Biodiversity and distribution patterns of planktonic cnidarians in San Matías Gulf, Patagonia, Argentina
Abstract
The special location (40–42°S in the Southwestern Atlantic Ocean) and the hydrodynamic regime (limited water exchange with open ocean) in San Matías Gulf (Argentina) seem to produce a particular fauna of planktonic cnidarians whose their abundances are mainly shaped by the Gulf circulation. Four oceanographic cruises, covering 93 stations in three different seasons during 2007 and 2008 were carried out to quantify species richness and abundance, as well as to analyse the distribution of these cnidarians. We identified 20 species of hydromedusae and one siphonophore, increasing the total number of hydromedusae for the area to 23. This value is similar to the one found in the abutting Argentine continental shelf (20), but with a different assemblage composition. Hydromedusae abundances found were low, except for a bloom of the Leptomedusa Obelia spp. during the cold season. The only siphonophore found in the area, Pyrostephos vanhoeffeni, has previously been thought to be endemic to Antarctic and sub-Antarctic waters, this being the first record for temperate waters of the Southwest Atlantic Ocean.
Introduction
Global patterns of biodiversity show that species richness generally increases towards lower latitudes (Gaston 2000), including marine planktonic cnidarians (Macpherson 2002). In the South Atlantic Ocean, species richness of Hydromedusae and Siphonophorae increases from the pole to c. 40°S, but further north shows little variation towards the equator (Macpherson 2002). Genzano et al. (2008a) observed a marked decrease in the number of hydromedusan species with increases in latitude in the continental shelf of Argentina and Uruguay (from 33° to 55°S). More locally, there is a positive relationship between cnidarian richness and coastal ecosystems, seasonality and inputs of allochthonous water masses. Gili & Hughes (1995) stated that the number of hydroids species is higher in shallow coastal waters, probably due to their greater environmental heterogeneity, and the number decreases with depth. Seasonality produces a continuous change in the composition of hydroid and medusa populations, which proliferate or stay as resting stages according to the optimal season of each species (Boero 1984; Boero & Bouillon 1993). As regards inputs of allochthonous water masses, gelatinous zooplankton is particularly susceptible to mesoscale advective processes, which tend to increase their density and diversity (Gili et al. 1991; Pagès & Gili 1991).
San Matías Gulf is a coastal ecosystem with a marked seasonality and important intrusions of nutrient rich sub-Antarctic water coming from the adjoining continental shelf (Rivas & Beier 1990; Gagliardini & Rivas 2004; Tonini et al. 2006, 2007). However, before this report, only nine species of planktonic cnidarians have been recorded in the area (Ramírez & Zamponi 1980; Ramírez 1996, 2007; Genzano et al. 2008a; C. Rodriguez unpubl. data). This is a low value compared to the at least 20 species cited for adjacent areas (Genzano et al. 2008a). Further away, given the three factors cited above, we hypothesize that the planktonic cnidarian richness in the Gulf should be even higher at this latitude (40°–42°) in the adjacent continental shelf. Likewise, we expect not just a positive influence of sub-Antarctic inputs into the Gulf on biodiversity and density, but that the oceanography of the area should shape the distribution of cnidarians as well.
To test this hypothesis we comprehensively sampled San Matías Gulf and adjacent waters in three different seasons over 1 year. We analysed planktonic cnidarians biodiversity as number of species, quantified their abundance, and measured and analysed physical parameters (temperature and salinity). Afterwards, we evaluated relationships between these biological and physical parameters and the hydrography of the area as well as the species composition.
Material and Methods
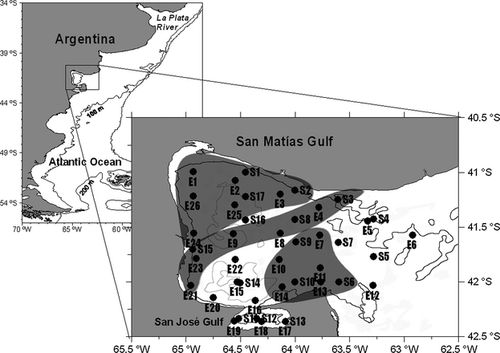
San Matías Gulf is located on the temperate Atlantic coast of South America, between 40°50′–42°15′S and 63°05′–65°10′W (Fig. 1). It is the second largest gulf in Argentina, covering an area of 19,700 km2 (Canessa 1976 in Mazio & Vara 1983), and with maximum depths of over 200 m (Piola & Scasso 1988). Its mouth, which opens to the east onto the Argentine continental shelf, is approximately 100 km wide and has a sill at 70 m depth. Towards the east, the continental shelf is about 80 m deep.
A thermohaline frontal system, situated near 41°50′S, is formed during the austral spring and summer months. This frontal system divides the Gulf into a northwestern sector with warmer and saltier waters, and a southeastern sector with colder and less saline waters with ingress from the Patagonian Coastal Current coming from the south (Gagliardini & Rivas 2004).
