Benthic hydroids from off Low Island (Southern Ocean, Antarctica)
Abstract
A survey of marine benthic community biodiversity off Low Island (Southern Ocean) was carried out by the Spanish Antarctic Expedition Bentart 2006, using the BIO Hespérides. Samples from the expedition included an important collection of hydroids. A total of 39 species was recorded, two of them being new to science (Eudendrium bentart sp. nov., and Orthopyxis curiosa sp. nov.), belonging to the subclasses Anthoathecata and Leptothecata. Anthoathecates are represented by only two species, both referable to the genus Eudendrium. The 37 leptothecate species belong to the families Campanulinidae, Lafoeidae, Haleciidae, Schizotrichidae, Kirchenpaueriidae, Sertulariidae and Campanulariidae. Sertulariidae is the most diverse family with 16 species (41%), followed by Campanulariidae with five species (13%) and Haleciidae, Schizotrichidae and Kirchenpaueriidae with four species (10%) each. At the generic level, the predominant genera are Symplectoscyphus with nine species (23%) and Halecium, Oswaldella and Schizotricha with four species (10%) each. Fifteen of the 39 species represent new records for the waters surrounding Low Island, as also are three (Lafoeina, Opercularella and Orthopyxis) of the 16 genera. In all, 33 species (c. 81%) are endemic to Antarctic waters, either with a circum-Antarctic (20 species, c. 49%) or West Antarctic (13 species, c. 32%) distribution. Thirty-eight species (c. 93%) are restricted to Antarctic or Antarctic/sub-Antarctic waters; only three species have a wider distribution. The Low Island hydroid fauna is composed of typical representatives of the Antarctic benthic hydroid fauna, with good representation of some of the most diverse and widespread Antarctic genera such as Antarctoscyphus, Oswaldella, Schizotricha and Staurotheca.
Introduction
Low Island, situated between the South Shetland Islands and the Antarctic Peninsula, is 14.4 km long and 8 km wide. A relatively rich benthic hydroid fauna is known to occur in waters surrounding it. Hydroids from the area were first reported by Blanco (1977a,b, 1982, 1984), all of them based on samples from a single station. In the first three of her papers, descriptions are provided of four new species. The last paper reported another 20 species, bringing the total number from the area to 24. Later, in a series of papers based on material collected by the United States Antarctic Research Program (USARP), Peña Cantero & Vervoort (2003, 2004, 2005) reported three species of Staurotheca, four species of Oswaldella and one species of Schizotricha from off Low Island. Five of these eight species were new records. Finally, Peña Cantero & Vervoort (2009) reported 18 species from material collected in the area by Brazilian expeditions, with six of them constituting new records. The total number of species known from off Low Island thus increased to 35.
As part of the Spanish Antarctic expedition Bentart 2006, undertaken with the Spanish polar research vessel BIO Hespérides, samples were taken from sea bottoms off Low Island to survey local benthic communities. These benthic samples contained a rich collection of hydroids, discussed herein. Fifteen of the 39 species found in the collection constitute new records for waters surrounding Low Island (including Eudendrium bentart sp. nov. and Orthopyxis curiosa sp. nov.), raising the known number of species from the area to 50. Three of the 16 genera present in the collection are also reported from the study area for the first time.
Material and Methods
In February 2006, during the Spanish Antarctic Expedition Bentart 2006, four benthic samples were taken with an Agassiz trawl from bottoms off Low Island (Southern Ocean). Samples were collected at typical shelf depths (between 82 and 115 m). Sampling stations of the expedition (Table 1) and those of previous collections from the study area, are shown in Fig. 1. Hydroids in the samples were fixed in 70% ethanol. Information on the ecology and distribution of most of these species has been provided in recent publications (cf. Peña Cantero & Vervoort 2003, 2004, 2005, 2009; Peña Cantero et al. 2004; Peña Cantero 2006, 2008, 2010a; Peña Cantero & Ramil 2006) and is not repeated here. Only new information from studies of the collection is presented here, together with data from previous records of species from the area by Blanco (1977a,b, 1982, 1984) and Peña Cantero & Vervoort (2003, 2004, 2005, 2009). All species recorded from off Low Island, including those absent in this collection [except Blanco's (1984) records considered doubtful], were included in a final general discussion on biodiversity, bathymetric distribution and biogeography of the hydroid fauna inhabiting the study area. Groups established by Peña Cantero (2004) were used in bathymetric analyses, and distributional models considered by Peña Cantero & García Carrascosa (1999) were employed in biogeographic studies.
| Station | Latitude (S) | Longitude (W) | Depth (m) |
|---|---|---|---|
| Low 44 | 63.43009 | 62.2038 | 82 |
| Low 45 | 63.43171 | 62.2116 | 86 |
| Low 46 | 63.43699 | 62.2450 | 97 |
| Low 47 | 63.46682 | 62.2151 | 115 |
Results
Taxonomic account
[(*) Species reported from off Low Island but absent in the Bentart 2006 collection]
Family Eudendriidae L. Agassiz, 1862
Eudendrium bentart sp. nov
(Fig. 2 and Fig. 3A,B)
Material examined: Low 44, one stem, c. 110 mm high, on sponge; Low 45, one basally broken stem, c. 100 mm high, with female gonophores (Holotype, MNCN 2.03/440; Museo Nacional de Ciencias Naturales, Madrid, Spain); Low 46, three stems, up to 135 mm high, on pebbles, basibiont of Filellum antarcticum, Halecium delicatulum, Symplectoscyphus curvatus and Symplectoscyphus hero.
Description (Holotype): Basally broken stem c. 100 mm high (Fig. 2A), polysiphonic in the first 60 mm and practically deprived of branches up to that point. Main stem completely annulated, giving rise to well developed primary branches spirally arranged and strongly directed upwards (Fig. 2A); a whorl every five branches. Primary branches usually only annulated at base, but frequently with annulations also marked on upper side of branch. Primary branches giving rise to second-order branches alternately in two planes forming an acute angle; secondary branches on upper side of primary ones. Secondary branches giving rise to pedicels, usually alternately in two planes making a very acute angle (clear tendency to unilateral arrangement). Some secondary branches with up to six pedicels. Polyp c. 500 μm high and with a crown of c. 20 tentacles (Fig. 3A).
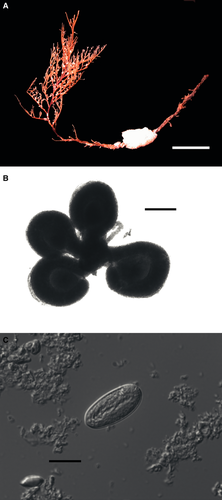
Gonozooids on distinctly shorter pedicels, reduced, but with tentacles, with up to five female gonophores on polyp body (Figs 2B and 3B). Eggs large (c. 430 μm in diameter), surrounded by a large simple spadix (Fig. 2B).
Cnidome consisting of small microbasic euryteles (7–8 × 3 μm), present everywhere, and large ? isorhizas (not seen discharged) (Fig. 2C), concentrated on the hypostome, frequent on pedicel coenosarc, and scarce and dispersed on spadix and ova. Measurements (in μm): (20.9 ± 0.6) × (8.5 ± 0.5) (10) (range: 20–21.5 × 8–9.5); ratio 2.5 ± 0.2 (10) (range 2.2–2.7).
Remarks: The same colony structure is found in the remaining material, where E. bentart sp. nov. also forms polysiphonic, little-branched stems with a clear tendency to unilateral arrangement of the polyp-bearing pedicels. Some of the stems are completely annulated.
At first glance, E. bentart sp. nov. is characterized by the completely annulated stems. This feature is also found in Eudendrium californicum Torrey, 1902, Eudendrium pocaruquarum Marques, 1995; Eudendrium ritchiei Millard, 1975 and Eudendrium vaginatum Allman, 1863 [see Marques et al. 2000; Schuchert 2008 (revision of E. vaginatum)]. Eudendrium californicum, E. pocaruquarum and E. vaginatum have female gonophores with simple spadix as is also characteristic for E. bentart sp. nov.
Biogeographically, the closest of those four species is E. ritchiei. However, E. bentart sp. nov. is clearly distinguishable from that species by having a simple spadix, as E. ritchiei is characterized by the bifid spadix supporting the egg (Millard 1975). In addition, the complementary nematocysts in E. ritchiei, which are microbasic eutyteles, are distinctly larger (23.4–27.6 × 8.4–10.8 μm) than those in E. bentart sp. nov. Furthermore, E. ritchiei forms stiff and spiky colonies, with stems branching irregularly in all planes and branches often re-branching. The perisarc is strongly annulated almost throughout, with just occasional smooth areas on some of the youngest pedicels. By contrast, E. bentart sp. nov. forms relatively high and thin, little-branched colonies (cf. Fig. 2A), with up to second-order branches. The strong annulations are restricted to the main stem in the holotype, but some stems are completely ringed in the material from Low 46.
