Succession patterns of polychaetes on algal-dominated rocky cliffs (Aegean Sea, Eastern Mediterranean)
Abstract
Ecological succession has been scarcely investigated on sublittoral rocky cliffs. The few relevant studies deal with the structure of the developing community and are limited to higher taxa or sessile forms. The objective of the present study was to examine succession patterns on algal-dominated rocky cliffs both at the structural (species composition) and functional (feeding guild composition) level, using Polychaeta, a dominant taxon in this marine habitat, as a reference group. Cement panels were seasonally installed on the rocky substratum (25–30 m depth) and sampled every 3 months over a 1-year period. Twenty-nine polychaete species were recorded, previously reported from the surrounding benthic community, and classified into eight feeding guilds. Most species were assigned as sessile filter-feeders; this guild dominated in abundance and biomass. A strong effect of the length of immersion and of the seasonal onset of succession on the developed communities was assessed: species composition analyses suggested convergence into a similar organization as succession proceeds, whereas the impact of starting season on succession was stronger when analysing feeding guilds. In both cases succession was faster on panels installed in winter. The main emerging patterns were in agreement with relevant surveys of the entire benthic fauna, thus supporting the efficacy of polychaetes as a surrogate group for studying ecological succession in the benthic marine environment.
Introduction
Sublittoral rocky cliffs are prominent habitats in the Mediterranean, sustaining species-rich benthic communities (Garrabou et al. 1998; Terlizzi et al. 2007) due to their high structural complexity, i.e. physical structure (Matias et al. 2010). Rocky cliffs are highly heterogeneous in topographic relief (Riggs et al. 1996) and are colonized to a varying extend by algae and sessile invertebrates of various growth forms, ranging from thin encrusting layers to massive branching shapes. These interacting organisms create a biotic complex where sediment is often trapped (Antoniadou & Chintiroglou 2005). In this way, physical structure further expands and, accordingly, a greater diversity of niches or exploitable resources is produced (Matias et al. 2010). These habitats are considered vulnerable (Ballesteros 2006), being strongly affected by natural disturbances and anthropogenic pressure (see Antoniadou et al. 2011a), which can be also classified under the recently proposed term ‘affectors’ (Montefalcone et al. 2011). However, disturbances are thought to be the main causes of increased biodiversity on rocky cliffs; they create open areas in these space-limited habitats (Jackson 1977; Sousa 1984; Benedetti-Cecchi & Cinelli 1996; Maughan & Barnes 2000), triggering colonization and subsequent succession (Connell & Slatyer 1977; Underwood & Anderson 1994). Both ecological processes are highly variable, unpredictable, and stochastic (Underwood & Chapman 2006), at least during the early periods of succession (Antoniadou et al. 2010, 2011a; Pacheco et al. 2011; Rico et al. 2012); they operate in diverse spatial and temporal scales and are affected by various factors, such as larval supply, competition and predation. Accordingly, benthic communities consist of a puzzling mixture of assemblages at different successional stages (Sousa 1984; Bulleri & Benedetti-Cecchi 2006; Chapman 2007), thus enhancing regional biodiversity.
Colonization and ensuing succession, despite being fundamental processes in benthic ecology, have been inadequately studied on sublittoral rocky cliffs (see Antoniadou et al. 2010, 2011a). They both induce significant changes in community structure and function but the relevant mechanisms are highly complex, as large numbers of species are involved and thus not understood completely. Biota information usually focuses on higher taxa, or only sessile forms are taken into consideration, whereas other aspects of succession related mainly to community function, such as biomass or life and feeding traits, have been largely overlooked.
Along the Mediterranean sublittoral zone, rocky cliffs are occupied by algal or animal-dominated communities according to the prevailing environmental conditions, mainly illumination (Garrabou et al. 2002). In these communities, Polychaeta is among the most dominant invertebrate groups of macrobenthos, usually constituting over one-third of both species richness and numerical abundance (Antoniadou et al. 2004; Chintiroglou et al. 2004; Giangrande et al. 2005). Furthermore, feeding biology has been recognized to be one of the primary aspects of community function (Bremner et al. 2003) for polychaete communities in particular (Pagliosa 2005; Antoniadou & Chintiroglou 2006; Domínguez Castanedo et al. 2012). This animal group has been extensively studied under the ‘feeding guild’ concept, which is much broader than simple feeding interactions (Fauchald & Jumars 1979). With this concept, differences in the (i) food particle size and composition (microphages and macrophages), (ii) mechanism of food intake (herbivores, carnivores, filter feeders, surface deposit feeders, and burrowers), and (iii) motility patterns associated with feeding (sessile, discretely motile, and motile) are integrated. Therefore, polychaetes constitute a representative animal group for thorough studies of macrobenthic communities on hard-substratum habitats, at both structural and functional levels.
