Diversity and temporal fluctuations of epiphytes and sessile invertebrates on the rhizomes Posidonia oceanica in a seagrass meadow off Tunisia
Abstract
The aim of this work is to study the temporal dynamics of rhizome epiphytes and sessile animals living on the rhizomes of the seagrass Posidonia oceanica in the east of Tunisia. Surveys were conducted in October 2009, and in January, April and August 2010 on a fringing reef located in Chebba. Rhizomes were sampled by SCUBA diving at three stations. Samples were examined with a microscope to estimate the cover of macroinvertebrate and macroalgal organisms on the top 10 cm of each rhizome. Results revealed a high diversity of epiphytes on P. oceanica rhizomes with a dominance of red and brown algae, ascidians, and bryozoans. Distinct temporal changes were observed in Oued Lafrann, with a high January cover (winter period) for all groups. These winter increases can be attributed to: (i) the low phenological parameters of P. oceanica in winter that reduce the effects of shading, (ii) life cycles of the epiphytes and invertebrates, (iii) water motion and (iv) grazing.
Introduction
The seagrass Posidonia oceanica supports one of the most important marine ecosystems of the Mediterranean. Posidonia oceanica meadows play an important role in ecological production and they export large quantities of plant material (Romero et al. 1992). They are places of spawning, nursery and habitat for thousands of associated species. Indeed, the leaves and rhizomes of P. oceanica are preferred substrates for many epiphytic species that contribute to the primary production of the seagrass and are a direct as well as indirect food source for many animal species (Mazzella et al. 1989). Seagrass rhizomes are long-lived structures, whereas leaves represent a relatively exposed and unstable substrate controlled by the life cycle of the plant. Colonization of epiphytes and macroinvertebrates on leaves depends on a combination of stochastic events and factors acting locally in the meadow, such as differences in shoot density, local hydrodynamics, dispersal of propagules, grazing and competition among sessile organisms (Trautman & Borowitzka 1999; Borowitzka et al. 2006; Mabrouk et al. 2011). On the rhizomes, the community structure is influenced by the life cycle of the epiphytes and invertebrates (Alcoverro et al. 1997), hydrodynamic conditions (Borowitzka et al. 2006), distance from rocky reefs (Van Elven et al. 2004), substratum type, and morphological characteristics of the meadow (Giovannetti et al. 2008). These communities can be adversely affected by anchoring (Francour et al. 1999), hyper-sedimentation (Boudouresque et al. 1984; Gambi et al. 2005) and fish farming (Pergent et al. 1999).
Seagrass epiphytes may be more sensitive to environmental changes than their host plants (Giovannetti et al. 2010). Changes in water quality (eutrophication, human disturbance) have been related to modifications of epiphyte assemblages, including changes in species composition and abundance on the leaves (Balata et al. 2008, 2010; Ben Brahim et al. 2010) and the rhizomes (Mabrouk et al. 2013). In addition, competition between the introduced and native species also plays an important role in structuring the rhizome algal assemblages and their spatial and temporal patterns (Piazzi et al. 2002).
In Tunisia, P. oceanica is the most abundant and widely distributed seagrass species. Studies relating to seagrass meadows in Tunisia have defined their structure and their extent, but few of them have characterized the organisms living on seagrass leaves (Ben Brahim et al. 2010; Mabrouk et al. 2011, 2012) and, to the best of our knowledge, only one study on rhizome epiphytes has been conducted up to now (Mabrouk et al. 2013). In addition, many introduced species such as Caulerpa racemosa (Forsskal) J. Agardh (Hamza et al. 1995) and Caulerpa taxifolia (Vahl) C. Agardh (Langar et al. 2003) have been reported in Tunisia. We therefore studied the organisms living on rhizomes of P. oceanica in an understudied area (east of Tunisia) to access their diversity and their spatial variability. We attempted to test the following hypotheses:
- The rhizomes of Posidonia oceanica support a high diversity of epiphytes and sessile invertebrates.