Eudendrium pocaruquarum, known from Brazilian waters (cf. Marques 1995), also agrees with E. bentart sp. nov. in the number of female gonophores (three to five), the presence of a simple spadix and the radiate pattern of the branches. However, it differs by its monosiphonic stems, branched up to the third order. The perisarc of the main stem may be either completely annulated or with smooth parts. The origin of branches is extensively or completely annulated and the pedicels are completely annulated, or wrinkled. Marques's species also differs by the smaller size of the eggs (240–300 μm) and the complementary nematocysts (12.8–19.2 × 5.4–8.0 μm). The large microbasic euyteles are distributed over the hydranth body, hypostome and spadix of immature female gonophores. In E. bentart sp. nov. they are also seen everywhere, but they are concentrated on the hypostome.
Unlike E. bentart sp. nov., E. vaginatum is an Arctic to northern boreal species (Schuchert 2008), easily identifiable even in the absence of gonophores. It is distinguished by having perisarc arising from a circular groove located in the upper half of the hydranth body, giving the impression of a pseudohydrotheca. In addition, the complementary nematocysts, microbasic euryteles, have one small coil giving them a characteristic aspect. Like E. bentart sp. nov., E. vaginatum has unbranched spadix on normal polyps or polyps with shortened tentacles, and a similar size of the complementary nematocysts (20–23 × 9–9.5 μm). However, E. vaginatum differs in the number of gonophores (six or more), the presence of the pseudohydrotheca, and the type and aspect of the complementary nematocysts.
Finally, E. californicum differs from E. bentart sp. nov. in its large, much-branched colonies, the huge, generally squarish, peculiar polyp, with a ring of distinctly larger nematocysts [23–28 × 6.0–9.5 μm, atrichous isorhizas in Weill (1934) and Marques et al. (2000)] just above a deep basal circular groove. In addition the colony is tightly annulated throughout, from the stem and branches to the pedicels.
Ecology and distribution: Our material was collected at depths between 82 and 97 m.
Etymology: The specific name bentart honours the Bentart team, the group of Spanish scientists devoted to the study of the Antarctic benthos.
Eudendrium scotti Puce, Cerrano & Bavestrello, 2002 (*)
Eudendrium scotti – Peña Cantero & Vervoort 2009: p. 84.
Ecology and distribution: Species with circum-Antarctic distribution (Peña Cantero & Vervoort 2009). Reported from off Low Island, at a depth of 66 m, by Peña Cantero & Vervoort (2009).
Eudendrium sp
Material examined: Low 47, a few monosiphonic stems, up 12 mm high, with a few polyps, on sponges, axis of dead gorgonian and bryozoans.
Remarks: The material resembles young, monosiphonic colonies of Eudendrium scotti, with which it also shares the size of the large nematocysts [in μm: (21.8 ± 0.6) × (9.1 ± 0.7) (10), range 21–23 × 8–10; ratio 2.4 ± 0.2 (10), range 2.1–2.8]. However, in E. scotti the large nematocysts are disposed in two bands, on the base of the polyp and on the hypostome, whereas in our material there is only one band on the hypostome, just below the aperture. Anyway, the infertile condition of our material prevents us from providing a proper identification. Large nematocysts (not seen discharged) are provided with a long, thick and ornamented filament, but apparently no butt.
Family Campanulinidae Hincks, 1868
Lafoeina longitheca Jäderholm, 1904
(Fig. 3C)
Material examined: Low 47, a few hydrothecae, on bryozoans.
Ecology and distribution: Present material collected at a depth of 115 m, epibiotic on bryozoans. Species with a pan-Antarctic distribution (Peña Cantero & Vervoort 2009).
Opercularella belgicae (Hartlaub, 1904)
Material examined: Low 44, a few hydrothecae, on Symplectoscyphus glacialis; Low 46, a few hydrothecae, on bryozoans; Low 47, several hydrothecae, on Sertularella sanmatiasensis, Campanularia sp. and calcareous bryozoans.
Remarks: Hydrothecal pedicel up to 400 μm. Hydrothecal height (up to diaphragm) 350–400 μm; maximum diameter 120–130 μm.
Ecology and distribution: Present material collected at depths between 82 and 115 m, epibiotic on Campanularia sp., S. sanmatiasensis, S. glacialis and bryozoans. Species with uncertain distribution (Peña Cantero et al. 2004).
Family Lafoeidae Hincks, 1868
Abietinella operculata (Jäderholm, 1903) (*)
Abietinella operculata – Peña Cantero & Vervoort 2009: p. 84.
Ecology and distribution: Species with Antarctic–Patagonian distribution (Stepanjants 1979). Reported from off Low Island, at a depth of 135 m, by Peña Cantero & Vervoort (2009).
Filellum antarcticum (Hartlaub, 1904)
Material examined: Low 45, numerous hydrothecae, with coppinia, on Antarctoscyphus grandis; Low 46, a few hydrothecae, with coppinia, on E. bentart sp. nov.; Low 47, numerous hydrothecae, with coppiniae, on S. sanmatiasensis and gorgonian axis.
Ecology and distribution: Present material collected at depths between 86 and 115 m, epibiotic on A. grandis, E. bentart sp. nov., S. sanmatiasensis and gorgonian axis. Coppinia in February. Species mainly reported around Antarctica, but also from off South Africa (cf. Peña Cantero et al. 2004).
Lafoea dumosa (Fleming, 1820)
Lafoea fruticosa – Blanco 1984: p. 15–16, pl. 10 figs 22–23.
Lafoea dumosa – Peña Cantero & Vervoort 2009: p. 85.
Material examined: Low 44, several stems, up to 70 mm high, with coppinia, on stones, sponges, axis of dead gorgonian and bryozoans; Low 45, numerous stems, up to 40 mm high, on stones, sponges, axis of dead gorgonian, bryozoans and ascidians; Low 46, numerous stems, up to 80 mm high, with coppiniae, on gravel, algae, axis of dead gorgonian, bryozoans and ascidians; Low 47, numerous stems, up to 90 mm high, with coppiniae, on pebbles, bryozoans, Oswaldella stepanjantsae and S. sanmatiasensis.
Remarks: The coppiniae are characterized by having long, curved, unforked defensive tubes. However, there are some colonies in the material from Low 46 with coppiniae lacking defensive tubes. There are no differences in the size of the nematocysts between colonies with unforked defensive tubes in the coppinia (22.5–23.5 × 9–10 μm) and without them (22.5–25 × 9.5–10.5 μm). The latter seems to correspond to old coppiniae that have lost the defensive tubes. They are discoloured and the gonothecae are empty.
Ecology and distribution: Present material collected at depths between 82 and 115 m, epilithic on gravel, pebbles and stones and epibiotic on algae, sponges, axis of dead gorgonian, bryozoans, ascidians, O. stepanjantsae, S. sanmatiasensis. Coppinia in February. Previously found in the area by Blanco (1984), at a depth of 90–100 m, on ascidians, and by Peña Cantero & Vervoort (2009), between 66 and 135 m.
Family Haleciidae Hincks, 1868
Halecium delicatulum Coughtrey, 1876
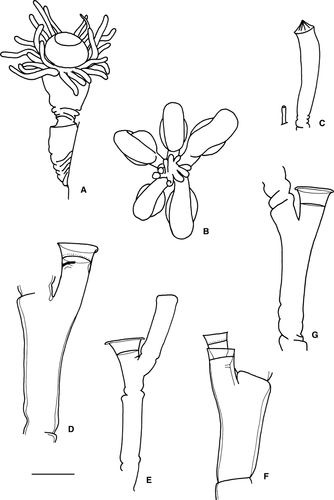
(Fig. 3D)
Material examined: Low 44, several polysiphonic stems, up to 80 mm high, with gonothecae; Low 45, several polysiphonic stems, up to 60 mm high, with gonothecae, on bryozoans; Low 46, several stems, up to 25 mm high, on bryozoans, E. bentart sp. nov. and Symplectoscyphus sp.; Low 47, a few monosiphonic stems, up to 19 mm high, on bryozoans.
Ecology and distribution: Present material collected at depths between 82 and 115 m, epibiotic on E. bentart sp. nov., Symplectoscyphus sp. and bryozoans. Gonothecae in February. Species with a reportedly worldwide distribution.
Halecium ovatum Totton, 1930
(Fig. 3E)
Halecium ovatum – Peña Cantero & Vervoort 2009: p. 85; Fig. 1f.
Material examined: Low 44, several stems, up to 30 mm high (largest slightly polysiphonic), on bryozoan, S. sanmatiasensis and S. glacialis; Low 45, four stems, up to 17 mm high, on S. sanmatiasensis.
