Inorganic nitrogen acquisition by the tropical seagrass Halophila stipulacea
Abstract
The tropical seagrass Halophila stipulacea is dominant in most regions of the Indo-Pacific and the Red Sea and was introduced into the Mediterranean Sea after the opening of the Suez canal. The species is considered invasive in the Mediterranean Sea and has been progressively colonizing new areas westward. Growth and photosynthetic responses of H. stipulacea have been described but no information is yet available on the nitrogen nutrition of the species. Here we simultaneously investigated the uptake kinetics of ammonium and nitrate and the internal translocation of incorporated nitrogen in H. stipulacea using 15N-labelled substrates across a range of Ni levels (5, 25, 50 and 100 μm). The ammonium uptake rates exceeded the nitrate uptake rates 100-fold, revealing a limited capacity of H. stipulacea to use nitrate as an alternative nitrogen source. The uptake rates of ammonium by leaves and roots were comparable up to 100 μm 15NH4Cl. At this concentration, the leaf uptake rate was 1.4-fold higher (6.22 ± 0.70 μmol·g−1 DW h−1) than the root uptake rate (4.54 ± 0.28 μmol·g−1 DW h−1). The uptake of ammonium followed Michaelis–Menten kinetics, whereas nitrate uptake rates were relatively constant at all nutrient concentrations. The maximum ammonium uptake rate (Vmax) and the half-saturation constant (Km) of leaves (9.79 μmol·g−1 DW h−1 and 57.95 μm, respectively) were slightly higher than that of roots (6.09 μmol·g−1DW h−1 and 30.85 μm, respectively), whereas the affinity coefficients (α = Vmax/Km) for ammonium of leaves (0.17) and roots (0.20) were comparable, a characteristic that is unique among seagrass species. No substantial translocation (<2.5%) of 15N incorporated as ammonium was detected between plant parts, whereas the translocation of 15N incorporated as nitrate was higher (40–100%). We conclude that the Ni acquisition strategy of H. stipulacea, characterized by a similar uptake capacity and efficiency of leaves and roots, favors the geographical expansion potential of the species into areas with variable water-sediment N levels throughout the Mediterranean.
Introduction
Nitrogen is an essential nutrient for seagrass growth and is available to seagrasses as ammonium and nitrate either in the water column or in the sediment porewater. In seagrass habitats lacking appreciable anthropogenic influence, such as agricultural land runoff or wastewater discharges, Ni concentrations in the water column are usually very low (<5 μm; Touchette & Burkholder 2000), whereas the concentration of ammonium in the anoxic sediments is much higher (up to 180 μm). Both the leaves and the roots of seagrasses are able to take up nitrogen. The few available studies on the role of leaves versus roots in the nitrogen uptake by seagrasses show that, in some species, inorganic nitrogen is mainly supplied through root absorption as ammonium (Iizumi & Hattori 1982; Short & McRoy 1984), whereas in others the leaf uptake of ammonium and nitrate can contribute considerably to the total Ni uptake of seagrasses (Hemminga et al. 1994; Terrados & Williams 1997; Alexandre et al. 2011).
The translocation of incorporated nitrogen from the leaves to the roots and vice versa was first suggested in the seagrass Zostera marina after the observation that leaf and root uptake rates were affected by the availability of nitrogen to the opposite plant part (Thursby & Harlin 1982, 1984). However, the internal movement of nitrogen following the distribution of incorporated 15N within the plant was only assessed directly in a few studies (Iizumi & Hattori 1982; Vonk et al. 2008; Alexandre et al. 2011). In those studies, the translocation rates and the direction of the labelled 15N varied significantly with the species and with the nitrogen source (i.e. ammonium or nitrate).
Halophila stipulacea is the dominant seagrass in most regions of the Indo-Pacific and the Red Sea (Short et al. 2001). The species migrated into the Mediterranean after the opening of the Suez canal in 1869, where it is considered invasive, and has been colonizing new areas westward (Procaccini et al. 1999; Gambi et al. 2009; Sghaier et al. 2011). Surprisingly, nothing is known about the nutritional physiology of H. stipulacea. Here we investigate the nitrogen acquisition strategy of this species by assessing (i) the uptake rates of ammonium and nitrate of leaves and roots, through the incorporation of 15N in the tissues, and (ii) the potential translocation of nitrogen between plant parts by following the distribution of 15N within the plant.