- The composition and abundance of the epiphytes and invertebrate species on the rhizomes vary over time.
Material and Methods
Study area
This study was conducted in the locality of Oued Lafrann (35°15′18″ N, 11°07′28″ E) in the region of Chebba (east of Tunisia). The climate is semi-arid (average precipitation: 350 mm per year) and sunny with strong northerly winds. This locality is characterized by barrier and fringing reefs in addition to an extensive plain meadow that has the highest cover and shoot densities in the east of Tunisia (Mabrouk 2012). This area is under consideration as a marine protected area (submitted in Italo-Tunisian project iTunES) because of its biocenotic richness and its distinctive ecosystems.
Surveying and sampling were conducted during October 2009 (autumn), January 2010 (winter), April 2010 (spring) and August 2010 (summer). To reduce habitat variability in the present study, all samples were collected in a continuous and high-density fringing reef meadow at a depth of 5 m, where there were well-developed mat structures. The presence of a mat may reduce differences related to different substrata and burial rates.
The sampling design was hierarchical. Three stations (500 m apart) were chosen at random (Fig. 1). At each station, three random replicate quadrats (1600 cm2 large and about 10 m apart) were sampled. Within each quadrat, five vertical shoots chosen at random were uprooted and preserved in 4% formalin seawater for laboratory observation. To estimate cover of each epiphyte and invertebrate species, the upper 10 cm of each rhizome was examined under a dissecting microscope. Animal and macroalgal organisms were identified at the level of species or genus and the abundance of each taxon was expressed as cover. Percentage cover was evaluated as the surface covered by the taxon in orthogonal projection (Appendix A) and referred to the total surface of the rhizomes sampled (Boudouresque 1971; Piazzi et al. 2002). Percentage cover of encrusting algae was measured after the removal of the erect organisms.
Data analysis
Data were tested for normality using the Kolmogorov–Smirnov goodness of fit test and for heterogeneity using Cochran's C Test, then transformed if necessary (Zar 1999).
Multivariate analysis of variance based on permutations (PERMANOVA) employing the PERMANOVA.exe program (Anderson 2005) was used to test the hypothesis that rhizome epiphytes showed different patterns of variation in composition and in percentage cover of species at different times. The analysis consisted of a one-way model between sampling times (four levels, random factor). PERMANOVA was conducted on a Bray–Curtis dissimilarity matrix, calculated from log(x+1)-transformed data to meet the assumption of homogeneity of variances (homogeneity confirmed by non-significant Cochran's C-tests).
Analyses of similarity (ANOSIM) randomization tests (with untransformed data) were used to test for differences in species abundance among sampling times (Clarke 1993). ANOSIM gives a P-value similar to an ANOVA, with values of P < 0.05 indicating significance. If differences were found using ANOSIM, then SIMPER analysis was used for identifying which species primarily accounted for observed differences in epiphytic assemblages between sampling times. SIMPER generates a ranking of the percent contribution of the species that are most important to the significant differences. These analyses used a matrix composed of Bray–Curtis similarity coefficients generated with log(x+1)-transformed data.
The similarity in composition and coverage among sampling times was analyzed by means of multi-dimensional scaling (MDS) based on Bray–Curtis indices of similarity derived from log(x+1)-transformed data. The calculations were performed using the statistical software PRIMER 5.0 (Clarke & Warwick 2001).
Univariate indices, species number (S) and diversity (Shannon–Weaver index H'), were calculated for each sampling time. The one-way ANOVA was used to test for overall differences between these indices and the Tukey HSD multiple comparison test was used in pairwise comparisons between sampling times.
One-way analysis of variance (ANOVA) was used to test the hypothesis that the percentage cover of each of the most abundant groups varies among sampling times. Tukey test HDS was employed for a posteriori multiple comparisons of means. The calculations were performed using the statistical software STATISTICA v10 (StatSoft Inc., Tulsa, OK, USA).