The main task of the present study was to assess the structure (i.e. species composition) and function (i.e. feeding guild composition) of polychaete communities, considering abundance and biomass data as well, during early succession (1-year period) on a sublittoral rocky cliff (Aegean Sea), using cement-constructed experimental panels. Cement panels were selected for conformity reasons, as they have been widely used in succession studies (see Antoniadou et al. 2010) and also because they consist of a suitable material to construct artificial reefs for restoring benthic communities (Baine 2001).
Study area
The study was carried out in Porto Koufo Bay located in the North Aegean Sea, Eastern Mediterranean (Fig. 1). The sea bottom consists of an almost vertical (80–90° inclination) limestone rocky cliff down to a depth of 60 m. Algal-dominated communities occur down to 35 m depth; beyond that depth, they are replaced by animal-dominated communities (Antoniadou & Chintiroglou 2005). Taking into account the scarcity of data on benthic succession in the subtidal zone, especially considering its deeper part (Antoniadou et al. 2010), the depth zone of 25–30 m was chosen to perform the field experiment. At this depth zone the turf-forming filamentous Rhodomelacea Womersleyella setacea and Polysiphonia spp. prevail, forming a dense carpet, sparsely interrupted by the presence of conspicuous sessile animals such as sponges, bryozoans, and ascidians (Antoniadou et al. 2010).
Materials and Methods
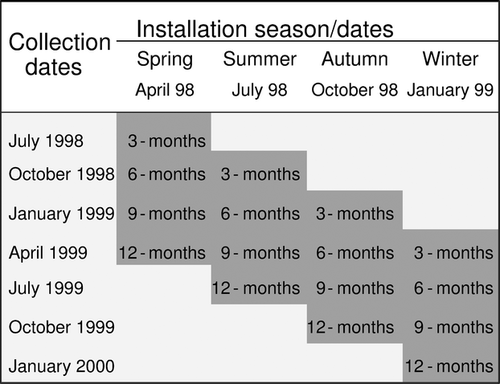
Experimental square panels (30 × 30 × 2 cm) constructed of rough cement were randomly deployed on the rocky cliff, at the chosen depth zone (25–30 m), by SCUBA diving. Four seasonal immersion experiments were carried out during which four sets, each consisting of 12 panels, were fixed with nails on the substratum in April 1998, July 1998, October 1998, and January 1999 (Fig. 2). The panels of each set were arranged on the rock, keeping a distance of 5 m from each other, at a relatively homogeneous area covered by the algal carpet, avoiding the presence of large animal species. The next set of panels was installed keeping a distance of 1 m from the previous one. Each set was surveyed at 3-month intervals. During each sampling, three replicate panels of each set were randomly collected. Panels were carefully detached from the rock and kept separately in net bags of 0.25 mm mesh size. Concurrently, the main physical and chemical parameters (i.e. temperature, salinity, pH, and dissolved oxygen) were measured in the water column with an autographic recorder (TOA-DKK WQC-24). Altogether 48 samples were obtained. In the laboratory the upper surface of each panel was photographed to estimate cover of sessile organisms (e.g. algae, sponges, bryozoans, ascidians), as percentage of the panel area, and then carefully scraped using a scalpel. The collected material was sieved (mesh opening 0.5 mm) and preserved in an 8% formaldehyde–seawater solution. After sorting, all polychaetes were identified to species level, counted, and the biomass of each individual was estimated as formalin wet weight (decalcified weight for tube-building species) using an electronic scale (0.01 mg precision). All specimens have been deposited in the Museum of the Department of Zoology in the Aristotle University of Thessaloniki. Polychaete species were classified to feeding guilds according to the seminal work of Fauchald & Jumars (1979) taking into account updated information on this topic (Gambi et al. 1995; Giangrande et al. 2000; Martin et al. 2000; Box et al. 2010; Castanedo et al. 2012; Mattos et al. 2013).