Methods
Plants of H. stipulacea were collected from a shallow muddy to sandy meadow (average water depth of 3 m) located in Akrotiri Bay, Limassol, Cyprus (34°42′22″°N, 33°07′26″°E) in April 2012. The average seawater salinity in the bay is 39 and the mean annual temperature is 20–21 °C (Forchino 2010). The average concentrations of ammonium and nitrate are 0.18 ± 0.16 μm (n = 19) and 0.16 ± 0.15 μm (n = 14), respectively. Average values were obtained from water samples collected in Akrotiri Bay (0–32 m depth) in 2005–2006 during the monitoring programmes of the Department of Fisheries and Marine Research of Cyprus (M. Marcou, personal communication).
After collection, the plants were immediately transported in moist tissues to the Centre of Marine Sciences, South Portugal, and kept for 2 weeks under a 12 h:12 h light:dark cycle in aerated natural seawater from Ria Formosa lagoon (35 PSU, 18 °C). Seawater was renewed every 2 days to keep oxygen levels at saturating levels (6.69 mg l−1; Mudge et al. 2007). During acclimation, the physiological condition of H. stipulacea plants was monitored by measuring the effective electron quantum yield of photosystem II (Y = Fv/Fm). Yield was measured on a daily basis using a pulse amplitude modulated (PAM) fluorometer (Diving-PAM; Heinz Waltz, Germany). Yield steadily increased during the acclimation to values of around 0.76, similar to those measured in situ by Sharon et al. (2009). The experiment was performed when this point was reached.
Ammonium and nitrate uptake rates of H. stipulacea were measured separately in leaves and roots by incubating the above- and belowground parts of whole plants in separate media. Two independent plastic containers (4 cm height × 5 cm diameter) were placed side by side to create two different incubation media, one for the leaves and one for the roots. The leaves of whole plants were submerged in the leaf container, and roots in the root container. Small pieces of cotton soaked in nitrogen-free artificial seawater and covered with a piece of plasticine were wrapped around the emerged part of the rhizome between the two containers to prevent tissue desiccation during incubation and contact between the leaf and root media via capillarity. Leaf uptake was measured by incubating the leaves in 100 ml nitrogen-free artificial seawater (35 PSU, pH 8.2) enriched with 15NH4Cl or 15KNO3 (atom% = 99, Cambridge Isotope Laboratories, Tewksbury, MA, USA) at four nutrient concentrations (5, 15, 50 and 100 μm). Roots were left without nutrients. Root uptake was determined using a similar procedure, i.e. the roots were incubated in nitrogen-enriched media while leaves were left in nitrogen-free solutions. The dry biomass of incubated leaves and roots averaged 0.0051 ± 0.002 g and 0.0016 ± 0.007 g, respectively. The aboveground: belowground biomass ratio was 3.18. Plants were incubated at a constant light intensity (50 μmol quanta m−2·s−1) and temperature (20 °C) for 12 h. During incubation, the nutrient concentration in the media did not vary noticeably. The media were stirred constantly using a shaking platform to ensure the mixing of the label. Even though the sediment porewater in seagrass habitats is mostly anoxic, the roots of H. stipulacea were incubated in oxygenated media because previous experiments showed no effect of oxygenation on the ammonium uptake by the roots of the seagrasses Zostera noltii (Alexandre et al. 2010, 2011) and Z. marina (A. Alexandre, R. Santos, unpublished data).
At the end of the incubation, the leaves and the roots were separated from the rhizomes and rinsed with deionized water to remove adherent salts and label. Tissues were dried at 60 °C for 48 h and reduced to a fine powder for isotopic analysis. Total nitrogen content and atom% of 15N of dried tissues were determined using a Flash EA 1112 Series elemental analyser coupled on line via Finningan conflo II interface to a Thermo delta VS mass spectrometer. 15N background levels in the tissues were measured in five replicate samples.
