Unravelling the Role of PANoptosis in Liver Diseases: Mechanisms and Therapeutic Implications
Funding: This work was supported by The National Natural Science Foundation of China (82360132), Gansu Province Joint Scientific Research Fund Program (24JRRA911), Gansu Provincial Key R&D Program Projects (24YFFA037), Gansu Province Key Research and Development Program Project (22YF7FA085), and Gansu Province Joint Scientific Research Fund Project (23JRRA1489).
Wanyuan Xiong and Junfeng Li are co-first authors.
Handling Editor: Luca
ABSTRACT
PANoptosis is a multimodal form of cell death that involves inflammatory, apoptotic, and necroptotic pathways, playing a key role in the development of liver diseases. This article first outlines the definition and characteristics of PANoptosis, and then explores its mechanisms of action in different types of liver diseases, including acute liver injury, liver failure, metabolic dysfunction-associated fatty liver disease, and hepatocellular carcinoma. Furthermore, this article analyses the molecular regulatory network of PANoptosis and potential therapeutic targets. Finally, this article summarises the current research on PANoptosis in liver diseases and future research directions, and it reviews the role of the emerging cell death mechanism of PANoptosis in liver diseases.
Summary
- This article provides a detailed exploration of the molecular mechanisms of PANoptosis, a multifaceted form of cell death, and its role in liver diseases, offering new insights into its significance in hepatic pathology.
- This article emphasises the connection between PANoptosis and liver diseases, such as acute liver injury and chronic liver disease, revealing the critical role of cell death in the pathological processes of the liver.
- This article explores the potential of targeting PANoptosis as a novel therapeutic strategy and suggests methods for improving treatment approaches for liver diseases. It emphasises the need for further investigation into the specific mechanisms of PANoptosis in various liver diseases and the necessity of targeting this pathway, highlighting the urgency and importance of future research.
1 Introduction
Liver diseases are a collective term for all diseases that occur in the liver, including viral hepatitis, chemical liver diseases (alcohol, drugs, toxins, etc.); autoimmune and cholestatic liver diseases; hereditary metabolic liver diseases; and vascular liver diseases. Liver diseases cover all stages from acute liver damage (including acute liver failure [ALF]), liver fibrosis, and cirrhosis (end-stage liver disease) to primary hepatocellular carcinoma (HCC). They can also be classified according to the course of the disease into acute and chronic stages, and further refined into diagnoses based on liver function, complications, laboratory test results, and other factors [1]. Globally, two million people die from liver diseases each year, accounting for about 4% of the total annual global deaths, with cirrhosis and liver cancer complications being the main causes of death for patients with liver disease [2]. The pathogenesis of liver diseases is complex, and at the microscopic level, cell death [3], mitochondrial dysfunction [4], and abnormal regenerative and repair functions of hepatocytes [5] are all part of the pathogenesis. Cell death, as the most widespread phenomenon in the development of liver diseases, plays an important role in the development of all kinds of liver diseases.
Cell death refers to a series of ordered biological processes designed to remove cells that are damaged, senescent, or no longer needed [6]. The commonly known forms of cell death include apoptosis, autophagy, and necrosis, as well as some alternative forms of cell death that have received widespread attention in recent years, including pyroptosis, necroptosis, ferroptosis, and cuproptosis [7]. These modes of cell death can influence each other under specific circumstances, and PANoptosis is an emerging and complex form of cell death resulting from the interplay of pyroptosis, apoptosis, and necroptosis [8]. PANoptosis was first reported in 2016 [9] and named in 2019 [10].
The pathogenesis and progression of many diseases are closely associated with PANoptosis, including cancer, infectious, and inflammatory diseases [11]. PANoptosis also plays a significant role in liver diseases [12-14]. PANoptosis primarily exerts its effects on the liver by activating inflammatory and immune responses [15, 16]. By uncovering the mechanisms of PANoptosis in liver diseases, new therapeutic targets or biomarkers can be identified to improve disease prognosis. To conclude, the study of PANoptosis is crucial for advancing our understanding of the pathophysiology of liver diseases, identifying new therapeutic targets, and developing novel treatment strategies.
2 Basic Concepts and Mechanisms of PANoptosis
2.1 Activation and Progression of PANoptosis
PANoptosis is a complex form of programmed cell death that integrates features of pyroptosis, apoptosis, and necroptosis, challenging traditional classification within these categories [17]. This intricate process is triggered by a variety of stimuli, particularly in response to infections or during cellular stress. The innate immune system detects invading pathogens through pathogen-associated molecular patterns (PAMPs), which are unique molecular structures foreign to the body. Concurrently, damage-associated molecular patterns (DAMPs), endogenous molecules released due to cellular damage, also signal the immune response. These signals are recognised by specific sensors that initiate PANoptosis by assembling the PANoptosome complex.
As depicted in Figure 1, under cellular stress or infection, the activation of sensors, such as Z-DNA binding protein 1 (ZBP1), absent in melanoma 2 (AIM2), receptor-interacting protein kinase(RIPK) 1, and nucleotide-binding leucine-rich repeat-containing receptor (NLRP) 12 leads to the formation of a PANoptosome complex. This complex sets off a cascade of events, including the activation of caspases (e.g., Caspase-3/7), the cleavage of gasdermin proteins (e.g., Gasdermin D and Gasdermin E), and the phosphorylation of mixed lineage kinase domain (MLKL). These events ultimately result in the formation of membrane pores and the rupture of the cell membrane. In addition, PANoptosis can be activated by mitochondrial damage and endoplasmic reticulum stress. In the later stages of PANoptosis, this rupture leads to the release of intracellular contents and inflammatory cytokines, thereby initiating an immune response.
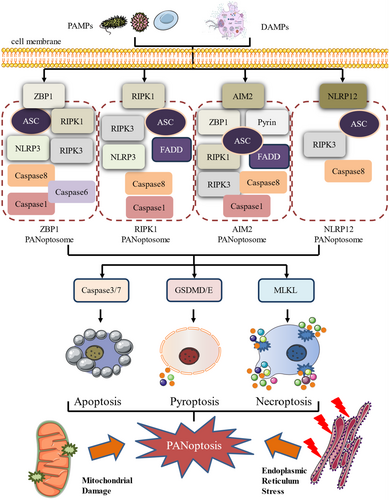
The resulting inflammatory reaction is crucial for controlling infections and maintaining tissue homeostasis, thus reinforcing the body's defence mechanisms and preserving cellular balance. The interplay between PANoptosis and immune response underscores the significance of this complex form of cell death in both physiological and pathological contexts. Understanding the activation and regulation of PANoptosis is essential for deciphering its role in disease progression and for developing targeted therapeutic strategies.