Τhe relationships of polychaete species richness, abundance, and biomass with the cover of the sessile component of the biota were estimated using a linear regression analysis to assess possible coactions.
Abundance biomass comparisons (ABC plots) were constructed per immersion period for each seasonal experiment to assess relevant accrual rates of the studied populations. Dominance curves for abundance and biomass were plotted by ranking species in terms of importance on x-axis and in terms of cumulative percentage of abundance or biomass on y-axis using Primer software package (Clarke & Gorley 2006).
Multivariate analyses were used to compare the similarity of polychaete communities developed on the panels according to immersion period (i.e. among samplings) for both species and feeding guild compositions. Non-metric multidimensional scaling (nMDS) via Bray–Curtis distances on root-transformed abundance and biomass data was used to visualize changes in species (or feeding guilds) composition across immersion periods. Permutational analysis of variance, PERMANOVA (Anderson 2005), was used to test for differences in community composition among the seasonal installations (four levels) and across periods of immersion (four levels). SIMPER was used to identify the species (or feeding guilds) responsible for any differences between the biotic patterns observed. Matching biotic to environmental patterns procedure, BIOENV, was used to identify the environmental parameters that were related to the polychaete community patterns during succession, and the degree of this relation. A further matching procedure (RELATE) using Spearman rank correlation was applied to compare multivariate patterns deriving from the data matrix of the entire fauna (Antoniadou et al. 2011a) with that of polychaetes, to assess the surrogacy of the latter animal group. Multivariate analyses were performed using the PRIMER software package (Clarke & Gorley 2006).
Results
Species diversity, abundance, and biomass
In all, 29 polychaete species were collected from the four sets of panels (Table 1). Spirobranchus triqueter was the most dominant species in terms of abundance and biomass and it was found settled on panels in all samplings. Five other species –Hydroides norvegicus, Nereis zonata, Polyophthalmus pictus, Spirobranchus polytrema, and Vermiliopsis infundibulum – were routinely collected from the panels (their frequency of appearance surpassed 65%), and contributed greatly to density and/or biomass.
| Polychaete species | Feeding guild | Spring installation | Summer installation | Autumn installation | Winter installation | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 6 | 9 | 12 | 3 | 6 | 9 | 12 | 3 | 6 | 9 | 12 | 3 | 6 | 9 | 12 | ||
| Aphroditidae | |||||||||||||||||
| Hermione sp. | CMJ | + | |||||||||||||||
| Chrysopetalidae | |||||||||||||||||
| Chrysopetalum debile (Grube, 1855) | CMX | + | |||||||||||||||
| Euphrosinidae | |||||||||||||||||
| Euphrosine foliosa Audouin & Milne-Edwards, 1833 | CMX | + | |||||||||||||||
| Eunicidae | |||||||||||||||||
| Eunice vittata (Delle Chiaje, 1828) | CMJ/HMJ/OM | + | + | + | + | + | + | + | |||||||||
| Lysidice ninetta Audouin & Milne-Edwards, 1833 | HMJ/CMJ/OM | + | |||||||||||||||
| Nematonereis unicornis (Grube, 1840) | HMJ/CMJ/OM | + | + | + | + | ||||||||||||
| Glyceridae | |||||||||||||||||
| Glycera tesselata Grube, 1840 | CDJ | + | + | + | + | + | + | + | + | ||||||||
| Hesionidae | |||||||||||||||||
| Kefersteinia cirrata Keferstein, 1862 | CMJ | + | + | + | + | ||||||||||||