The amount of 15N (g) in plant tissues after incubation was calculated by subtracting the post-incubation 15N level (%) from the initial background level (%), and multiplying by the total nitrogen in the tissue (g). Nitrogen uptake rates, expressed as μmol N g−1 DW h−1, were plotted against substrate concentration (μm). The ammonium uptake rates were fitted to the Michaelis–Menten model, V = (Vmax × S)/(S + Km), and the uptake kinetic parameters Vmax, Km and α were obtained. In the model, V is the uptake rate (μmol·g−1 DW h−1), Vmax is the maximum uptake rate (μmol·g−1 DW h−1), S is the substrate concentration (μm), and Km is the half-saturation constant (μm), i.e. the substrate concentration at which the uptake rate is half of Vmax. The leaf ammonium uptake rate, expressed as μmol·g−1 DW day−1, of H. stipulacea at the ambient ammonium concentration in Akrotiri Bay was calculated from the Michaelis–Menten equation obtained for the leaves, according to the equation Vamb = (Vmax × Samb)/(Km + Samb) × 24, where Vamb is the ammonium uptake rate at ambient nutrient concentration (μmol·g−1 DW h−1) and Samb is the ambient water column nutrient concentration (μm). The nitrogen requirement for growth (μmol N g−1 DW day−1) was estimated based on the species-specific growth rate obtained previously (0.0167 g·g−1 DW day−1) and multiplied by the total nitrogen content (1.05%) of leaves. The translocation of 15N-label from the leaves to the roots and vice versa was assessed by measuring the amount of 15N that was recovered in the opposite, non-incubated plant part and comparing this with the amount of 15N taken up by the incubated plant part.
Data analysis
Differences in the ammonium uptake rates between leaves and roots were tested using two-way analysis of variance, with plant part and nitrogen concentration as the main factors. Post hoc comparisons were performed using the Holm–Sidak method to determine where significant differences occurred. Differences in the nitrate uptake rates between leaves and roots could not be tested using two-way analysis of variance because the root uptake data were not normally distributed, even after data transformation. Consequently, one-way analysis of variance was used to test differences in the nitrate uptake rates of leaves among substrate concentrations, and differences in root uptake rates were tested using the Kruskal–Wallis non-parametric test. Differences were considered significant at a level of P = 0.05.
Results
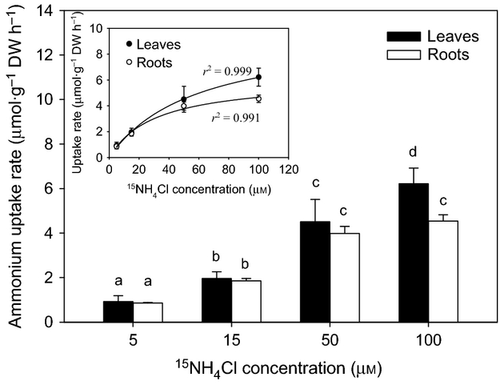
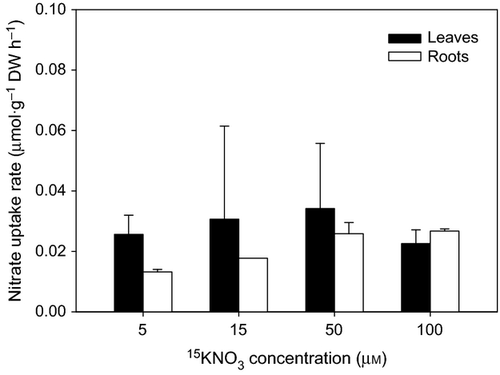
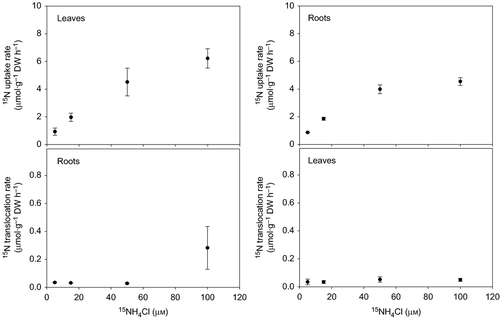
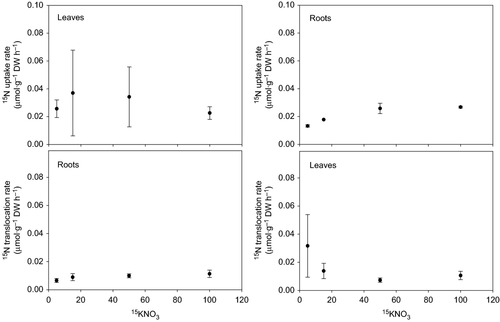
The ammonium uptake rates by leaves and roots of Halophila stipulacea increased with nutrient concentration (F = 111.10, P = 0.001; Fig. 1). Leaf uptake rates were not significantly different from root uptake rates except at 100 μm, when the leaf uptake was 1.4-fold greater (6.22 ± 0.70 μmol·g−1 DW h−1) than the root uptake (4.54 ± 0.28 μmol·g−1 DW h−1; F1,3 = 9.18, P = 0.008). The uptake of ammonium followed Michaelis–Menten kinetics (Fig. 1, inset graph). The maximum ammonium uptake rate (Vmax) and the half-saturation constant (Km) of leaves (9.79 μmol·g−1 DW h−1 and 57.95 μm, respectively) were higher than for roots (6.09 μmol·g−1DW h−1 and 30.85 μm, respectively). However, the affinity coefficients (α = Vmax/Km) for ammonium of leaves (0.17) and roots (0.20) were comparable. Overall, the nitrate uptake rates were 100 times lower than the ammonium uptake rates and these were relatively constant at all nutrient concentrations (Fig. 2). Leaf uptake rates did not vary significantly with nutrient concentration (F = 0.38, P = 0.771), whereas root uptake rates at 5 μm (0.013 ± 0.001 μmol·g−1 DW h−1) were significantly lower than at 50 μm (0.026 ± 0.004 μmol·g−1 DW h−1) and 100 μm (0.027 ± 0.001 μmol·g−1 DW h−1; H = 7.04, P = 0.024). No substantial translocation of 15N incorporated as ammonium (<2.5%) occurred between leaves and roots (Fig. 3). The translocation rates averaged 0.5 μmol·g−1 DW h−1 in either direction and did not vary with the nutrient concentration. The translocation of 15N incorporated as nitrate was higher (40–100%; Fig. 4) but the nitrate acquisition by the species was negligible when compared with the total ammonium uptake.
