Colony growth of corals transplanted for restoration depends on their site of origin and environmental factors
Abstract
We determined that growth differences among coral fragments transplanted for restoration were influenced by both source population and environmental factors. In two common garden experiments, storm-generated fragments of Acropora palmata were transplanted from two source populations in the British Virgin Islands to a restoration site (a ‘common garden’) that lacked A. palmata. In the first experiment, colonies from different sources grew at different rates in the first year after transplanting, suggesting either genetic differences among source populations or enduring acclimation to conditions at the source site. No differences in growth among source populations were detected in the second common garden experiment. To isolate environmental effects on growth, we subdivided fragments from three source populations to create genetically identical pieces that were attached separately at both source and restoration sites. Genetically identical pieces from all source populations grew slightly faster at their source than at the restoration site, implying a subtle home-site advantage. Overall, our results suggest that matching environmental conditions at source and restoration sites may increase the success of restoration projects.
Introduction
Corals are in decline worldwide (Gardner et al. 2003; Bellwood et al. 2004), and transplanting colonies from surviving populations to sites where species have become rare or eliminated is gaining popularity as a restoration method (Yap 2000; Rinkevich 2005; Precht 2006). The success of these restoration efforts depends partly on whether characteristics of source populations influence the performance of corals after they are transplanted (Shearer et al. 2009). Local adaptation might result in poor performance of individuals transplanted to a restoration site from elsewhere (Baums 2008). Mixing local and foreign genotypes could also have deleterious effects due to outbreeding depression or genetic swamping (Hufford & Mazer 2003). Conversely, translocating a range of genotypes might provide a reservoir of genetic variation, allowing the restored population to respond positively to future perturbations (Clewell & Rieger 1997). Effects of these processes on plant and seagrass restoration are beginning to be revealed (Hufford & Mazer 2003; Menges 2008) and there is potential for similar effects on the outcome of coral restoration efforts (Baums 2008). For example, reciprocal transplant and common garden experiments indicate that coral growth, mortality (Potts 1984; Raymundo 2001; Bowden-Kerby 2008; Smith et al. 2008), skeletal characteristics (Smith et al. 2007) and morphology (Foster 1979; Bruno & Edmunds 1997; Bowden-Kerby 2008) can differ among populations in a manner consistent with a mix of genetic differentiation and environmental effects.
We studied Acropora palmata (the Elkhorn coral), a major reef-building coral in the Caribbean that was formerly the dominant species in shallow wave-exposed areas (Goreau 1959). During the 1980s and 1990s, A. palmata declined in abundance by 85–98% and localized extirpations have occurred (Precht et al. 2004), making it a priority candidate for restoration. Acropora palmata reproduces both asexually and sexually. Asexual propagation occurs when branches break off in storms and then reattach to the substratum to form new colonies (Bak & Engel 1979; Dunne & Brown 1980; Highsmith et al. 1980; Fong & Lirman 1995). Broken fragments of A. palmata have been translocated in a number of restoration projects (Bruckner & Bruckner 2001; Garrison & Ward 2008; Williams & Miller 2010; Forrester et al. 2011). Acropora palmata reproduces sexually by the synchronized release of gametes into the water column once a year (Szmant 1986). The resulting larvae are planktonic for up to 20 days, suggesting the potential for wide dispersal. However, extant populations can show genotypic variation within a site (<100 m in extent) so there is potential for transplanting to mix novel combinations of genotypes (Baums et al. 2006; Reyes & Schizas 2010). Genotypes vary in factors that are likely to affect their transplant success, such as susceptibility to bleaching (Edmunds 1994) and disease resistance (Vollmer & Kline 2008), so there is also potential for local adaptation despite gene flow.