The circulation within the Gulf has distinct seasonal variations. During the spring–summer period there is a large and well defined cyclonic gyre in its central area, which limits water exchange between the Gulf and the continental shelf. During the autumn–winter period the Gulf is divided into an anticyclonic gyre in the west coast of the Gulf, intensified by zonal offshore winds, and a poor-defined cyclonic gyre in the rest of the Gulf area. The latter facilitates water exchange between the Gulf and the continental shelf during the cold season. In addition, northerly winds favour upwelling events on the west coast of San Matías Gulf during the cold season (Williams 2004; Tonini et al. 2006; Tonini 2010).
We conducted four oceanographic cruises during June 2007, October 2007, February 2008 and June 2008. The four surveys matched three different seasons throughout the year: late austral autumn (both June dates), spring (October), and summer (February). Surveys were conducted under a long-term oceanographical and biological program to provide information for the management of fishery resources carried out by the IBMPAS and the Centro Nacional Patagónico.
The locations of the 26 sampling stations, which covered the whole San Matías–San José gulf area and the nearest external region of influence, were primarily chosen to assess possible relationships between hydrodynamic processes and the distribution of planktonic cnidarians. During the October survey, only 17 stations were sampled due to limited ship availability; those stations were chosen so that all areas were well represented.
At each station, water temperature and conductivity (c. 3 m depth) were measured with a YSI® 556 multi-parameter probe (precision ± 0.15 °C and ±0.001 mS·cm−1). Salinity was calculated from conductivity and temperature using the probe software ECOWATCH® (YSI Inc., Yellow Springs, OH, USA). Measurements were made at a sampling rate of one scan every 2 s for 1–2 min. Data were processed to achieve sample station averages and standard deviations. The zooplankton sampling methodology (vertical tows) was adopted due to restricted manoeuvrability of the ship. At every station, we sampled the whole water column (from bottom to surface) with a 200-μm-mesh Hensen net of 39 cm mouth diameter, obtaining only one sample that integrated the entire water column. Samples were fixed immediately with 4% formalin–seawater solution.
To quantify planktonic hydrozoan abundance and community composition, the volume of water filtered was calculated from the vertical distance (m) covered by the mouth area of the net, assuming 100% filtration efficiency. Hydromedusae and asexual and sexual stages of siphonophorae were separated from the sample, counted, and identified under a stereo-microscope using mainly the following taxonomic references: Kramp (1959), Bouillon (1999), and Totton (1965). Nectophores of the physonect siphonophore were counted and divided by 10 according to Pugh (1984) to approximate the actual number of colonies sampled. Counts of hydromedusae and siphonophores were standardized to number of individuals per 1000 m3 to obtain the abundances. Small juvenile preserved ctenophores were identified, following Mianzan (1999), as belonging to the order Cydippida because of the round body and presence of tentacle sheaths. However, these data are not presented here because they may underestimate true abundances due to the collection and preservation methods used in this study. The lobate ctenophore Mnemiopsis leidyi A. Agassiz, 1865 also was observed in each survey and manually removed from samples before preservation.
For statistical comparisons the four surveys were fitted into the three seasons by averaging physical and biological data of both Junes (2007 and 2008), thus we have named the three seasons as autumn (2007, 2008), spring and summer. Besides, three different sectors within the Gulf were identified a priori taking into account station locations and the main literature describing the oceanography of the area (Piola & Scasso 1988; Gagliardini & Rivas 2004; Tonini 2010). Of the 26 sampling stations, six were established as the most representative of the north-western (NW) sector, five of the south-eastern (SE) sector and five of the Central sector. In the case of the 17 stations carried out in the October 2007 survey, three stations were stated for each of these three sectors (see Fig. 1). Temperature and salinity data fulfilled normality and variance homogeneity requirements. They were compared among sectors and seasons using two-way analysis of variance (ANOVA) with a factorial design. This design allowed us to compare the effects of each factor and also their interaction over the temperature and salinity data. When interactions were significant Bonferroni pair-wise comparisons were conducted. To evaluate differences among sectors and seasons, abundance and richness data were evaluated using generalized linear mixed models (GLMM); the package ‘lme4’ (Bates et al. 2011) was used with a Poisson error family and a log link (Bolker et al. 2009; Zuur et al. 2009). Since determining abundance involves counting (O'Hara & Kotze 2010), these models allow us to deal with other than normal or Gaussian data distributions. To eliminate the bias due to different sampling units (filtered volume of seawater by the net), we used the filtered volume as an offset inside the model (Penston et al. 2008; Zuur et al. 2009). Spatial autocorrelation of samples (near stations should be more similar than those far away) was eliminated, allowing stations to vary randomly, i.e. using a random intercept and slope for each station (Zuur et al. 2009; Bates et al. 2011). In addition, relationships between hydromedusae community indices (abundance and richness) and environmental variables (temperature and salinity) were evaluated using generalized additive mixed models (GAMM) applying the ‘gamm’ package (Wood 2006) in a similar way as in GLMM: (i) using Poisson family error distribution, with a log link, (ii) allowing the filtered volume to be an offset of the model, and (iii) eliminating the spatial autocorrelation of the sampling stations (Murase et al. 2009). For all models the amount of degrees of freedom for each term was restricted to four (equivalent to a polynomial regression term). In this case, when relationships with temperature were significant, two separate linear (GLMM) regressions were fitted to the data to evaluate the trends within the different ranges of the environmental variable.
