STING Activation in Various Cell Types in Metabolic Dysfunction-Associated Steatotic Liver Disease
Funding: This work was supported by the National Natural Science Foundation of China (81673750); 2023Pudong New Area Traditional Chinese Medicine Summit Discipline (YC-2023-0602); 2025 Pudong New Area's Peak and Plateau Discipline Development in Clinical Medicine for Novel and Special Diseases (2025-PWXZ-15); ‘Famous Traditional Chinese Medicine’ Talent Training Plan of the Seventh People's Hospital affiliated to Shanghai University of Traditional Chinese Medicine (MZY2021-01); ‘Medical Craftsman’ Talent Training Plan of the Seventh People's Hospital affiliated to Shanghai University of Traditional Chinese Medicine (GJ2021-06); Pudong New Area Traditional Chinese Medicine Brand Multiplication Plan—Chronic Nephropathy (PDZY-2021-0302); Construction of He Liqun's famous TCM studio (PDZY-2022-0703).
JingJing Wang and Yue Guo contributed equally to this study.
Handling Editor: Dr. Luca Valenti
ABSTRACT
Background
During the hepatic histological progression in metabolic dysfunction-associated steatotic liver disease (MASLD), the immunological mechanisms play a the pivotal role, especially when progressing to metabolic dysfunction-associated steatohepatitis (MASH). The discovery of the stimulator of interferon genes (STING) marked a significant advancement in understanding the immune system.
Methods
We searched literature on STING involved in MASLD in PubMed to summarise the role of intrahepatic or extrahepatic STING signal pathways and the potential agonists or inhibitors of STING in MASLD.
Results
Besides inflammation and type I interferon response induced by STING activation in the intrahepatic or extrahepatic immune cells, STING activation in hepatocytes leads to protein aggregates and lipid deposition. STING activation in hepatic macrophages inhibits autophagy in hepatocytes and promotes hepatic stellate cells (HSCs) activation. STING activation in HSCs promotes HSC activation and exacerbates liver sinusoidal endothelial cells (LSECs) impairment. However, it was also reported that STING activation in hepatic macrophages promotes lipophagy in hepatocytes and STING activation in HSCs leads to HSC senescence. STING activation in LSEC, inhibits angiogenesis. For extrahepatic tissue, STING signalling participates in the regulation of the intestinal permeability, intestinal microecology and insulin action in adipocytes, which were all involved in the pathogenesis of MASLD.
Conclusion
There're plenty of STING ligands in MASLD. How STING activation affects the intercellular conversation in MASLD deserves thorough investigation.
Abbreviations
-
- AKT
-
- protein kinase B kinase
-
- AMP
-
- adenosine monophosphate
-
- AMPK
-
- AMP-activated protein kinase
-
- ATG
-
- autophagy-related protein
-
- cGAMP
-
- cyclic GMP-AMP
-
- cGAS
-
- cyclic GMP-AMP synthase
-
- COP II
-
- coat protein complex II
-
- CREB
-
- cAMP response element binding
-
- DMXAA
-
- 5,6-dimethylxanthenone-4-acetic acid
-
- ER
-
- endoplasmic reticulum
-
- ERGIC
-
- ER-Golgi intermediate compartment
-
- FADS2
-
- fatty acid desaturase 2
-
- GMP
-
- guanosine monophosphate
-
- HFD
-
- high-fat diet
-
- HSC
-
- hepatic stellate cell
-
- IFN
-
- interferon
-
- IKK
-
- IκB kinase
-
- IRF3
-
- interferon regulatory factor 3
-
- IκB
-
- inhibitor of NF-κB
-
- JNK
-
- c-Jun N-terminal kinases
-
- LC3
-
- microtubule-associated protein 1 light chain 3
-
- LSEC
-
- liver sinusoidal endothelial cell
-
- MAPK
-
- mitogen-activated protein kinase
-
- MASH
-
- metabolic dysfunction-associated steatohepatitis
-
- MASLD
-
- metabolic dysfunction-associated steatotic liver disease
-
- MCD
-
- methionine- and choline-deficient
-
- mEV
-
- gut microbial DNA-containing extracellular vesicle
-
- NF-κB
-
- nuclear factor kappaB
-
- NICD
-
- NOTCH1 intracellular domain
-
- NLRP3
-
- NOD-like receptor family pyrin domain-containing 3
-
- NOTCH1
-
- notch receptor 1
-
- PERK
-
- protein kinase RNA-like endoplasmic reticulum kinase
-
- SCAP
-
- SREBP cleavage-activating protein
-
- SMA
-
- smooth muscle actin
-
- SREBP
-
- sterol regulatory element-binding protein
-
- STING
-
- stimulator of interferon genes
-
- TBK1
-
- TANK-binding kinase 1
-
- TGF-β
-
- transforming growth factor-β
-
- TRAF
-
- tumour necrosis factor receptor-associated factor
-
- TRIM
-
- tripartite motif
-
- ULK
-
- Unc51-like kinase
-
- UPR
-
- unfolded protein response
-
- WIPI
-
- WD repeat domain phosphoinositide-interacting protein
-
- YAP
-
- yes-associated protein
Summary
- Besides inflammation and type I interferon response induced by STING activation in the intrahepatic or extraphepatic immune cells, STING activation in hepatic macrophages inhibits autophagy in hepatocytes and promotes HSC activation.
- STING activation in hepatocytes leads to protein aggregates and lipid deposition.
- STING activation in HSCs promotes HSC activation and exacerbates LSEC impairment.
- STING activation in LSEC inhibits angiogenesis.
- The reported beneficial effects of STING in MASLD include STING activation in hepatic macrophages promoting lipophagy in hepatocytes and STING activation in HSCs leading to HSC senescence.
