closely resembling those reported from Eastern Antarctica (Faleeva & Gerasimchuk 1990). In late summer (March–April), adult specimens stopped the resting stage and showed an active gametogenesis, vitellogenic oocytes and primary spermatocytes being the most advanced gametocytes in females and males, respectively. According to Wallace & Selman (1990), at least two cohorts of oocytes can be distinguished in maturing ovaries, corresponding to a fairly synchronous population of larger oocytes to be spawned during the current breeding season, and a more heterogeneous population of smaller oocytes to be spawned in future breeding seasons. Two maturing females showed individual large oocytes at different stages of resorption, perhaps making up the fraction of unspawned eggs of the previous spawning season, as observed in the Eastern Antarctica (Faleeva & Gerasimchuk 1990; present data). Assuming that this species is a winter spawner (Kock & Kellermann 1991), ovarian follicle atresia is therefore a very slow, long-lasting process.
Population structure and reproductive potential
In contrast, fecundity estimates showed evidence of differentiation geographically. Populations from the Weddell Sea showed maximum values of 12,000–13,000 eggs per female and 190–200 eggs·g−1 (Hubold 1992), similar to our data, whereas absolute and relative fecundity estimates in the Mawson Sea were approximately 4000–18000 eggs per female and 70–160 egg·g−1, respectively (Gerasimchuk 1987). Nevertheless, reproductive traits observed in fishes collected from Marguerite Bay and Charcot showed little evidence of differentiation: in particular, absolute and relative fecundity and its positive relationship with fish size were similar between the two areas, indicating similar parental investment in reproduction. Although significantly lower gonadosomatic index and egg size in fishes from Charcot Island suggested a slight temporal shift in female gonad maturation between the two areas, the difference was very small. Consequently, the effect was unlikely to have been biologically meaningful, especially as fish were still far from being in reproductive condition. As a result, we were unable to refute the hypothesis of a single southern wAP population (Ferguson 2012) based on the reproductive data.
Length–frequency distributions were also consistent with a single population (Ashford et al. 2010; Ferguson 2012). Thus, specimens caught in Marguerite Bay and near Charcot Island showed almost the same size range, consisting solely of adult fish 150–240 mm in size. In contrast, those sampled near Joinville Island contained only a few adult specimens, and consisted almost exclusively of juveniles smaller than 160 mm. Length at first maturity appeared therefore to be in the range of 150–160 mm, at approximately 65–70% of maximum size. Converting to age using the von Bertalanffy curve published by Hubold & Tomo (1989), age-at-first maturity occurred at approximately 50% of maximum age, suggesting a significant delay in energy expenditure for reproduction. Moreover, although egg size remained constant, fecundity rose rapidly to reach 10,000–12,000 eggs per female after 200 mm length, highlighting the importance of larger, older fish in maintaining reproductive potential.
The demographic differences indicated that very different population constraints operate spatially. Thus, in the southern wAP, a large proportion (>30%) of fish were 200 mm or larger, consistent with substantial reproductive potential, but the lack of smaller fish suggested a failure in recent recruitment, potentially as a result of continuing sea-ice retreat. Conversely, at the tip of the AP, the large numbers of juveniles demonstrated recent recruitment, which could be partially explained by a longer persistence of sea ice in the Antarctic Sound than in the southern wAP. However, the low relative number of adults found in our sampling suggested that the mortality rate may be higher and reproductive potential consequently lower than in the southern wAP. Alternatively, connectivity may be important, with juveniles transported from spawning areas in the Western Weddell Sea.
Comparing reproductive investment with other high-Antarctic nototheniids, Pleuragramma antarctica exhibited the highest absolute fecundity except for the related Antarctic toothfish (Dissostichus mawsoni), which spawns up to 1 million eggs (Kock & Kellermann 1991). Even so, taking into account relative fecundity, P. antarctica is overwhelmingly the species with the highest maternal contribution, as a result of the trade-off between small body size and reproductive effort in terms of potential fecundity and egg size. Yet, Antarctic silverfish also currently appear to be one of the species most vulnerable to climate change (La Mesa & Eastman 2012). Several effects may be implicated. Geographical fragmentation and reproductive isolation implied by the lack of catches recorded from historically abundant areas near Renaud and Anvers Islands may have led to a sharp loss in connectivity along the wAP and, with it, the stabilizing effect of immigration as a subsidy to local self-recruitment (Pulliam 1988).
Inshore, the northward dispersal or transport of juvenile Pleuragramma from the Charcot Island/Marguerite Bay population is prevented for most of the year by the southward flow of the APCC, which is triggered by the melt water input in spring and persists until June, before the onset of the sea-ice formation. The flow is virtually absent in winter, rising again in early November at the sea-ice retreat, concomitantly with the hatching peak of Pleuragramma (Moffat et al. 2008; La Mesa & Eastman 2012). Offshore, Lagrangian particle simulations indicated a potential transport by the Antarctic Circumpolar Current (ACC) from Marguerite Bay north as far as Anvers Island, with trajectories further north passing along the outer shelf of South Shetlands away from the Northern Peninsula, consistent with the lack of mixing between the tip of the AP and southern wAP (Ferguson et al. 2011).
Sea ice melting and increases in seawater temperature may also reduce the duration of larval stages of P. antarctica (Vacchi et al. 2004; O'Connor et al. 2007), in turn reducing larval dispersal and connectivity. Most important, however, is their dependence on sea ice throughout their life history (La Mesa & Eastman 2012), and in particular the loss of critical spawning habitat due to sea-ice retreat. The substantial reproductive potential of silverfish may mitigate the effect of isolation and moderate reductions in habitat availability but it cannot ultimately offset catastrophic loss of spawning habitat in a rapidly changing system.
Acknowledgements
We gratefully acknowledge the support of the crew of the R/V Nathaniel B. Palmer; the field technicians on board from Raytheon Inc.; and J. Ferguson, E. Bortolotto and G. Santovito, who assisted with collecting the samples. We also are indebted to Dr Joseph Torres of the University of South Florida for his advice and encouragement. Funding for Dr Ashford was provided by the United States National Science Foundation (Grant no. 0741348). Funding for Dr La Mesa was provided by the Programma Nazionale di Ricerca in Antartide (PNRA).