Planktonic community structure were compared among season and areas, using the analysis of similarities (ANOSIM) under the ‘vegan’ package (Oksanen et al. 2012). Data were previously standarized to account for differences in variability due to high abundance species (Clarke 1993).
All analyses were done using the free-statistical software R, version 2.15.0 (R Development Core Team 2012).
Results
Hydrography
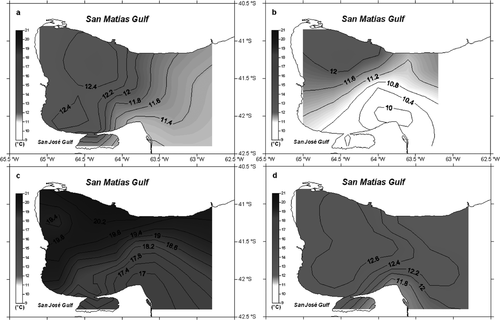
San Matías Gulf sea-surface temperature distributions in autumn were more homogeneous than those in summer and spring (Fig. 2, Table 1). For the three sectors, higher temperatures were recorded during summer, followed by autumn and last by spring (NW: F2 = 1537.0, P-value <0.01; Central: F2 = 1719.9, P-value <0.01; SE: F2 = 193.2; P-value <0.01). Only in the NW sector were autumn and spring temperatures not significantly different. For all seasons the SE sector was significantly colder than the NW one (autumn: F2 = 6.3, P-value <0.01; spring: F2 = 66.8, P-value <0.01; summer: F2 = 27.9, P-value <0.01) (Table 1). The sea-surface salinity distribution showed a consistent pattern for all sampling seasons (no significant differences among seasons), being constantly saltier in the NW sector and less saline in the SE one (autumn: F2 = 19.5, P-value <0.01; spring: F2 = 35.1, P-value <0.01; summer: F2 = 7.4, P-value <0.01). No differences were found between NW and Central sectors.
| Autumn | Spring | Summer | ||||
|---|---|---|---|---|---|---|
| T (°C) | S | T (°C) | S | T (°C) | S | |
| NW | 12.51 ± 0.41 | 34.23 ± 0.09 | 12.20 ± 0.16 | 34.37 ± 0.05 | 19.95 ± 0.48 | 34.24 ± 0.06 |
| Central | 12.37 ± 0.26 | 34.20 ± 0.06 | 11.37 ± 0.22 | 34.34 ± 0.03 | 19.82 ± 0.23 | 34.21 ± 0.13 |
| SE | 12.01 ± 0.50 | 33.98 ± 0.13 | 9.98 ± 0.29 | 33.87 ± 0.13 | 17.56 ± 0.87 | 34.04 ± 0.09 |
Species richness composition
We identified 21 planktonic cnidarian species, 20 hydromedusae and one siphonophore (Table 2). Of the hydromedusa species, nine belonged to the Anthomedusae subclass and 11 to the Leptomedusae. The siphonophore belonged to the order Physonectae. It was found throughout all sampled seasons, although it never occurred in the San José Gulf. Species richness was significantly higher in spring (Z-value = 6.6, P-value <0.01) when 16 species were found, contrasting with the four species found in autumn 2008. In total, 19 species were found in the San Matías Gulf area, and at least seven [Bougainvillia muscus (Allman, 1863); Turritopsis nutricula McCrady, 1857; Proboscidactyla mutabilis; Euphysa aurata Forbes, 1848; Cosmetirella davisi (Browne, 1902); and Clytia and Obelia genera] in the San José Gulf. Only B. muscus appeared exclusively in the San José Gulf. No differences were found among sectors.