1 Introduction
Metabolic dysfunction-associated steatotic liver disease (MASLD) is a chronic liver condition linked with metabolic syndrome and not attributable to alcohol consumption. The histology of MASLD is characterised by widespread hepatic steatosis and inflammation, evolving from simple steatosis to metabolic dysfunction-associated steatohepatitis (MASH), which may progress to MASH-related fibrosis, cirrhosis, and ultimately hepatocellular carcinoma. MASLD has become the most common chronic liver disease [1]. Recently, resmetirom has been approved for the treatment of noncirrhotic MASH [2], but its safety of long-term use still needs to be evaluated.
Growing evidence has supported that immune cell-mediated inflammatory processes are involved in the pathogenesis and progression of MASLD. The interaction among immune cells, hepatocytes, hepatic stellate cells (HSCs) and liver sinusoidal endothelial cells (LSECs) intensifies hepatic lipid accumulation, inflammation, fibrosis and even hepatocellular carcinoma [3]. The discovery of the stimulator of interferon genes (STING) marked a significant advancement in understanding the immune system [4]. In this review, we summarised the latest advancements in the role of STING signalling pathways in MASLD, including STING in various liver cell types and the extrahepatic STING, and the potential agonists and inhibitors of STING reported in MASLD. Since the role of STING in cancer has been well summarised in other review articles [5], the studies on the roles of STING in hepatocellular carcinoma were excluded from this article.
2 The Molecular Structure of STING
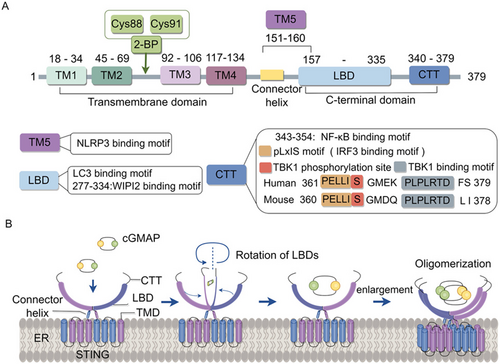
STING is a conserved transmembrane protein consisting of 379 amino acids and situated in the endoplasmic reticulum (ER). It is primarily composed of a short N-terminal cytoplasmic segment, a four-span transmembrane domain localising to the ER, and a C-terminal domain facing the cytosol. The C-terminal domain includes the ligand-binding domain with the C-terminal tail appended. In a resting state, STING mainly exists as a symmetrical dimer in the ER and the ligand-binding domain (contains four α-helices and five β-strands) of STING generates a V-shaped ligand-binding pocket to accommodate cyclic guanosine monophosphate (GMP)-adenosine monophosphate (AMP) (cGAMP) or other ligands [6]. Besides, a synthetic human STING agonist C53 was revealed to bind to the transmembrane domain of STING, suggesting the second ligand pocket in STING [7]. The C-terminal tail of STING harbours conserved motifs, including the PLPLRT/SD motif responsible for binding to TANK-binding kinase 1 (TBK1) [8] and pLxIS motif responsible for binding to interferon regulatory factor 3 (IRF3) upon phosphorylation [9] (Figure 1).
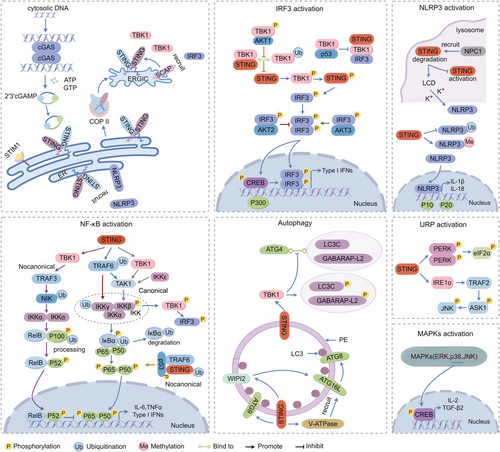
3 Signalling Mediated via STING (Figure 2)
3.1 STING-IRF3 Pathway
STING-TBK1-IRF3 signalling is the classic STING activation pathway. When the cytosolic DNA is detected by cyclic GMP-AMP synthase (cGAS), the second messenger, noncanonical 2′-3′cGAMP, is produced. cGAMP binds to STING, triggering STING oligomerisation, which disrupts its association with stromal interaction molecule 1 [10] (an ER retention protein) and enhances its interaction with the cytoplasmic coat protein complex II (COP II, exporting newly synthesised proteins from ER) [11]. This process allows the oligomerised STING transition through ER-Golgi intermediate compartment (ERGIC) into the Golgi apparatus, where the two Cys88/91 residues on STING undergo palmitoylation [12, 13]. Sterol regulatory element-binding protein (SREBP) cleavage-activating protein (SCAP) was identified to interact directly with STING and serve as a scaffold adaptor to recruit IRF3 to the perinuclear Golgi foci [14]. The STING oligomeric platform brings together multiple TBK1 dimers, facilitating the trans-autophosphorylation near the Ser172 of TBK1 [8]. Once being catalytically activated, the TBK1 molecules then phosphorylate the pLxIS motif of adjacent STING dimers at Ser366 not the STING dimers they bounding to. The N-terminal of the C-terminal tail within IRF3 accommodates the phosphorylated pLxIS motif in STING and another IRF3's cLxIS motif [15]. In this way, IRF3 is recruited to the STING oligomers, forming dimers and being phosphorylated within the cLxIS motif by TBK1 [6]. Phosphorylated IRF3 dimers translocate into the nucleus to induce Type I interferon (IFN), facilitated by cAMP response element-binding (CREB) protein/p300 coactivators [16].