| Lumbrineridae | |||||||||||||||||
| Scoletoma funchalensis (Kinberg, 1865) | HMJ/CMJ/OM | + | + | + | |||||||||||||
| Nereidae | |||||||||||||||||
| Nereis zonata Malmgren, 1867 | CDJ | + | + | + | + | + | + | + | + | + | + | + | + | + | |||
| Platynereis dumerilli (Audouin & Milne-Edwards, 1834) | HMJ | + | + | ||||||||||||||
| Opheliidae | |||||||||||||||||
| Polyophthalmus pictus (Dujardin, 1839) | HMX/BMX | + | + | + | + | + | + | + | + | + | + | ||||||
| Phyllodocidae | |||||||||||||||||
| Phyllodoce madeirensis Langerhans, 1880 | CMX | + | + | + | + | + | + | + | |||||||||
| Polynoidae | |||||||||||||||||
| Harmothoe areolata (Grube, 1860) | CMJ | + | + | + | + | + | + | ||||||||||
| Sabellidae | |||||||||||||||||
| Amphiglena mediterranea (Leydig, 1851) | FST | + | + | + | + | + | + | + | |||||||||
| Sabella pavonina Savigny, 1822 | FST | + | |||||||||||||||
| Serpulidae | |||||||||||||||||
| Hydroides norvegicus Gunnerus, 1768 | FST | + | + | + | + | + | + | + | + | + | + | ||||||
| Placostegus crystallinus (non Scacchi, 1836) sensu Zibrowius, 1968 | FST | + | + | ||||||||||||||
| Protula sp. | FST | + | |||||||||||||||
| Serpula concharum Langerhans, 1880 | FST | + | + | + | |||||||||||||
| Spirobranchus polytrema (Philippi, 1844) | FST | + | + | + | + | + | + | + | + | + | + | + | + | ||||
| Spirobranchus triqueter (Linnaeus, 1758) | FST | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Vermiliopsis infundibulum (Philippi, 1844) | FST | + | + | + | + | + | + | + | + | + | + | ||||||
| Spirorbidae | |||||||||||||||||
| Spirorbis sp. | FST | + | + | ||||||||||||||
| Syllidae | |||||||||||||||||
| Salvatoria limbata (Claparède, 1868) | HMJ | + | |||||||||||||||
| Sphaerosyllis pirifera Claparède, 1868 | ΗΜJ | + | + | + | + | ||||||||||||
| Syllis hyalina Grube, 1863 | CMJ/HMJ/ΟΜ | + | + | + | |||||||||||||
| Syllis prolifera Krohn, 1852 | CMJ/HMJ/ΟΜ | + | |||||||||||||||
| Syllis vittata Grube, 1840 | CMJ | + | + | + | |||||||||||||
| Mean density (individuals · m−2) | 41 | 189 | 104 | 152 | 104 | 59 | 411 | 533 | 304 | 667 | 537 | 507 | 78 | 389 | 915 | 1348 | |
| Mean biomass (mg · m−2) | 36 | 1064 | 270 | 1685 | 252 | 169 | 2048 | 2096 | 744 | 1734 | 2557 | 2112 | 213 | 2579 | 2206 | 6749 | |
- FST, filter feeders, sessile, tentaculated; CMX, carnivores, motile, unarmed pharynx; CMJ, carnivores, motile, jawed pharynx; CDJ, carnivores, discretely motile, jawed pharynx; HMJ, herbivores, motile, jawed pharynx; HMX, herbivores, motile, unarmed pharynx; BMX, burrowers, motile, unarmed pharynx; OM, omnivores; 3–12 = immersion periods in months.
Filamentous algae and colonial bryozoans constituted the sessile biota settled on panels; their cover was very low at first (10% at 3-month immersion) and reached less than 45% at the end of the experiment (1-year immersion). Very strong relationships were assessed between the cover of panels and polychaete species richness (determination coefficient R2 = 96%), abundance (R2 = 98%), and biomass (R2 = 99%).
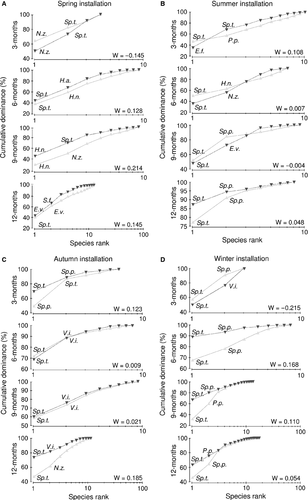
ABC plots produced for each seasonal experiment of succession (Fig. 3) showed the prevalence of biomass curves in most cases, especially at more advanced immersion periods. Seven and nine species constituted the two most dominant ones in terms of abundance and biomass, respectively; seven species dominated on panels when succession started in summer, six in spring, and four in autumn and winter experiments. In most cases, different species dominated abundance and biomass.