Several researchers have argued that restoration projects should be designed as ‘common garden’ or reciprocal transplant experiments to identify population and environmental influences on the growth of transplanted corals (Clewell & Rieger 1997; Rinkevich 2005; Baums 2008). The classic method of determining whether observed differences among populations are genetically based is the common garden study (Turesson 1922). To isolate differences among source populations that might affect growth, we performed two common garden experiments, in which storm-generated fragments were transplanted from different source populations to a single restoration site. Reciprocal transplant experiments are the most informative design to separate genetically based effects of population of origin from environmental effects due to local conditions (Hufford & Mazer 2003). However, they are not compatible with restoration projects when the focal species is extinct at the restoration site. Local extinction can be both the motivation for selecting a site for restoration and the reason why transplants cannot be reciprocal; individuals can be transplanted into the restoration site but none are available to transplant out. As an alternative test for environmental effects on growth, compatible with the goal of restoring a depopulated site, we performed experiments in which fragments were collected from source populations and split to create two genetically identical sub-fragments. One sub-fragment was then attached at its site of origin and the other at the restoration site, so any site-specific differences in growth can likely be attributed to the local environment.
Material and Methods
Study sites
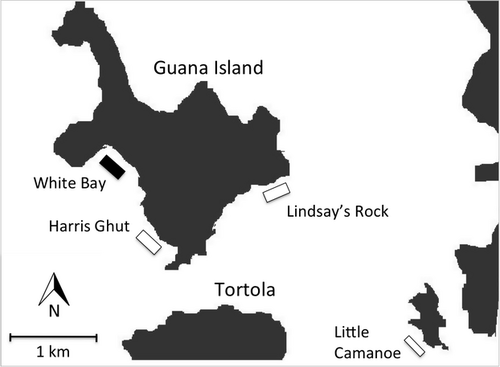
The common restoration site, White Bay, is a set of patch reefs on the leeward south side of Guana Island. Acropora palmata has been absent from White Bay for at least 19 years but, based on the presence of skeletal remains and anecdotal reports, it was present in the 1970s and 1980s (Forrester et al. 2011). Storm-generated A. palmata fragments were collected for restoration from three source populations in the British Virgin Islands (Fig. 1) (18°29′ N, 64°35′ W). Our objective was not to isolate the effect of specific environmental factors, but we selected source sites that differed from White Bay in one or more of the following conditions under normal conditions: depth, tidal flow, water clarity and wave exposure (Table 1). We selected sites differing in these conditions because they are known to influence coral growth by altering light availability, sedimentation rates, and the delivery of particulate food.
| Depth (range in m) | Max. tidal velocity (range in m·s−1) | Water clarity (range of visibility in m) | Wave exposure (relative ranking) | |
|---|---|---|---|---|
| Source sites | ||||
| Lindsay's Rock | 1.6–3.2 | 0.2–04 | 6–9 | Exposed |
| Harris Ghut | 2.5–6.4 | <0.1 | 5–8 | Protected |
| Little Camanoe | 0.6–1.6 | 0.3–0.6 | 8–10 | Intermediate |
| Restoration site | ||||
| White Bay | 0.4–1.6 | <0.1 | 5–8 | Protected |
Common garden experiments: is there variation in growth among source populations?
To test for differences in growth among source populations, we performed two common garden experiments. For the first experiment, fragments collected in 2007 from Harris Ghut (n = 31) and Lindsay's Rock (n = 7) were transplanted to White Bay. For the second experiment, fragments collected in 2010 from Harris Ghut (n = 45) and Little Camanoe (n = 23) were relocated to White Bay.
All fragments were transplanted between late July and mid-August. Divers located fragments at the source sites and brought them to the surface. Each piece was then submerged in a bin of fresh seawater on a boat and taken to the restoration site. Before corals were attached to the reef, we secured a plastic identification tag nearby and scraped the attachment site with a wire brush to remove some of the macroalgae. In 2007, fragments were attached to the reef using either cable ties or marine epoxy (Z-spar A788 splash zone compound; Carboline Company, St. Louis, MO, USA). Cable ties were used to affix fragments to projections on the reef, mostly standing dead Acropora palmata skeletons, and epoxy was used where the reef surface was flat. The growth of A. palmata transplanted using these methods is indistinguishable (Williams & Miller 2010; Forrester et al. 2011) but, to avoid confounding attachment method with the other treatments the use of epoxy and cable ties was equalized among sites, and we tested for effects of attachment method in our analysis. In 2010, only cable ties were used to attach fragments.