Results
High numbers of epiphytic and invertebrate species on the rhizomes were identified (Table 1). Dominant groups included red and brown algae, ascidians, bryozoans and sponges. Cover on rhizomes was highest in January and lowest in August (Fig. 2).
| groups | species |
|---|---|
| Green algae | Anadyomene stellata (Wulfen) C. Agardh |
| Caulerpa prolifera (Forsskål) J. V. Lamouroux | |
| Codium bursa (Agardh) | |
| Halimeda tuna (J. Ellis & Solander) J. V. Lamouroux | |
| Flabellia petiolata (Turra) Børgesen | |
| Red algae | Amphiroa rigida (Lamouroux) |
| Cryptonemia lomation (Bertoloni) J. Agardh | |
| Hydrolithon farinosum (Lamouroux) D. Penrose & Y. M. Chamberlain | |
| Hypnea musciformis (Wulfen) J. V. Lamouroux | |
| Jania rubens (Linnaeus) J. V. Lamouroux | |
| Lithophyllum incrustans (Philippi) | |
| Lithophyllum racemus (Lamarck) | |
| Peyssonnelia squamaria (S. G. Gmelin) Decaisne | |
| Phymatolithon calcareum (Pallas) W. H. Adey & D. L. McKibbin | |
| Phymatolithon lenormandii (J.E. Areschoug) W. H. Adey | |
| Pneophyllum fragile (Kützing) | |
| Polysiphonia elongata (Hudson) Sprengel | |
| Rhodymenia sp. | |
| Brown algae | Cutleria multifida (Turner) Greville |
| Cystoseira crinita (Duby) | |
| Cystoseira foeniculacea f. schiffneri (Hamel) Gómez Garreta, Barceló, Ribera & Rull Lluch | |
| Dictyopteris polypodioides (A. P. De Candolle) J. V. Lamouroux | |
| Dictyota dichotoma (Hudson) Lamouroux | |
| Dictyota linearis (C. Agardh) Greville | |
| Padina pavonica (Linnaeus) Thivy | |
| Sphacelaria cirrosa (Roth) C. Agardh | |
| Bryozoa | Aetea truncata (Landsborough) |
| Alcyonidium gelatinosum (Linnaeus) | |
| Amathia lendigera (Linnaeus) | |
| Beania hirtissima (Heller) | |
| Calpensia nobilis (Esper) | |
| Cellaria sinuosa (Hassall) | |
| Puellina radiata (Moll) | |
| Frondipora verrucosa (Lamouroux) | |
| Lichenopora radiata (Audouin et Savigny) | |
| Membranipora membranacea (Linnaeus) | |
| Schizobrachiella sanguinea (Norman) | |
| Tubulipora flabellaris (Fabricius) | |
| Tubulipora plumosa Thompson in Harmer | |
| Porifera | Clathrina coriacea (Montagu) |
| Dysidea avara (Schmidt) | |
| Haliclona simulans (Johnston) | |
| Hemimycale columella (Bowerbank) | |
| Ircinia dendroides (Schmidt) | |
| Ircinia variabilis (Schmidt, 1862) | |
| Ircinia oros (Schmidt) | |
| Scalarispongia sp. | |
| Ascidians | Aplidium conicum (Olivi) |
| Clavelina lepadiformis (Muller) | |
| Didemnum maculosum (Milne-Edwards) | |
| Diplosoma listerianum (Milne-Edwards) | |