Protein kinase B (AKT) kinase family (AKT1, AKT2, AKT3) was found to be involved in STING-IRF3 signalling. AKT1 directly phosphorylates TBK1, preventing the association of TBK1-STING and TBK1 K63-linked ubiquitination, and ultimately attenuating STING signalling [17]. AKT2 negatively regulates type I IFN expression by phosphorylating IRF3 at Thr207 and inhibiting its nuclear translocation [18]. AKT3 promotes the activation of IRF3 by phosphorylating the Ser385 residue [19]. The oncogenic mutant p53 was also found to bind to TBK1, preventing the STING-TBK1-IRF3 complex formation, resulting in immune evasion [20].
3.2 STING- Nuclear Factor KappaB (NF-κB) Pathway
NF-κB is also observed to be activated by stimulation with the ligand or agonist of STING, resulting in the production of cytokines and type I IFN [21]. Nonetheless, the precise mechanism is not entirely understood.
The mammalian NF-κB family consists of NF-kB1 (p105/p50), NF-kB2 (p100/p52), RelA (p65), RelB and c-Rel. The activation of NF-κB involves canonical and noncanonical signalling pathways.
The canonical NF-κB activation begins with the inducible degradation of the inhibitor of NF-κB (IκB) triggered through its site-specific phosphorylation by IκB kinase (IKK) complex. IKK is composed of two catalytic subunits, IKKα and IKKβ, and a regulatory subunit, IKKγ. Upon activation, IKK phosphorylates IκBα at two N-terminal serines, triggering ubiquitin-dependent IκBα degradation in the proteasome, resulting in rapid and transient nuclear translocation of canonical NF-κB members, predominantly the p50/RelA and p50/c-Rel dimers [22]. IKKγ, IKKβ and RelA were demonstrated to be crucial for the NF-κB activation in the cGAS-STING pathway, and IKKγ is necessary for the full activation of TBK1 and IRF3 [23]. The amino acid 343–354 of the C-terminal tail in STING is identified as a minimal functional subdomain mediating NF-κB activation independent of TBK1 and IRF3 binding [24]. The canonical IKK activation begins with tumour necrosis factor receptors-associated factor (TRAF) oligomerisation and activation of their ubiquitin-ligase activity, leading to IKKγ-K63-linked ubiquitin chain interaction and K63 polyubiquitination of TRAFs themselves [25]. Ubiquitinated TRAFs promote transforming growth factor-β (TGF-β)-activated kinase 1 activation that phosphorylates IKKβ, resulting in IKK activation [25]. TRAF6 K63-linked ubiquitin chains were reported to be essential for IKKγ activation [25]. E3 ligases tripartite motif (TRIM) 32 and TRIM56 are required for cGAS-STING-mediated IKKγ activation via synthesising ubiquitin chains that bind to IKKγ to activate NF-kB [23]. In STING-deficient cells, IKKγ was not found to bind ubiquitin chains after dsDNA stimulation [23], indicating that cGAS-STING activation is the prerequisite for IKKγ-ubiquitin chain interaction. STING's C-terminal tail module was reported to directly recruit TRAF6 to activate NF-κB Signalling [26]. These results confirmed that TRAF6-IKKγ-NF-κB activation works downstream of cGAS-STING activation. However, a recent study reported that TGF-β-activated kinase 1 and IKKβ, but not TRAF6, are required for STING-NF-κB activation [27]. TBK1 [28] and its homologue IKKi (IKKϵ) [29] were identified as the two IKKβ-related kinases. A recent study demonstrates that they act redundantly to mediate STING-induced NF-κB activation in myeloid cells [27]. In a noncanonical activation of STING independent of cGAS, the assembly of an alternative STING signalling complex involves the tumour suppressor p53 and TRAF6. TRAF6 catalyses the formation of K63-linked ubiquitin chains on STING, leading to the activation of NF-κB and the induction of an alternative STING-dependent gene expression program [30].
The noncanonical NF-κB activation begins with the activation of IKKα by NF-κB-inducing kinase to mediate p100 phosphorylation, and in turn induces p100 ubiquitination and degradation of its C-terminal IκB-like structure, resulting in the generation of mature NF-κB2 p52 and nuclear translocation of the noncanonical NF-κB complex p52/RelB [22]. In radiotherapy, noncanonical NF-κB signalling was found to be activated by the STING-TBK1 axis in dendritic cells, and antagonised ionising radiation-induced antitumour effects by suppressing recruitment of the RelA onto the IFN-β promoter [31]. TRAF3 participates in the noncanonical NF-κB signalling activated by the STING-TBK1 axis by acting as the E3 ubiquitin ligase to degrade NF-κB-inducing kinase to induce phosphorylation-dependent ubiquitination and processing of p100 [32].
3.3 STING-Mitogen-Activated Protein Kinases (MAPKs) Pathway
With stimulation of STING ligands, the MAPKs including extracellular signal-regulated kinases 1 and 2, c-Jun N-terminal kinases (JNK), and p38 were observed to be activated independently of TBK1 [21]. The translocation of STING from the ER to the Golgi activates MAPK p38 activity, which subsequently triggers the transcription factor CREB activation. The activation of the STING-MAPK-CREB signalling pathway induces the expression of many cytokine genes, including interleukin-2 and TGF-β2, to promote the regulatory T-cell differentiation [33].
3.4 STING-Unfolded Protein Response (UPR) Signalling Pathway
STING is also found to be required for the ER stress response. In some cancer cells (including triple-negative breast cancer, colorectal adenocarcinoma and melanoma), the tunicamycin-stimulated ER stress signalling was blunted by STING knocking out [34]. In cecal ligation and puncture-induced sepsis, ER stress was markedly blocked by STING deletion [35].