Feeding guild diversity, abundance, and biomass
Overall seven feeding guilds were detected: three carnivore guilds (CMJ, CDJ, CMX), two herbivore ones (HMJ and HMX), one of burrowers (BMX) and one of filter-feeders (FST) (see Table 1 for list of acronyms). FST was the most speciose guild (10 species) followed by CMJ (four species). Six species following diversified feeding modes by consuming both algal and animal material, along with Polyophthalmus pictus, which consumes algae or deposited material, were classified as omnivorous (OM). FST dominated in abundance and biomass, reaching 14,500 individuals · m−2 and 30812.2 mg · m−2, respectively. OM and CDJ had a density of 2489 and 1345 individuals · m−2, respectively; the above guilds contributed significantly to biomass, reaching 4916.66 and 2214.45 mg · m−2, respectively. The remaining guilds had a very low contribution to both abundance and biomass (fewer than 650 individuals · m−2 and 1825.55 mg · m−2, respectively).
The strongest relationships between the cover of sessile biota and feeding modes (various guilds were pooled over main feeding modes) were assessed for herbivores (R2 = 91%, R2 = 94%, and R2 = 76% for species number, abundance, and biomass, respectively) and carnivores (R2 = 82%, R2 = 96%, and R2 = 96%). The abundance (R2 = 96%, and R2 = 97%) and biomass (R2 = 98%, and R2 = 75%) of filter and deposit feeders were strongly related, whereas no significant relationships were found for species diversity.
Early succession patterns of polychaete communities: species and feeding guild composition
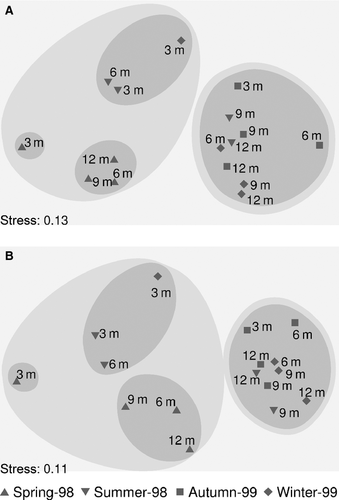
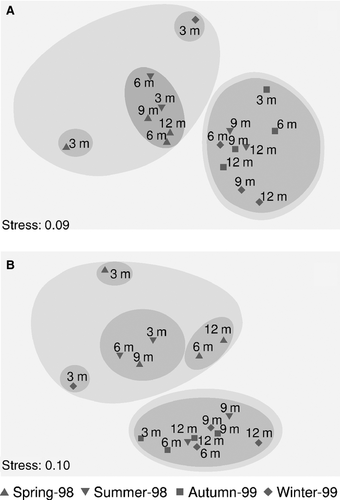
Multidimensional analysis of species abundance or biomass data over immersion periods (Fig. 4A,B) produced a rather similar pattern: samples from the succession experiment that started in spring were separated together with early stages from the summer (3- and 6-month immersions) and the winter experiments (3-month immersion) (Group I), whereas almost all other samples were arranged in a second group (Group II). When analysing community structure at a functional level, the same overall pattern was found; the same single group of samples diverged (Group II), whereas samples within Group I were arranged differently into subgroups, as the very early stage (3-month immersion) was separated when succession started in winter (Fig. 5A,B). When feeding guild abundance was analysed, the early stages (3- and 6-month immersions) from the succession experiment started in summer were placed together with the more advanced stages of the relevant spring experiment (Fig. 5A). When feeding guild biomass was analysed, the 6- and 12-month immersion samples of the spring experiment were placed in a separated sub-group within Group I (Fig. 5B). SIMPER analysis showed that seven species (Spirobranchus polytrema, Spirobranchus triqueter, Polyophthalmus pictus, Vermiliopsis infundibulum, Nereis zonata, Hydroides norvegicus, and Eunice vittata) contributed 60% of the average dissimilarity of polychaete assemblages during succession in terms of abundance; the above species, excluding P. pictus, contributed the same percentage of average dissimilarity in terms of biomass. Applying the same analysis on feeding guild data, two guilds contributed 60% to the average dissimilarity for both abundance and biomass (i.e. FST and OM). Two-way PERMANOVA showed that polychaete community structure was significantly affected by both the immersion period and the season of installation, considering abundance (pseudo-F = 2.32, P < 0.05 and pseudo-F = 5.67, P < 0.05) and biomass (pseudo-F = 2.37, P < 0.05 and pseudo-F = 2.14, P < 0.05) as well. In both cases a significant interaction between these two factors was detected (pseudo-F = 2.11, P < 0.05 and pseudo-F = 1.83, P < 0.05 for abundance and biomass data, respectively), making further comparisons impossible. Applying the same analysis on feeding guild abundance data, similar results were found: immersion period (pseudo-F = 3.68, P < 0.05) and starting season of succession (pseudo-F = 5.79, P < 0.05) significantly affected the trophic structure of polychaete assemblages, with a significant interaction between the studied factors (pseudo-F = 2.94, P < 0.05). Considering biomass data, only the starting season of succession had a significant effect (pseudo-F = 1.83, P < 0.05) on feeding guild composition. BIOENV analysis showed that none of the measured environmental variables was related to the biotic pattern of the developing polychaete communities, either for species or feeding guild composition (rs < 0.05). RELATE analysis showed that the similarity matrices derived from the entire fauna abundance matrix and from polychaete abundance matrix were highly correlated (rs = 0.68).
