Short-term temporal variation in gelatinous zooplankton populations over 48 hours in a coral reef at Redang Island, Malaysia
Abstract
Gelatinous zooplankton abundance and species composition were investigated at 3-h intervals for a 48-h period at a fringing reef in Malaysia. A total of 20 gelatinous zooplankton species were observed; the community was dominated by the calycophoran siphonophore Diphyes chamissonis (79.9%), followed by the trachymedusdae Aglaura hemistoma (5.6%) and Liriope tetraphylla (4.8%). The gelatinous zooplankton were not collected in the water column during most of the daytime hours (1200, 1500 and 1800 h) but were common during the night. However, an abrupt peak in abundance was found at 0900 h on the second day. The times of appearance at night were different depending on the species, and the number of species was also different depending on the hour of sampling. Sampling at 3-h intervals over a 48-h period revealed that the temporal variation (or sampling availability) was large in this study. Careful consideration should be given to the sampling variability in handling the gelatinous zooplankton samples in coral reef areas.
Introduction
Zooplankton are central players in coral reef food webs as a trophic link between primary producers and higher trophic levels including benthic planktivores such as corals (Glynn 1973). Knowledge of their behavior and distribution are essential for a complete understanding of community dynamics in these important systems (Alldredge & King 2009). The nocturnal concentrations and biomass of coral reef zooplankton have been demonstrated to be dramatically higher than daytime levels (e.g. Yahel et al. 2005a,b). There are also several reports of coral reef zooplankton showing a peak abundance at various times throughout the night, e.g. soon after sunset or before sunrise (e.g. Nakajima et al. 2009; Soroki & Sorokin 2010).
To date, short-term temporal variation in the bulk zooplankton community as well as various zooplankton species including copepods and other invertebrate larvae in coral reef ecosystems have been investigated in several studies (reviewed by Nakajima et al. 2008); however, such studies on gelatinous zooplankton are scarce despite their ubiquitous presence in reef systems (Emery 1968). Gelatinous zooplankton are a component of the planktonic community that has an important role as a source of energy for reef inhabitants, including fish (Arai 2005) and scleractinian corals (Alamaru et al. 2009) in coral reef ecosystems. Our knowledge on the short-term variations of gelatinous zooplankton in coral reef ecosystems is restricted to a very few studies, all at the community level (e.g. Goswami & Goswami 1990; Nakajima et al. 2008), with no studies on the temporal patterns at the species level. Further studies on the behavior of gelatinous zooplankton will contribute to an understanding of their significance in coral reef food webs.
In this study, we collected gelatinous zooplankton at 3-h intervals for a 48-h period at a coral reef in Malaysia. This paper presents results on the short-term variations in abundance, diversity and community composition of gelatinous zooplankton on a diel basis to examine gelatinous zooplankton behavior over the coral reef.
Study area
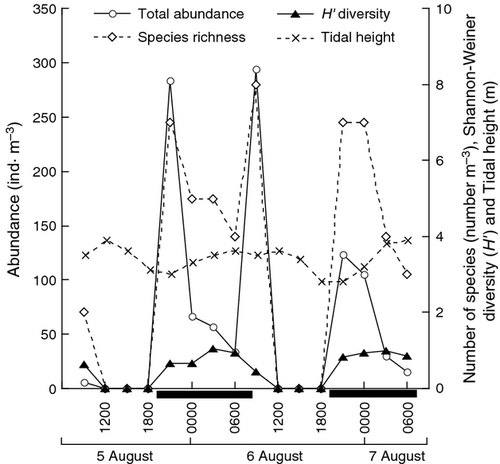
This study was carried out during 5–7 August 2003 at a fringing coral reef (5°44΄49˝ N, 102°59΄60˝ E) at Pinang Island, a satellite island of Redang Island, off the east coast of Peninsular Malaysia (See Fig. 1 of Nakajima et al. 2008 for a map of the sampling site). Gelatinous zooplankton sampling was conducted at a jetty in the marine park. The maximum depth of the sampling site was 3.9 m during high tide. Live coral coverage of the sampling site was 49%, with 8% dead corals and 43% other bottom substrata (e.g. sand and rock) (Kok 2003). There was no distinct reef crest separating the open sea and back reef zones, allowing water from the open sea to freely enter the nearshore area. Sea conditions at the sampling site were calm with no strong winds or rainfall during the study period. Mean water temperature was 29.4 ± 0.3 °C, mean salinity was 32.7 ± 0.7, and the water column was homogeneous throughout the study period (Nakajima et al. 2008). Minimum tidal height was observed at 2100 h on 5 August (3.0 m) and at 1800 and 2100 on 6 August (2.8 m). Maximum tidal height was observed at 1200 h on 5 August (3.9 m), at 0600 and 1200 h on 6 August (3.6 m), and at 0600 h on 7 August (3.9 m; Fig. 1).