| Species | June 2007 | October 2007 | February 2008 | June 2008 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MN | SD | n | D | F | MN | SD | n | D | F | MN | SD | n | D | F | MN | SD | n | D | F | |
| Hydromedusae | 4259 | 11,789 | 2990 | 99.40 | 88.0 | 1760 | 1503 | 1655 | 75.51 | 88.2 | 822 | 1224 | 634 | 98.72 | 92.3 | 130 | 231 | 91 | 83.33 | 48.0 |
| Unidentifiable | 59 | 1.96 | 107 | 4.86 | 264 | 41.03 | 24 | 21.74 | ||||||||||||
| Bougainvillia muscus | – | – | – | – | – | 27 | 79 | 16 | 0.75 | 11.8 | – | – | – | – | – | – | – | – | – | – |
| Turritopsis nutricula | 18 | 49 | 18 | 0.60 | 16.0 | – | – | – | – | – | 7 | 33 | 4 | 0.64 | 3.8 | 15 | 52 | 8 | 7.25 | 8.0 |
| Hydractinia cf. areolata | – | – | – | – | – | 7 | 21 | 16 | 0.75 | 11.8 | 11 | 41 | 12 | 1.92 | 7.7 | – | – | – | – | – |
| Proboscidactyla mutabilis | 155 | 175 | 177 | 5.88 | 60.0 | 514 | 616 | 541 | 24.67 | 70.6 | 54 | 92 | 70 | 10.90 | 34.6 | 63 | 175 | 40 | 36.23 | 24.0 |
| Halitiara formosa | 5 | 25 | 5 | 0.15 | 4.0 | – | – | – | – | – | 19 | 72 | 12 | 1.92 | 7.7 | – | – | – | – | – |
| Corymorpha januarii | – | – | – | – | – | – | – | – | – | – | 5 | 18 | 8 | 1.28 | 7.7 | – | – | – | – | – |
| Sarsia sp. | – | – | – | – | – | 28 | 80 | 25 | 1.12 | 11.8 | 38 | 68 | 29 | 4.49 | 26.9 | – | – | – | – | – |
| Euphysa aurata | – | – | – | – | – | 224 | 367 | 213 | 9.72 | 52.9 | 10 | 52 | 4 | 0.64 | 3.8 | – | – | – | – | – |
| Hybocodon chilensis | 20 | 74 | 14 | 0.45 | 8.0 | 35 | 69 | 41 | 1.87 | 23.5 | – | – | – | – | – | – | – | – | – | – |
| Aequorea coerulescens | – | – | – | – | – | 26 | 77 | 41 | 1.87 | 17.6 | – | – | – | – | – | – | – | – | – | – |
| Laodicea undulata | 4 | 19 | 5 | 0.15 | 4.0 | 98 | 229 | 74 | 3.36 | 35.3 | 166 | 555 | 119 | 18.59 | 19.2 | – | – | – | – | – |
| Eucheilota ventricularis a | 4 | 19 | 5 | 0.15 | 4.0 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Cosmetirella davisi | – | – | – | – | – | 66 | 105 | 57 | 2.62 | 35.3 | – | – | – | – | – | – | – | – | – | – |
| Halopsis ocellata | – | – | – | – | – | 8 | 32 | 16 | 0.75 | 5.9 | – | – | – | – | – | – | – | – | – | – |
| Mitrocomella brownei | 13 | 45 | 14 | 0.45 | 8.0 | 7 | 30 | 8 | 0.37 | 5.9 | 71 | 206 | 58 | 8.97 | 23.1 | – | – | – | – | – |
| Mitrocomella frigida | – | – | – | – | – | 43 | 178 | 57 | 2.69 | 5.9 | – | – | – | – | – | – | – | – | – | – |
| Clytia sp. (young specimens) | 7 | 27 | 9 | 0.30 | 8.0 | 60 | 205 | 33 | 1.50 | 11.8 | – | – | – | – | – | – | – | – | – | – |
| Clytia gracilis a | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 2 | 11 | 4 | 3.62 | 4.0 |
| Clytia lomae | 5 | 24 | 5 | 0.15 | 4.0 | 27 | 63 | 41 | 1.87 | 17.6 | 26 | 88 | 16 | 2.56 | 11.5 | – | – | – | – | – |
| Clytia simplex | – | – | – | – | – | 10 | 29 | 16 | 0.75 | 11.8 | – | – | – | – | – | – | – | – | – | – |
| Obelia spp. | 3973 | 11,772 | 2682 | 89.14 | 36.0 | 458 | 922 | 352 | 16.07 | 70.6 | 63 | 136 | 37 | 5.77 | 26.9 | 29 | 115 | 16 | 14.49 | 8.0 |
| Siphonophorae | 16 | 38 | 18 | 0.60 | 16.0 | 310 | 616 | 537 | 24.49 | 23.5 | 5 | 16 | 8 | 1.28 | 7.7 | 14 | 36 | 18 | 16.67 | 16.0 |
| Pyrostephos vanhoeffeni | 16 | 38 | 18 | 0.60 | 16.0 | 310 | 616 | 537 | 24.49 | 23.5 | 5 | 16 | 8 | 1.28 | 7.7 | 14 | 36 | 18 | 16.67 | 16.0 |
| Total | 4275 | 11,804 | 3008 | 100 | 88.0 | 2069 | 1595 | 2192 | 100 | 88.2 | 827 | 1222 | 642 | 100 | 92.3 | 143 | 237 | 109 | 100 | 52.0 |
- MN = mean abundance (ind. 1000 m−3); SD = standard deviation; n = total abundance (ind. 1000 m−3); D = dominancy (total abundance percentage of one species versus the entire species in total); F = frequency (percentage of samples in which the species occurs).