Ecology and distribution: Present material collected at depths between 82 and 86 m, epibiotic on S. sanmatiasensis, S. glacialis and bryozoans. Previously found in the area at a depth of 66 m (Peña Cantero & Vervoort 2009). Species with a circum-Antarctic distribution (Peña Cantero 2008).
Halecium pallens Jäderholm, 1904
(Fig. 3F)
Material examined: Low 46, a large, strongly polysiphonic stem, c. 135 mm high, basibiont of Filellum sp.
Ecology and distribution: Present material collected at a depth of 97 m. Used as substratum by colonies of Filellum sp. Species with a circum-Antarctic distribution (Peña Cantero 2006).
Halecium sp
(Fig. 3G)
Material examined: Low 44, a few monosiphonic stems, up to 8 mm high, on calcareous bryozoan and tube of polychaete; Low 45, several monosiphonic stems, up to 24 mm high, on bryozoan; Low 47, a few monosiphonic stems, up to 9 mm high, on bryozoans.
Remarks: This material agrees with Halecium delicatulum in the long hydranthophores and the everted rim, but it differs in lacking pseudodiaphragm. Internodes are also arranged in a distinct zigzag pattern, whereas in H. delicatulum they are roughly straight.
Ecology and distribution: Present material collected at depths between 82 and 115 m, on bryozoans and tube of polychaete.
Family Schizotrichidae Peña Cantero, Sentandreu & Latorre, 2010
Schizotricha crassa Peña Cantero & Vervoort 2004
Schizotricha crassa – Peña Cantero & Vervoort, 2009: p. 85.
Material examined: Low 47, eight stems, up to 200 mm high, with gonothecae (two of the smallest stems with a secondary one).
Ecology and distribution: Present material collected at a depth of 115 m. Gonothecae in February. Previously found in the area at a depth of 135 m by Peña Cantero & Vervoort (2009). Species with a West Antarctic distribution (Peña Cantero & Vervoort 2004).
Schizotricha falcata Peña Cantero, 1998
Schizotricha anderssoni – Blanco, 1984: p. 46–48; pl. 42, figs 98–99; pl. 43, fig. 100.
Material examined: Low 45, three unbranched stems, up to 370 mm high, with female gonothecae; Low 46, three unbranched stems, up to 680 mm high, with female gonothecae; Low 47, six unbranched stems, up to 540 mm high, with male and female gonothecae, basibiont of S. sanmatiasensis, Symplectoscyphus sp. and L. dumosa.
Remarks: The hydrocladial apophyses sometimes have a barely visible, little-marked node at the basal part, forming an inconspicuous ahydrothecate internode bearing the nematotheca.
Ecology and distribution: Present material collected at depths between 86 and 115 m. Used as substratum by colonies of L. dumosa, S. sanmatiasensis and Symplectoscyphus sp. Gonothecae in February. Previously found in the area at a depth of 90–100 m by Blanco (1984). Species with a West Antarctic distribution (Peña Cantero & Vervoort 1999).
Schizotricha turqueti Billard, 1906
Material examined: Low 45, two stems, 325 and 270 mm high, with female gonothecae; Low 47, three stems, up to 200 mm high, with male and female gonothecae.
Remarks: This species is characterized by the unbranched stems, though sometimes it is possible to find a secondary stem (as happens here). Frequently, there are double, unforked hydrocladial internodes.
Ecology and distribution: Present material collected at depths between 86 and 115 m. Gonothecae in February.
Schizotricha vervoorti Peña Cantero, 1998
Schizotricha unifurcata – Blanco, 1984: p. 48–50; pl. 43, fig. 101; pl. 44, figs 102–103; pl. 45, figs 104–105; pl. 46, figs 106–107; pl. 47, figs 108–109.
Schizotricha vervoorti – Peña Cantero & Vervoort, 2005: p. 814–815; 2009: p. 86.
Material examined: Low 44, several branched stems, up to 270 mm high, with male and female gonothecae; Low 45, numerous stems, 43–340 mm high, with male and female gonothecae; Low 46, several stems, up to 225 mm high, with male and female gonothecae; Low 47, one stem fragment, c. 83 mm long, with male gonothecae.
Remarks: The unforked hydrocladial internodes are relatively short and are present in great number in each hydrocladium. Occasionally, there are double, unforked hydrocladial internodes. There are usually two infrathecal nematothecae, approximately at the same level, but sometimes there is only one, particularly at distal internodes. Typically the stems are branched, but there are also clearly unbranched stems. The latter may be mistakenly identified as S. turqueti. However, a careful examination reveals the presence of unforked hydrocladial internodes with two infrathecal nematothecae, whereas those internodes are always provided with a single infrathecal nematotheca in Billard's species. The shape of the hydrotheca is also clearly different: in S. vervoorti the abcauline wall is strongly directed outwards, distinctly diverging from the internode, the rim of hydrothecal aperture is strongly directed backwards adcaudally, and the hydrotheca strongly widens distally, so that it becomes wider than the internode.
Ecology and distribution: Present material collected at depths between 82 and 115 m. Blanco (1984) found it at a depth of 90–100 m, Peña Cantero & Vervoort (2005) between 91 and 95 m, with gonothecae in February, and Peña Cantero & Vervoort (2009) at 66 m.
Family Kirchenpaueriidae Millard, 1962
Oswaldella blanconae El Beshbeeshy, 1991 (*)
Oswaldella antarctica – Blanco, 1984: p. 41; pl. 38, figs 86–88.
Ecology and distribution: Species with circum-Antarctic distribution (Peña Cantero & Vervoort 2004). Reported from off Low Island, at a depth of 90–100 m, by Blanco (1984).
Oswaldella frigida Peña Cantero & Vervoort, 2004 (*)
Oswaldella frigida Peña Cantero & Vervoort, 2004: p. 830–832, fig. 8.
Ecology and distribution: West Antarctic distribution. Reported from off Low Island, at depths from 119 to 124 m, by Peña Cantero & Vervoort (2004).
Oswaldella grandis Peña Cantero, Svoboda & Vervoort, 1997
Oswaldella bifurca – Blanco, 1984: p. 43–44; pl. 39, figs 89–90; pl. 40, figs 91–93; pl. 41, fig. 94.
Material examined: Low 46, one stem, c. 420 mm high.
Ecology and distribution: Present material collected at a depth of 97 m. Previously reported in the area, at a depth of 90-100 m, by Blanco (1984). Species with a West Antarctic distribution (Peña Cantero & Vervoort 1998).
Oswaldella incognita Peña Cantero, Svoboda & Vervoort, 1997
Oswaldella incognita – Peña Cantero & Vervoort, 2004: 837–839, fig. 11.
Material examined: Low 44, one basally broken stem, c. 70 mm high, with female gonothecae, and one complete stem, c. 40 mm high, with male gonothecae.
Ecology and distribution: Present material collected at a depth of 82 m. Previously reported in the area, at a depth of 91–95 m, by Peña Cantero & Vervoort (2004). Species with a circum-Antarctic distribution (Peña Cantero 2009).
Oswaldella shetlandica Stepanjants 1979
Oswaldella billardi – Blanco & De Redolatti, 1977: p. 1–8, pls 1–4.
Oswaldella billardi – Blanco, 1984: p. 45–46; pl. 41, fig. 95 (in part).
Oswaldella shetlandica – Peña Cantero & Vervoort, 2004: p. 845–847; fig. 14; 2009: 86.
Material examined: Low 44, numerous stems, up to 95 mm high, with gonothecae; Low 45, several colonies with many stems, up to 150 mm high, with gonothecae; Low 46, five stems, up to 135 mm high, with gonothecae; Low 47, several stems, up to 80 mm high.
Ecology and distribution: Present material collected at depths between 82 and 115 m. Previously reported off Low Island by Blanco & de Redolatti (1977) and Blanco (1984), at a depth of 90–100 m, by Peña Cantero & Vervoort (2004), at 91–95 m, and by Peña Cantero & Vervoort (2009), at 135 m. Species with a West Antarctic distribution (Peña Cantero & Vervoort 2004).
Oswaldella stepanjantsae El Beshbeeshy, 1991
Oswaldella stepanjantsae – Peña Cantero & Vervoort, 2004: p. 847–850, fig. 15.
Material examined: Low 47, several branched stems, up to 380 mm high, with gonothecae, basibiont of Halecium sp., L. dumosa and S. sanmatiasensis.
Ecology and distribution: Present material collected at a depth of 115 m. Previously reported in the area by Peña Cantero & Vervoort (2004) at a depth of 119–124 m. Species with a circum-Antarctic distribution (Peña Cantero & Vervoort 1998).