Material and Methods
We collected gelatinous zooplankton every 3 h, starting at 0900 h on 5 August 2003 with the last sampling at 1200 h on 7 August. On 6 August sunrise was at 0703 h and sunset at 1925 h. The total number of sampling events (n) was 16 (samples collected every 3 h over a 48-h period). Of the 16 sampling events, eight were done during the day (0900, 1200, 1500 and 1800 h over the two days of sampling) and the other eight at night (2100, 0000, 0300 and 0600 h).
Gelatinous zooplankton were collected by five gentle vertical tows of a plankton net (mesh size 100 μm, diameter 30 cm, length 100 cm), equipped with a flowmeter, from 1 m above the bottom to the surface. The net was left at maximum depth for 5 min before retrieval. The samples were pooled and immediately fixed in 5% formalin seawater. The net-collected gelatinous zooplankton samples were identified to the lowest taxonomic division possible and counted under a dissecting microscope.
The statistical differences in the abundances, number of species and Shannon–Weiner diversities (H′) of gelatinous zooplankton between day (0900–1800 h) and night (2100–0600 h) were determined by a two-sided Mann–Whitney U-test. The statistical differences in the abundance of gelatinous zooplankton at four tidal phases (L, low tide; LH, low to high tide; H, high tide; and HL, high to low tide) were examined with a Kruskal–Wallis test. Although the tidal height at 0900 h on 6 August was slightly lower than in the three adjacent hours before and after (Fig. 1), we consider the tide was high (H) taking into account the general trend of the tidal phase. Likewise, the tidal height at 1800 h on 6 August was considered as HL. Differences with P < 0.05 were considered significant for all the statistical tests.
Bray–Curtis similarity (Bray & Curtis 1957) was calculated on 4th-root transformations of species abundance each hour to define the relationships of the species composition between all sampling times. The purpose of this transformation is to accentuate the effect of rare species. The intra-location resemblance was subjected to group-average linkage cluster analysis. A similarity profile (SIMPROF) test was performed on the null hypothesis that a specific sub-cluster can be recreated by permuting the entry species and samples. The significant branch (SIMPROF, P < 0.05) was used as a prerequisite for defining the species composition zones. The analyses were conducted using primer v6 (Clarke & Gorley 2006).
Results
A total of 20 gelatinous zooplankton species were identified in this study (Table 1). The gelatinous zooplankton community was dominated by the calycophoran siphonophore Diphyes chamissonis, constituting (79.9%) of the total gelatinous zooplankton abundance, followed by the trachymedusdae Aglaura hemistoma (5.6%) and Liriope tetraphylla (4.8%).
