Representation of Sex, Race and Ethnicity in MASH Randomised Controlled Trials: A Systematic Review and Meta-Analysis
Handling Editor: Luca Valenti
Alessandro Mantovani and Cristiane Alves Villela-Nogueira share co-senior authorship.
Funding: The authors received no specific funding for this work.
ABSTRACT
Background and Aims
Randomised controlled trials (RCTs) have historically underrepresented female, racial and ethnic minorities across various fields. This systematic review and meta-analysis aims to examine the global distribution, reporting and participation of diverse groups based on sex, race and ethnicity in trials focused on metabolic dysfunction-associated steatohepatitis (MASH).
Methods
PubMed and Cochrane Library databases were systematically searched for MASH RCTs (through December 13, 2024) that included any pharmacotherapy as an intervention arm. RCTs were qualitatively reviewed to assess their global distribution and reporting of populations. A meta-analysis of proportions was performed using a generalised linear mixed model.
Results
One hudred and nine studies were identified, reporting data from 112 RCTs and 19 516 MASH participants. Of the 49 countries that conducted trials, 34 were high-income countries (69.4%). Sex, race and ethnicity were reported in 111 (99.1%), 69 (61.6%) and 56 (50.0%) of the 112 RCTs, respectively, with reporting improving in recent years. We found no reporting of sexual and gender minorities. The pooled proportions of female, White, Asian, Black and Hispanic/Latino groups were 54.23% (95% confidence interval [CI]: 51.31–57.12), 87.63% (95% CI: 85.37–89.58), 4.95% (95% CI: 3.42–7.10), 2.27% (95% CI: 1.89–2.71) and 31.42% (95% CI: 26.61–36.66), respectively. Meta-regressions showed a trend toward more female, White and Hispanic/Latino participants in RCTs over time.
Conclusions
Although female and Hispanic/Latino representation has increased over time, racial minorities are underrepresented in MASH trials. These data provide an overview of participant representation in MASH trials and call for collaborative efforts among researchers, sponsors, regulators and other relevant stakeholders to improve diversity in these trials.
Summary
- Randomised controlled trials of metabolic dysfunction-associated steatohepatitis (MASH) are highly concentrated in high-income countries.
- Reporting of data on race and ethnicity in MASH trials is suboptimal, although it has improved in recent years.
- Female and Hispanic/Latino representation has improved in recent years, but White participation remains high and is increasing over time, resulting in low representation of racial minorities.
- Future clinical trials should address disparities in the enrollment of racial minorities and promote diversity in MASH trials.
Abbreviations
-
- CI
-
- confidence interval
-
- HIC
-
- high-income country
-
- LMIC
-
- lower-middle-income country
-
- MASH
-
- metabolic dysfunction-associated steatohepatitis
-
- MASLD
-
- metabolic dysfunction-associated steatotic liver disease
-
- PRISMA
-
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
-
- PROSPERO
-
- International Prospective Register of Systematic Reviews
-
- RCT
-
- randomised controlled trial
-
- SDOH
-
- social determinants of health
-
- SGM
-
- sexual and gender minority
-
- UMIC
-
- upper-middle-income country
1 Introduction
Metabolic dysfunction-associated steatotic liver disease (MASLD), formerly known as non-alcoholic fatty liver disease, is the leading cause of chronic liver disease [1]. Affecting an estimated 38% of adults worldwide (ranging from 34% to 42%), it is a major global health problem with a significant social and economic burden [1, 2]. MASLD ranges from simple steatosis to more severe conditions, including metabolic dysfunction-associated steatohepatitis (MASH), cirrhosis and ultimately hepatocellular carcinoma [3]. The complexity of MASLD involves a systemic metabolic milieu where insulin resistance and metabolic dysfunction contribute to the disease, leading to extrahepatic complications such as cardiovascular disease, chronic kidney disease and extrahepatic cancers, which significantly impact patient morbidity and mortality [4-6].