UPR is triggered by three key sensors: inositol-requiring enzyme 1, protein kinase RNA-like endoplasmic reticulum kinase (PERK) and activating transcription factor 6 [36]. It was demonstrated that the STING-dependent inositol-requiring enzyme 1α/TRAF2/apoptosis signal-regulating kinase 1/JNK signalling pathway exerts neuroinflammation and neuronal death in traumatic brain injury [37]. STING was reported to mediate the PERK-eukaryotic initiation factor-2α pathway to enable an innate immunity control of cap-dependent messenger RNA translation. Upon cGAMP binding, STING at the ER binds and directly activates the ER-located kinase PERK, resulting in the phosphorylation of eukaryotic initiation factor-2α and forming an inflammatory- and survival-preferred translation program, which precedes TBK1-IRF3 activation [38].
3.5 STING-Mediated Autophagy
Autophagy is another output of the STING-dependent signalling. But the exact mechanism remains uncertain. In mammalian cells, cellular materials to be degraded by autophagy are sequestered into double-membrane vesicular autophagosomes and transported to lysosomes to form the autolysosome [39]. Several multi-subunit protein complexes are involved in the biogenesis of autophagosomes. The nucleation of autophagosomes is initiated by the Unc51-like kinase (ULK) complex (ULK1/2, focal adhesion kinase family interacting protein of 200 kD, autophagy-related protein [ATG]13, and ATG101) and ATG9-containing vesicles. ULK1 recruits the phosphoinositide 3-kinase complex (Vps34, Beclin-1, ATG14 and Vps15) to generate phosphatidylinositol 3-phosphate on growing phagophores, which recruits WD repeat domain phosphoinositide-interacting proteins (WIPIs) and double FYVE-containing protein 1 via their phosphatidylinositol 3-phosphate binding domains. WIPI2 then binds ATG16L1 to promote the conjugation of mammalian ATG8 proteins [microtubule-associated protein 1 light chain 3 (LC3)A, B and C, and GABA receptor associated proteins L1 and L2] to phosphatidylethanolamine in the autophagosome membrane (termed lipidation) [40]. For this conjugation, ATG4 first cleaves ATG8-family proteins in the C-terminal tail to expose their glycine residues. Then, ATG7, ATG3, and the ATG16L1 complex act as E1, E2 and E3 enzymes, respectively [39]. Lipidation of ATG8s is a hallmark of autophagosome formation.
After dsDNA stimulation, STING colocalises with LC3 and ATG9 [41]. TBK1 was reported to phosphorylate LC3C and GABA receptor associated protein-L2 on surface-exposed serine residues to impede their binding to ATG4 and thus protect from ATG4-mediated premature removal from nascent autophagosomes, ensuring autophagosome formation [42].
STING was also reported to activate autophagy independent of TBK1 activation and IFN induction. STING harbours LC3 interacting regions in the ligand-binding domain and interacts with LC3 directly [43]. Upon binding cGAMP, STING translocates to ERGIC. The STING-containing ERGIC serves as a membrane source for LC3 lipidation, which is dependent on WIPI2 and ATG5 but independent of the ULK and VPS34-beclin kinase complexes [44]. However, the endolysosomal localisation of the STING ligand-binding domain did not induce LC3B lipidation despite strong induction of STING phosphorylation [45]. STING was also found to interact directly with WIPI2 to induce LC3 lipidation and autophagosome formation [46].
STING is also known to restrict microbial infection through noncanonical autophagy. The conjugation of LC3/ATG8 to single membranes is a form of noncanonical autophagy, being mediated through the direct recruitment of ATG16 by the vacuolar-type ATPase, a proton pump that senses organelle deacidification [47]. Ligand-bound STING forms an electron-sparse pore in its transmembrane domain that causes proton efflux from post-Golgi STING vesicles and subsequent vesicle deacidification [45, 47]. It has been demonstrated that STING activation induces vacuolar-type ATPase-dependent LC3B lipidation onto single-membrane perinuclear vesicles mediated by ATG16L1 via its WD40 domain [40].
3.6 STING Promotes NOD-Like Receptor Family Pyrin Domain-Containing 3 (NLRP3) Activation
STING promotes the activation of the NLRP3 inflammasome in various ways independent of Type I IFN. Inflammasomes are a group of cytoplasmic protein complexes that mediate host immune responses to microbial infection and cell damage. The most characteristic inflammasome is the NLRP3 inflammasome, consisting of a sensor (NLRP3), an adaptor (apoptosis-associated speck-like protein, ASC) and an effector (caspase-1). NLRP3 is a tripartite protein containing an aminoterminal pyrin domain, a central NACHT domain and a carboxy-terminal leucine-rich repeat domain [48]. ASC has a caspase recruitment domain. NLRP3 and ASC together promote pro-Caspase-1 cleavage to produce active subunits p20 and p10 that regulate interleukin-1β maturation, contributing to sterile inflammation [48].
STING was found to recruit NLRP3 to promote its localisation within the ER, facilitating the formation of the NLRP3 inflammasome [49]. STING promoted histone methylation in the NLRP3 promoter region via WDR5/DOT1L, thereby recruiting IRF3 to enhance NLRP3 transcription [50]. Further studies showed that the STING transmembrane domain (amino acid 151–160) interacts with the NACHT and leucine-rich repeat domains of NLRP3, inhibiting K48-and K63-linked polyubiquitination of NLRP3 and keeping NLRP3 activation [49]. In the later stages of STING activation, the lysosomal membrane protein Niemann-Pick type C1 interacts with STING and recruits it to the lysosome for degradation [51], serving as a negative feedback mechanism to ensure cascade termination and prevent continued activation. In certain cells, such as HEK293T or BLaER1 monocytes, activated STING is transported to the lysosome, where it triggers membrane permeabilisation instead of being degraded and consequently leads to lysosomal cell death, promoting potassium efflux to activate NLRP3 [45, 52].The precise mechanism under STING-induced lysosomal cell death is unclear, but it is independent of IRF3 and downstream IFN response [53, 54].