Family Sertulariidae Hincks, 1868
Antarctoscyphus asymmetricus Peña Cantero, García Carrascosa & Vervoort, 1997
Material examined: Low 44, four stems, up to 90 mm high, basibiont of S. sanmatiasensis; Low 45, one stem, c. 120 mm high, with gonothecae.
Ecology and distribution: Present material collected at depths between 82 and 86 m. Species with a West Antarctic distribution (Peña Cantero 2006).
Antarctoscyphus grandis (Blanco, 1977)
Symplectoscyphus grandis Blanco, 1977a: p. 6; pl. 4, figs 14–16; pl. 5, figs 17–18.
Material examined: Low 45, two stems, c. 230 and 140 mm high, basally broken, with female gonothecae, basibiont of F. antarcticum and S. curvatus; Low 46, three stems, up to 200 mm high, with gonothecae.
Ecology and distribution: Present material collected at depths between 86 and 97 m. Gonothecae in February. Basibiont for colonies of F. antarcticum and S. curvatus. Previously reported off Low Island by Blanco (1977a) at a depth of 90-100 m. Species with a circum-Antarctic distribution (Peña Cantero et al. 1997).
Antarctoscyphus spiralis (Hickson & Gravely, 1907)
Symplectoscyphus spiralis – Blanco, 1984: p. 31–34; pl. 27, fig. 62; pl. 28, fig. 63.
Antarctoscyphus spiralis – Peña Cantero & Vervoort, 2009: p. 87.
Material examined: Low 44, one stem, c. 90 mm high, with gonothecae, on bryozoans; Low 45, six stems, up to 90 mm high, with gonothecae; Low 46, numerous stems, up to 105 mm high, with stolons attached to pebbles, axis of dead gorgonians, bryozoans and ascidians; Low 47, six stems, up to 75 mm high, with gonothecae, on bryozoans.
Ecology and distribution: Present material collected at depths between 82 and 115 m, on pebbles, axis of dead gorgonians, bryozoans and ascidians. Gonothecae in February. Previously reported in the area by Blanco (1984), at a depth of 90–100 m, and by Peña Cantero & Vervoort (2009), between 66 and 135 m. Species with a circum-Antarctic distribution (Stepanjants 1979).
Sertularella sanmatiasensis El Beshbeeshy, 1991
Sertularella polyzonias – Blanco, 1984: p. 37–39; pl. 31, figs 69–71; pl. 32, figs 72, 73; pl. 33, figs 74, 75; pl. 34, figs 76, 77; pl. 35, figs 78, 79; pl. 36, figs 80, 81.
Sertularella sanmatiasensis – Peña Cantero & Vervoort, 2009: p. 87; fig. 2b.
Material examined: Low 44, numerous stems, up to 90 mm high, with gonothecae, on sponges, axis of dead gorgonian, bryozoans, polychaete tube, Cnemidocarpa sp., L. dumosa and S. glacialis, basibiont of Symplectoscyphus nesioticus; Low 45, numerous stems, up to 110 mm high, with gonothecae, on pebbles, sponges, axis of dead gorgonian, bryozoans and Staurotheca pachyclada, basibiont of L. dumosa and S. glacialis; Low 46, numerous stems, up to 100 mm high, with gonothecae, on algae, sponges, axis of dead gorgonian, tube of benthic organism, bryozoans, ascidians and S. glacialis, basibiont of Obelia bidentata and S. glacialis; Low 47, numerous stems, up to 70 mm high, with gonothecae, on stones, sponges, tube of polychaete, bryozoans, L. dumosa and hydrorhiza of O. stepanjantsae.
Ecology and distribution: Present material collected at depths between 82 and 115 m, epilithic on pebbles and stones and epibiotic on algae, sponges, axis of dead gorgonians, bryozoans, polychaete tubes, ascidians, Cnemidocarpa sp., L. dumosa, S. pachyclada, S. glacialis and hydrorhiza of O. stepanjantsae. Basibiont for colonies of L. dumosa, O. bidentata, S. glacialis, and S. nesioticus. Gonothecae in February. Previously reported in the area by Blanco (1984) at a depth of 90–100 m. Species with a West Antarctic–Patagonian distribution (Peña Cantero 2006).
Sertularella sp. (*)
Sertularella sp.2 Peña Cantero & Vervoort, 2009: p. 87; Fig. 2d.
Ecology and distribution: Found by Peña Cantero & Vervoort (2009), at a depth of 168 m.
Staurotheca antarctica Hartlaub, 1904
Material examined: Low 44, a few colony fragments, up to 20 mm long; Low 46, one fragment, c. 23 mm long, with two male gonothecae.
Ecology and distribution: Present material collected at depths between 82 and 97 m. Species with a circum-Antarctic distribution (Peña Cantero & Vervoort 2003).
Staurotheca compressa Briggs, 1938
Staurotheca tubifera – Blanco, 1984: p. 40–41; pl. 37, fig. 82.
Staurotheca compressa – Peña Cantero & Vervoort, 2009: p. 87.
Material examined: Low 44, one palmate colony, c. 100 × 70 mm, on axis of dead gorgonian, plus two colony fragments, up to 60 mm long, with male and female gonothecae; Low 45, three masses, up to 180 × 180 mm, with male and female gonothecae, basibiont of S. glacialis; Low 46, a palmate colony, c. 90 × 50 mm, with male gonothecae; Low 47, two masses, c. 80 and 30 mm in diameter, with female gonothecae.
Ecology and distribution: Present material collected at depths between 82 and 115 m, on axis of dead gorgonian. Basibiont for colonies of S. glacialis. Gonothecae in February. Previously reported in the area by Blanco (1984), at a depth of 90–100 m, and by Peña Cantero & Vervoort (2009), between 66 and 135 m. Species with a circum-Antarctic distribution (Stepanjants 1979).
Staurotheca glomulosa Peña Cantero, Svoboda & Vervoort, 1997(*)
Staurotheca glomulosa – Peña Cantero & Vervoort, 2003: p. 2687–2689, fig. 9.
Ecology and distribution: Circum-Antarctic distribution (Peña Cantero & Vervoort 2003). Reported from off Low Island by Peña Cantero & Vervoort (2003), at depths of 156–253 m.
Staurotheca pachyclada (Jäderholm, 1904)
Staurotheca pachyclada – Peña Cantero & Vervoort, 2003: p. 2698–2702, figs 14 and 15.
Material examined: Low 45, three stems, up to 250 mm high, with female gonothecae, basibiont of S. sanmatiasensis, S. curvatus and S. glacialis; Low 46, several stems, up to 220 mm high, with male and female gonothecae, basibiont of S. sanmatiasensis; Low 47, several stems, up to 220 mm high, with gonothecae.
Remarks: The incipient stem from Low 47 allows us to understand the development of the stems in this species. The hydrothecae are firstly arranged in pairs, forming two longitudinal rows, but later verticils of three hydrothecae are formed. On the other hand, the material from Low 46 shows how the pinnae are firstly arranged in one plane, but in several planes distally. The stems are sinuous and the perisarc is transversally striated.
Ecology and distribution: Present material collected at depths between 86 and 115 m. Basibiont for colonies of S. sanmatiasensis, S. curvatus and S. glacialis. Gonothecae in February. Reported from off Low Island by Peña Cantero & Vervoort (2003), at depths of 119–124 m. Species with a circum-Antarctic distribution (Peña Cantero & Vervoort 2003).
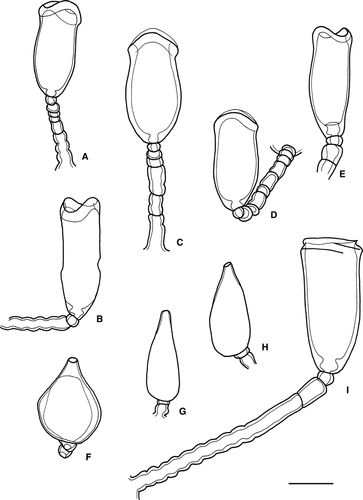
Symplectoscyphus anae Peña Cantero, Svoboda & Vervoort, 2002
(Fig. 4A)
Material examined: Low 47, a few stems, up to 12 mm high, on ascidian pedicel.
Remarks: Measurements of large nematocysts (in μm): 13.9 ± 0.8 × 3.3 ± 0.3 (10), range 12.5–15 × 3–3.5; ratio 4.3 ± 0.4, range 3.7–4.8.
Ecology and distribution: Present material collected at a depth of 115 m, epibiotic on ascidian pedicel. Species with a circum-Antarctic distribution (Peña Cantero et al. 2002).
Symplectoscyphus cumberlandicus (Jäderholm, 1905)
(Fig. 4B)
Symplectoscyphus cumberlandicus – Blanco, 1984: p. 23–24; pl. 17, figs 39, 40; pl. 18, figs 41 and 42; pl. 19, figs 43 and 44; pl. 20, fig. 45.