There is an increasing need to identify and treat this disease promptly to reverse its progression and associated comorbidities. In recent decades, substantial efforts have been made to understand the pathophysiological mechanisms of MASLD, leading to advances in the development of pharmacotherapies, particularly through the MASH randomised controlled trials (RCTs) [7, 8]. A milestone in this journey was the US Food and Drug Administration's (FDA) conditional approval of resmetirom for the treatment of non-cirrhotic MASH patients with moderate to advanced liver fibrosis [9]. While promising new therapies are on the horizon, addressing the lack of diversity in trial populations remains a challenge, limiting insights into treatment efficacy across different groups and the broader applicability of trial results [10]. We therefore conducted a systematic review and meta-analysis to assess the global distribution of sites, reporting, and representativeness of diverse populations by sex (i.e., female), race (i.e., White, Asian, Black, American Indian/Alaska Native and Native Hawaiian/Pacific Islander) and ethnicity (i.e., Hispanic/Latino) in MASH RCTs. In addition, we aimed to examine factors associated with the participation of these groups, with further analysis of whether this participation varied over time and by trial size.
2 Materials and Methods
2.1 Protocol Registration
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [11]. The research protocol was a priori registered in the International Prospective Register of Systematic Reviews (PROSPERO) database (CRD42024522709).
2.2 Search Strategy and Study Selection
PubMed and Cochrane Library databases were systematically searched from inception to December 13, 2024. The full search strategy is provided in Appendix S1: Methods. In addition, we manually screened the references of primary articles and reviews to ensure a comprehensive search. After removing duplicate records, two authors (MS and LA-S) screened titles/abstracts and then independently reviewed full-text articles for inclusion based on the eligibility criteria. Disagreements were resolved by consensus. Studies were selected for inclusion in this meta-analysis if they met the following criteria: (i) RCTs published as original articles; (ii) studies in which patients with MASH were randomised to any pharmacotherapy and control groups; and (iii) studies that provided information on baseline characteristics of the population. Exclusion criteria were as follows: (i) studies including patients without MASH; (ii) studies with a sample size < 30 patients with MASH; (iii) studies focused on paediatric populations (< 18 years); (iv) post hoc analyses or studies with the same population but with longer follow-up; and (v) studies in which lifestyle modification (e.g., diet or exercise) was the only intervention. Abstracts, case reports, reviews, meta-analyses, commentaries, editorials, and practice guidelines were also excluded.
2.3 Data Extraction and Quality Assessment
Two authors (MS and LA-S) independently extracted data on baseline characteristics using a standardised format. Details about data extraction are described in Appendix S1: Methods. Four authors (ID, SMK, MS and XHL) independently assessed the risk of bias using the Cochrane Risk-of-Bias 2 Tool (RoB 2) [12]. Each domain was judged as low risk, some concerns, or high risk of bias [12]. Disagreements were resolved by consensus.
2.4 Data Synthesis and Analysis
The primary outcomes were the proportions of the various groups by sex (i.e., female), race (i.e., White, Asian, Black) and ethnicity (i.e., Hispanic/Latino) in the MASH trials in which they were reported. In addition, we calculated the proportion of American Indians/Alaska Natives and Native Hawaiians/Pacific Islanders. Qualitative data on the geographic distribution of MASH trials were provided by descriptive statistics. We grouped countries based on the World Bank's classification of income levels for 2024–2025, including lower-middle-income (LMIC), upper-middle-income (UMIC) and high-income countries (HIC) [13]. Details on the analyses of race and ethnicity reporting are described in Appendix S1: Methods.
A meta-analysis using a generalised linear mixed model with Clopper-Pearson intervals was conducted to obtain pooled proportions with 95% confidence intervals (CIs). A random effects model was used in all analyses, irrespective of heterogeneity [14]. Statistical heterogeneity was assessed using the Cochran Q test and the I2-statistics, with significance for heterogeneity indicated by a Cochran Q test p-value < 0.10 and I2 > 50%. I2 values of ~25%, ~50% and ~75% were representative of low, moderate and high heterogeneity, respectively. Prespecified subgroup, meta-regression and sensitivity analyses are described in Appendix S1: Methods. Publication bias was not assessed since it falls outside the scope of our study. A two-sided p-value of < 0.05 was used as the threshold to determine statistical significance. All analyses were performed using R software (version 4.2.3, R Foundation for Statistical Computing, Vienna, Austria) with the “meta” and “metafor” packages.