| Gelatinous zooplankton taxa | Life history type | Abundance (ind. m−3) | Day/night Tidal phase | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall ± SD | n | % | Day ± SD | n | % | Night ± SD | n | % | P | P | ||
| Siphonophora | ||||||||||||
| Agalma elegans | H | 0.1 ± 0.5 | 16 | 0.2 | 0.0 ± 0.0 | 8 | 0.0 | 0.0 ± 0.0 | 8 | 0.0 | 0.67 | 0.23 |
| Bassia bassensis | H | 0.1 ± 0.5 | 16 | 0.2 | 0.0 ± 0.0 | 8 | 0.0 | 0.0 ± 0.0 | 8 | 0.0 | 0.67 | 0.23 |
| Diphyes chamissonis | H | 50.8 ± 85.4 | 16 | 79.9 | 34.1 ± 94.8 | 8 | 93.2 | 67.5 ± 77.5 | 8 | 75.3 | 0.04 | 0.03 |
| Polygastric stage | (25.3) | (30.8) | (22.0) | |||||||||
| Eudoxid stage | (74.7) | (69.2) | (78.0) | |||||||||
| Lensia subtiloides | H | 1.3 ± 3.7 | 16 | 2.1 | 0.5 ± 0.9 | 8 | 1.4 | 2.2 ± 5.2 | 8 | 2.5 | 0.83 | 0.02 |
| Polygastric stage | (77.3) | (100.0) | (71.9) | |||||||||
| Eudoxid stage | (22.7) | (0.0) | (28.1) | |||||||||
| Sphaeronectes koellikeri | H | 0.2 ± 0.6 | 16 | 0.2 | 0.0 ± 0.0 | 8 | 0.0 | 0.3 ± 0.9 | 8 | 0.3 | 0.67 | 0.07 |
| Sulculeolaria chuni | H | 0.1 ± 0.5 | 16 | 0.2 | 0.0 ± 0.0 | 8 | 0.0 | 0.3 ± 0.8 | 8 | 0.3 | 0.67 | 0.64 |
| Hydromedusae | ||||||||||||
| Aglaura hemistoma | H | 3.6 ± 6.6 | 16 | 5.6 | 0.0 ± 0.0 | 8 | 0.0 | 7.2 ± 8.0 | 8 | 8.0 | 0.01 | 0.12 |
| Amphogona apsteini | H | 0.3 ± 1.1 | 16 | 0.4 | 0.0 ± 0.0 | 8 | 0.0 | 0.5 ± 1.5 | 8 | 0.6 | 0.67 | 0.64 |
| Anthomedusa sp. 1 | M | 0.6 ± 1.2 | 16 | 1.0 | 0.0 ± 0.0 | 8 | 0.0 | 1.3 ± 1.4 | 8 | 1.4 | 0.09 | 0.18 |
| Anthomedusa sp. 2 | M | 0.3 ± 0.8 | 16 | 0.5 | 0.0 ± 0.0 | 8 | 0.0 | 0.6 ± 1.1 | 8 | 0.6 | 0.4 | 0.27 |
| Bougainvillia sp. | M | 0.1 ± 0.5 | 16 | 0.2 | 0.0 ± 0.0 | 8 | 0.0 | 0.0 ± 0.0 | 8 | 0.0 | 0.67 | 0.23 |
| Cunina sp. | H | 0.6 ± 1.7 | 16 | 1.0 | 0.0 ± 0.0 | 8 | 0.0 | 1.2 ± 2.3 | 8 | 1.4 | 0.4 | 0.27 |
| Cytaeis sp. | M | 0.6 ± 1.3 | 16 | 0.9 | 0.0 ± 0.0 | 8 | 0.0 | 1.1 ± 1.8 | 8 | 1.2 | 0.21 | 0.26 |
| Cytaeis aff. adherans | M | 0.2 ± 0.6 | 16 | 0.2 | 0.0 ± 0.0 | 8 | 0.0 | 0.3 ± 0.9 | 8 | 0.3 | 0.67 | 0.07 |
| Euphysora bigelowi | M | 0.1 ± 0.6 | 16 | 0.2 | 0.0 ± 0.0 | 8 | 0.0 | 0.3 ± 0.8 | 8 | 0.3 | 0.67 | 0.64 |
| Liriope tetraphylla | H | 3.1 ± 4.7 | 16 | 4.8 | 0.7 ± 2.1 | 8 | 2.0 | 5.4 ± 5.6 | 8 | 6.1 | 0.01 | 0.03 |
| Obelia sp. | M | 0.1 ± 0.5 | 16 | 0.2 | 0.0 ± 0.0 | 8 | 0.0 | 0.0 ± 0.0 | 8 | 0.0 | 0.67 | 0.23 |
| Solmundella bitentaculata | H | 0.9 ± 2.5 | 16 | 1.4 | 1.2 ± 3.5 | 8 | 3.4 | 0.6 ± 1.1 | 8 | 0.7 | 0.75 | 0.40 |
| Ctenophora | ||||||||||||
| Ctenoplana sp. | D | 0.3 ± 1.1 | 16 | 0.4 | 0.0 ± 0.0 | 8 | 0.0 | 0.6 ± 1.6 | 8 | 0.6 | 0.67 | 0.64 |
| Pleurobrachia sp. | H | 0.1 ± 0.5 | 16 | 0.2 | 0.0 ± 0.0 | 8 | 0.0 | 0.2 ± 0.7 | 8 | 0.3 | 0.67 | 0.64 |
- n signifies number of samples. Values in parentheses pertain to the relative percentage of the polygastric and eudoxid stages to the total number of Diphyes chamissonis or Lensia subtiloides. P values pertain to either the abundance differences between day and night or the abundance differences between tidal phase. P values in bold mean statistical significance (P < 0.05).