Discussion
The results of this study clearly show that ammonium is the major inorganic nitrogen source for the tropical seagrass Halophila stipulacea. Ammonium uptake rates by both leaves and roots were much higher than nitrate uptake rates, which were nearly zero. A compilation of the few available studies on the acquisition of ammonium versus nitrate by seagrasses showed that ammonium is generally preferred over nitrate as the main source of Ni (Short & McRoy 1984; Hemminga et al. 1994; Terrados & Williams 1997; Lee & Dunton 1999; Cornelisen & Thomas 2004; Hasegawa et al. 2005; Alexandre et al. 2011). This preference is usually ascribed to the lower energetic cost associated with the assimilation of ammonium comparated with nitrate, which must first be reduced via nitrite into ammonium (Turpin 1991; Bloom et al. 1992). Halophila stipulacea demonstrated a very limited capacity to use nitrate. The amount of nitrate taken up by the species corresponded to less than 1% of the total Ni incorporated, which represents a much lower fraction of the total Ni compared with other seagrass species (40% in Z. marina, Iizumi & Hattori 1982 5% in Z. noltii, Alexandre et al. 2011). The low nitrate uptake rates determined here for H. stipulacea are consistent with the extremely low nitrate reductase activity of leaves and roots previously reported for the species (Doddema & Howari 1983). Nitrate reductase is the enzyme responsible for the reduction of nitrate into nitrite, which is the first step in the nitrate assimilation process (Campbell 1999). Although the relationship between enzyme activity and uptake rate is not always evident, the low enzyme activity and nitrate uptake rates of H. stipulacea are both indicative of the poor use of nitrate by the species. The limited capacity to use nitrate as an alternative nitrogen source represents an ecological disadvantage for H. stipulacea, as growth and survival may be compromised in areas where ammonium availability becomes infrequent or non-existent.
The leaves and roots of H. stipulacea took up ammonium at comparable rates, in contrast to most seagrass species, which show much higher ammonium uptake rates by the leaves (Thursby & Harlin 1984; Pedersen et al. 1997; Hasegawa et al. 2005; Alexandre et al. 2011). The affinity coefficient (α) for ammonium uptake, i.e. the efficiency to take up ammonium at low concentrations (Harrison et al. 1989), was also similar for leaves and roots. This is an exceptional characteristic of H. stipulacea compared with other tropical and temperate seagrass species (Fig. 5), which show much higher affinities for ammonium uptake in the leaves. Our results indicate that the leaves and roots of H. stipulacea are both capable of significant ammonium uptake and are equally efficient at removing the nutrient when available at low concentrations either in the water column or in the sediment porewater. The similar uptake capacity and efficiency of both leaves and roots represents an asset for H. stipulacea, as ammonium can be efficiently acquired from the environment independently of its location.