3 Results
3.1 Characteristics of the Included Studies
Figure S1 illustrates the search and selection processes. Our initial literature search yielded 8360 records. After removing duplicates and unrelated records based on abstract/title, 274 records were screened for inclusion in the full text. Finally, 109 studies were included in the meta-analysis, reporting data from 112 RCTs and 19 516 participants with MASH. Of note, 3 of these studies reported data from 2 RCTs each [15-17]. Table 1 provides a brief overview of the RCTs (detailed in Table S1), summarising their characteristics and reporting. Based on RoB 2, most studies had a low risk of bias (89 studies), 14 studies had some concerns, and 6 studies had a high risk of bias (Table S2).
| RCTs, n (%) | |
|---|---|
| Phase | |
| 1 | 4 (3.6) |
| 1/2 | 2 (1.8) |
| 2 | 63 (56.2) |
| 2/3 | 2 (1.8) |
| 3 | 10 (8.9) |
| 4 | 5 (4.5) |
| Uncleara | 26 (23.2) |
| Year of publication | |
| ≤ 2010 | 12 (10.7) |
| 2011–17 | 31 (27.7) |
| 2018–24 | 69 (61.6) |
| Multicenter | 83 (74.1) |
| Double-blinded | 93 (83.0) |
| Placebo-controlled | 91 (81.3) |
| Funding | |
| Involves a pharmaceutical company | 71 (63.4) |
| No pharmaceutical company | 41 (36.6) |
| Sample size | |
| ≤ 50 | 27 (24.1) |
| 51–100 | 34 (30.4) |
| 101–150 | 16 (14.3) |
| 151–200 | 12 (10.7) |
| > 200 | 23 (20.5) |
| Sex | |
| Reported | 111 (99.1) |
| Not reported | 1 (0.9) |
| Race | |
| Reported | 69 (61.6) |
| Not reported | 43 (38.4) |
| Ethnicity | |
| Reported | 56 (50.0) |
| Not reported | 56 (50.0) |
- Abbreviations: MASH, metabolic dysfunction-associated steatohepatitis; RCT, randomised controlled trial.
- a We considered the trial phase as ‘unclear’ if this information was not available in the manuscript or its registration.
3.2 Geographic Distribution
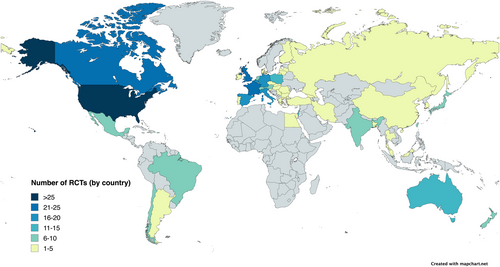
The continental distribution of RCTs was as follows: 41 (36.6%) were conducted in North America only, 18 (16.1%) in Asia only, 14 (12.5%) in Europe only, 4 (3.6%) in South America only, 2 (1.8%) in Africa only, and 33 (29.5%) were multicontinental. Among the 49 countries, the US was the country with the highest number of trials (74 RCTs; 66.1%), followed by France (26 RCTs; 23.2%) and the United Kingdom (25 RCTs; 22.3%), as shown in Figure 1 (the corresponding list is provided in Table S3). According to the World Bank classification, when looking at individual countries, most sites were in HICs (34/49; 69.4%), with the remainder in UMICs (12/49; 24.5%) and LMICs (3/49; 6.1%).
3.3 Sex Representation
Sex was reported in 111 (99.1%) of the 112 included RCTs. We did not identify any trials reporting on sexual and gender minorities (SGMs). Among 111 RCTs (19 476 participants), the overall pooled proportion of female participants was 54.23% (95% CI: 51.31–57.12), with a high degree of heterogeneity (I2 = 85.7%) (Figure S2). Subgroup analysis showed no significant differences when stratified by trial phase (p = 0.23) and risk of bias (p = 0.15), but a significant difference was observed according to funding: trials supported by pharmaceutical companies had a higher proportion of female participants compared to those without such involvement (57.13% [71 RCTs, 16 861 participants] vs. 48.77% [40 RCTs, 2615 participants], p = 0.03) (Table S4). Meta-regression showed an increase in female participation in trials over time (β = 0.0324, p = 0.002), while sample size had no impact on female participation (p = 0.301) (Figure S3). Similarly, a sensitivity analysis including only trials conducted in the US yielded an estimate of 56.59% (95% CI: 51.92–61.15, I2 = 82.3%) female participants.
3.4 Race Representation
Race was reported in 69 (61.6%) of the 112 RCTs, with an increasing trend from 1 (8.3%) of 12 RCTs before 2010, to 12 (38.7%) of 31 RCTs in 2011–17, to 56 (81.2%) of 69 RCTs in 2018–24. Of the 69 RCTs that reported race, 48 (69.6%) RCTs did not clearly report race data for racial minority participants.