Because a coral colony is a collection of genetically identical modules (polyps) growing in a single layer, colony surface area is a good measure of its size (Hughes 1984). We estimated the surface area of live tissue using the leaf area index (LAI) (Williams & Miller 2010). To estimate LAI, we took photographs of each major surface (Bythell et al. 2001). We placed a ruler in the frame to provide a scale, used image analysis software (IMAGEJ, National Institutes of Heath, Washington DC, USA) to measure the area of each surface, and then summed the areas to get the LAI for the entire fragment (Abramoff et al. 2004). LAI was measured immediately after transplanting, after 3 months and again after 12 months. We used the % change in LAI after 12 months as an index that captures colony growth (recorded as a positive change), shrinkage or partial mortality (recorded as a negative change) and death (a change of −100%). We used a separate analysis of covariance (ANCOVA) for each experiment to test for differences among source populations (a categorical variable). We also included terms in the model for initial fragment size (a covariate) and attachment method (cable tie or epoxy: a categorical variable) to account for their possible effects on colony growth.
Split-fragment experiments: are there environmental effects on growth?
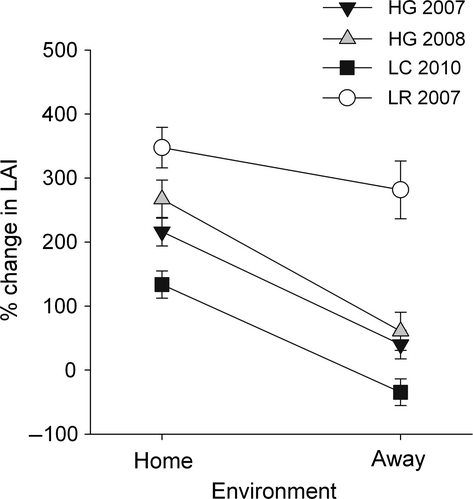
To isolate environmental effects on growth we performed experiments in which fragments were divided into two sub-fragments. One sub-fragment was reattached at its site of origin (home) and the other was transplanted to White Bay (away). Because paired sub-fragments are genetically identical, a difference between those growing ‘home’ and ‘away’ would indicate a phenotypic response to local conditions. We tested four different source groups of fragments, differing in when and where they were collected: (i) Harris Ghut 2007 (n = 28), (ii) Harris Ghut 2008 (n = 16), (iii) Lindsay's Rock 2007 (n = 14), (iv) Little Camanoe 2010 (n = 32).
Methods for this experiment were as described for the common garden studies, with two additions. First, after each fragment was collected, it was split under water using a hammer and chisel to produce two sub-fragments. Secondly, to keep the average amount of time fragments were kept in the boat roughly equal, ‘home’ fragments were sometimes transplanted first, whereas on other occasions the ‘away’ fragments were transplanted first. The mean time held in the boat (home: n = 10 collections, mean ± SD = 0.6 ± 0.5 h; away: n = 11 collections, mean ± SD = 0.7 ± 0.3 h) did not differ significantlybetween the two groups (t-test: df = 19, t = 0.69, P = 0.50).
The data were analyzed using an ANCOVA model. Our primary interest was in the effect of environment (home versus away: a categorical variable). To account for other sources of variation in growth, we also tested for differences between source groups (a categorical variable) and fragments (a categorical variable: nested within source group), and for the interaction between source group and environment. We also included a term in the model for initial fragment size (a covariate).