4 The Expression of STING in Liver
STING is extensively expressed in immune-associated tissues in humans, including the thymus, heart, spleen, placenta, lung and kidney [55]. STING is widely expressed in various cell types, including immune cells (macrophages, T cells, B cells, dendritic cells and natural killer cells), epithelial cells (intestinal epithelial cells, lung epithelial cells, skin keratinocytes), endothelial cells, fibroblasts, neuronal cells and cancer cells [56].
In liver, STING is mainly expressed in macrophages, including monocyte-derived macrophages and Kupffer cells, but is rarely expressed in LSECs, dendritic cells and HSCs [57]. Multiple studies have shown that STING is not expressed in human and mouse liver parenchymal cells [57-60]. However, several other studies have shown that STING is expressed in hepatocytes at low levels [50, 58, 61].
5 Function of STING in Various Liver Cells in MASLD (Figure 3)
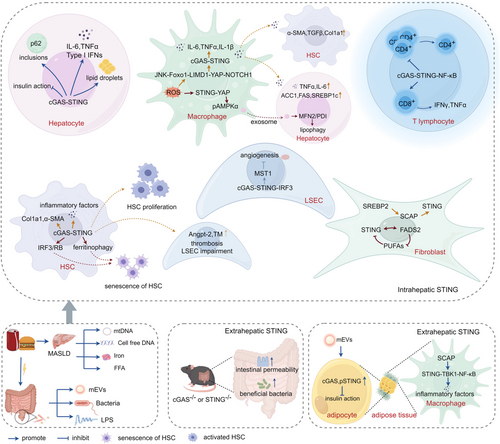
Increased STING was found in MASLD patients. Liver biopsy in a cohort of women with morbid obesity disclosed that STING expression in the liver tissue increased with the occurrence of MASLD, specifically in the simple steatosis stage (mild or moderate steatosis) [62]. In the liver sections of MASLD patients with hepatic steatosis and fibrosis, the intensity of STING-positive staining was much stronger than that in subjects without MASLD. The STING-positive cells were mainly immune cells, including macrophages/Kupffer cells and endothelial cells [60]. Chronic exposure to the STING agonist (5,6-dimethylxanthenone-4-acetic acid, DMXAA) led to hepatic steatosis and inflammation in wild-type mice [63]. Less severe hepatic steatosis, inflammation, and/or fibrosis induced by a high-fat diet (HFD) or a methionine- and choline-deficient (MCD) diet was found in STING-knockout mice and mice with STING disruption only in myeloid cells [60, 63]. STING deficiency alleviated insulin resistance and weight gain, decreasing levels of cholesterol, triglycerides and low-density lipoprotein-cholesterol, while increasing high-density lipoprotein-cholesterol levels in serum [63].
STING disruption alleviates MASLD in mice fed with short-term HFD (3 months) but not in long-term HFD (7 months) feeding mice, which is probably due to the proinflammatory activation of multiple types of liver cells in long-term HFD feeding to offset the effect of STING disruption [64]. However, golden ticket mice lacking the STING protein did not show any differences in hepatic steatosis, inflammation and fibrosis induced by a short-term (4 weeks) high fructose diet or fructose–palmitate–cholesterol diet (1, 4 or 16 weeks), which indicated a limited role of STING in MASH [65].
5.1 STING Activation in Hepatocyte
Comparing with the healthy subjects, increased levels of cGAS and phosphorylated STING were found to suppress the insulin action in the obese human hepatocytes, which was caused by the gut microbial DNA-containing extracellular vesicles (mEVs) of obese patients [66]. Hepatocyte-specific loss of IRF3 reversed insulin resistance and restored glucose homeostasis in obese mice induced by a high-fat diet. In hepatocytes, Ppp2r1b was identified as a direct IRF3 target, and the subsequent dephosphorylation of AMP-activated protein kinase (AMPK)α and AKT aggravated dysglycemia [67]. mEVs of MASH mice increased levels of proinflammatory cytokines in the wild-type hepatocytes but not in cGAS−/− hepatocytes [68]. In MASLD models induced by a high-fat high-sucrose diet or a choline-deficient high-fat diet, DNA lesions activate the cGAS/STING pathway in hepatocytes [69]. MASH is associated with the accumulation of insoluble protein aggregates composed of ubiquitinated proteins and ubiquitin adaptor p62/sequestosome 1. The cGAS-STING-TBK1 axis participates in the progression of liver pathologies in MASLD by facilitating the formation of p62 inclusions in hepatocytes. Upon saturated fatty acid-induced lipotoxicity, cGAS and STING are involved in the phosphorylation of p62 by TBK1 and the formation of large protein inclusions in hepatocytes. TBK1 inhibition prevented the formation of ubiquitin-p62 aggregates not only in cultured hepatocytes but also in mouse models of obesity and MASH [70]. With stimulation of free fatty acids, STING was activated, leading to an increased expression of inflammatory cytokines and type I IFN in L02 cells [61]. STING overexpression enhanced lipid deposition and inflammatory response in the primary hepatocytes stimulated by palmitate [71]. Knocking down either STING or IRF3 led to a significant reduction in free fatty acid-induced inflammation and apoptosis and enhanced glycogen storage and alleviated lipid accumulation in L-02 cells [61]. Iron overload is common in MASLD patients. Iron treatment enhanced the expression of cGAS, STING and their downstream targets, including TBK1, IRF-3 and NF-κB in HepG2 cells and mouse liver [72]. Gut-derived LPS increased the expression of IRF3 in the primary hepatocyte isolated from MASLD mice induced by HFD [73].
However, some other reports demonstrated that STING is not expressed in murine hepatocytes, supported by immunohistochemistry results in the liver tissue sections and western blot results in the primary hepatocytes [63]. Another report also disclosed that human and murine hepatocytes do not express STING, which was supported by the fact that hepatocytes do not produce Type 1 IFN in response to foreign DNA or hepatitis B virus infection, and mice lacking STING or cGAMP exhibit unaltered ability to control infection in an adenovirus–hepatitis B virus model [74].