Discussion
Polychaete diversity in algal-dominated Mediterranean communities ranges from 25 to 152 species with a decreasing trend as depth or pollution increases, or as habitat complexity decreases (see Antoniadou & Chintiroglou 2005 for a thorough review on sublittoral hard substrata Mediterranean biodiversity). The field experiments carried out revealed the presence of 29 polychaete species classified into eight feeding guilds, previously reported to live in the surrounding algal-dominated community (Antoniadou & Chintiroglou 2005), confirming the role of the latter as the basic pool of colonizers (Antoniadou et al. 2010, 2011a). However, these numbers correspond to just 37% of the species richness and 57% of the feeding guild richness reported from the neighboring community (Antoniadou et al. 2004; Antoniadou & Chintiroglou 2006). Thus, an impoverished community was detected at both the structural and functional level, especially considering each seasonal succession experiment separately. These results can be indicative of the low ‘adjustment stability’ (Menge 1975) or ‘engineering resilience’ (Montefalcone et al. 2011), i.e. the low ability of algal-dominated communities on sublittoral rocky cliffs to recover after a perturbation. Certainly the current study covers a relatively short time of succession, as only early patterns were surveyed; further long-term studies are crucial to follow the fate of the developing communities and estimate the time needed for recovery.
Under the recent definition of resilience by Folke (2006), emphasis is given to the ability of a biotic system to maintain its original function, through either recovery of the original state or reorganization in a new context (see also Montefalcone et al. 2011 for a comprehensive review of the term). Therefore, functional aspects of succession are mandatory to understand ecosystem change for conserving and restoring purposes. According to the results of the present study, the developing polychaete community had poor functional organization. Surface deposit feeders were absent, although highly represented by four guilds in the surrounding benthic community, where they exploited resources from the sediment entrapped among the algal turfs (Kelaher et al. 2001; Gorgula & Conell 2004; Antoniadou & Chintiroglou 2006). Another difference is the very low abundance of herbivores, which appeared only at more advanced immersion periods, despite their increased density in the algal turfs (Antoniadou & Chintiroglou 2006).
Apart from some tube-building, filter-feeding polychaetes, which directly settle on panels after the formation of biofilm, most species require the development of some structural elements prior to their settlement. This implies a strong relation between the development of the sessile component and the associated motile fauna during succession (Dean & Connell 1987b; Antoniadou et al. 2010, 2011a). This strong relationship is supported by the results of the present study. During early succession, about 10% of the panel surface was covered by sessile biota (mostly algae and encrusting bryozoan) and this percentage increased with immersion period; the associated polychaete fauna responded to this trend by increasing diversity, abundance, and biomass at both structural (species) and functional levels (feeding guilds). The strongest relationship considering functional diversity was assessed for herbivores. So, as algae gradually covered experimental panels they attracted herbivore polychaetes on their turfs; the latter probably respond to increasing habitat complexity taking advantage of the newly available ecological niche offering food, refuge, and living space.