Results
Common garden experiments: variation in growth among source populations
In the 2007 garden experiment, there was no significant difference in colony growth between fragments originating from Lindsay's Rock and Harris Ghut (ANCOVA: F1,34 = 2.07, P = 0.16; Fig. 2). There was also no indication of bias introduced by effects of initial colony (ANCOVA: F1,34 = 2.03, P = 0.16) size or the method used to attach fragments to the reef (ANCOVA: F1,34 = 1.78, P = 0.19).
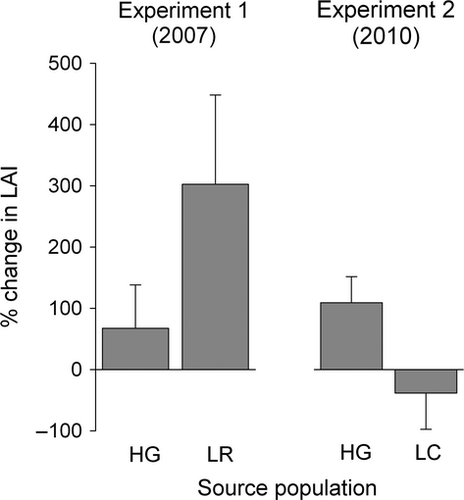
In contrast, the 2010 common garden experiment revealed a significant effect of source population on colony growth (ANCOVA: F1,65 = 4.08, P = 0.047). After a year in the common garden, fragments originating from Harris Ghut exhibited significantly better colony growth than those from Little Camanoe (Fig. 2). As in 2007, there was no detectable effect of initial fragment size on colony growth (ANCOVA: F1,65 = 1.32, P = 0.25).
Split-fragment experiments: environmental effects on growth
To isolate environmental effects (home versus away) on colony growth we needed to account for possible effects of initial size, attachment method and differences between fragments. None of these factors, however, had a significant influence on colony growth (Table 2). Fragments from the four source groups differed significantly in growth rate (Table 2, Fig. 3). Most importantly, though, when fragments were divided in two to test for phenotypic plasticity, the piece attached at the source site (home) grew significantly faster than the piece attached at in White Bay (away) (Table 2, Fig. 3). There was no significant interaction between the effect of environment and source group, indicating that the pattern of faster growth at the home site was consistent across source groups (Table 2, Fig. 3).
| Source | SS | df | MS | F | P |
|---|---|---|---|---|---|
| Environment | 451,363 | 1 | 451,363 | 16.21 | <0.0004 |
| Source group | 693,918 | 3 | 231,306 | 8.30 | <0.0004 |
| Environment × Source group | 44,254 | 3 | 14,751 | 0.53 | 0.664 |
| Fragment (source group) | 1,373,978 | 41 | 33,522 | 1.20 | 0.280 |
| Initial fragment size | 13,788 | 1 | 13,788 | 0.49 | 0.502 |
| Attachment method | 12,329 | 1 | 12,329 | 0.44 | 0.592 |
| Error | 1,113,754 | 38 | 27,843 |
Discussion
We cannot be certain why coral growth rates differed significantly in the 2010 common garden experiment but not in the 2007 experiment. Two obvious possibilities are the small sample size and limited statistical power of the 2007 experiment or simply that we used different source populations each year. The fact that, in 2010, growth differences based on source were detectable after 1 year of growth at the restoration site (common garden) has one of two possible causes. First, the differences might reflect genotypic differences among source populations (Bowden-Kerby 2008). A second possibility is that fragments were all the same genotype but were acclimated to conditions at their source site and 1 year was not sufficient time to express a phenotypic response to new conditions at the restoration site. We consider this second possibility very unlikely because our experiments with genetically identical subdivided fragments clearly demonstrated that a year was sufficient time for a phenotypic response to differences in conditions between the source and restoration sites. Moreover, 1 year was also sufficient time for another coral species, Madracis mirabilis, to express a phenotypic response to a change in conditions (Bruno & Edmunds 1997). The most parsimonious interpretation of the combined results of the 2010 common garden experiment and the split-fragment experiments is, therefore, that colony growth of Acropora palmata at the restoration site was influenced both by local conditions and by the source population from which fragments were drawn.