| Phallusia nigra (Savigny) | |
| Polysyncraton lacazei (Giard) | |
| Trididemnum cereum (Giard) | |
| Annelida | Serpula vermicularis (Linnaeus) |
| Spirobranchus triqueter (Linnaeus) | |
| Spirorbis sp. | |
| Vermiliopsis infundibulum (Philippi) | |
| Rhizopoda | Miniacina miniacea (Linnaeus) |
| species number | |
| Green algae | 5 |
| Red algae | 13 |
| Brown algae | 8 |
| Bryozoa | 13 |
| Porifera | 8 |
| Ascidian | 7 |
| Annelida | 4 |
| Rhizopoda | 1 |
| Total | 59 |
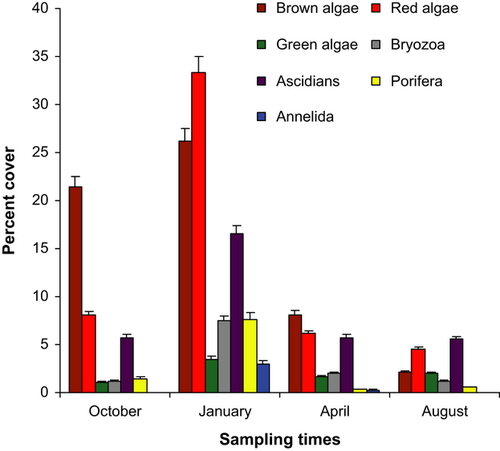
PERMANOVA revealed a significant effect of sampling time (F = 7.577; Pper = 0.002: Table 2). The analysis of variance of the rhizome cover followed by Tukey post hoc tests (Table 3) showed the existence of significant variation between sampling times with high January cover for all groups.
| source | df | MS | F | P(perm) | P(MC) |
|---|---|---|---|---|---|
| Time | 3 | 1574.380 | 7.577 | 0.002 | 0.002 |
| Residual | 20 | 207.778 |
- df, degrees of freedom; F, F value; MS, mean square; P(MC), Monte Carlo P-value; P(perm), probability level.
| source | df | MS | F | P | Tukey test |
|---|---|---|---|---|---|
| Green algae: Cochran C-test, C = 0.647; P = 0.002, transformation log(x+1) | |||||
| Time | 3 | 0.27 | 8.98 | <0.01 | January = August > April = October |
| Residuals | 32 | 0.03 | |||
| Red algae: Cochran C-test, C = 0.832; P = 0.000, transformation log(x+1) | |||||
| Time | 3 | 1.38 | 12.26 | <0.01 | January > April = October = August |
| Residuals | 32 | 0.11 | |||
| Brown algae: Cochran C-test, C = 0.667; P = 0.000, transformation log(x+5) | |||||
| Time | 3 | 1.374 | 22.326 | <0.01 | January = October > April = August |
| Residuals | 32 | 0.062 | |||
| Bryozoa: Cochran C-test, C = 0.976; P = 0.005, transformation log(x+1) | |||||
| Time | 3 | 3.66 | 130.95 | <0.01 | January > April > August = October |
| Residuals | 32 | 0.03 | |||
| Ascidians: Cochran C-test, C = 0.471; P = 0.067 transformation:none | |||||
| Time | 3 | 2406.8 | 24.94 | <0.01 | January> April = October = August |
| Residuals | 32 | 96.507 | |||
| Spongiaires: Cochran C-test, C = 0.922; P = 0.000, transformation log(x+1) | |||||
| Time | 3 | 2.16 | 14.47 | <0.01 | January > April = October = August |
| Residuals | 32 | 0.15 | |||
| Annelida: Cochran C-test, C = 0.936; P = 0.55; transformation none | |||||
| Time | 3 | 172.028 | 20.76 | <0.01 | January > April = October = August |
| Residuals | 32 | 8.278 | |||
| Total algae: Cochran C-test, C = 0.735; P = 0.000, transformation log(x+1) | |||||