2.2 PANoptosome Assembly and Regulation in PANoptosis
In PANoptosis, sensors detect different PAMPs or DAMPs, initiating the assembly of PANoptosomes in a context-dependent manner. This assembly activates pyroptosis, apoptosis, and necroptosis simultaneously through various catalytic effectors, highlighting the PANoptosome complex as the central component of PANoptosis regulation [18]. Four distinct types of PANoptosomes have been identified, the ZBP1 PANoptosome [19, 20], the RIPK1 PANoptosome [21, 22], the AIM2 PANoptosome [23], and the NLRP12 PANoptosome [24], each with its unique sensors and regulators.
The ZBP1 PANoptosome, which assembles in response to IAV infection or NEI and Interferon (IFN) stimulation, includes components, such as ZBP1, apoptosis-associated speck-like protein (ASC), RIPK1, RIPK3, NLRP3, Caspase-1, Caspase-6, and Caspase-8 [20, 25]. The AIM2 PANoptosome forms following HSV1 or Francisella infection, with AIM2 as the sensor, and includes AIM2, Pyrin, ZBP1, ASC, Caspase-1, Caspase-8, RIPK3, RIPK1, and FADD [23]. The RIPK1 PANoptosome assembles in the absence or inhibition of Transforming growth factor-β-activated kinase 1 (TAK1) upon Yersinia infection, consisting of RIPK1, NLRP3, ASC, Caspase-1, RIPK3, FADD, and Caspase-8 [26]. Finally, the NLRP12 PANoptosome assembles in response to heme plus PAMP stimulation, composed of NLRP12, ASC, RIPK3, and Caspase-8 [24, 27].
Each PANoptosome, while uniquely composed, generally consists of three components: (a) Sensors that detect PAMPs or DAMPs, including not only the aforementioned sensors but also others, such as NLRP3 [28], NLR Family CARD domain containing (NLRC) 5 [29], and NLRC4 of the NLR family [30], Pyrin [23]. (b) Adaptors that serve as connecting bridges, with adapter proteins like ASC containing a caspase recruitment domain, facilitating the assembly of the PANoptosome complex [31]. (c) Catalytic effectors or executioners, including RIPK1, RIPK3, and Caspase-1/8 [23, 25], which mediate the downstream signalling events leading to cell death [32].
2.3 Endoplasmic Reticulum Stress in PANoptosis
In addition to the PANoptosome, endoplasmic reticulum stress and mitochondrial damage are also not negligible in the development of PANoptosis. Endoplasmic reticulum stress is a response mechanism triggered by endoplasmic reticulum dysfunction, which is usually caused by a variety of factors, such as hypoxia, nutritional deficiencies, pathogen infections, and responses to certain drugs, and leads to the accumulation of unfolded proteins. When the endoplasmic reticulum senses abnormally folded proteins, it initiates the unfolded protein response in an attempt to restore endoplasmic reticulum function and avoid cell death. However, if endoplasmic reticulum stress is too severe to be effectively regulated, cell death can result [33]. Endoplasmic reticulum stress-induced cellular injury and metabolic disturbances can activate signalling pathways associated with PANoptosis [34]. These signalling pathways may involve the release of endogenous molecules that activate sensors required for PANoptosis for cell death [35]. In ALF, the endoplasmic reticulum stress inhibitor 4-phenylbutyric acid weakened the promotion effect of the deacetylated mechanism of deacetylated malate dehydrogenase 1 (MDH1) K118 and isocitrate dehydrogenase 1 (IDH1) K93 on PANoptosis [36]. The relationship between endoplasmic reticulum stress and pan-apoptosis exhibits interactive and dependent properties. By modulating the endoplasmic reticulum stress response, cells determine different modes of death in response to dysfunctions and pathological states. It is important to consider the interrelationship between the two when studying the mechanisms of PANoptosis and its role in disease.
2.4 Mitochondrial Damage in PANoptosis
Mitochondria are essential organelles within the cell, with a broad range of functions that encompass energy production, metabolic regulation, reactive oxygen species (ROS) signalling, calcium homeostasis, and various aspects of cell death mechanisms. They also serve as the hub of the intrinsic apoptotic pathway induced by a variety of stimuli [37]. Mitochondrial damage can lead to metabolic dysregulation, which in turn can trigger cellular stress responses that provide the inducing conditions for PANoptosis. Zhang et al. discovered that when damaged mitochondria are released into the circulation, specific mitochondrial components are recognised as PAMPs by pattern recognition receptors of the innate immune system, thereby driving inflammatory responses [38]. Mitochondrial damage results in the release of intrinsic pro-apoptotic factors, such as cytochrome c (Cyt c) from the mitochondria into the cytoplasm; these factors can activate caspases, enhance apoptotic signalling, and interact with PANoptosis signalling pathways, increasing the risk of PANoptosis [39]. Additionally, mitochondrial damage can trigger inflammatory responses within the cell, leading to the release of DAMPs [40], which can activate various modes of cell death, including apoptosis and pyroptosis [41]. When mitochondria are severely damaged, they may initiate the PANoptosis pathway rather than being limited to a single death program. PANoptosis integrates these diverse death signals, providing a mechanism for cells to cope with extreme stress. Overall, mitochondrial damage is closely related to PANoptosis through its impact on cellular energy metabolism, the release of pro-apoptotic factors, and inflammatory responses. A deeper understanding of the relationship between mitochondrial damage and PANoptosis can help reveal the mechanisms of cell death and their roles in various diseases, offering potential targets for treatment.
3 Important Factors of PANoptosis
The development process of PANoptosis involves the activation of many key components and signalling pathways. In the PANoptosome, there is primarily the activation of Caspase-8, and downstream of it, there is mainly the involvement of Gasdermin proteins and the action of MLKL.
3.1 Caspase-8 in PANoptosis
Caspase-8 is the central regulatory molecule of PANoptosis, and its activation is a key step in the PANoptotic pathway [42]. Under certain conditions, such as the loss of function of TAK1 kinase, Caspase-8 can promote the assembly of PANoptosomes, leading to the occurrence of PANoptosis [16, 43]. The role of Caspase-8 in PANoptosis is not only involved in the execution of cell death but may also be involved in the crosstalk and regulation between cell death pathways. Under specific conditions, such as viral infection, Caspase-8 can be activated through various receptors (including death receptors). Once activated, Caspase-8 initiates a cascade of events leading to the activation of other caspases and promoting cell death. As a key protease, Caspase-8 mediates apoptosis, but it is not only responsible for initiating the apoptotic process; it can also activate other cell death pathways [44].