3.4.1 White Race
Among 69 RCTs (16 543 participants), the overall pooled proportion of White participants was 87.63% (95% CI: 85.37–89.58), with a high degree of heterogeneity (I2 = 92.3%) (Figure S4). Subgroup analysis showed no significant differences when stratified by risk of bias (p = 0.13), but significant differences were observed according to trial phase and funding. Phase 1–2 trials had a higher proportion of White participants compared to phase 3–4 trials (88.64% [58 RCTs, 8860 participants] vs. 80.58% [10 RCTs, 7637 participants], p = 0.03), and trials supported by pharmaceutical companies had a higher proportion of White participants compared to those without such involvement (88.75% [59 RCTs, 15 714 participants] vs. 76.22% [10 RCTs, 829 participants], p = 0.01) (Table S4). Meta-regression indicated an increase in the number of White participants in the trials over time (β = 0.0546, p = 0.030), while sample size had no effect on White participation (p = 0.428) (Figure S5). Similarly, a sensitivity analysis including only trials conducted in the US yielded an estimate of 88.85% (95% CI: 84.82–91.91, I2 = 90.8%) White participants.
3.4.2 Asian Race
Among 38 RCTs (12 107 participants), the overall pooled proportion of Asian participants was 4.95% (95% CI: 3.42–7.10), with a high degree of heterogeneity (I2 = 95.2%) (Figure S6). Subgroup analysis showed no significant differences when stratified by trial phase (p = 0.30) and funding (p = 0.48), but a significant difference was observed according to risk of bias: trials with low risk of bias had a higher percentage of Asian participants compared to trials with some concerns (5.49% [35 RCTs, 11 579 participants] vs. 1.70% [3 RCTs, 528 participants], p < 0.01) (Table S4). Meta-regression showed no effect of publication year (p = 0.962) and sample size (p = 0.445) on Asian participation (Figure S7). A sensitivity analysis including only trials conducted in the US yielded a lower estimate of 2.15% (95% CI: 0.94–4.85, I2 = 69.1%) Asian participants.
3.4.3 Black Race
Among 42 RCTs (12 456 participants), the overall pooled proportion of Black participants was 2.27% (95% CI: 1.89–2.71), with a moderate degree of heterogeneity (I2 = 35.9%) (Figure S8). Subgroup analysis showed no significant differences when stratified by trial phase (p = 0.96), funding (p = 0.33) and risk of bias (p = 0.99) (Table S4). Meta-regression showed no effect of publication year (p = 0.761) and sample size (p = 0.431) on Black participation (Figure S9). Similarly, a sensitivity analysis including only trials conducted in the US yielded an estimate of 3.26% (95% CI: 2.49–4.26, I2 = 3.7%) Black participants.
3.4.4 American Indian/Alaska Native and Native Hawaiian/Pacific Islander Races
Among 14 RCTs (4460 participants) and 11 RCTs (3773 participants), the overall pooled proportions of American Indian/Alaska Native and Native Hawaiian/Pacific Islander were 0.83% (95% CI: 0.60–1.14, I2 = 0%) and 0.42% (95% CI: 0.26–0.69, I2 = 0%), respectively (Figure S10).
3.5 Ethnicity Representation
Ethnicity was reported in 56 (50.0%) of the 112 RCTs, with an increasing trend from 2 (16.7%) of 12 RCTs before 2010, to 11 (35.5%) of 31 RCTs in 2011–17, to 43 (62.3%) of 69 RCTs in 2018–24. Of note, 3 RCTs reported “Caucasian” as ethnicity and one RCT reported “White” as ethnicity.
Among 52 RCTs (13 695 participants), the overall pooled proportion of Hispanic/Latino participants was 31.42% (95% CI: 26.61–36.66), with a high degree of heterogeneity (I2 = 94.1%) (Figure S11). Subgroup analysis showed no significant differences when stratified by funding (p = 0.15) and risk of bias (p = 0.12), but a significant difference was observed according to trial phase: phase 1–2 trials had a higher proportion of Hispanic/Latino participants compared to phase 3–4 trials (33.97% [43 RCTs, 6321 participants] vs. 21.02% [9 RCTs, 7374 participants], p = 0.02) (Table S4). Meta-regression indicated an increase in the number of Hispanic/Latino participants in trials over time (β = 0.0813, p = 0.006), with sample size inversely impacting Hispanic/Latino participation (β = −0.0006, p = 0.026) (Figure S12). Similarly, a sensitivity analysis including only trials conducted in the US yielded an estimate of 37.30% (95% CI: 29.10–46.29, I2 = 92.5%) Hispanic/Latino participants.