- H, holoplankton; M, meroplankton; D, demersal plankton.
Although the highest total gelatinous zooplankton abundance was recorded at 0900 h on the second day (morning of 6 August; Fig. 1), the average abundance was significantly higher during the night (P = 0.011), being 2.2 times higher (90 ± 87 ind. m−3) than during the day (38 ± 104 ind. m−3). Focusing on the nighttime value only, the total gelatinous zooplankton abundance attained a maximum in the early hours of the night (2100 h) on both nights, reaching 284 and 123 ind. m−3 on the first and second night, respectively (Fig. 1). The peaks at 2100 h on both nights were mainly due to the addition of D. chamissonis (80.0–84.3%), L. tetraphylla (6.1–6.7%), and Lensia subtiloides (2.2–5.2%; Fig. 2d). On the first night, the peak at 2100 h declined sharply at 0000 h and continued to decline at a slower pace thereafter. On the second night, the peak at 2100 h declined slightly at 0000 h and then sharply at 0300 h. The number of species and Shannon–Weiner diversity (H’) were also significantly higher during the night (P = 0.011 and 0.00068, respectively), being 4.2 and 6.4 times higher (5.3 ± 1.6 for species number and 0.87 ± 0.15 for H’ diversity) than during the 2 daytimes (1.3 ± 2.8 and 0.14 ± 0.26), respectively. The highest number of species (eight species) was observed at 0900 h on the second day when the total gelatinous zooplankton abundance was at a maximum but H’ diversity was relatively low (0.44) due to the overwhelming dominance of D. chamissonis (91.3%; Fig. 2d). For nighttime values only, the number of species attained maximum peaks at 2100 h (seven species) on both nights, coinciding with the maximum abundance at night, whereas H’ diversity reached a maximum later in the night at 0300 h when the number of species declined to four to five species and the abundance of the overwhelmingly dominant species (D. chamissonis) was zero or minute (Fig. 2d).
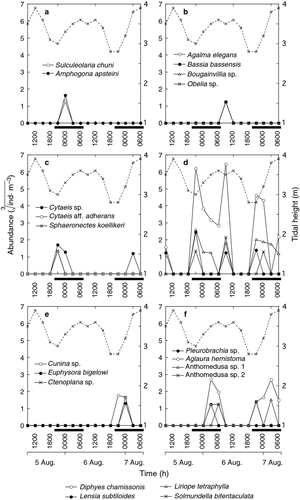
At 0900 h on the second day, there was an abrupt peak in total gelatinous zooplankton abundance, with the highest abundance found during the study (294 ind. m−3, Fig. 1). The abrupt peak was mainly caused by the addition of D. chamissonis (91.3% of total abundance, polygastric stage: eudoxid stage =3:7), Solmundella bitentaculata (3.4%), and L. tetraphylla (2.0%) – all holoplanktonic species with no benthic stage (Fig. 2d). However, there was no clear peak at 0900 h on the previous day (6 ind. m−3; Fig. 1).