When Caspase-8 is enzymatically active, it primarily drives apoptosis, a process that does not cause tissue damage and is essential for the removal of unwanted cells, such as infected or senescent cells. However, when Caspase-8 lacks enzymatic activity, it can lead to embryonic lethality and inflammatory tissue destruction by inducing necroptosis and pyroptosis [45, 46]. In the context of PANoptosis, the enzymatic activity of Caspase-8 is crucial for inhibiting pyroptosis, a highly inflammatory form of cell death that is activated in response to microbial pathogens and is crucial for enhancing antimicrobial immunity [47]. Interestingly, when necroptosis is blocked, enzymatically inactive Caspase-8 can act as a protein scaffold to form an inflammasome complex, which ultimately leads to pyroptosis [48]. Moreover, the study by Hamid Kashkar's team revealed that the expression of enzymatically inactive Caspase-8 induces necroptosis and pyroptosis, causing embryonic lethality in mice [42]. This indicates that Caspase-8, regardless of its enzymatic activity, plays a significant role in the regulation of cell death mechanisms. In summary, the function of Caspase-8 is multifaceted; with enzymatic activity, it drives apoptosis, while without enzymatic activity, it can induce both necroptosis and pyroptosis, highlighting its role as a critical regulatory node in cell death pathways.
3.2 Gasdermin Proteins in PANoptosis
Gasdermin proteins are a family of pore-forming proteins capable of mediating a highly inflammatory form of cell death known as pyroptosis, which is a component of PANoptosis. Gasdermin proteins typically require cleavage by inflammasomes or caspase proteases for activation. For instance, Gasdermin D (GSDMD) can be cleaved by Caspase-1 or Caspase-11, releasing its N-terminal domain, which oligomerizes on the cell membrane to form pores, leading to cell death and the release of inflammatory cytokines [49]. The activated N-terminal domain of Gasdermin proteins oligomerizes on the cell membrane, forming pores that compromise the integrity of the cell membrane, a characteristic event of pyroptosis [50, 51]. The pore formation mediated by Gasdermin leads to the release of cellular contents that can act as DAMPs, activating inflammatory responses [52]. In PANoptosis, pyroptosis mediated by Gasdermin proteins may act in concert with apoptosis and necroptosis to collectively promote cell death [53]. The activity of Gasdermin proteins is tightly regulated. The activation and function of Gasdermin proteins are regulated by various mechanisms, including post-translational modifications and protein–protein interactions. In addition, their activation and function are crucial for controlling cell death and inflammatory responses [54]. Overall, Gasdermin proteins contribute to PANoptosis by mediating membrane pore formation and promoting inflammatory responses, synergizing with other cell death mechanisms to participate in the cell death process.
3.3 MLKL in PANoptosis
MLKL plays a crucial role in the molecular mechanisms of PANoptosis, particularly in mediating necroptosis, which is one of the components of PANoptosis. MLKL, identified as a downstream target of RIPK3, acts as the executioner of necroptosis [55]. MLKL is activated through phosphorylation by RIPK3, which is triggered by various stimuli, including death receptor ligands and PAMPs. Upon phosphorylation, MLKL undergoes a conformational change that leads to its translocation from the cytosol to cellular membranes, where it induces membrane permeabilisation. The translocated MLKL causes loss of membrane integrity, resulting in the release of cellular contents and inflammatory responses characteristic of necroptosis [56]. MLKL contributes to the cell death pathway through interactions with other components of the apoptotic, pyroptotic, and necroptotic pathways [57-59]. The role of MLKL in PANoptosis is many-sided, and its activation and function are tightly regulated, making it a potential therapeutic target in diseases where PANoptosis plays a significant role.
4 The Regulators of PANoptosis
The regulation of PANoptosis involves a variety of molecular mechanisms, and therefore research on inhibitors and agonists is still ongoing. Although specific inhibitors and agonists have not been fully identified, several molecules and drugs are currently known to potentially affect PANoptosis. The characteristics and mechanisms of action of pan-apoptosis-related activators and inhibitors are summarised in Table 1.
| Regulators | Typology | Features/functions | Mechanisms of action | Outcome |
|---|---|---|---|---|
| IRF1 | Activator | Transcription factors | Promotes PANoptosis by regulating ZBP1 expression | Promotion of PANoptosis in colorectal cancer involved in cell death regulation |
| TNF-α and IFN-γ | Activator | Inflammatory factors | Activation of JAK/STAT1/IRF1 axis induces nitric oxide production and caspase-8/FADD-mediated PANoptosis | Triggers PANoptosis in SARS-CoV-2 infection |
| LPS | Activator | PAMP | Initiates PANoptosis via pattern recognition receptors | Promotes PANoptosis, leading to inflammatory cell death |
| Viral RNA | Activator | PAMP | Initiates PANoptosis via pattern recognition receptors | Promotes PANoptosis, participates in antiviral response |
| HMGB1 | Activator | DAMP | Released upon cell injury or death to promote PANoptosis | Promotes PANoptosis, involved in inflammatory and cell death signalling |
| YBX1 | Activator | RNA-binding protein | Promotes ZBP1 expression by stabilising ZBP1 mRNA and exacerbates PANoptosis | Enhances PANoptosis, involved in cell death regulation |
| Gefitinib, Navitoclax | Activator | Chemotherapeutic agents | Activates PANoptosis through induction of cellular stress signalling pathways, including antiviral drugs and environmental pollutants | Activates PANoptosis for cancer therapy |
| Diamidobenzimidazole | Activator | Trigger of the STING pathway | STING agonist triggered PANoptosis, causing IFN-dependent acute lung inflammation, PANoptosis-mediated cell death, and inflammatory cytokine production | Triggers PANoptosis in acute respiratory distress syndrome |
| Eukaryotic translation initiation factor 2 alpha kinase 2 | Activator | A sensor for double-stranded DNA plays a crucial role in various conditions | Eukaryotic translation initiation factor 2 alpha kinase 2 can target activation of AIM2 to form a complex and mediate renal cell PANoptosis by activating the inflammasome | Treat the sepsis-associated AKI. |