- a Only a single specimen was found.
Abundance
Hydromedusae numerically dominated the catches, representing between 75.5% and 99.4% of the total abundance (spring and autumn 2007, respectively). Their abundance peaked in autumn 2007 (4259 ± 11,789 ind. 1000 m−3) and gradually decreased until autumn 2008 (130 ± 231 ind. 1000 m−3; Table 2, Fig. 3). This peak was mainly due to the Leptomedusa Obelia spp., which reached maximum abundances of 43,530 ind. 1000 m−3, mostly immature individuals recently liberated from the polyp stage.
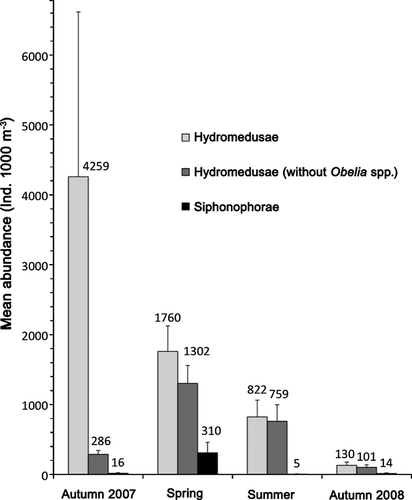
The most frequently occurring species were P. mutabilis and Obelia spp., with frequencies of occurrence of 24–71% and 8–71%, respectively. These were the only species present in every survey; the other species occurred mainly in either one or two surveys (see Table 2). Only one specimen each was found of the species Eucheilota ventricularis McCrady, 1859 and Clytia gracilis (M. Sars, 1850).
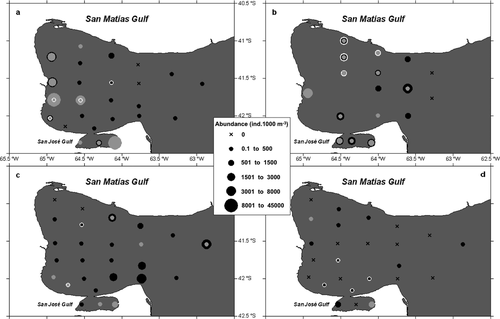
Peak densities of the three most abundant species occurred for Obelia spp. in autumn 2007 (3973 ± 11,772 ind. 1000 m−3), P. mutabilis (Browne, 1902) in spring (514 ± 616 ind. 1000 m−3) and Laodicea undulata (Forbes & Goodsir, 1851) in summer (166 ± 555 ind. 1000 m−3; see Table 2, Figs 4 and 5).
All colony components of the physonect siphonophore Pyrostephos vanhoeffeni Moser 1925 were found. Only ‘adult-like’ nectophores were used for calculating abundance values, which reached the highest mean abundance (310 ± 616 colonies 1000 m−3) and occurrence frequency (24%) in spring. Most of the gastrozoids caught during this season contained prey (e.g. fish eggs and larvae, copepods, and euphausiids) with one or two items per gastrozoid.
Spatio-temporal distributions and relationships with physical variables
As seen in Fig. 3, Obelia bloom abundances greatly influenced the total hydromedusa abundance; therefore, we decided to analyse the total hydromedusa abundance without taking into account Obelia spp. densities (Penston et al. 2008). The highest density of hydromedusae was found in spring (Z-value = 3.0, P-value <0.01), followed by summer and autumn (Z-value = −14.8, P-value <0.01). Only during summer were there significant differences among the three sectors, with the highest abundance in the SE (1204 ± 653 ind. 1000 m−3; Z-value = 9.6, P-value <0.01) and the lowest abundance in the NW (86 ± 41 ind. 1000 m−3; Z-value = 56.6, P-value <0.01). The percentage of explained hydromedusa abundance deviance was 63%.
Obelia spp. was more abundant in the Central sector in autumn (4234 ± 4194 ind. 1000 m−3) and spring (1343 ± 1282 ind. 1000 m−3) than in the other areas; although the differences were not significant. This image is mainly promoted by the bloom that occurred in the west coast stations (see Fig. 4). This species hardly occurred in the SE sector, ranging from 0 in autumn to 41 ± 41 ind. 1000 m−3 in spring (Z-value = −2.1, P-value <0.05). Likewise, Obelia spp. was less abundant during summer than during the rest of the year (Z-value = −96.3, P-value <0.01). The percentage of explained deviance for Obelia spp. abundance was 69%.