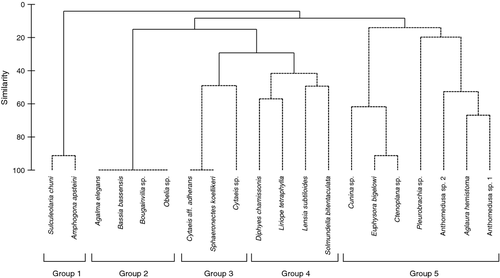
Of the 20 gelatinous zooplankton species found, 12 species appeared in the water column exclusively during the night, four species occurred only during the day, and the other four species appeared during both day and night (Table 1). Hierarchical cluster analysis and the SIMPROF test revealed five significant groups (Fig. 3; P < 0.05). Group 1 comprised Sulculeolaria chuni and Amphogona apsteini (both holoplanktonic), which appeared in the water column only at 0000 h on the first night (Fig. 2a). Group 2 comprised the four species that were observed during the day only at 0900 h on the second day (i.e. holoplanktonic species Agalma elegans, Bassia bassensis and meroplanktonic species Bougainvillia sp. and Obelia sp.; Fig. 2b). Group 3 consisted of three species (i.e. holoplanktonic species Sphaeronectes koellikeri and meroplanktonic species Cytaeis sp. and Cytaeis aff. adherans) that were observed only during the night with a peak in abundance at 2100 h on the first night for the three species and at 0300 h on the second night for Cytaeis sp. (Fig. 2c). Group 4 comprised four species (i.e. holoplanktonic species D. chamissonis, L. tetraphylla, L. subtiloides and S. bitentaculata), which exhibited their peaks at 2100 h on both nights but also at 0900 h (Fig. 2d). Group 5 consisted of seven species (i.e. holoplanktonic species Cunina sp., Pleurobrachia sp., A. hemistoma, meroplanktonic species Euphysora bigelowi, Anthomedusa sp. 1 and 2, and demersal species Ctenoplana sp.), which were observed only during the night and peaked in abundance mainly later in the night (0000–0600 h) but Cunina sp. and Anthomedusa sp. also showed a peak at 2100 h (Fig. 2e and f).
A significant day/night difference in each gelatinous zooplankton species population was found for the three most abundant species (i.e. D. chamissonis, A. hemistoma and L. tetraphylla; Table 1; P < 0.05). There were significant differences in the abundances of D. chamissonis, L. subtiloides and L. tetraphylla between the tidal phases (Table 1). However, the increase/decrease patterns of the abundance of these three species did not coincide with tidal phase as they were abundant during both low and high tides (Fig. 2d).
Discussion
This paper provides information over a scale of hours on the variations in gelatinous zooplankton communities that can exist on a coral reef. We collected gelatinous zooplankton by vertical tows through the water column from 1 m above the bottom to the surface, so the net did not collect gelatinous zooplankton deeper than 1 m above the bottom in the benthopelagic layer, and the abundance results may therefore be underestimated.
Although the study period spanned only 48 h, gelatinous zooplankton exhibited a substantial diel variation in abundance. They were not collected in the water column during most of the day (i.e. 1200, 1500 and 1800 h except for 0900 h) but were common during the night. The times of appearance at night were different among the species, and the number of species was also different depending on the hour of sampling. For example, species in Groups 3 and 4 exhibited their nocturnal peaks in abundance at 2100 h, whereas those of Groups 1 and 5 showed their peaks later in the night (0000–0300 h). The nocturnal appearance could be attributed to two possible factors: (i) offshore vertical migrations of oceanic species and subsequent advection into the reef by horizontal currents and (ii) upward movement of demersal or bottom swarms of gelatinous zooplankton into the water column (e.g. Morgado et al. 2003). During the night, if the tide is lowest when external influence is minimal, zooplankton samples collected over patch reefs and reef flats contain almost exclusively reef-inhabiting species which reside in the bottom environment during the day and disperse at night (Sorokin 1993). In the present study, minimum tidal heights were observed at 2100 h, coinciding with maximum nocturnal appearances of various gelatinous zooplankton species. For example, Diphyes chamissonis, Liriope tetraphylla, Lensia subtiloides, and Solmundella bitentaculata in Group 4 exhibited a high abundance during the night at 2100 h. Although D. chamissonis, L. tetraphylla and L. subtiloides exhibited a significant difference in abundance between the tidal phases, their nocturnal peaks coincided with low tide, suggesting the peaks of these species at 2100 h were due more to their ascent from the bottom to the upper water column than to horizontal advection from oceanic water. Indeed, L. tetraphylla is known to form swarms, active aggregation of individuals, near the bottom during daytime and to disperse at night (Ueno & Mitsutani 1994). Considering that they were not collected during the day (1200–1800 h) with our net, they may inhabit the reef bottom environment during the daytime. In addition, four gelatinous zooplankton species from Group 3 and Cunina sp. in Group 5 exhibited their peak in abundance at 2100 h and their appearance in the water column at 2100 h could also be attributed to vertical migration from the bottom environment. On the other hand, the species in Groups 1 and 5 (except Cunina sp.) exhibited their peaks later in the night, coinciding with the flooding tide phase, suggesting some of them may have been advected inshore with the incoming tide. However, some may also concurrently migrate into the water column from the bottom environment later in the night, since Ctenoplana sp., a demersal species that usually resides on the bottom, was also observed later in the night (0000 h).