Material examined: Low 46, one stem, c. 25 mm high, on bryozoan, basibiont of Symplectoscyphus sp.
Remarks: Measurements of large nematocysts (in μm): 8.6 ± 0.4 × 2.3 ± 0.2 (10), range 8.5–9 × 2–2.5; ratio 3.8 ± 0.5, range 3.2–4.5.
Ecology and distribution: Present material collected at a depth of 97 m, on bryozoans. Basibiont of S. glacialis. Previously reported in the area by Blanco (1984), at a depth of 90–100 m. Species with a circum-Antarctic distribution (Peña Cantero et al. 2002).
Symplectoscyphus curvatus (Jäderholm, 1917)
Symplectoscyphus curvatus – Blanco, 1984: p. 24–26; pl. 20, fig. 46; pl. 21, figs 47 and 48; pl. 22, figs 49 and 50; Peña Cantero & Vervoort 2009: p. 87.
Material examined: Low 44, a few stems, up to 25 mm high, on axis of dead gorgonian, basibiont of S. glacialis; Low 45, a few stems, up to 15 mm high, on sponges, axis of dead gorgonians, bryozoans, A. grandis and S. pachyclada, basibiont of O. bidentata and S. glacialis; Low 46, several stems, up to 14 mm high, on axis of dead gorgonian and ascidians, basibiont of Symplectoscyphus sp.; Low 47, several stems, up to 20 mm high, on axis of dead gorgonian, tube of polychaete, ascidian pedicel and O. stepanjantsae, basibiont of S. nesioticus.
Remarks: Measurements of large nematocysts (in μm): 13.7 ± 0.6 × 3.4 ± 0.2 (10), range 12.5–14.5 × 3–3.5; ratio 4.1 ± 0.2, range 3.7–4.5.
Ecology and distribution: Present material collected at depths between 82 and 115 m, on sponges, axis of dead gorgonians, tube of polychaete, bryozoans, ascidians, A. grandis, O. stepanjantsae and S. pachyclada. Basibiont for colonies of O. bidentata, S. glacialis, S. nesioticus and Symplectoscyphus sp. Previously reported in the area by Blanco (1984), at a depth of 90–100 m, and by Peña Cantero & Vervoort (2009), between 66 and 135 m. Species with a circum-Antarctic distribution (Stepanjants 1979).
Symplectoscyphus exochus Blanco, 1982
(Fig. 4C)
Symplectoscyphus exochus Blanco, 1982: p. 39–41; figs 1–7; Peña Cantero & Vervoort 2009: p. 88, Fig. 2f.
Material examined: Low 44, two masses, c. 30 mm in diameter, with gonothecae, basibiont of Filellum sp.; Low 45, a mass, c. 30 mm in diameter, plus another mixed with A. asymmetricus and S. glacialis, with gonothecae; Low 46, several planar masses, up to 160 × 110 mm, with gonothecae, on stones, gravel and algae; Low 47, two palmate colonies, up to 100 × 70 mm, with immature gonothecae.
Remarks: Gonothecae with c. eight rings. Funnel-shaped neck, overpassing distal ring. Measurements (in μm): length c. 1100, maximum diameter c. 700, neck length c. 230, diameter at aperture c. 200. Measurements of large nematocysts (in μm): 9.6 ± 0.3 × 3 ± 0.2 (10), range 9–10 × 2.5–3; ratio 3.3 ± 0.2, range 3.2–3.8.
Ecology and distribution: Present material collected at depths between 82 and 115 m, epilithic on gravel and stones and epibiotic on algae. Basibiont for colonies of Filellum sp. Gonothecae in February. Previously reported in the area by Blanco (1982), at a depth of 90–100 m, and Peña Cantero & Vervoort (2009) at 135 m. Species with a West Antarctic distribution (Peña Cantero 2010b).
Symplectoscyphus glacialis (Jäderholm, 1904)
(Fig. 4D)
Symplectoscyphus glacialis – Blanco, 1984: p. 26–28; pl. 23, figs 51 and 52; pl. 24, fig. 53; Peña Cantero & Vervoort 2009: p. 88; fig. 2g.
Material examined: Low 44, numerous palmate colonies, up to 140 × 200 mm, with gonothecae, on stones, pebbles, sponges, axis of dead gorgonian and bryozoans; Low 45, numerous palmate masses, up to 100 × 150 mm, with gonothecae, on stones, gravel, axis of dead gorgonian, polychaete tube, bryozoans, ascidians, O. shetlandica, S. compressa, S. pachyclada and S. curvatus, basibiont of L. dumosa; Low 46, numerous masses of stems, up to 170 × 120 mm, with gonothecae, on pebbles, stones, algae, axis of dead gorgonian, bryozoans, ascidians, A. grandis, L. dumosa and S. sanmatiasensis; Low 47, two masses, c. 30 and 50 mm in diameter, with gonothecae.
Remarks: This is the most abundant species in the collection and commonly was found growing mixed with S. sanmatiasensis. Frequently, with distal stolons attaching to several substrates. Measurements of large nematocysts (in μm): 11.3 ± 0.4 × 3.5 ± 0.0 (10), range 10.5–12 × 3.5; ratio 3.2 ± 0.1, range 3.0–3.4.
Ecology and distribution: Present material collected at depths between 82 and 115 m, epilithic on gravel, pebbles and stones and epibiotic on algae, sponges, axis of dead gorgonians, polychaete tubes, bryozoans, ascidians, A. grandis, L. dumosa, O. shetlandica, S. sanmatiasensis, S. compressa, S. pachyclada and S. curvatus. Basibiont for L. dumosa. Gonothecae in February. Previously reported in the area by Blanco (1984), at a depth of 90–100 m, and by Peña Cantero & Vervoort (2009) at 135 m. Species with an Antarctic–Kerguélen distribution (Peña Cantero et al. 2002).
Symplectoscyphus hero Blanco, 1977
(Fig. 4E,F)
Symplectoscyphus hero Blanco, 1977a: p. 4–6; figs 1–13, 19 and 20; Peña Cantero & Vervoort, 2009: p. 88; fig. 2h.
Material examined: Low 44, one stem, c. 37 mm high; Low 45, one stem, c. 32 mm high, distally attached to a bryozoan; Low 46, many stems, up to 40 mm high, with gonothecae, on ascidian pedicel and Eudendrium bentart sp. nov.; Low 47, one monosiphonic stem, c. 45 mm high, with gonothecae.
Remarks: The stems are monosiphonic and branch irregularly, although sometimes branching is alternate in two planes or unilateral. The 32-mm-high stem from Low 45 has lateral branches stolonized distally and attached to a bryozoan where they become hydrorhizal stolons, giving rise to a few smaller stems. The gonothecal wall is slightly striated transversally. Measurements of the gonothecae (in μm): height 1900–1970, maximum diameter 750–800, height of distal neck c. 130, diameter at the aperture 250–270. Measurements of large nematocysts (in μm): 13.6 ± 0.5 × 3.6 ± 0.2 (10), range 12.5–14 × 3.5–4; ratio 3.8 ± 0.2, range 3.5–4.
The general shape of the hydrotheca of this species resembles that of S. anae. However, they can be clearly identified because in S. hero the internodes are straight and separated by very oblique, strongly marked nodes, whereas in S. anae the internodes are arranged in a distinct zigzag pattern (cf. Fig. 4A,E). Moreover, the hydrothecae in S. anae are free for a longer portion and they are clearly curved outwards.
Ecology and distribution: Present material collected at depths between 82 and 115 m, on bryozoans, ascidian pedicel and E. bentart sp. nov. Gonothecae in February. Previously reported in the area by Blanco (1977a), at a depth of 90–100 m, and by Peña Cantero & Vervoort (2009) at 135 m. Species with a West Antarctic distribution (Peña Cantero & Vervoort 2009).
Symplectoscyphus liouvillei (Billard, 1914)
(Fig. 4G,H)
Material examined: Low 46, two stems, c. 60 and 135 mm high, with gonothecae.
Remarks: Measurements of large nematocysts (in μm): 10.5 ± 0.3 × 2.7 ± 0.2 (10), range 10–11 × 2.5–3; ratio 3.9 ± 0.4, range 3.3–4.2.
Ecology and distribution: Present material collected at a depth of 97 m. Fertile in February. Species with a West Antarctic–Patagonian distribution (Peña Cantero et al. 2002).
Symplectoscyphus nesioticus Blanco, 1977
Symplectoscyphus nesioticus Blanco, 1977b: p. 1–12; pl. 1, figs 1–6; pl. 2, figs 7–10; pl. 3, fig. 11.