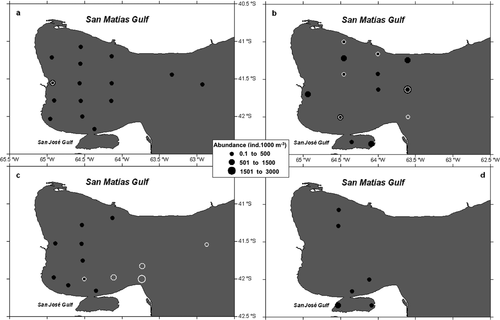
Pyrostephos vanhoeffeni appeared only in the NW sector during spring and in the Central and SE sectors during autumn. Maximum abundance occurred during spring (1366 ± 351 colonies 1000 m−3) and the species barely was present in summer (9 ± 9 ind. 1000 m−3) only present in two distant stations (Fig. 4).
Hydromedusa abundance (without Obelia) showed a negative trend (non-significant) with temperature and a slight positive trend (non-significant) with salinity. In the same way, richness was observed to be inversely correlated with temperature (F2 = 6.51, P-value <0.01) but no appreciable relationship with salinity. As the temperature values measured were clearly differentiated into two ranges (from 9.82 to 12.74 °C for spring and autumn and from 16.49 to 20.30 °C for summer) they were analysed separately. With low explained deviance (12% and 2% for the colder and warmer temperature ranges, respectively) GLMMs showed that hydromedusa richness was always higher in colder waters: Richness = 8.3966 − 0.8514 × Temperature; Z-value = −5.1, P-value <0.01 and Richness = 5.077 − 0.367 × Temperature; Z-value = −2.2, P-value <0.05, respectively. In both cases, these lowest temperatures corresponded to those registered in the SE area.
Analysis of similitude showed non-significant differences in the hydromedusae community composition among sectors and among seasons (Global R = 0.196; P-value >0.05 and 0.111; P-value >0.05, respectively).
Discussion
Our hydrographical data agree with previous descriptions of San Matías Gulf hydrodynamics (Piola & Scasso 1988; Gagliardini & Rivas 2004; Tonini 2010). The Gulf can essentially be divided into a NW sector, with warmer and saltier water, and a colder and less saline SE sector, the differences being more evident in spring, when the thermohaline front starts to become conspicuous, and in summer, when the front is completely developed.
The present work increases the list of planktonic cnidarian species from the San Matías Gulf area by 20 hydromedusae and one siphonophore species. The total number of hydromedusan species reported for the area is therefore now 23, including the 20 from the present study plus two previous records: Dipurena reesi Vannucci, 1956 (Genzano et al. 2008a) and Malagazzia carolinae (Mayer, 1900) (Ramírez 2007) and Olindias sambaquiensis Müller, 1861, collected during other coastal surveys in the northern part of the (E. G. Gulf, pers. obs.). This hydromedusa richness for the area is similar to that of the neighbouring shelf (20 valid species; Table 3), but lower (45 valid species) than records on northern areas of Argentine–Uruguayan shelf (Genzano et al. 2008a). These data agree with the decrease in number of hydromedusan species with increased latitude previously observed in the temperate Southwestern Atlantic Ocean (Genzano et al. 2008a). In fact, San Matías Gulf and surrounding areas might be located where Macpherson (2002) observed a strong decrease in species richness for Hydromedusae and Siphonophorae in the Atlantic Ocean, around 40°S.
| Argentine shelf (40°–44°S) | Sc | Author(s) | Present study |
|---|---|---|---|
| Bougainvillia macloviana | A | Zamponi (1983) | – |
| Bougainvillia muscus | A | Zamponi (1983) | Present |
| Oceania armata a | A | Ramírez & Zamponi (1980); Zamponi (1983) | – |
| Amphinema dinema | A | Zamponi (1983) | – |
| Halitholus intermedius a | A | Zamponi (1983) | – |
| Proboscidactyla mutabilis | A | Genzano et al. (2008a) | Present |
| Moerisia inkermanica a | A | Zamponi (1983) | – |
| Coryne eximia | A | Genzano et al. (2008a) | – |
| Aequorea coerulescens | L | Genzano et al. (2008a) | Present |
| Aequorea forskalea | L | Genzano et al. (2008a) | – |
| Eucheilota ventricularis | L | Genzano et al. (2008a) | Present |
| Laodicea undulata | L | Genzano et al. (2008a) | Present |
| Staurophora mertensii | L | Zamponi (1983) | – |
| Malagazzia carolinae | L | Zamponi (1983) | – |
| Cosmetirella davisi | L | Ramírez & Zamponi (1980); Zamponi (1985) | Present |
| Mitrocomella brownei | L | Genzano et al. (2008a) | Present |
| Mitrocomella frigida | L | Ramírez & Zamponi (1980); Zamponi (1983); Genzano et al. (2008a) | Present |
| Clytia simplex | L | Ramírez & Zamponi (1980); Genzano et al. (2008a) | Present |
| Obelia sp. | L | Ramírez & Zamponi (1980); Zamponi (1983); Genzano et al. (2008a) | Present |
| Obelia longissima | L | Genzano et al. (2008a,b) | Genera present |
| Olindias sambaquiensis | Li | Mianzan (1989) | Present |
| Solmundella bitentaculata | N | Zamponi (1985) | – |
| Halitrephes maasi | T | Ramírez & Zamponi (1980); Zamponi (1983) | – |
| Rhopalonema velatum | T | Zamponi (1983) | – |
- Sc = Hydromedusae subclass; A = Anthomedusae; L = Leptomedusae; Li = Limnomedusae; N = Narcomedusae; T = Trachymedusae.