| Time | 3 | 1.19 | 30.01 | <0.01 | January > October > April = August |
| Residuals | 32 | 0.04 | |||
| Total cover: Cochran C-test, C = 0.748; P = 0.000, transformation log(x+1) | |||||
| Time | 3 | 1.04 | 58.61 | <0.01 | January > October > April > August |
| Residuals | 32 | 0.02 | |||
| Shannon–Weaver (H'): Cochran C-test, C = 0.405; P = 0.176, transformation none | |||||
| Time | 3 | 1.478 | 36.468 | <0.01 | January > August > April = October |
| Residuals | 32 | 0.041 | |||
| Number of species: Cochran C-test, 0.780; P = 0.000, transformation log(x+1) | |||||
| Time | 3 | 0.307 | 56.946 | <0.01 | January> August = April> October |
| Residuals | 32 | 0.005 | |||
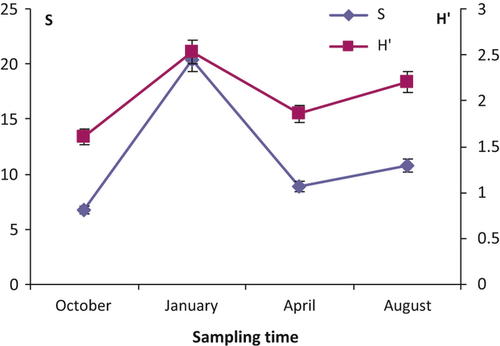
Species numbers showed strong temporal variations, with the highest values in January and the lowest in October (Fig. 3, Table 3). Shannon–Weaver indices ranged between 1.60 ± 0.18 in October and 2.53 ± 0.25 in January (Fig. 3) and the difference between the two extremes was significant (Table 3).
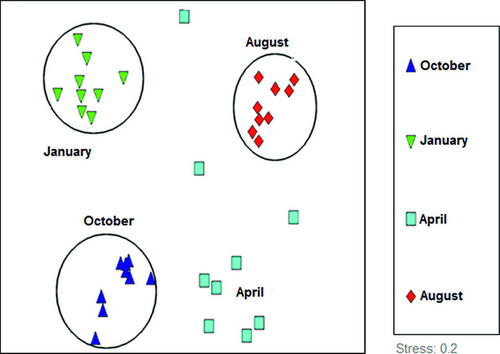
Interpretation of the MDS (Fig. 4) shows that samples of each sampling time grouped together except for April.
Analysis of similarities (ANOSIM) showed highly significant differences for specific percentage covers according to time (Table 4).
| R | P | |
|---|---|---|
| Global | 0.914 | 0.001 |
| Pairwise comparison | ||
| October, January | 0.993 | 0.001 |
| October, April | 0.754 | 0.001 |
| October, August | 0.999 | 0.001 |
| January, April | 0.873 | 0.001 |
| January, August | 0.999 | 0.001 |
| April, August | 0.816 | 0.001 |
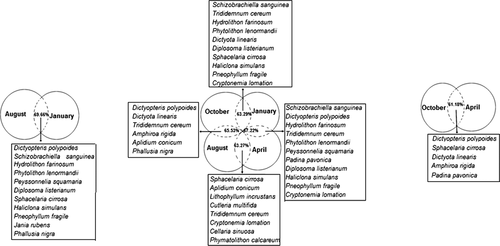
The analysis of similarity percentages (SIMPER) shows high dissimilarities of epiphytes and invertebrates between sampling times (October and January = 63.26%; October and April = 61.18%; January and April = 67.22%; October and August = 65.53%; January and August = 69.66%; April and August = 63.27%). Species that contribute to these dissimilarities are the bryozoan Schizobrachiella sanguinea, the ascidians Diplosoma listerianum and Phallusia nigra, the sponge Haliclona simulans, and algae Dictyopteris polypodioides, Sphacelaria cirrosa, Peyssonnelia squamaria, Phytolithon lenormandii, Hydrolithon farinosum, Pneophyllum fragile and Jania rubens, which are especially abundant in January, and Dictyopteris polypoides, Dictyota linearis, Sphacelaria cirrosa and Amphiroa rigida, which are abundant in October and January (Fig. 5).