Although most of the gelatinous zooplankton were common during the night, the maximum abundance of gelatinous zooplankton was found at 0900 h on the second day; this was due to the addition of eight species from Groups 3 and 4. Only two meroplanktonic species were sampled at 0900 h on the second day (Bougainvillia sp. and Obelia sp.), the remaining six species being holoplanktonic. The appearance of meroplanktonic species almost exclusively at night (Table 1) points to them being released from their benthic hydroid stage or becoming detached from the bottom under the cover of darkness. With regard to the holoplanktonic species, it is known that they can form intense aggregations or swarms in patches during the day and that the patch movement depends largely on horizontal movement of the water column (Omori & Hamner 1982; Graham et al. 2001). We may have caught such a swarm, which resulted in the high apparent abundance at 0900 h on August 6th.
We have previously observed at the present study site that many zooplankton taxa, including copepods and other invertebrate larvae, ascend into the water column in the early hours of the night (2100 h; Nakajima et al. 2008), probably due to their active avoidance of sedentary zooplanktivores (Yahel et al. 2005a,b) and these increases coincide with the nocturnal increases in many gelatinous zooplankton of Groups 3 and 4 in this study. Dispersal into the upper water column at night presumably facilitates feeding in the plankton. Unfortunately, full gastrozooids were not found associated with the colonies and this hypothesis could therefore not be checked. However, since D. chamissonis of Group 4 was the most abundant gelatinous zooplankton sampled in the present study, this species could be expected to be a major consumer of zooplankton prey at the present coral reef site.
This paper described data on changes in gelatinous zooplankton populations over short time intervals at a coral reef. Although the 48-h sampling period includes only 2 days and nights, the consecutive sampling at 3-h intervals revealed that the temporal variations were large, with great variability in the abundance during both the day (38 ± 104 ind. m−3) and at night (90 ± 87 ind. m−3), being 96.6–273.7% of the average values. Careful consideration should be given to the sampling variability in interpreting reef gelatinous zooplankton samples. Further investigations in situ using SCUBA diving (Hamner et al. 1975) or depth-discrete sampling (e.g. Alldredge & King 2009; Heidelberg et al. 2010) are proposed in order to obtain a full understanding of temporal variations in coral reef gelatinous zooplankton.
Acknowledgements
The authors thank M. Y. Ng, F. L. Alice Ho and S. Takeda for field assistance; W. Kinjyo for help in microscopy; and two anonymous reviewers for helpful comments. This work was partially funded by the Asian CORE Program of the Japan Society for the Promotion of Science (JSPS), by the UKM Research Grant UKM-GUP- ASPL-08-04-231, by JSPS KAKENHI Grant Number 22380108, 23405031 and 24248032, and by the Environment Research and Technology Development Fund (S9) of the Ministry of the Environment, Japan. This study is a contribution from the Census of Marine Zooplankton (CMarZ) ocean realm field project of the Census of Marine Life (CoML).