- a Doubtful species identification (Genzano et al. 2008a; C. Rodriguez, unpubl. data) not taken into account in the calculations.
The aforementioned factors – coastal ecosystem (Gili & Hughes 1995), seasonality (Boero 1984; Boero & Bouillon 1993) and allochthonous water inputs (Gili et al. 1991; Pagès & Gili 1991) – do not appear to be promoting a higher biodiversity in San Matías Gulf compared with the contiguous shelf. Nevertheless, the species composition inside the Gulf is rather different to the one found offshore in the continental shelf (Table 3). Both areas showed high similarity in Limnomedusae (100%) and Leptomedusae (80%), but low coincidence of Anthomedusae (25%) and none of Narco- and Trachymedusae (0%). In the Southwestern Atlantic Ocean, the two latter subclasses mostly have been found in oceanic and shelf break front environments (Genzano et al. 2008a) about 300 miles offshore from the Gulf mouth, which could explain the limited occurrence of oceanic cnidarians along the wide Patagonian continental shelf. It may also be possible that some medusae of these taxa entered the Gulf but failed to survive due to specific inner-Gulf environmental conditions. Carreto et al. (1974), Ramírez (1996) and Williams (2004) defined the seasonal cycle of primary and secondary productivity within the Gulf by short and limited pulses. In fact, Carreto et al. (1974) described the northern sector as an area where nutrient exhaustion prevents continuous primary production. On the other hand, there is an important population of filter-feeding bivalve molluscs, especially in the northern part of the Gulf (Narvarte et al. 2007). These factors, among others, may be limiting food availability in the pelagic realm, meroplanktonic species being strongly favoured over holoplanktonic ones. We think the absence of calycophoran siphonophores in San Matías Gulf might result from factors similar to the ones for the holoplanktonic hydromedusan species.
Oceanographically, San Matías Gulf has been described as an ecosystem with limited communication with the open sea (Piola & Scasso 1988; Rivas & Beier 1990; Gagliardini & Rivas 2004). Latest studies in the area (Tonini et al. 2006, 2007; Tonini 2010) stated that the Gulf inner circulation limits water exchange, especially during the spring–summer period, whereas during the autumn-winter period only, the shallowest western coast keeps more isolated. This marked isolation would support the particular composition of planktonic cnidarians, as has been observed for other zooplankton groups (Ramírez 2007). This phenomenon has been also cited as responsible for the development of local sub-populations in several mollusc species (Narvarte et al. 2007) and for the Argentine hake (Sardella & Timi 2004; Machado Schiaffino et al. 2011), the latter being the major species of the main fishery in San Matías Gulf. The siphonophore Pyrostephos vanhoeffeni, the only holoplanktonic cnidarian found in San Matías Gulf, seems to be entering the Gulf with continental shelf water intrusions and staying inside for variable periods of time. During autumn, when the greater Gulf–shelf connexion takes place, the siphonophore, placed in the SE and Central sectors, could be entering the Gulf with the colder and nutrient richer sub-Antarctic water masses of the Patagonian Coastal Current (Gagliardini & Rivas 2004). During the spring season, then, the siphonophores may be confined in the NW area, trapped by the cyclonic gyre (Tonini et al. 2006, 2007; Tonini 2010). We do not have evidence of P. vanhoeffeni reproductive events inside the Gulf; however, maximum densities were found during spring, when it occupied the main spawning area for hake (Mercado et al. 1993; González et al. 2010), and, as seen in their gastrozoid content, the siphonophore should be favoured because of high prey availability. During summer, when surface temperatures reached over 20 °C, its abundance was scarce and scattered (Fig. 4c). Pyrostephos vanhoeffeni has been described as endemic to Antarctic and sub-Antarctic waters (Moser 1925; Totton 1965; Alvariño et al. 1990); we think this species may have a preference for colder water, with summer temperatures probably causing high mortalities. Actually, P. vanhoeffeni is considered to be one of the few carnivorous gelatinous species well adapted to cold waters that has oleocysts with lipid reserves (Pagès & Schnack-Schiel 1996), which may allow their survival longer than other holoplanktonic hydromedusan and siphonophores in periods of low planktonic food availability in San Matías Gulf.