| RIPK1/RIPK3/MLKL pathway | Activator | Signalling pathways activated by viruses | Direct activation of PANoptosis through the RIPK1/RIPK3/MLKL signalling pathway | Direct activation of PANoptosis, involved in cell death regulation |
| NF-κB pathway | Activator | A protein complex that controls transcribed DNA, cytokine production, and cell survival | Influence the expression of PANoptosis-related genes through the NF-κB pathway. Chemicals that activate the NF-κB pathway may indirectly promote PANoptosis | Indirectly promotes PANoptosis and affects inflammation and immune response. |
| JNK/p38 MAPK | Activator | Activator of specific signalling pathways | Influence PANoptosis through JNK or p38 MAPK signalling pathway | Promotes PANoptosis, involved in stress response and inflammation regulation |
| ADAR1 | Inhibitor | An enzyme that acts on double-stranded RNA | Interacts with the Zα2 domain of ZBP1 to limit the interaction between ZBP1 and RIPK3 and inhibit PANoptosis | Restricts PANoptosis in colorectal cancer and melanoma and promotes tumour development |
| TRIM 56 | Inhibitor | An endogenous inhibitor of YBX1 | Inhibits ZBP1 expression and PANoptosis by promoting YBX1 degradation | Inhibits PANoptosis, ameliorates spinal cord injury |
| Cysteine desulphurase | Inhibitor | Rate-limiting enzyme in iron–sulphur (Fe-S) cluster biogenesis | Blocks PANoptosis in a S293 phosphorylation-dependent manner | Inhibits PANoptosis in cisplatin treatment |
| Dickkopf-related protein 1 | Inhibitor | Typical inhibitor of the Wnt/B-catenin signalling pathway | Blocks GSDMD, caspase-3, RIPK3 | Inhibits PANoptosis in diabetic retinopathy |
| FUNDC1 | Inhibitor | A mitochondrial outer membrane protein | Interacts with Tu translation elongation factor to inhibit cytoplasmic release of mitochondrial DNA and PANoptosome activation | Prevention of PANoptosis in DOX cardiotoxicity |
| Melatonin | Inhibitor | One of the hormones secreted by the pineal gland of the brain. | Reduces expression of NLRP3, ASC, cleaved caspase-1, GSDMD, and cleaved GSDMD | Inhibition of PANoptosis in acute intraocular pressure elevation |
| Baicalin | Inhibitor | A flavonoid compound isolated from Scutellaria baicalensis root. | By blocking mitochondrial Z-DNA formation and ZBP1-PANoptosome assembly | Inhibits PANoptosis protects against inflammatory diseases and has anti-inflammatory effects |
| Cucurbitacin E | Inhibitor | A natural product of plant origin | Inhibits cyclin-dependent kinases1 through interaction with the PANoptosome in a ZBP1-dependent manner | Regulation of PANoptosis in adrenocortical carcinoma |
| Echinacea polyphenols | Inhibitor | A source of bioactive chemicals with several pharmacological activities | Regulating the expression of NLRP 3 inflammasome, gasdermin D, caspase-8, caspase-3, and MLKL. Reducing the activity of inducible nitric oxide synthase and the production of NO during acute liver injury | Alleviate LPS-induced acute liver injury by inhibiting PANoptosis |
| 3,4-Methylenedioxy-β-nitrostyrene | Inhibitor | A tyrosine kinase inhibitor | Specific inhibition of NLRP3 | Reduces PANoptosis in renal ischaemia–reperfusion injury |
4.1 Activators of PANoptosis
PANoptosis can be triggered or amplified by various activators, which may be endogenous or exogenous in nature. Endogenous activators include intracellular molecules or signals, such as PAMPs like bacterial lipopolysaccharide (LPS) and viral RNA, which are recognised by pattern recognition receptors to initiate PANoptosis [60]. Transcription factor Interferon regulatory factor 1 (IRF1) can promote PANoptosis by regulating ZBP1, thus playing a role in cell death regulation in diseases like influenza virus infection and colorectal cancer [61, 62]. In addition, some pro-inflammatory factors, including tumour necrosis factor (TNF)-α, IFN-γ can also promote cell death and may induce PANoptosis under specific conditions [63]. Pro-inflammatory factors can activate the JAK/STAT1/IRF1 axis, inducing nitric oxide (NO) production and caspase-8/FADD-mediated PANoptosis, particularly evident in SARS-CoV-2 infection [63]. DAMPs, such as high mobility group box 1 (HMGB1) and heat shock proteins, are released during cell injury or death and also contribute to the activation of PANoptosis [15, 64]. Y-box-binding protein 1 (YBX1) is a multifunctional protein in the RBP family that promotes ZBP1 expression by stabilising the Zbp1 mRNA, thereby exacerbating ZBP1-mediated PANoptosis [65]. Moreover, eukaryotic translation initiation factor 2 alpha kinase 2 can activate PANoptosis by inducing AIM2 upregulation [66]. The activation of death receptors like Fas and TNF receptor 1 can also initiate PANoptosis, either through caspase-dependent or -independent pathways [67, 68].
Exogenous activators encompass chemotherapeutic agents, antiviral drugs, environmental factors, and certain chemicals that induce cellular stress [69]. Viral infections, particularly those that activate the RIPK1/RIPK3/MLKL pathway, can directly trigger PANoptosis [23, 70]. Some chemical substances that induce cellular stress may facilitate the signalling pathways associated with PANoptosis. For instance, specific activators of signalling pathways, such as NF-κB, JNK, and p38 MAPK, may indirectly promote PANoptosis by influencing the expression of PANoptosis-related genes and modulating inflammatory and immune responses [71-73]. Diamidobenzimidazole acts as a stimulator of interferon genes pathway agonist, leading to PANoptosis through IFN-dependent mechanisms accompanied by acute lung inflammation and inflammatory cytokine production [74]. Genetic mutations affecting PANoptosis-related proteins may lead to abnormal activation of PANoptosis and immune activation through T-cell receptors or B-cell receptors can promote PANoptosis in certain contexts. The activation of PANoptosis is a multifaceted process involving numerous signalling pathways and molecular interactions, and the specific activators may vary depending on cell type and environmental conditions. Understanding these activators is crucial for elucidating the role of PANoptosis in diseases and for developing novel therapeutic strategies.