Material examined: Low 44, numerous stems, up to 15 mm high, with gonothecae, on axis of dead gorgonian, bryozoans, L. dumosa and S. sanmatiasensis; Low 45, several stems, up to 12 mm high, with immature gonothecae, on sponges, bryozoans and ascidians; Low 46, numerous stems, up to 4 mm high, with gonothecae, on algae, axis of dead gorgonian, bryozoans and ascidians; Low 47, several stems, up to 12 mm high, with gonothecae, on bryozoans and S. sanmatiasensis.
Remarks: Measurements of large nematocysts (in μm): 11.8 ± 0.3 × 2.9 ± 0.2 (10), range 11.5–12 × 2.5–3; ratio 4.2 ± 0.4, range 3.8–4.8.
Ecology and distribution: Present material collected at depths between 82 and 115 m, on algae, sponges, axis of dead gorgonian, bryozoans, ascidians, L. dumosa and S. sanmatiasensis. Gonothecae in February. Previously reported in the area by Blanco (1977b), at a depth of 90–100 m. Species with a West Antarctic distribution (Peña Cantero 2010b).
Symplectoscyphus plectilis (Hickson & Gravely, 1907)
Material examined: Low 47, a few stems, up to 15 mm high, on bryozoans.
Ecology and distribution: Present material collected at a depth of 115 m, on bryozoans. Species with a circum-Antarctic distribution (Stepanjants 1979).
Symplectoscyphus subdichotomus (Kirchenpauer, 1884) (*)
Symplectoscyphus subdichotomous – Blanco, 1984: p. 34; pl. 29, figs 64–66.
Remarks: Species with uncertain distribution because of the presence of a group of closely related species (Vervoort 1972). Reported from off Low Island, at a depth of 90–100 m, by Blanco (1984).
Blanco's (1984) material consists of a single fragment c. 20 mm long with a gonotheca. From the description, figures and measurements provided by that author, the material could also belong to S. exochus, widely represented in the area.
Symplectoscyphus vanhoeffeni Totton, 1930 (*)
Symplectoscyphus vanhöeffeni – Blanco, 1984: p. 36–37; pl. 30, figs. 67 and 68.
Ecology and distribution: Species with a circum-Antarctic distribution (Peña Cantero & García Carrascosa 1995). Reported from off Low Island, at a depth of 90–100 m, by Blanco (1984).
Family Campanulariidae Hincks, 1868
Billardia subrufa (Jäderholm, 1904)
Billardia subrufa – Blanco, 1984: p. 17–18; pl. 11, figs 24 and 25; pl. 12; figs 26–28; Peña Cantero & Vervoort 2009: p. 89.
Material examined: Low 44, a few stems, up to 30 mm high, with gonothecae; Low 45, a few stems, up to 25 mm high, with gonothecae, on gravel and ascidians; Low 46, several stems, up to 30 mm high, with gonothecae, on bryozoans; Low 47, three stems, up to 80 mm high, with gonothecae.
Ecology and distribution: Present material collected at depths between 82 and 115 m, epilithic on gravel and epibiotic on bryozoans and ascidians. Gonothecae in February. Previously reported in the area by Blanco (1984), at a depth of 90–100 m, and by Peña Cantero & Vervoort (2009), between 135 and 168 m. Species with an Antarctic–Patagonian distribution (Peña Cantero et al. 2004).
Campanularia hicksoni Totton, 1930 (*)
Campanularia hicksoni – Blanco, 1984: p. 18–20; pl. 13, figs 29–31.
Ecology and distribution: Species with a circum-Antarctic distribution (Peña Cantero et al. 2004). Reported from off Low Island, at a depth of 90–100 m, by Blanco (1984).
Campanularia sp
Material examined: Low 45, several hydrothecae, on bryozoans; Low 46, numerous hydrothecae, on bryozoans; Low 47, numerous hydrothecae, on S. sanmatiasensis.
Remarks: Dark-brown coenosarc. Approximately 32 tentacles. Measurements (in μm): hydrothecal length 800–950, diameter at aperture 450–600, height of basal chamber 90–110, diameter at diaphragm 130–150.
Measurements of nematocysts (in μm): Larger microbasic mastigophore: mean ± SD (n) 9.3 ± 0.3 × 1.9 ± 0.2 (10), range 9–10 × 1.5–2; ratio mean ± SD (n) 4.9 ± 0.6 (10), range 4.5–6. Smaller microbasic mastigophore: 6–7 × 1.5.
This species differs from C. hicksoni by having much smaller hydrothecae and, particularly, much smaller large nematocysts (17–18.5 × 4–4.5 μm in C. hicksoni).
Ecology and distribution: Present material collected at depths between 86 and 115 m, on bryozoans and S. sanmatiasensis.
Obelia bidentata Clark, 1875
Obelia bidentata – Peña Cantero & Vervoort, 2009: p. 89.
Material examined: Low 44, a few stems, up to 14 mm high, with gonothecae, on sponges, bryozoans, S. sanmatiasensis, S. exochus and S. glacialis; Low 45, several stems, up to 15 mm high, on S. curvatus and S. glacialis; Low 46, several stems, up to 16 mm high, on bryozoans and S. sanmatiasensis; Low 47, several stems, up to 25 mm high, on S. sanmatiasensis.
Remarks: Measurement of the hydrothecae (in μm): height 550–730, diameter at the aperture 250–350, height of basal chamber 60–90, diameter at diaphragm 100–130.
Ecology and distribution: Present material collected at depths between 82 and 115 m, on sponges, bryozoans, S. sanmatiasensis, S. curvatus, S. exochus and S. glacialis. Gonothecae in February. Previously reported in the area by Peña Cantero & Vervoort (2009), at a depth of 66 m. Species with a worldwide distribution.
Orthopyxis norvegiae (Broch, 1948)
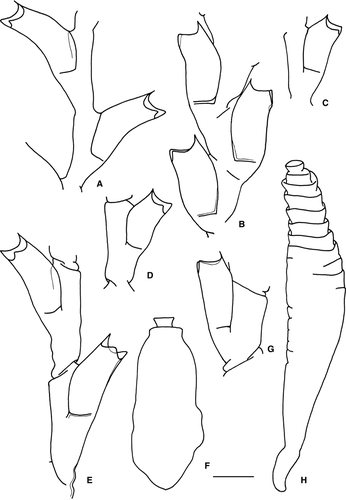
(Fig. 5A and Fig. 6I)
Material examined: Low 44, several hydrothecae, on algae; Low 46, three hydrothecae on S. sanmatiasensis.
Remarks: The hydrotheca is relatively high and has a strong perisarc development. The rim of the hydrothecal aperture is even and frequently lengthened by a collar of perisarc.
Measurements (in μm): Large microbasic mastigophores (shaft with spines, c. 23 μm): 18.5–20 × 4–4.5 (range), 19.6 ± 0.5 × 4.1 ± 0.2 (10) [mean ± SD (n)]; 4.4–5.0 (ratio − range), 4.8 ± 0.2 (10) [ratio − mean ± SD (n)]. Smaller microbasic mastigophores: 5.5–6.5 × 1.5–2.
Ecology and distribution: Present material collected at depths between 82 and 97 m, on algae and S. sanmatiasensis.
Orthopyxis curiosa sp. nov
(Fig. 5B,C and Fig. 6A–H)
Campanularia norvegiae – Stepanjants, 1979: p. 30–31; pl. 2, Fig. 2.
Material examined: Low 44, numerous hydrothecae, with male gonothecae, on algae (Holotype, MNCN 2.03/441; Museo Nacional de Ciencias Naturales, Madrid, Spain).
Description: Hydrorhiza creeping, branched and anastomosing. Hydrothecal pedicels erect, unbranched, variable in length, usually annulated throughout (Figs 5B and 6A–E). Pedicel followed by a sub-hydrothecal spherule of smaller diameter than pedicel (Fig. 6–E). Hydrothecae tubiform, relatively high, flattened and bilateral. Hydrothecal diameter increasing from basal part upwards, reaching maximum diameter by the middle of hydrothecal length, later slightly decreasing to widen again at hydrothecal aperture (Figs 5B and 6A–E). Hydrothecal aperture compressed, forming two lip-like extensions (Figs 5B and 6A–E), oval in upper view, rim smooth. Perisarc of hydrotheca fairly developed, particularly at basal part, delimiting a basal chamber. Perisarc development also conspicuous at the hydrothecal aperture, forming a sort of band all around the rim (Figs 5B and 6A–E). Hydrothecal rim frequently lengthened by a collar of perisarc (Figs 5B and 6C,D), usually shorter than the perisarc band around the aperture.