4 Discussion
This systematic review and meta-analysis of 112 RCTs involving 19 516 participants highlights progress and gaps in the diversity of populations enrolled in MASH trials. MASH trials are predominantly conducted in HICs, with limited participation from LMICs. Although almost all trials reported sex and female representation has improved over time, information on race and ethnicity was reported in 61.6% and 50.0% of trials, respectively. In addition, our meta-analysis showed a predominance of White participants (~88%) and an underrepresentation of racial minorities. Hispanic/Latino participation increased over time but decreased in larger trials.
The global distribution of MASH trials reflects existing economic and social disparities around the world, with most RCTs concentrated in wealthier countries. There are significant challenges to further decentralisation, including regulatory, ethical, infrastructure and human resource barriers, logistical and technological issues, and sociocultural factors that limit the inclusion of regions such as Africa, South America and parts of Asia [18]. These can be addressed through international collaboration, novel methodologies and capacity-building efforts. In the global context of MASH research, emphasising diversity can increase the generalisability of trial results and ensure their relevance to patient populations with diverse backgrounds. MASH is a heterogeneous disease with a complex interplay of genetic factors, environmental exposures and social determinants of health (SDOH) [19]. Hence, the inclusion of different patient populations in research has important clinical implications. It may help to identify potential disparities in treatment outcomes as well as to understand the role of biological and sociocultural factors in drug treatment. In particular, SDOH assessment could be incorporated to understand treatment response and provide holistic and patient-centred care for MASLD [20-22]. Additionally, it may be useful for collecting data about its impact on disadvantaged groups, thereby helping to identify high-risk populations and develop culturally sensitive interventions [21].
In 2023, a global multidisciplinary panel organised by the Healthy Livers, Healthy Lives coalition proposed a research agenda for steatotic liver disease with 28 priorities [23]. One is the need to investigate factors that contribute to disparities in steatotic liver disease, with a specific call to improve the reporting of data by categories such as “sex, race, ethnicity, age, socioeconomic status, education level and other variables related to inequities” [23]. Reporting of race and ethnicity is also supported by recommendations from the FDA and the National Institutes of Health (NIH) [24, 25]. We found that the reporting of race and ethnicity in MASH RCTs remains suboptimal. Furthermore, as reported in Table S1, race and ethnicity are often reported together, contrary to the NIH recommendation that suggests that race and ethnicity should be reported as a separate category. Indeed, race refers to physical traits, whereas ethnicity refers to cultural aspects of identity [24]. For this reason, future clinical trials should improve transparency with granular data on the demographics of the enrolled population.
Clinical trials have lacked participant diversity, reflecting health disparities due to unequal access to healthcare [10, 26, 27]. The increasing representation of female sex in MASH trials over time is a significant achievement, in contrast to their historical underrepresentation in clinical research [28]. Specifically, this group has a higher risk of advanced fibrosis [29], possibly due to sex differences in the pathogenesis of MASLD [30]. In addition, we found a lack of reporting of SGMs, leading to uncertainty about the representativeness of these groups. Identifying LGBTQIA+ people is a critical step in incorporating their experiences and addressing their needs in MASH trials [31, 32].
White participants were overrepresented in the MASH trials, with an increasing trend in participation over time, suggesting underrepresentation of minority races. Notably, racial differences in response to MASH drug treatment have not been extensively studied. Recent evidence from a meta-analysis of placebo-treated patients with MASH showed that African Americans tended to have a lower rate of MASH resolution as well as a ≥ 1-point reduction in fibrosis [33]. Diversifying the participant populations in MASH trials may be the starting point for further research in this area. Racial minorities are more likely to live in conditions of socioeconomic disadvantage, including limited access to healthcare, lower rates of insurance coverage and food insecurity [21, 34]. These factors contribute to worsening MASLD disparities [35] and lead to greater susceptibility to unhealthy lifestyle choices that are compounded by poor awareness of liver disease [36, 37]. There are also disparities in awareness of clinical trials, which may be exacerbated by limited access to healthcare, thereby reducing opportunities for minority participation [38, 39]. Beyond patient-related factors, implicit treatment biases on the part of healthcare providers contribute to unconscious discriminatory practices (e.g., physicians may be less likely to enrol racial minorities in clinical trials [40]). These need to be recognised in clinical practice and further addressed through education and training to create a more inclusive environment for all groups.