4.2 Inhibitors of PANoptosis
Many factors can activate PANoptosis, yet at the current stage, no specific inhibitor is widely recognised. The occurrence of PANoptosis is closely related to apoptosis, pyroptosis, and necroptosis, and many factors play an important role in its progression. Therefore, the inhibitors related to these factors can be considered as references. Inhibitors of PANoptosis comprise various enzymes, compounds, and proteins that restrict this form of cell death through different mechanisms. ZBP1 is a key sensor in PANoptosis, and its inhibitors may suppress PANoptosis by blocking the assembly of the ZBP1-PANoptosome. Karki et al. showed that adenosine deaminase acting on RNA-1 interacts with the Zα2 domain of ZBP1 to inhibit its interaction with RIPK3, thus suppressing PANoptosis and contributing to tumorigenesis in colorectal cancer and melanoma [75]. Tripartite motif containing 56 (TRIM 56) is an endogenous inhibitor of YBX1. In spinal cord injury, the interaction between TRIM 56 and YBX1 is a key molecular event that promotes the degradation of YBX1, thereby suppressing ZBP1 expression and mitigating PANoptosis, improving spinal cord injury [65]. Apart from ZBP1, the absence of other key factors of PANoptosis can also inhibit PANoptosis. Studies have shown that deleting the core components of the PANoptosome, Caspase-8, and RIPK3, can inhibit PANoptosis [76]. It can be reasonably speculated that knocking out key regulatory factors of PANoptosis through gene-editing technologies, such as CRISPR/Cas9, can directly suppress PANoptosis.
It was also found that some plant extracts also have an inhibitory effect on PANoptosis. Baicalin inhibits PANoptosis in macrophages by blocking the formation of mitochondrial Z-DNA and the assembly of the ZBP1-PANoptosome, thereby protecting against inflammatory diseases [16]. Cucurbitacin E can inhibit cyclin-dependent kinases1 through interaction with the PANoptosome in a ZBP1-dependent manner [77]. In addition, it was found that NO was identified as a novel upstream signalling molecule for PANoptosis [63]. Echinacea polyphenols may inhibit pan-cellular apoptosis by decreasing inducible NO synthase activity and NO production [78]. Other inhibitors include cysteine desulphurase, which blocks PANoptosis during cisplatin treatment in a phosphorylation-dependent manner [79]. Dickkopf-related protein 1 inhibits PANoptosis by blocking molecules like GSDMD and caspase-3 [80]. FUN14 domain containing 1 interacted with mitochondrial Tu translation elongation factor to prevent cytoplasmic mitochondrial DNA release and PANoptosom [81]. Melatonin can inhibit PANoptosis by reducing the expression of various pro-inflammatory factors, such as NLRP3 and ASC [82]. 3,4-Methylenedioxy-β-nitrostyrene can specific inhibit NLRP3 and reduces PANoptosis in renal ischaemia–reperfusion injury [83].
The ongoing development of inhibitors for PANoptosis holds promise for future therapeutic applications, as the identification of specific inhibitors targeting this process could significantly impact the treatment of a range of diseases, particularly those involving aberrant cell death pathways. Understanding both activators and inhibitors of PANoptosis is crucial for elucidating its roles in various diseases and for developing novel therapeutic strategies.
PANoptosis represents a unique inflammatory programmed cell death pathway characterised by the intricate interplay and activation of various cell death-associated molecules, culminating in the formation of PANoptosome complexes. These complexes play a pivotal role in regulating PANoptosis and are implicated in a diverse array of pathological conditions, including microbial infections, cancers, metabolic dysfunction-related steatotic liver disease, ALF, ischaemia–reperfusion injury, and organ failure. The regulatory nature of PANoptosis arises from the complex interactions of multiple molecular mechanisms within the cell, generating a distinct inflammatory response that is essential for pathogen elimination and the maintenance of tissue homeostasis. Elucidating the molecular basis and the regulatory approach of PANoptosis is critical to advancing therapeutic strategies aimed at modulating cell death in a variety of disease conditions, including liver disease.
5 The Role of PANoptosis in the Development of Liver Disease
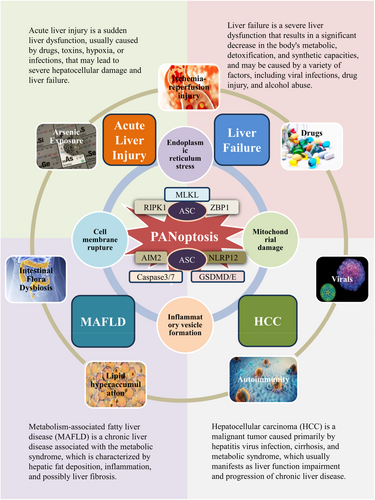
PANoptosis plays an important role in various liver diseases, such as acute liver injury (ALI), liver failure, metabolic dysfunction-associated steatotic liver disease (MASLD), and HCC, as shown in Figure 2. Additionally, the importance of PANoptosis should not be underestimated in other liver diseases, as its presence is part of the complex pathogenesis of liver disorders. Research on PANoptosis can further elucidate the occurrence and progression of various liver diseases, as well as their diagnosis and treatment.
5.1 PANoptosis in ALI
ALI is a common disease characterised by a rapid deterioration of liver function due to massive hepatocyte death over a short period, typically within 2 weeks [84]. It poses a serious threat to patient health and can be caused by various aetiologies, including drug-induced hepatotoxicity, ischaemia–reperfusion injury, autoimmunity, and viral hepatitis [85]. PANoptosis plays a significant role in ALI, particularly in the response of hepatocytes to various stressors and insults. In the context of ALI, hepatocytes can undergo PANoptosis due to the activation of specific signalling pathways [73]. PAMPs or DAMPs can trigger the pathways leading to PANoptosis [86].
In a study on ALI induced by excessive acetaminophen (APAP), it was found that APAP overdose triggers multiple forms of programmed cell death in hepatocytes, including pyroptosis, apoptosis, and necroptosis, while also inducing neutrophil activation. Activated neutrophils migrate to the site of injury and participate in hepatocyte damage by forming neutrophil extracellular traps (NETs), contributing to the progression of APAP-induced ALI. In the ALI mouse model induced by APAP, PANoptosis occurs alongside a significant increase in NETs markers (such as myeloperoxidase and citrullinated histone H3) in liver tissue and serum. Preinjection of DNase1 significantly inhibits NETs formation, reduces PANoptosis, and alleviates APAP-induced ALI. Additionally, AIM2 is involved in this process, as knocking down AIM2 eliminates NETs-induced PANoptosis in HepaRG cells. In AIM2 knockout mice, the ALI associated with excessive APAP is significantly reduced, and the occurrence of PANoptosis is also less frequent. This suggests that neutrophils, through NETs, participate in the hepatocyte damage and PANoptosis processes, potentially serving as a new therapeutic target for treating ALI [14]. PANoptosis contributes to the inflammatory response observed in ALI. The release of pro-inflammatory cytokines and danger signals during PANoptosis can activate immune cells, further propagating inflammation within the liver. Activated immune cells, including Kupffer cells and infiltrating lymphocytes, may contribute to a cycle of inflammation and cell death, exacerbating liver injury [87]. Understanding the role of PANoptosis in ALI opens up potential therapeutic avenues. Research into inhibitors of specific molecules involved in the PANoptosis pathway, such as caspases and other cellular effectors, may have therapeutic relevance for conditions like ALF and acute hepatitis.