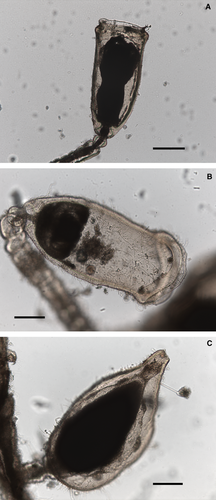
Gonotheca originating from hydrorhizal stolon, pear-shaped in lateral view, resting on a short pedicel with a ring under gonotheca (Figs 5C and 6F–H).
Measurements (in μm): hydrothecal height 550–630, maximum diameter 230–260, diameter at aperture 230–250; gonothecal height 500–540, diameter 200–360, diameter at aperture 50–60. Nematocysts (in μm): Large microbasic mastigophores: 10–12 × 2 (range), 11.0 ± 0.7 × 2.0 ± 0.0 (10) [mean ± SD (n)], 5.0–6.0 (ratio − range), 5.5 ± 0.3 (10) [ratio − mean ± SD (n)]. Smaller microbasic mastigophores: 5 × 1.5.
Remarks: Orthopyxis curiosa sp. nov. was found growing along with O. norvegiae. Both species agree in having relatively high hydrothecae and in O. norvegiae even a slight perisarc band may be noted all around the hydrothecal aperture; the distal part of its hydrotheca is also frequently lengthened by a collar of perisarc (cf. Fig. 5A). However, both species are perfectly distinguishable by the shape and size of the hydrothecae (cf. Figs 5 and 6) and by the size of the large nematocysts (18.5–20 × 4–4.5 μm in O. norvegiae). The hydrorhizal stolons are also larger in O. norvegiae.
Orthopyxis curiosa sp. nov. is unique in the peculiar shape of its hydrotheca, being easily distinguishable from the other species of the genus. In having a smooth rim at the hydrothecal aperture it is close to a number of species of the genus [e.g. Orthopyxis angulata Bale, 1914, O. asymmetrica Stechow, 1919, O. caliculata (Hincks, 1853), O. clytioides (Lamouroux, 1824), O. compressa (Clark, 1876), O. compressima Kubota & Yamada, 1992, O. integra (MacGillivray, 1842), O. macrogona (von Lendenfeld, 1885), O. pacifica Stechow, 1919, O. pedunculata Jäderholm, 1904, O. platycarpa Bale, 1914 and O. wilsoni Bale, 1914], but in all those species the hydrotheca is cup- to bell-shaped and the hydrothecal aperture is not compressed. The remaining species of the genus [e.g. O. affabilis Vervoort & Watson, 2003, O. australis (Stechow, 1924), O. crenata (Hartlaub, 1901), O. sargassicola (Nutting, 1915), O. tincta (Hincks, 1861)] have cusps at the hydrothecal rim and a completely different hydrothecal shape.
On the other hand, the material from the South Shetland Islands assigned to O. norvegiae by Stepanjants (1979) could belong to O. curiosa sp. nov. They agree in shape and size of the hydrotheca (cf. pl. 5, fig. 2A in Stepanjants 1979).
Ecology and distribution: Present material collected at a depth of 82 m, epibiotic on algae. Gonothecae in February. The material studied by Stepanjants (1979) was collected at a depth of 30–35 m off King George Island.
Etymology: The specific name curiosa is taken from the latin adjective ‘curiosus’ meaning odd, curious, and refers to the uncommon shape of the hydrotheca.
Remarks on other species recorded from Low Island
Several species reported from off Low Island by Blanco (1984) have not been considered above because they are considered doubtful records.
Blanco's record of Filellum serratum (Clarke, 1879) was based on infertile material. At present, however, it is acknowledged that it is not possible to carry out the proper identification of the species of Filellum in the absence of coppinia (cf. Peña Cantero et al. 1998), so it must be considered doubtful.
The record of Halecium antarcticum Vanhöffen, 1910 is likely based on mixed material as it comes from different localities and is composed of mono- and polysiphonic stems up to 80 mm high. According to Blanco (1984: p. 10) there is one pseudodiaphragm, or even two, on both sides of the hydranthophore, as is characteristic of H. delicatulum but not H. antarcticum. In any case, many authors consider both species conspecific. In addition, Blanco's (1984) figures of H. antarcticum show hydranthophores much longer than those of either H. delicatulum or H. antarcticum. Peña Cantero (2010a) already indicated that part of the material could belong to Halecium frigidum Peña Cantero 2010a2010a, particularly the material represented in pl. 4, figs 10 and 11 and pl. 5, fig. 13. For all those reasons, Blanco's record from off L>ow Island must be considered doubtful until the revision of her material.
Blanco's material of Halecium tenellum Hincks, 1861 could actually belong to H. ovatum, with which it agrees in the size and shape of the hydrothecae (cf. pl. 6, fig. 15 in Blanco 1984) and in the presence of geniculate stems that can give rise to paired branches.
The record of Hincksella (Sertularella) fallax (Hartlaub, 1904) was based on a single infertile fragment c. 10 mm long. Sertularella fallax Hartlaub, 1904 is a poorly characterized species, likely belonging to the genus Staurotheca. Blanco's (1984) material probably belongs also to a species of Staurotheca, but it is not possible to identify it properly.
Discussion
Table 2 shows a list of the benthic hydroids found in the sea bottoms off Low Island. Thirty-nine species (in bold in Table 2), including two new species to science, were collected during the Spanish Antarctic expedition Bentart 2006. The species belong to the subclasses Anthoathecata Cornelius 1992 and Leptothecata Cornelius 1992, eight families and 16 genera. Anthoathecates were only represented by two species of Eudendrium. The 37 leptothecate species belong to the families Campanulinidae, Lafoeidae, Haleciidae, Schizotrichidae, Kirchenpaueriidae, Sertulariidae and Campanulariidae. Fifteen of the 39 species constitute new records for the waters surrounding Low Island (indicated with * in Table 2), as do three of the 16 genera.
| Depth | Known range | Substrate | Epibionts | Distribution | Records | |
|---|---|---|---|---|---|---|
| Eudendrium bentart sp. nov. * | 82–97 | – | Pebbles, sponges | F. antarcticum, H. delicatulum, S. curvatus, S. hero | WA | PS |
| E. scotti | – | 10–135 | – | – | CA | PV09 |
| Eudendrium sp. | 115 | – | Sponges, axis of dead gorgonians, bryozoans | – | – | PS |
| Lafoeina longitheca * | 115 | 5–470 | Bryozoans | – | PA | PS |
| Opercularella belgicae * | 82–115 | 0–650 | Bryozoans, Campanularia sp., S. sanmatiasensis, S. glacialis | – | ? | PS |
| Abietinella operculata | – | 63–1500 | – | – | AP | PV09 |
| Filellum antarcticum * | 86–115 | 14–423 | Gorgonian axis, A. grandis, E. bentart, S. sanmatiasensis | – | CA+ | PS |
| Filellum sp. | – | – | – | – | – | B84, PV09 |
| Lafoea dumosa | 82–115 | 12–520 | Gravel, pebbles, stones, algae, sponges, axis of dead gorgonian, bryozoans, ascidians, O. stepanjantsae, S. sanmatiasensis | – | W | B84, PV09, PS |
| Halecium antarcticum a | – | – | B84 | |||
| H. delicatulum * | 82–115 | 12–385 | Bryozoans, E. bentart, Symplectoscyphus sp. | – | W | PS |
| H. ovatum | 82–86 | 3–471 | Bryozoans, S. sanmatiasensis, S. glacialis | – | CA | PV09, PS |
| H. pallens * | 97 | 20–401 | – | Filellum sp. | CA | PS |
| H. tenellum a | – | – | – | – | – | B84 |
| Halecium sp. | 82–115 | – | Bryozoans, tube of polychaete | – | – | PS |
| Schizotricha crassa | 115 | 93–238 | – | – | WA | PV09, PS |
| S. falcata | 86–115 | 73–248 | – | L. dumosa, S. sanmatiasensis, Symplectoscyphus sp. | WA | B84, PS |
| S. turqueti * | 86–115 | 0–1890 | – | – | CA | PS |
| S. vervoorti | 82–115 | 50–1152 | – | – | WA+ | B84, PV05, PV09, PS |
| Oswaldella blanconae | – | 90–351 | – | – | CA | B84 |
| O. frigida | – | 44–124 | – | – | WA | PV04 |
| O. grandis | 97 | 109–922 | – | – | WA | B84, PS |
| O. incognita | 82 | 20–952 | – | – | CA | PV04, PS |
| O. shetlandica | 82–115 | 24–182 | – | – | WA | B84, PV04, PV09, PS |
| O. stepanjantsae | 115 | 36–1890 | – | Halecium sp., L. dumosa, S. sanmatiasensis | CA | PV04, PS |
| Antarctoscyphus asymmetricus * | 82–86 | 70–429 | – | S. sanmatiasensis | WA | PS |
| A. grandis | 86–97 | 15–380 | – | F. antarcticum, S. curvatus | CA | B77a, PS |
| A. spiralis | 82–115 | 6–720 | Pebbles, axis of dead gorgonians, bryozoans, ascidians | – | CA | B84, PV09, PS |