Although no comparable studies have been performed in the context of coral restoration, several reciprocal transplant experiments have examined genetic and environmental effects on coral colony growth. Consistent with our results, effects of both source population and environment on growth were reported for Acropora palifera, Acropora cuneata and Porites attenuata, although environmental effects were of greater magnitude for the two acroporids (Potts 1984; Raymundo 2001). In contrast, Pocillopora eydouxi showed phenotypic plasticity in response to local conditions, but no differences in growth among source populations. Lastly, neither environmental nor genetically based differences in growth were detected in a reciprocal transplant study of Porites lobata (Smith et al. 2008). Although the importance of these effects is thus likely to be species- and context-dependent, a practical implication of our results is that matching environmental conditions at source and restoration sites may increase the success of A. palmata restoration projects.
We did not closely monitor differences in conditions between home and away sites during the split-fragment studies, and it was not our intent to isolate the effects of specific environmental factors on coral growth. The reasons for the consistent home site advantage are thus not clear, but they may reflect chronic differences among sites such as depth, flow rates or sedimentation that are well known to influence coral growth (Sheppard et al. 2010). It may thus be worthwhile in future to test the effects of matching source and restoration sites using specific criteria, particularly because our results illustrate how, in some cases, the mis-match can result in poor performance of the transplants. Although coral fragments from Harris Ghut and Lindsay's Rock displayed positive growth when transplanted, it was striking that coral fragments from Little Camanoe experienced net tissue loss after 1 year. If Little Camanoe were the only source site, the restoration would hardly be considered successful. Future experiments could compare restoration success using source sites that differ systematically in one factor (e.g. flow rate) but are similar in other regards, in order to determine which factors are the most important to match.
Although A. palmata populations can show genetic differentiation among local populations (Baums et al. 2006; Reyes & Schizas 2010), it was nonetheless surprising that the 2010 common garden experiment showed inherent differences in colony growth between source populations separated by less than 4 km. This finding provides empirical support for the hypothesis that the choice of source population may influence the outcome of coral restoration projects (Baums 2008). Like most transplant studies, we measured the performance of the overall holobiont (the coral animal, its endosymbiotic zooxanthellae, and other associated microorganisms). Acropora palmata tends to associate with just one clade of zooxanthellae (Goulet 2006; Thornhill et al. 2006; Baker & Romanski 2007). In P. eydouxi, however, zooxanthellae types differed among source populations used in a reciprocal transplant study. In this case, between-population differences in zooxanthellae genotypes had no effect on colony growth after transplanting (Smith et al. 2007). It will be valuable to isolate further the heritable contribution of each component of the holobiont to the performance of transplanted corals (Baums et al. 2010). In addition, it will be important to identify the mechanisms underlying the success of transplanted corals by isolating the relative effects of local adaptation, founder effects, heterosis and outbreeding depression, and genetic swamping (Hufford & Mazer 2003).
The experimental design we used is a good compromise between achieving scientific and conservation objectives because its allows the testing of environmental and population influences on coral performance while still concentrating most effort on the relocation of corals to a restoration site (Raymundo 2001). This approach may thus be a useful component of future work exploring environmental and genetic influences on the success of restoration projects.
Acknowledgements
For help with field work we thank Fiona Forrester, Katherine Forrester, Linda Forrester, Sandra Giovannini, Lindsay Harmon, Rebecca Karis and Jason Krumholz. We thank Lianna Jarecki and the staff on Guana Island for valuable logistical support. Erinn Mueller and two anonymous reviewers provided helpful comments on an earlier draft of the manuscript. Financial support from the Falconwood Foundation, National Science Foundation (OCE 0222087), and the University of Rhode Island (Coastal Fellowship Program, Cobb Endowment Independent Study Award, and Undergraduate Research Initiative Award) is gratefully acknowledged.