5.2 PANoptosis in Liver Failure
Liver failure is a common clinical syndrome of severe liver disease. ALI can be transformed into liver failure in severe cases, and liver failure often results in severely impaired or dysfunctional hepatic synthesis, detoxification, metabolism, and biotransformation, with an extremely high mortality rate. Liver failure can be divided into four categories: ALF, subacute liver failure, acute-on-chronic (subacute) liver failure (ACLF), and chronic liver failure. Viruses, drugs, and autoimmune diseases are the main causes of liver failure in normal individuals. In recent years, alcohol abuse and metabolic syndrome have also become significant causes of liver failure [88, 89]. The high mortality rate of ALF creates an enormous health and economic burden worldwide. Currently, there is still a lack of effective treatment options for ALF, and liver transplantation remains the only recommended therapeutic approach. However, the application of liver transplantation is limited due to the scarcity of donor organs and the constraints of organ rejection. Therefore, in-depth research into the pathophysiological mechanisms of ALF may provide new strategies for its clinical treatment [90].
In ALF or ACLF, PANoptosis involves the activation of the immune system and the regulation of inflammatory responses. Acute triggers can activate immune cells and inflammatory cytokine pathways, leading to hepatocyte damage and necrosis. Research indicates that in an LPS/D-Gal-induced cell injury model, decreased cell viability, increased lactate dehydrogenase release, and higher cell death observed by PI staining are accompanied by increased levels of PANoptosis-related molecules in the LPS/D-Gal group compared to controls, indicating elevated PANoptosis levels in ALF. Furthermore, deacetylated MDH1 and IDH1 have been found to exacerbate PANoptosis in ALF through endoplasmic reticulum stress signalling. In ALF, deacetylated MDH1 and IDH1 weaken the inhibitory effect of the histone deacetylase inhibitor ACY1215 on PANoptosis, suggesting that the acetylation status of MDH1 and IDH1 impacts PANoptosis. In a mouse model, the deacetylated forms of MDH1 and IDH1 exacerbated liver tissue damage and increased PANoptosis levels in the liver tissue of ALF mice [36]. It has also been found that intestinal endotoxaemia could inhibit the expression of TAK1 by binding to TLR4 molecules and promote hepatocyte PANoptosis during ALF. In this context, promoting TAK1 expression could effectively relieve LPS-induced hepatocyte PANoptosis. Therefore, if we can effectively promote the expression of TAK1, it may serve as an effective target for the treatment of ALF, alleviating PANoptosis in hepatocytes induced by intestinal endotoxaemia [91].
Research into selectively inhibiting components of the PANoptosis pathway may yield new treatments for ALF. A deeper grasp of its role in liver failure could lead to more effective therapies and better patient outcomes. PANoptosis is critical to the pathophysiology of liver failure through its impact on inflammation and cell death, and exploring these mechanisms may inform new treatment strategies and enhance clinical management.
5.3 PANoptosis in Metabolic Dysfunction-Associated Fatty Liver Disease
MASLD is a group of fatty liver diseases associated with systemic metabolic disorders, the prevalence of MASLD is 33.0% [92, 93]. The pathogenesis of MASLD is complex and mainly includes lipid metabolism abnormalities, inflammatory responses, hepatocellular injury and cell death, genetic and environmental factors, and gut microbiome dysbiosis.
In the context of MASLD, hepatocytes undergo metabolic stress due to excessive lipid accumulation, insulin resistance, and oxidative stress. These factors collectively activate the process known as PANoptosis [13, 94]. The accumulation of surplus lipids within liver cells results in the release of DAMPs, which can initiate inflammatory signalling through pathways, such as the TNF-α axis critical for the onset of PANoptosis [63, 95]. In MASLD, apoptosis, pyroptosis, and necroptosis encompassed by PANoptosis can occur concurrently. Numerous factors trigger apoptosis in MASLD conditions, most of which are linked to mitochondrial dysfunction. Damage to the mitochondria can lead to mitochondrial outer membrane permeabilisation, resulting in the release of Cyt c into the cytoplasm. Once in the cytoplasm, Cyt c binds to Apaf-1 and pro-Caspase-9, facilitating the formation of apoptosomes that activate downstream caspases, ultimately leading to apoptotic cell death [96]. In patients with non-alcoholic steatohepatitis, excessive lipid accumulation promotes the generation of ROS. Elevated ROS levels, combined with excessive calcium ion (Ca2+) accumulation, can result in sustained mitochondrial permeability transition pore activation and the release of potent pro-apoptotic factors, such as Cyt c [97, 98]. Additionally, pyroptosis plays a significant role in hepatocyte death associated with MASLD. Here, TNF-α activates downstream signalling pathways via TNF receptor 1, which contributes to the activation and maturation of the NLRP3 inflammasome, culminating in the release of inflammatory cytokines IL-1β and IL-18 [99]. This cascade exacerbates the inflammatory response and leads to the swelling and rupture of hepatocytes. Moreover, necrotic apoptosis a programmed cell death mechanism contributes to pathological hepatocyte death in non-alcoholic steatohepatitis. This form of cell death results in the release of DAMPs, further triggering an inflammatory cascade through the activation of Kupffer cells, ultimately leading to hepatocyte swelling and subsequent cell death [100, 101].
In summary, PANoptosis represents a critical mechanism involved in the progression of MASLD. Further research is essential to fully elucidate the role of PANoptosis in MASLD and its potential as a target for treatment.
5.4 PANoptosis in HCC
Liver cancer was the eighth most common cause of cancer responsible for 485 000 deaths, which accounted for 0.86% of total global deaths [102]. The most common of all liver cancers is primary liver cancer, HCC accounts for 80%–90% of primary liver cancers. There are many causes of primary liver cancer, with the spread of the hepatitis B virus vaccine and the improvement of people's living environment, metabolic alterations have gradually become a major factor in these factors leading to primary liver cancer [103].