| Sertularella sanmatiasensis | 82–115 | 30–500 | Pebbles, stones, algae, sponges, axis of dead gorgonians, bryozoans, tube of polychaete, ascidians, L. dumosa, O. stepanjantsae, S. pachyclada, S. glacialis | L. dumosa, O. bidentata, S. glacialis, S. nesioticus | WAP | B84, PV09, PS |
| Sertularella sp. | – | 168 | – | – | – | PV09 |
| Staurotheca antarctica * | 82–97 | 55–661 | – | – | CA | PS |
| S. compressa | 82–115 | 45–1042 | Axis of dead gorgonian | S. glacialis | CA | B84, PV03, PV09, PS |
| S. fallax a | – | – | – | – | – | B84 |
| S. glomulosa | – | 55–870 | – | – | CA | PV03 |
| S. pachyclada | 86–115 | 42–1405 | – | S. sanmatiasensis, S. curvatus, S. glacialis | CA | PV03, PS |
| Symplectoscyphus anae * | 115 | 20–640 | Ascidians | – | CA | PS |
| S. cumberlandicus | 97 | 8–380 | Bryozoans | S. glacialis | CA | B84, PS |
| S. curvatus | 82–115 | 49–799 | Sponges, axis of dead gorgonian, tube of polychaete, bryozoans, asicidians, A. grandis, O. stepanjantsae, S. pachyclada | O. bidentata, S. glacialis, S. nesioticus, Symplectoscyphus sp. | CA | B84, PV09, PS |
| S. exochus | 82–115 | 15–634 | Gravel, stones, algae | Filellum sp. | WA | B82, PV09, PS |
| S. glacialis | 82–115 | 5–922 | Gravel, pebbles, stones, algae, sponges, axis of dead gorgonian, polychaete tube, bryozoans, ascidians, A. grandis, L. dumosa, O. shetlandica, S. sanmatiasensis, S. compressa, S. pachyclada, S. curvatus | L. dumosa | AK | B84, PV09, PS |
| S. hero | 82–115 | 70–135 | Bryozoans, ascidians, E. bentart | – | WA | B77a, PV09, PS |
| S. liouvillei * | 97 | 65–443 | – | – | WAP | PS |
| S. nesioticus | 82–115 | 56–522 | Algae, sponges, axis of dead gorgonian, bryozoans, ascidians, L. dumosa, S. sanmatiasensis | – | WA | B77b, PS |
| S. plectilis * | 115 | 7–457 | Bryozoans | – | CA | PS |
| S. subdichotomous | – | 3–750 | – | – | ? | B84 |
| S. vanhoeffeni | – | 6–390 | – | – | CA | B84 |
| Billardia subrufa | 82–115 | 25–1030 | Gravel, bryozoans, ascidians | – | AP | B84, PV09, PS |
| Campanularia hicksoni | – | 10–385 | – | – | CA | B84 |
| Campanularia sp. * | 86–115 | – | Bryozoans, S. sanmatiasensis | – | – | PS |
| Obelia bidentata | 82–115 | 3–377 | Sponges, bryozoans, S. sanmatiasensis, S. curvatus, S. exochus, S. glacialis | – | W | PV09, PS |
| Orthopyxis curiosa sp. nov. * | 82 | – | Algae | – | WA | PS |
| O. norvegiae * | 82–97 | 21–40 | Algae, S. sanmatiasensis | – | WA | PS |
- In bold, species found in the present collection (*new records). Depth and known range in meters. Distribution: AK, Antarctic-Kerguélen; AP, Antarctic-Patagonian; CA, Circum-Antarctic; PA, Pan-Antarctic; WA, West Antarctic; WAP, West Antarctic-Patagonian; W, Wider Distribution. References: B77a, Blanco (1977a); B77b, Blanco (1977b); B82, Blanco (1982); B84, Blanco (1984); PV03, Peña Cantero & Vervoort (2003); PV04, Peña Cantero & Vervoort (2004); PV05, Peña Cantero & Vervoort (2005); PV09, Peña Cantero & Vervoort (2009); PS, Present Study. 1Doubtful records.
At the family level, Sertulariidae is by far the most diversified family, accounting for almost half of the species diversity (16 species, 41%). It is followed by Campanulariidae with five species (13%) and Haleciidae, Schizotrichidae and Kirchenpaueriidae with four (10%). These five families embrace 84% of the species diversity.
At the generic level, the predominant genera are Symplectoscyphus with nine species (23%) and Halecium, Schizotricha and Oswaldella with four species each (10%). The dominance of these four genera is remarkable, representing only 25% of the generic diversity but 53% of the species.
The indirect sampling method used in the present study does not allow us to obtain a complete picture of the substrata on which the species live. Only general impressions of substrate types are possible, particularly in relation to species with large colonies directly attached to the bottom, which usually come on board unattached or basally broken. This problem is clearly apparent in the present collection (cf. Table 2), and the substrate for most of the largest species (e.g. Staurotheca pachyclada and the species of Schizotricha and Oswaldella) is unknown. The situation is different for species living on other organisms or on small inorganic substrates, and some information can be obtained. Although a few species were observed on a single substratum (e.g. Lafoeina longitheca, Symplectoscyphus cumberlandicus and Symplectoscyphus plectilis on bryozoans, Symplectoscyphus anae on ascidians, and Orthopyxis curiosa, sp. nov., on algae), most species studied appeared to colonize and grow on a wide range of both non-living and living substrates. Lafoea dumosa, Sertularella sanmatiasensis and Symplectoscyphus glacialis occurred on the broadest spectrum of substrates, having been found epilithic on gravel, pebbles and stones and epibiotic on a wide range of basibionts (algae, sponges, gorgonians, polychaete tubes, bryozoans, ascidians and several species of hydroids) (cf. Table 2). In general, hydrozoans did not constitute a suitable hydroid substrate (cf. Table 2). For example, the low number of basibionts amongst the species forming large colonies is remarkable (e.g. only Schizotricha falcata and Oswaldella stepanjantsae amongst the species of Schizotricha and Oswaldella). The number of epibiotic species used, in turn, as a substrate by other species of hydroids is slightly higher (cf. Table 2). The most important were Eudendrium bentart sp. nov., S. sanmatiasensis and Symplectoscyphus curvatus, with four species each.
Bathymetric distributions of local species, both within the particular study area and across their total ranges, are shown in Table 2. Four of the six bathymetric groups proposed by Peña Cantero (2004) were recognizable in the study. The most important of these comprised species restricted to shelf waters (59%), a group consisting of two assemblages with one embracing species absent from the shallowest waters (26%) and the other including species distributed throughout the whole continental shelf (33%). The other two groups included eurybathic species, extending across the entire bathymetric range from shallowest waters to bathyal or even abyssal depths (19%), and species present in deep waters as well as on the continental shelf but excluding shallowest waters (22%). The other two groups proposed by Peña Cantero (2004), namely, the groups formed by species exclusively inhabiting either the shallowest sublittoral zone or deep ocean bottoms, were not represented because samples were taken only at depths between 82 and 115 m. Thus, species from the shallowest waters, and from strictly deep waters, were absent in the collection.
Table 2 also shows the biogeographic distribution model assigned to each species. Both circum-Antarctic (20 species, 49%) and West Antarctic (13 species, 32%) representatives constitute a group of endemic species which is by far the most dominant (81%). In any case, the dominant group is that formed by circum-Antarctic species. Another interesting group is that formed by species restricted to both Antarctic and sub-Antarctic waters. In this group, species with an Antarctic–Kerguélen, Antarctic–Patagonian or West Antarctic–Patagonian distribution are included. Thus, most species (93%) are restricted to Antarctic or Antarctic/sub-Antarctic waters and only three species are found outside those waters.
Acknowledgements
I wish to thank Dr José Luis Sanz Alonso from the Instituto Español de Oceanografía for his help in the elaboration of Fig. 1. The Bentart 2006 campaign was funded by the Ministerio de Ciencia y Tecnología (Ref. CGL2004-01856) of Spain. This study was developed thanks to a research project (Ref. CTM2009-11128ANT) funded by the Ministerio de Ciencia e Innovación of Spain and the Fondo Europeo de Desarrollo Regional (FEDER). The publication of this paper is supported by CONISMA, the Italian National Interuniversity Consortium for Marine Sciences.
Conflicts of Interest
None of the authors have any potential conflicts of interest.