The same overall species and feeding guilds have been reported to colonize experimental panels in another, organically rich area of the Aegean Sea (Antoniadou et al. 2011b) but with big differences in the abundance and contribution of carnivores. This implies that pioneer species in early succession of sublittoral hard substrata, such as Spirobranchus triqueter and Spirobranchus polytrema, are persistent regardless of the studied habitat; the latter, however, severely influences quantitative aspects of community structure and function, such as the abundance and biomass.
Focusing on the parallel examination of species abundance and biomass, the dominance of very few species is apparent. In most cases S. triqueter prevailed when considering both parameters and this pattern persisted in all immersion periods. Serpulid worms were particularly favoured when succession started in winter. In general, the biomass curve lay above the abundance curve as succession proceeded. However, this trend was not consistent in all seasonal experiments or immersion periods. For example, the two curves intermingled at the more advanced 12-month immersion period when succession started in winter. It seems that successful colonizers experience different rates of abundance and biomass growth as succession progresses, according to their unique life history traits. These traits, especially reproduction output and recruitment success, are subjected to severe temporal fluctuations (Fraschetti et al. 2003; Watson & Barnes 2004); for instance, the most dominant polychaete S. triqueter has been reported to show great seasonal and inter-annual variability in recruitment (Castric-Fey 1983; Cotter et al. 2003a,b).
Polychaetes are one of the three dominant taxa in succession studies examining vagile fauna, mollusks and crustaceans being the others (Dean & Connell 1987a; Kocak et al. 1999; Olabarria 2002). In relevant studies (Antoniadou et al. 2010, 2011a), although not considering the functional aspects of the developed community, a similar pattern of increasing diversity and abundance through time has been reported. Furthermore, a good agreement of the ordinations derived from the entire benthic fauna and the polychaete fauna separately was found in the present study. This conformity supports the role of polychaetes as a surrogate group in succession studies.
Seasonal changes of polychaetes associated with algal-dominated communities on sublittoral rocky cliffs have been demonstrated in the composition of taxa (Antoniadou et al. 2004) and feeding guilds (Antoniadou & Chintiroglou 2006). The season in which succession starts has been recognized as among the important factors modulating its outcome; larval supply is linked to season, determining in this way the availability of first colonizers (Anderson & Underwood 1994; Qvafordt et al. 2006), which after having occupied available space, interact with new, potential colonizers, facilitating or inhibiting their settlement (Dean & Hurd 1980). Our results showed that the starting season severely affected polychaete community structure and function. Colonization was favored when succession started in winter, as more species and individuals successfully settled on these panels and the developing communities supported higher functional diversity. A similar trend was observed when the whole benthic fauna was analysed (Antoniadou et al. 2011a), whereas another study reported strong seasonal effects only on species abundance (Underwood & Anderson 1994).
Whether succession in marine environment follows predictable, sequential stages remains questionable. Most studies suggest convergence towards a common structure as succession proceeds, despite strong initial differences (Foster et al. 2003; Underwood & Chapman 2006; Antoniadou et al. 2010, 2011a; Pacheco et al. 2011; but see also Brown & Swearingen 1998 reporting the lack of any unidirectional sequence of succession). Increased divergence of the developed polychaete communities during very early succession stages has been assessed, in conformity with previous works (Antoniadou et al. 2011a). The impact of starting season on succession was stronger when analysing feeding guilds; thus, convergence towards a common structure cannot be suggested in the latter case, at least not during the temporal scale of the present study. Further experiments for much longer periods are therefore required to address functional issues of succession in the marine environment.
Conclusions
The results of the present study showed that polychaetes can be used as a surrogate group in marine benthic succession studies. Three main patterns emerged: (i) an increase of diversity, abundance, and biomass of colonizers as succession proceeded; (ii) a profound effect of the seasonal onset of colonization on benthic succession at initial stages with convergence towards a similar structure as succession progressed; and (iii) a faster rate of succession when started in winter. These were in agreement with relevant works in which total benthic fauna was treated. The analyses of the developed polychaete communities showed strong conformity between structural and functional levels of organization, with a higher impact of seasonality in the latter.