PANoptosis's role in cancer is intricate. It can prevent tumorigenesis by clearing damaged cells, maintaining homeostasis, and protecting against malignancy [104]. However, overactivation in the tumour microenvironment may inadvertently promote cancer by killing immune cells that suppress tumours, aiding immune evasion and inflammation-driven oncogenesis [105]. With regard to HCC, PANoptosis is activated by a variety of stimuli, particularly during infection or cellular stress responses. It may play a crucial role in the tumour microenvironment and cancer progression [106, 107]. The molecular mechanisms underlying PANoptosis in HCC involve the activation of multiple cell death pathways, which can be co-activated within the same cell. This complex interplay is mediated by a multiprotein complex known as the PANoptosome, which engages multiple modes of cell death. The PANoptosome complex includes key proteins, such as ZBP1, caspases, NLRP3, RIPK1, and RIPK3, these proteins are involved in significant crosstalk events within PANoptosis, and their dysregulation has been observed in HCC [108-111]. The activation of PANoptosis in HCC can lead to the release of DAMPs, which can trigger an inflammatory response and further contribute to the disease progression. Moreover, the expression levels of many PANoptosis-related genes, such as NLRP3, Caspase-8, and TNFAIP3, are aberrant in HCC, with significant mutations detected [109, 112].
Targeting PANoptosis in HCC is a promising therapeutic approach, as it allows for the selective modulation of key molecules in the PANoptosis pathway, potentially leading to the effective elimination of cancer cells. This process is crucial in HCC progression, and further research is needed to explore its role and therapeutic potential fully.
5.5 PANoptosis in Other Liver Diseases
PANoptosis also occupies an indelible place in other liver injuries and liver diseases, including sodium sulphite (SS) exposure, mechanical ventilation, and arsenic exposure. SS is a common additive in food and pharmaceuticals, but excessive exposure can induce PANoptosis in hepatocytes. Research indicates that SS leads to the accumulation of mitochondrial ROS, activating the BAX/Bcl-2/Caspase-3 pathway, which triggers apoptosis and activates RIPK1/RIPK3/p-MLKL, resulting in cell necrosis. Furthermore, SS may cause the release of cathepsin B due to p-MLKL-mediated lysosomal membrane permeabilisation, activating NLRP3-dependent pyroptosis [12]. Mechanical ventilation can cause extrapulmonary organ damage, including liver injury, by negatively affecting the lungs and increasing inflammatory cytokine secretion. HMGB1 protein acts as a pro-inflammatory mediator in ventilator-associated lung injury and triggers NETs formation and PANoptosis in the liver via the TLR4/MyD88/TRAF6 pathway, resulting in liver damage. Inhibiting NETs formation may alleviate liver damage, suggesting that HMGB1 is a potential therapeutic target for liver injury associated with mechanical ventilation [113]. Arsenic exposure leads to environmental pollution known as arsenic contamination and increases the risk of multi-organ toxicity. Studies indicate that arsenic-induced liver toxicity in chickens is linked to PANoptosis. Arsenic treatment elevates ZBP1 protein levels, resulting in abnormal expression of apoptosis-related factors, pyroptosis-related factors, and necrosis-related factors [114].
In conclusion, PANoptosis plays a role in liver diseases by modulating inflammatory responses, promoting cell clearance, and influencing metabolism and tumorigenesis in a variety of ways. To show more clearly the role of PANoptosis in various liver diseases, we summarised in Table 2. An in-depth study of the specific mechanisms of PANoptosis may provide new targets for the treatment of liver diseases and help develop more effective therapeutic strategies to improve the prognosis of patients.
| Diseases | Type of study | Major factor | Specific mechanism | Outcome | Reference |
|---|---|---|---|---|---|
| HCC | Basic research | Caspase-2 | Caspase-2 significantly remodels the HCC immune microenvironment and affects the response rate to immunotherapy | Caspase-2, a gene associated with PANoptosis, significantly inhibits cell proliferation, invasion, and migration of HCC cells | [106] |
| HCC | Bioinformatics analysis and experimental validation | Caspase-8, FADD, Caspase-6, NLRP3, Caspase-7, GSDMD, MLKL, IRF1, AIM2, ZBP1, Caspase-1, RIPK1, RIPK3, NLRC4 etc | Expression and mutation patterns of PANoptosis-related genes affect the prognosis and tumour microenvironment of HCC | Construction of a prognostic PANoptosis risk scoring model that helps predict patient survival and the complexity of the tumour microenvironment | [107] |
| HCC | Bioinformatics analysis and experimental validation | RIPK1, NLRP3, RIPK3, FADD, ZBP1, Caspase-8, Caspase-6, Caspase-1 | A prognostic model was constructed by the expression and mutation patterns of PANoptosis-related genes, revealing the molecular heterogeneity of HCC and predicting patient survival and tumour microenvironment | Provided the first evidence of the role of PANoptosis in HCC, offering new ideas for individualised and precise treatment of HCC | [109] |
| HCC | Bioinformatics Analysis | Caspase-8 | In the tumour microenvironment, Caspase-8 was correlated with various immune cell types, and mutational analysis showed that patients with elevated Caspase-8 expression had a higher frequency of TP53 mutations. In addition, Caspase-8 regulates YEATS Domain Containing 2 in HCC | The multifaceted role of Caspase-8 in HCC emphasises its prognostic and therapeutic significance | [112] |
| HCC | Bioinformatics analysis and experimental validation | GSDME, Polo Like Kinase 1, Death Associated Protein 3 and Protein Phosphatase 2 Regulatory Subunit B'Beta | The reliability of quantifying the individual risk level and prognosis of each HCC patient using PANscore and single-cell analysis revealed that the PANoptosis model was associated with tumour immune cell infiltration | Knockdown or silencing of the Death Associated Protein 3 and Polo Like Kinase 1 gene inhibits the growth of most HCC cells | [115] |
| ALF | Basic research | RIPK1, GSDMD, Caspase-3, MLKL, IL-18, IL-1β | Deacetylation of MDH1 and IDH1 may exacerbate PANoptosis in ALF, possibly through endoplasmic reticulum stress signalling | Targeting MDH1 and IDH1 acetylation modifications, as well as PANoptosis-associated molecules, can be therapeutic for ALF | [36] |