Discussion
Among rhizome colonists, algae are very diverse (26 species) with a clear dominance of crustose and filamentous species such as Peyssonnelia squamaria and Padina pavonica. The algal diversity in the rhizomes of P. oceanica found in this study is similar to that found in Tuscany (Italy) (Balata et al. 2007) and higher than that (15 species) found off Cap Bon in Tunisia (Piazzi et al. 2002). However, in those studies, algal communities were dominated by filamentous, crustose and foliose species. Posidonia oceanica seagrass is distinguished from other marine Magnoliophyta by a larger assemblage of algae (Borowitzka et al. 2006) characterized mainly by sciaphilous and long-living organisms (Borowitzka & Lethbridge 1989; Mazzella et al. 1992). Panayotidis (1980) described this algal assemblage as a coralligenous sciaphilous assemblage of the sublittoral zone without characteristic taxa. Boudouresque (1974) has suggested that rhizomes should be regarded simply as the media on which latent state groups of subtidal habitats are found.
The rhizome epiphytic fauna represents bryozoans, poriferans and ascidians. These groups have been identified in several studies as important colonists of the rhizomes of Posidonia (Colmenero & Lizaso 1999; Nesti et al. 2009; Balata et al. 2007, 2008, 2010; Mabrouk et al. 2013). Bryozoans of the seagrass meadows of P. oceanica are more numerous on the rhizomes than on leaves (Kocak et al. 2002). The overgrowth by bryozoans (such as Calpensia nobilis) produced important changes in P. oceanica rhizomes including a significant increase in rhizome growth rate and a decrease in its weight/length ratio. These effects are similar to those of hyper-sedimentation (Colmenero & Lizaso 1999).
As hypothesized, distinct temporal patterns of organisms on rhizomes was observed in Oued Lafrann, with a high January cover (winter period) for all groups. Significantly high epiphytic algae cover in winter has been found in the Western Mediterranean of France (Boudouresque 1974) and in Gorgona (Italy) (Piazzi et al. 2002). Piazzi et al. (2002) did not find any significant temporal variation in the areas surveyed, including Cap Bon (Tunisia) and around Sicily and Tuscany. The lack of significant seasonality may be linked to the stability of the algal colonists (Piazzi et al. 1996, 2002).
The high diversity and cover of rhizome epiphytes in January can be attributed to the effect of shading, which is less important in winter when the phenological parameters are low; when winter leaf length is low (Gobert et al. 2003; Mabrouk et al. 2009), light is more likely to penetrate rhizomes, promoting epiphyte development. Thus, the composition and coverage of epiphytes depends on shoot density (Trautman & Borowitzka 1999; Vanderklift & Lavery 2000). Moreover, artificial shading carried out by Ruiz & Romero (2001) has proved that the absence of light would cause the decline of more than 80% of epiphyte biomass. Leaves are observed to attenuate the penetration of light and water movement, creating a shady habitat in the rhizomes (Borowitzka & Lethbridge 1989; Chimenz et al. 1989; Balata et al. 2007). Thus, irradiances and water movements in rhizome layers are linked to changes in the P. oceanica canopy during the year, following the seasonal trend of leaf production (Buia et al. 1992).
Epiphyte and invertebrate assemblages are different on leaves and rhizomes, as they are mainly composed of ephemeral and long-lived species, respectively (Borowitzka & Lethbridge 1989; Borowitzka et al. 2006; Balata et al. 2007, 2008). Seagrass rhizomes are relatively long-lived structures (Marba et al. 2002), whereas leaves represent a deciduous substrate, subject to a growth-senescence-renewal cycle yearly (Gobert et al. 2003).
Water motion may play a role in pumping nutrients out of the sediment, making them available for epiphytes in the canopy (Koch & Huettel 2000). When the phenological parameters of P. oceanica are high in summer, the leaf canopy reduces flow speed and bottom friction (Gacia et al. 1999), so reduction of water movements in rhizomes in August causes a decrease in the circulation of nutrients and therefore a decline in epiphyte development (Trautman & Borowitzka 1999). On other hand, storms between mid-September and late October cause nutrient enrichment of the water column (Duarte et al. 1999), favoring epiphyte development. In this context, changes in water quality and nutrient enrichment (due to human disturbance) have been related to modifications in species composition and abundance of epiphyte assemblages on the leaves (Nesti et al. 2009; Ben Brahim et al. 2010; Giovannetti et al. 2010) and rhizomes of P. oceanica (Balata et al. 2010; Mabrouk et al. 2013).