5.2 STING Activation in Hepatic Macrophage
Hepatic macrophages mainly comprise tissue-resident Kupffer cells and monocyte-derived macrophages. In inflammatory settings, the pool of liver macrophages is augmented by a robust influx of bone marrow-derived monocytes that give rise to monocyte-derived macrophages [75].
In a cohort composed of 98 MASLD subjects and 8 controls, STING was found to be mainly expressed in macrophages, including monocyte-derived macrophages (CCR2+, S100A9+), Kupffer cells (CD68+) and CD163+ macrophages. Compared with controls, the numbers of STING+ cells in livers from MASH patients were increased. The numbers of STING+/CCR2+ and STING+/S100A9+ cells positively correlated with liver inflammation grade and fibrosis stage. The numbers of STING+/CD68+ and STING+/CD163+ cells positively correlated with the aggravation of fibrosis stage only [57]. HFD-induced oxidative stress increased JNK-dependent forkhead box protein O 1 transcriptional activity and LIM domain-containing protein-mediated Yes-associated protein (YAP) nuclear translocation and activated notch receptor 1 (NOTCH1) signalling in hepatic macrophages. YAP directly interacts with the NOTCH1 intracellular domain (NICD) to form a YAP-NICD complex that is recruited to the promoter region of cGAS to enhance the expression of cGAS and then the cGAS-STING signalling [76]. Activation of STING signalling in macrophages was reported to increase the expression of inflammatory mediator genes, inhibiting the occurrence of autophagy flow and lipophagy in hepatocytes and aggravating lipid droplets deposition and MASH progression [77].
In a co-culture system of THP1 macrophages and LX-2 cells, the expression of α-smooth muscle actin (α-SMA), Type I collagen and TGFβ1 in LX2 cells was significantly increased upon STING activation in macrophages [57]. Hepatocytes and HCSs co-cultured with STING-knockout macrophages stimulated with a STING agonist had decreased lipid deposition and inflammatory cytokines compared with those in the co-culture with wild-type macrophages [60]. The mitochondrial DNA from hepatocytes of HFD-fed mice induced inflammatory cytokines in cultured Kupffer cells, which were attenuated by STING deficiency [63]. These results indicated that STING functions as a mitochondrial DNA sensor in the Kupffer cells of the liver under lipid overload in MASH.
However, some reports demonstrated beneficial STING in hepatic steatosis. HFD-induced oxidative stress activates STING-YAP pathways in hepatic macrophages. The macrophage STING-mediated YAP promotes lipophagy induction in hepatocytes by the release of p-AMPKα from macrophages to hepatocytes via exosomes and activates the mitofusin 2/protein disulfide isomerase pathway [78]. In lean MASLD mice induced by a Paigen diet, the expression of SCAP increased in macrophages in the liver tissue. SCAP was disclosed to specifically recruit STING and TBK1 onto the Golgi to activate NF-κB, leading to the production of inflammatory factors in macrophages [79].
5.3 STING Activation in HSCs and Fibroblasts
HSCs and portal fibroblasts are the main populations of resident mesenchymal cells in the liver. At steady state, HSCs are in contact with hepatocytes and sinusoids, and portal fibroblasts are found surrounding the portal vein and bile ducts in the portal region. HSCs appear to be the main source of myofibroblast-like cells following most injuries, having a central role in hepatic fibrosis.
Stimulation with STING agonist caused significant increases in markers of fibrosis in LX2 cells [60]. In humans and mice, MASH development was concomitant with a marked bacterial DNA enrichment in HSCs. Isolation of mEVs from MASH mice to stimulate HSCs leads to increased expression of the key genes associated with fibrogenic activation in wild-type HSCs, but not in the HSCs isolated from cGAS−/− mice. Similarly, the knockdown of cGAS in healthy human HSCs blunted the effects of MASH mEVs. These results supported that mEVs aggravate HSC activation via the role of cGAS/STING signalling [68].
With stimulation of TGF-β, the expression of profibrotic genes and inflammatory cytokines was reduced in cGAS-knocking down or STING-knocking down LX2 cells compared with that in wild-type LX2 cells. Overexpression of cGAS or STING in LX2 cells significantly upregulated the profibrotic genes and inflammatory cytokines with TGF-β stimulation. In a co-culture system of LX-2 and human liver LSECs, the activation of the cGAS-STING signalling pathway in HSCs exacerbates LSECs impairment. The injured LSECs release vasoactive substances, including angiopoietin-2 and thrombomodulin, to disrupt coagulatory homeostasis in the microcirculation and promote the onset of thrombosis [80]. IRF3 was significantly increased in HSCs in response to TGF-β1 stimulation. Knockdown of IRF3 in LX-2 cells significantly decreases the expression of Type I collagen and α-SMA and cell proliferation in activated LX-2 induced by TGF-β1, increasing HSCs apoptosis. Overexpression of IRF3 upregulates the expression of Type I collagen and α-SMA in LX-2 cells and further promotes HSC proliferation [81].
However, there is also a study demonstrating that the activation of STING in HSCs leads to the senescence of HSCs and alleviation of fibrosis. The cGAS-STING pathway is required to activate ferritinophagy in HSCs by oroxylin A, which leads to HSC senescence [50]. STING-IRF3-retinoblastoma signalling plays a notable role in HSCs within various murine models, pushing activated HSCs toward senescence. IRF3 produced by STING activation forms substantial endogenous complexes in the nucleus with the key cell cycle regulator, retinoblastoma, which attenuates cyclin-dependent kinase 4/6-mediated retinoblastoma hyperphosphorylation that mobilises retinoblastoma to deactivate E2 family transcription factors, thereby driving HSCs into senescence [82].