The Hispanic/Latino population is of particular interest in the MASH field. This ethnicity has the highest burden of MASLD [35], in part because the I148M variant in the PNPLA3, a major genetic factor for MASLD severity, is more common in this group [41, 42]. PNPLA3 variant may also influence treatment response [43, 44]. A retrospective study showed an increased reduction in alanine aminotransferase levels with semaglutide treatment in carriers of the PNPLA3 variant [43]. Collectively, these findings suggest that the Hispanic/Latino population may respond differently to drug treatment, but this should be validated in future clinical trials. A previous systematic review and meta-analysis of clinical trials through 2019 focused on MASLD, MASH, or cirrhosis and evaluating various interventions found low enrollment (11.6%) of Hispanics/Latinos in the 45% of trials reporting data on this population [45]. With caveats regarding methodological differences, our study contrasts with these findings and shows higher participation of this population (~31%), reflecting increased participation in recent years. Nevertheless, the trend of underrepresentation of the Hispanic/Latino population in larger RCTs suggests logistical factors underlying the limited scalability of representation for this population, such as geographic distribution. There are also multiple barriers (e.g., financial constraints, language/communication, transportation, lower trust in the healthcare system, underrepresentation of minority groups among physicians and access to information about clinical trials) that are often cumulative and could limit the representation of this population in RCTs [46-50]. Interestingly, we also found reports of “Caucasian” ethnicity, likely as a synonym for “White” However, this term technically refers to people from the Caucasus region and should be avoided when referring to people from other regions [51]. Although we found three MASH trials conducted in Africa (Egypt), there were no reports of African ethnicity.
To our knowledge, this is the first systematic review and meta-analysis to describe the global landscape, reporting and participation of diverse groups by sex, race and ethnicity in MASH RCTs. However, this study has important limitations. First, the categorization of race and ethnicity is a social construct, arbitrarily chosen without biological evidence. Nevertheless, there is compelling evidence of how these categories affect outcomes in chronic liver disease (including MASLD) [21, 35, 52, 53]. Second, we focused only on the Hispanic/Latino ethnicity, with limited reporting observed for non-Hispanic/Latino ethnic groups; further research needs to account for ancestry in each region. Third, a significant number of studies did not report data on race and ethnicity, so our analysis was limited to data that were reported. Non-reporting was particularly common in studies conducted exclusively in Asia, and we believe this may underestimate the representation of Asian race. However, when limited to a specific US setting, our sensitivity analysis showed that this disparity in the representation of Asian race remained. In addition, we found unclear reporting of racial minorities, with most trials reporting only the White race and not clearly reporting other races. Fourth, we found high heterogeneity in most of our analyses, suggesting underlying differences that were further explored in subgroup and meta-regression analyses. Yet, caution is required when interpreting these findings. Fifth, few trials using the new MASH definition were found to assess the potential impact of the terminology shift on participant representation in MASH trials. Finally, we were unable to assess how variations in MASH epidemiology (e.g., the inclusion of lean MASH) influenced the inclusion criteria and further representativeness of the included trials [2, 54].
In conclusion, this systematic review and meta-analysis show significant progress in the representation of women and Hispanics/Latinos in MASH trials but also reveal disparities in the distribution of trial sites worldwide, suboptimal reporting of race and ethnicity, and racial disparities in enrollment. Trends in changing representation over time and trial size for Whites and Hispanics/Latinos were also identified. These data provide an overview of the representativeness of MASH trials and help guide future clinical trials to reduce racial disparities in enrollment, promote inclusivity and ultimately improve patient care.
Author Contributions
Matheus Souza: conceptualization, data curation, formal analysis, writing – original draft, writing – review and editing, methodology. Lubna Al-Sharif: formal analysis, data curation, methodology. Ivanna Diaz: formal analysis, data curation, methodology. Samira Mohamad Khalil: formal analysis, data curation, methodology. Xiu-He Lv: formal analysis, methodology, writing – review and editing. Alessandro Mantovani: writing – review and editing, supervision. Cristiane Alves Villela-Nogueira: writing – review and editing, supervision. All authors have read and approved the final version of the manuscript for submission.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that supports the findings of this study are available in the Supporting Information of this article.