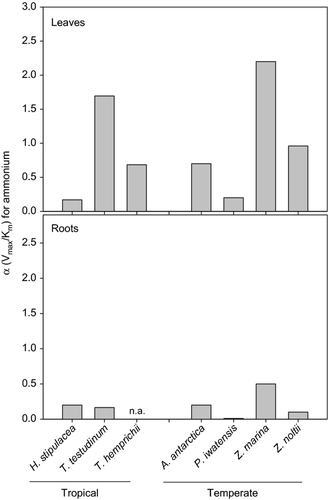
In seagrass habitats, the ammonium concentrations are usually much lower in the water column than in the sediment porewater (Touchette & Burkholder 2000), particularly in ultra-oligotrophic waters such as those of tropical regions and the Mediterranean Sea (Crombet et al. 2011). In Akrotiri Bay, where H. stipulacea was collected, the leaf ammonium uptake rate at the ambient nutrient concentration (0.18 μm) was 0.73 μmol·g−1 DW day−1, which is much lower than the estimated nitrogen requirement for growth of the species (12.5 μmol N g−1 DW day−1). This shows that leaf uptake alone is insufficient to sustain the growth of H. stipulacea in the bay and, consequently, that the major Ni supply for the species is porewater ammonium absorbed through the roots. The higher nitrogen content found in H. stipulacea roots (1.50 ± 0.10%) compared with leaves (1.05 ± 0.12%) supports this hypothesis. On the other hand, H. stipulacea may capitalize on the large inputs of ammonium resulting from the drastic growth of mariculture and fish farming practices off the coasts of the Mediterranean Sea (Karakassis et al. 2000; Pusceddu et al. 2007), which are increasing the ambient nutrient concentrations several-fold. In impacted sites, the ammonium concentrations in the water column may reach 2.5 μm (Apostolaki et al. 2010) and sustain growth of H. stipulacea. This may be one of the reasons for the successful expansion of this species in the region.
After nitrogen is taken up by plants, it can be translocated to other plant parts to be reduced and assimilated. In terrestrial plants, the internal movement of nitrogen occurs mainly towards leaves because of the lower capacity of roots and rhizoids to reduce and assimilate nitrogen (Williams 1984; Williams & Fisher 1985; Vermeer et al. 2003). In seagrasses, the internal nitrogen cycling is poorly investigated and the movement of 15N between leaves and roots has only been studied in a few species (Iizumi & Hattori 1982; Vonk et al. 2008; Alexandre et al. 2011). Of the six species examined, only Z. marina showed a significant translocation of 15N, incorporated as ammonium and nitrate, between plant parts after 24 h (Iizumi & Hattori 1982). In the present study, a small percentage (2–2.5%) of the 15N incorporated as ammonium by the leaves and roots of H. stipulacea was recovered by the opposite plant part after 12 h. Similar percentages (<1%) were reported for the tropical seagrasses Thalassia hemprichii, Halodule uninervis and Cymodocea rotundata (Vonk et al. 2008) and the temperate seagrass Z. noltii (Alexandre et al. 2011) after shorter incubation periods (1–4 h). Such low translocation rates of 15N, as those reported here for H. stipulacea, suggest that leaves and roots are both primary sites of ammonium assimilation. In fact, it is even possible that the 15N is translocated to the opposite plant part as assimilated organic nitrogen, such as in terrestrial plants (Hill-Cottingham & Lloyd-Jones 1979; Pate 1980). If 15N was translocated as ammonium to the opposite plant part, then the translocation rates of 15N should have followed the same pattern of the ammonium uptake rates, i.e. the translocation rates should have increased with nutrient concentration. This was not the case in H. stipulacea, which indicates that nitrogen was probably translocated as organic nitrogen and that the translocation rates were limited by some physiological process that is independent of the nutrient concentration (e.g. assimilation). Even though nitrate uptake represented a negligible fraction of the total Ni incorporated by H. stipulacea, it is interesting to note that a substantial part of 15N taken up as nitrate was internally translocated (40–100%). In Z. marina, half of the nitrogen taken up by the leaves as nitrate was translocated to the roots (Iizumi & Hattori 1982), whereas only 8–20% of the 15N was translocated to the leaves in other seagrass species (Vonk et al. 2008).
In summary, this study showed that H. stipulacea depends exclusively on ammonium as a source of Ni, taken up by the leaves and roots at similar rates. The similarity found in the uptake capacity and efficiency of leaves and roots indicates that H. stipulacea is adapted to acquire ammonium efficiently from environments where nitrogen is available either in the water or in the sediment. This trait may confer an advantage to H. stipulacea in relation to other Mediterranean seagrass species that may well contribute to its spread throughout the Mediterranean.
Acknowledgements
The authors are grateful to D. Kletou (University of Plymouth, UK) for his diving assistance during plant sampling in Cyprus, and to Joana Almeida for her dedicated assistance during the experiment. We also thank two anonymous reviewers for their valuable comments on the manuscript.