Fibroblasts are activated in the inflammatory stage of wound healing in response to cytokines and growth factors and differentiate into myofibroblasts. Strong evidence suggests that signals between bile duct epithelia and portal fibroblasts are instrumental in the progression of fibrosis. It has been disclosed that there is a direct correlation between the intensity of the ductular reaction and the severity of fibrosis in human MASLD [83]. A negative regulatory feedback loop between STING and fatty acid desaturase 2 (FADS2) has been demonstrated in fibroblasts. In fibroblasts, STING directly interacts with FADS2, inhibiting the FADS2-dependent desaturation of polyunsaturated fatty acids. polyunsaturated fatty acids, in turn, inhibit STING, thereby regulating antiviral responses and contributing to resolving STING-associated inflammation [84]. As the transcription factor regulating the synthesis of cholesterol, SREBP2 needs to be processed to mature nuclear forms in the Golgi apparatus, which requires SCAP trafficking SREBPs to the Golgi [85]. It was reported that SREBP2 trafficking by SCAP primes STING translocation to the Golgi and activates STING signalling in fibroblasts [51].
5.4 STING Activation in T Lymphocytes
In CD4+ T cells, STING activation reduces cell proliferation, which requires a distinct C-terminal domain of STING, activating NF-κB and causing mitotic errors [24]. In MASH, there is an imbalance between excess T helper 1-derived cytokines such as IFNγ and a deficiency in Th2-derived cytokines [73]. In a cohort of human patients, CD8+ T cells represent a dominant intrahepatic immune cell population that links to glucose dysregulation. CD8+ T cells and IFN-I responses correlate with MASLD activity in human patients. In MASH mice induced by 32 weeks of HFD, the frequency and number of IFNγ- and tumour necrosis factor alpha-producing CD8+ T cells increased significantly [73]. Fatty acid binding proteins are a family of lipid chaperones required to facilitate uptake and intracellular lipid trafficking. Genetic or pharmacologic inhibition of Fatty acid binding protein 5 leads to mitochondrial DNA release and activation of the cGAS-STING-dependent Type I IFN signalling in regulatory T cells [86].
5.5 STING Activation in LSECs
In LSECs stimulated with palmitic acid, mitochondrial DNA releases to the cytosol and activates cGAS-STING-IRF3 signalling. IRF3 upregulates the expression of mammalian Ste20-like kinases 1 by binding to the promoter of the mammalian Ste20-like kinases 1 gene, which inactivates Hippo-YAP and inhibits angiogenesis [87].
5.6 The Extrahepatic STING Activation
5.6.1 Intestine
The role of the gut–liver axis in MASLD has been extensively confirmed. In MASH mice induced by a high-fat, high-cholesterol, high-sucrose diet, globally knocking out cGAS or STING resulted in exacerbated liver damage, steatosis and fibrosis, which were closely correlated with significantly diminished thickness of the small intestine's mucosal layer, increased endotoxin levels, and induced notable structural alterations in the ileum leading to enhanced intestinal permeability [88].
But in another report, STING deletion mice showed decreased lipid accumulation and liver inflammation induced by HFD compared with wild-type mice, accompanied by elevated relative abundance of beneficial bacteria (such as Prevotellaceae) in the gut microbiota of STING-deficient mice compared to that of wild-type mice [89].
5.6.2 Adipose Tissue
Treatment with mEVs from obese patients elevated the levels of cGAS expression and STING phosphorylation in adipocytes. Deletion of cGAS in mice prevented the suppressive effect of obese mEVs on insulin action in adipocytes [66]. In lean MASLD mice induced by Paigen diet, the expression of SCAP increased in macrophages in the adipose tissues, which recruits STING and TBK1 onto the Golgi to activate NF-κB leading to the production of inflammatory factors [79].
6 STING Agonists and Inhibitors in MASLD
The STING signalling pathway has been identified as a key target for the treatment of inflammation and autoimmune diseases. Many agonists and inhibitors of STING have been identified and have been described in previously published review articles [90, 91]. Here, we summarised the ligands and inhibitors of STING validated in MASLD.
In MASLD, 2′3′-cGAMP [57], DMXAA [63], FFA [92], mitochondrial DNA [93, 94], mEVs [66], LPS [79], diABZI [95] and ISD [95] have been demonstrated to activate the STING signalling pathway in hepatocytes and nonparenchymal cells. On the other hand, several medications have been found to alleviate MASLD via inhibiting the STING signalling pathway, including Remdesivir [71], Sorafenib [96], probiotic triple live bacteria powder [97], the herbal formula Ling-Gui-Zhu-Gan-Tang and its components (Cinnamaldehyde, Atractylodin II and Glycyrrhizin) [98], flavonoid extracted from Epimedium [95], C176 [59, 98] and H151 [95, 99] (Table 1).