In general, hydromedusa abundance in San Matías Gulf was lower than that of other coastal ecosystems (Gili et al. 1988; Palma et al. 2007). The exception to that was the Obelia spp. bloom (Figs 3 and 4a) located mainly on the west coast of the Gulf during autumn 2007. Upwelling waters, together with the anticyclonic gyre described for this area during the cold season (Williams 2004; Tonini et al. 2006; Tonini 2010), might have influenced this event due to resuspension of organic matter and the isolation caused by the recirculation. Previous works (Boero et al. 2007) describe Obelia as a microphagous and filter-feeding medusa, at least at the onset of its medusan stage, and there is evidence of hydroid species of the same genus (Obelia geniculata; Orejas et al. 2000) achieving high ingestion rates (113% of the hydranth biomass per day) in upwelling areas. Therefore, in conditions of high productivity in San Matías Gulf, Obelia spp. could produce an explosive increase of the hydroid and medusa population. In fact, strong interannual variability was detected for Obelia longissima medusae, which bloomed during October 2003 in the El Rincón area in the northern Argentine Sea, followed by massive hydroid shoreline accumulations (Genzano et al. 2008b). Following this line of reasoning, Gili et al. (1998) highlighted that some hydrozoan species take full advantage of episodes of temporary high prey availability to feed intensely and, furthermore, that hydro- and scyphomedusan jellyfish blooms might be due to efficient incorporation of planktonic matter by the benthic stages, reflecting their feeding success over the preceding months. This could also explain why this area presented the highest species richness of other meroplankton hydromedusae in that season. The absence of such a bloom the following year during the same period could indicate that the bloom requires rather unusual or very specific conditions to occur.
Reviews such as Haury et al. (1978) and Denman & Powell (1984) attribute the control of spatial heterogeneity of zooplankton to hydrodynamic factors, and the regulation of temporal variability to biological factors. In the case of San Matías Gulf the highest abundances (without Obelia) and richness (see Results section) took place during spring, coinciding with high primary and secondary productivity values (Carreto et al. 1974; Ramírez 1996; Williams 2004). Furthermore, the negative relationship between hydromedusae abundance and richness with temperature in San Matías Gulf shows that hydromedusae are more abundant and rich related to the colder and nutrient rich sub-Antarctic water inputs occurring in the SE sector (Gagliardini & Rivas 2004; Tonini et al. 2007). Therefore, this input may be locally favouring the development of hydromedusa populations, both the medusa stage as well as the polyp, the last growing and increasing or triggering the liberation of medusae (Gili et al. 1988). Because no different hydromedusa species assemblages were detected among sectors, a poor or null contribution of allochthonous species within this water mass into the Gulf seems probable.
In conclusion, we may conclude that San Matías Gulf tends to have a particular composition of planktonic cnidarian species, favouring a meroplanktonic over a holoplanktonic life strategy, and the density and diversity distribution of these species is mainly shaped by hydrodynamic processes of the area.
- the first records of two hydromedusae: Hybocodon chilensis Hartlaub, 1905, a species previously recorded in Chile and New Zealand (Galea 2006), and one species of the genus Hydractinia (Hydractinia cf. areolata Alder, 1862);
- the first record of the siphonophore P. vanhoeffeni in temperate Atlantic waters.
The latter record together with new records for the Argentine continental shelf (E. M. Araujo, unpubl. data) and the expansion of its geographical distribution into the Chilean coast from 41°30′ to 56°S (Palma & Aravena 2001) suggest that P. vanhoeffeni species should not be considered Antarctic and sub-Antarctic endemic.
Acknowledgements
The authors would like to thank Prefectura Naval Argentina, especially the crew of the Coast Guard ships Río Paraná and La Plata. This work was funded by the projects PID 2003#371 and PICT-2006 Start up #1575 (Agencia Nacional de Promoción Científica y Tecnológica); EXA 465/11 (Universidad Nacional de Mar del Plata) and PIP – CONICET 112 201101 00152. E. Guerrero acknowledges financial support from AECI (Agencia Española de Cooperación Internacional-Ministerio Asuntos Exteriores). C. Rodriguez is supported by CONICET fellowship. E. M. Araujo acknowledges the fellowship support by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), Brazil. A. Canepa was funded by CONICYT (PFCHA/Doctorado al Extranjero 4ª Convocatoria, 72120016). The authors wish to thank three anonymous reviewers for their comments, which greatly improved the first manuscript. Special thanks go to Jennifer Purcell for revising the manuscript and for her valuable comments on it. This paper is dedicated to Francesc Pagès who introduced and taught the first author (E.G.) the art of planktonic cnidarians identification as well as he transmitted his enthusiasm for the gelatinous plankton ecology and systematics to many other researchers and friends. The publication of this paper is supported by CONISMA, the Italian National Interuniversity Consortium for Marine Sciences.
Conflicts of Interest
None of the authors have any potential conflict of interest.