Previous studies have shown that the community structure is influenced by the life cycle of the epiphytes (Alcoverro et al. 1997). In this context, Boudouresque (1974) suggested that the waning of summer epiphytes on rhizomes can be expected, as rhizomes are dominated by Ceramiales that includes numerous species that proliferate in winter and die or perish in summer.
Rhizome assemblages on P. oceanica appear to have no significant spatial variability. Balata et al. (2007) suggest that those assemblages appeared homogeneous within the same geographic area, independent of the habitat in which they develop and with the highest variability among shoots. Conversely, colonizing organisms of the rhizomes show a highly variable percentage cover at both the smallest (i.e. among rhizomes) and the largest spatial scales considered (i.e. among sites hundreds of meters apart) around the Maltese islands (Acunto et al. 2006a) and coast of Calabria (Acunto et al. 2006b). Piazzi et al. (2002) found no significant differences between depths in rhizome assemblages when studied solely on the basis of the abundance of morphological algal groups, whereas Nesti et al. (2009), who analyzed both algae and animal species, showed significant differences for rhizome assemblages, stating that the response of organisms to depth is species-specific.
The effect of grazing is also an important factor that contributes to the variation of the organismal cover on rhizomes, as many species feed on epiphytes of Posidonia, including the echinoid Paracentrotus lividus, some decapods and amphipods (Zakhama-Sraieb et al. 2011; Belgacem et al. 2013). Preference of sea urchins for epiphytes can be attributed to their high nutritional value (Alcoverro et al. 2000). In addition, about 50 species of fish are encountered in P. oceanica beds (Harmelin-Vivien 1989). Grazers, whose abundance varies seasonally, may modify the composition and coverage of rhizome- and leaf-colonizing organisms (Alcoverro et al. 1997; Keuskamp 2004; Prado et al. 2007).
Borowitzka et al. (2006) have suggested that our understanding of the factors controlling seagrass epiphyte diversity and abundance is still limited by the nature of the complex interactions between physical factors (e.g. light, temperature, water movement, nutrients) and biological interactions (competition for space, grazing and predation) such as competition between algae and indirect interactions between fauna and epiphytes. Thus, the interspecific competition imposed by introduced species may threaten the diversity of assemblages on rhizomes. Piazzi et al. (2002) found that the functional diversity of rhizome assemblages is low in sites where introduced species are present. Competition between the introduced and native species plays an important role in structuring the rhizome algal assemblages and their spatial and temporal patterns, and this may lead to the absence of seasonal variation (Piazzi et al. 2002). In our study area, only Halophila stipulacea (Forsskål) Ascherson in Anon have been reported, but its occupied surface does not exceed a few meters (Mabrouk 2012).
The present study demonstrates a high diversity of epiphytic and animal assemblages on the rhizomes of P. oceanica in the region studied and also showed a temporal fluctuation with a high cover in January. Many authors have bemoaned the lack of data in some Mediterranean regions, particularly North Africa (Ruiz et al. 2009). Our data will help remedy this lack of information for regional-scale studies while also helping to define best management practices to preserve P. oceanica in Oued Lafrann and its associated epiphytes with the recent establishment of several fish farms. A study by FAO (2008) recommends that aquaculture installation on Posidonia beds should be avoided whenever possible and our data support this recommendation.
Acknowledgements
This study was supported by the research project EBHAR ‘Etat du Benthos et des Habitats Remarquables’. We thank Prof. Craig Young, Prof. Nuria Marba and reviewers for their comments, which improved the quality of the manuscript.