| Drug | Inhibitor/agonist | Dosage | Model/cell line | Mechanism | References |
|---|---|---|---|---|---|
| DMXAA | Agonist | Mice: 25 mg/kg for 2 days, i.p [63]; cell: 75 μg/mL [98] |
HFD-induced MASH in mice for 26 weeks; MCD induced MASH in mice for 8 weeks [63]; Cell: primary liver macrophages isolated from healthy mice [98]; BMDMs [98] |
Induce hepatic steatosis and inflammation [63]; Induce STING-TBK1-NF-κB pathway and increase the release of TNF-α, IL-1β, IL-6 and IFN-β [98] |
[63, 98] |
| 2′3′cGAMP | Agonist | 20 μg/mL for 24 h | THP-1 cell line | Increase macrophage cytokine production and stimulate HSCs activation | [57] |
| Palmitate | Agonist | 1 mM for 24 h | THP-1 cell line | Increase macrophage cytokine production and stimulate HSCs activation | [57] |
| Oleate and Palmitate | Agonist | 1 mM for 24 h | L02 cell line | Promote lipids deposition | [92] |
| mtDNA | Agonist | 10 μg/mL for 12 h | BMDM | Induce activation of STING/NF-κB pathway | [94] |
| mEVs | Agonist | 5 × 109 EVs/mouse injection into tail vein or jejunum section twice per week for 4 weeks; 5 × 108 EVs for 24 h | HFD-induced MASH in mice for 4 or 16 weeks; 0.5 × 106 3T3-L1 cells or hepatocytes | Activate cGAS/STING-mediated inflammatory signalling | [66] |
| LPS | Agonist | 100 ng/mL for 24 h | primary liver macrophages isolated from healthy mice; BMDMs | Induce STING-TBK1-NF-κB pathway and increasethe release of TNF-α, IL-1β, IL-6 and IFN-β | [98] |
| ISD | Agonist | For 2 or 4 h | BMDMs; PBMCs isolated from peripheral blood of healthy volunteers | Induce STING signalling pathway | [95] |
| diABZI | Agonist | For 2 or 4 h | PBMCs isolated from peripheral blood of healthy volunteers | Induce STING signalling pathway | [95] |
| Remdesivir | Inhibitor | 20 mg/kg/d for 16 weeks (i.g) |
HFD for 16 weeks in mice Primary hepatocyte induced by palmitate |
Reduce liver inflammation and lipid accumulation by inhibiting the STING-IRF3/NF-κB signalling pathway in hepatocytes | [71] |
| Sorafenib | Inhibitor | 10 μmol/L for 8 h or 24 h | THP-1 cell line | Inhibit STING dimerisation and the recruitment of TBK1 and IRF3 | [96] |
| Probiotic triple live bacteria powder | Inhibitor | (1.0 × 107 CFU/mL) 0.4 mL/d(i.g.)for 4 weeks | HFD-diet for 16 weeks in mice | Inhibit the STING-NF-κB pathway to suppress the release of TNF-α, IL-1β, IL-6 and IFN-β. | [97] |
| Flavonoid extracted from Epimedium | Inhibitor |
Mice: 600 mg/kg/d (i.g) for 5 weeks Cell: 0.4 mg/mL for 1 h |
MCD-diet for 6 weeks in mice; BMDMs isolated from MASLD mice; THP-1; PBMCs isolated from peripheral blood of healthy volunteers | Inhibit STING signalling pathway to reduce liver inflammation | [95] |
| H-151 | Inhibitor |
Mice: 10 mg/kg(i.p) every other day for 5 weeks [95] Cell: 0.5 μM for 12.5 h [94] |
MCD-diet for 6 weeks in mice [95]; BMDM [94] |
Inhibit palmitoylation of STING at Cys91 | [94, 95] |
| Licorice extract | Inhibitor |
Cell: 2.5 mg/mL Mice: 500 mg/kg (i.g) for every other day for 6 weeks |
MCD-diet for 6 weeks in mice BMDM THP-1 cell line |
Inhibit the oligomerisation of STING | [59] |
| C-176 | Inhibitor |
Mice: 15 mg/kg (i.p) for every other day for 6 weeks [59] Mice: 6.7 mg/kg(i.g)for 9 weeks [98] |
MCD-diet for 6 weeks in mice [59] HFD-diet for 17 weeks in mice [98] Primary hepatocytes and liver macrophages were isolated from healthy mice [98] |
Inhibit palmitoylation of STING at Cys91 | [59, 98, 99] |
| Ling-Gui-Zhu-Gan-Tang | Inhibitor |
Mice: 22 g/kg/d(i.g)for 9 weeks Cell: 1 mg/mL |
HFD-diet for 17 weeks in mice; primary hepatocytes and liver macrophages isolated from healthy mice; AML12 | Inhibit STING-NF-κB pathway to suppress the release of TNF-α, IL-1β, IL-6 and IFN-β | [98] |
| Zbiotics | Inhibitor | 109 CFU/kg/d(i.g)for 4 weeks | HSHF diet for 14 weeks in Wistar rats | Restore the integrity of the gut–liver axis through modulation of the cGAS-STING signalling pathway | [100] |
| Patchouli alcohol | Inhibitor | 40 mg/kg for 5 weeks | HFD-induced MASH in rats for 8 weeks | Inhibit STING-signalling pathway-mediated inflammatory response | [101] |
| Aucubin | Inhibitor |
Mice: 10 mg/kg/d for 6 weeks Cell: 50 or 100 μM for 12.5 h |
HFD-induced MASH in mice for 12 weeks; BMDM | Suppress STING/NF-κB pathway and downregulate the gene expression of proinflammatory cytokines in BMDM | [94] |
- Abbreviations: BMDM, bone marrow-derived macrophage; HSHF, high sucrose and high fat; IL, interleukin; PBMC, peripheral blood mononuclear cell; TNF-α, tumour necrosis factor alpha; i.g, intragastric infusion; i.p, intraperitoneal injection.
7 Conclusion
Besides classic TBK1-IRF3 signalling, the activation of STING also interacts with multiple signal pathways and affects various biological functions, including Type I IFN and inflammatory cytokine secretion, autophagy and NLRP3 activation. So far as we know, there are plenty of STING ligands in MASLD, and STING activation was found in nonparenchymal cells and hepatocytes. However, the various functions of STING in different cell types and, especially, how STING activation affects the intercellular conversation in MASLD still remain unknown and deserve thorough investigation. We believe that as we gradually understand the role of STING in MASLD, the strategies targeting STING will have broader prospects.
Author Contributions
JingJing Wang: investigation, writing – original draft, visualisation. Yue Guo: investigation, writing – original draft. Jing Hu: conceptualisation, funding acquisition. Jinghua Peng: conceptualisation, writing – review and editing, project administration, funding acquisition.
Ethics Statement
The authors have nothing to report.
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data availability is not applicable to this article as no new data were created or analyzed in this study.