| ALF | Basic research | MLKL, RIPK1, GSDMD, Caspase-1, Caspase-3 and Caspase-7 | Intestinal endotoxaemia inhibited the expression of TAK1 by binding to TLR4 molecules and promoting hepatocyte PANoptosis during ALF | Promoting TAK1 expression is effective in alleviating LPS-induced PANoptosis in hepatocytes both in vitro and in vivo, suggesting that TAK1 may be an effective target for ALF treatment | [91] |
| ACLF | Bioinformatics analysis and basic research | NLRP3, Caspase-1, GSDMD, Caspase-8, Caspase-3, Caspase-7 and MLKL | The PANoptosome can radically integrate cell death patterns to drive robust inflammatory cell death under pathological conditions, which is highly consistent with the intense inflammatory pathogenesis of ACLF | Presence of PANoptosis-like cell death in ACLF | [116] |
| ALI | Basic research | AIM2, GSDMD, Caspase-3 and MLKL | Acetaminophen-induced ALI-derived mitochondrial DNA induces the formation of extracellular traps in neutrophils, and double-stranded DNA, a key component of NETs, is involved in the activation of the DNA sensor AIM2 and its downstream signalling molecules, which ultimately induces PANoptosis | Neutrophil extracellular traps promote acetaminophen-induced acute liver injury in mice by inducing PANoptosis through AIM2 | [14] |
| MASLD | Review | Caspase-8, Caspase-3, 6, 7, FADD, GSDMD, RIPK1, RIPK3, MLKL | The pro-inflammatory response triggered by PANoptosis-mediated immune cell regulation is a major driver of metabolic liver inflammation | Elucidating the regulatory mechanism of PANoptosis provides a solid foundation for addressing MASLD and advancing drug development | [117] |
| MASLD | Basic research | Caspase-1, 3, and 8, NLRP3, GSDMD, and MLKL | Fibronectin type III domain-containing protein 4 acts as a hepatocyte survival factor favouring mitochondrial homeostasis and decreasing PANoptosis via Adenosine 5-monophosphate-activated protein kinase α | Fibronectin type III domain-containing protein 4 prevents the initiation of TNF α-induced PANoptosis and the release of HMGB1 into the extracellular space of hepatocytes | [13] |
| MASLD | Basic research | ZBP1, RIPK3, Caspase-8, Caspase-6 | Siwutang profoundly repaired mitochondrial dysfunction, blocked mitochondrial permeability transition and Mitochondrial DNA (mtDNA) release into the cytoplasm, and subsequently reversed choline-deficient diet-stimulated PANoptosis and macrophage polarisation in hepatocytes in mice and in vitro | Siwutang prevents hepatitis-mediated hepatocyte apoptosis and macrophage M1 polarisation and ameliorates MASLD by affecting the intrahepatic synthesis, release, and intercellular transfer of mtDNA | [118] |
| MASLD | Basic research | Caspase-8, Caspase-6, GSDMD, MLKL | Liproxstatin-1 treatment markedly inhibited cleavages of PANoptosis-related Caspase-8 and Caspase-6 in MASLD mouse liver | Iron death inhibitors alleviate steatosis and steatohepatitis by blocking PANoptosis | [94] |
| Cholestatic liver fibrosis | Basic research | ZBP1, Caspase-8, Caspase-3, RIPK3, MLKL, GSDMD, Caspase-1 | Apigenin protects cholangiocytes from bile acid-stimulated PANoptosis, thereby attenuating damage-associated molecular pattern-mediated TANK binding kinase 1-NF-κB activation in macrophages | Apigenin treats cholestatic liver fibrosis by reversing the expression pattern of key genes associated with PANoptosis | [119] |
| Liver injury | Basic research | Caspase-3, RIPK1, RIPK3, MLKL, NLRP3 | Sodium sulphite leads to mitochondrial reactive oxygen species accumulation, activation of the BAX/Bcl-2/Caspase-3 pathway, and activation of RIPK1/RIPK3/p-MLKL, leading to PANoptosis | Sodium sulphite-induced PANoptosis can be regulated by p-MLKL | [12] |
| Liver injury | Basic research | Caspase-3, RIPK3, GSDMD | Mechanical ventilation-induced HMGB1 induces NETs formation and PANoptosis in the liver via the TLR4/MyD88/TRAF6 pathway | Inhibition of NETs Formation by DNase I or peptidyl arginine deiminase 4 Inhibitors or HMGB1 Neutralisation Ameliorates Liver Injury | [113] |
| Liver injury | Basic research | ZBP1, Caspase-8, Caspase-7, Caspase-3, NLRP3, ASC, GSDMD, RIPK1, RIPK3, MLKL | Arsenic exposure induces hepatotoxicity in chickens, mainly through activation of PANoptosis | PANoptosis is involved in arsenic-induced hepatotoxicity in chickens | [114] |
6 Clinical Significance of PANoptosis in Liver Diseases
The role of PANoptosis in liver diseases is garnering significant attention due to its implications for diagnosis, treatment, and prognosis. PANoptosis is a complex form of programmed cell death offering novel therapeutic targets for liver diseases [120]. By targeting crucial components, such as caspases, gasdermins, and MLKL, there is potential to alleviate liver injury and enhance outcomes in ALF and chronic liver diseases [121].
From a diagnostic perspective, molecules associated with PANoptosis, such as ZBP1 [122], RIPK1 [123], and AIM2 [124, 125], could serve as valuable biomarkers for the early detection and staging of liver diseases. Their detection not only aids in monitoring disease progression but also assesses treatment efficacy. Moreover, understanding the underlying mechanisms of PANoptosis in liver diseases sheds light on the complex relationships between cell death, inflammation, and immune responses, leading to a richer comprehension of disease pathogenesis.
PANoptosis also plays a crucial role in predicting treatment responses; certain genes and proteins linked to this pathway could inform how patients may react to specific therapies, facilitating more personalised medicine approaches [126, 127]. Additionally, elevated PANoptosis levels might indicate a worse disease state and poorer prognosis, guiding clinical judgement and decision-making [128].
It is important to recognise the dual nature of the inflammatory response initiated by PANoptosis. While it can contribute to host defence, excessive inflammation may further damage liver tissues, making it essential to strike an appropriate balance. The diversity of liver diseases, with varying roles of PANoptosis across conditions like viral hepatitis and non-alcoholic steatohepatitis, adds complexity to the development of targeted therapies and the interpretation of clinical outcomes [94, 117, 129].
In summary, the exploration of PANoptosis in liver diseases is multifaceted, encompassing scientific, medical, ethical, and practical dimensions. As research continues to unfold, our understanding of PANoptosis is poised to transform the landscape of liver disease diagnosis and treatment.
7 Perspectives
Despite the critical role of PANoptosis in liver diseases, challenges remain in translating its findings into clinical applications. A thorough investigation into the molecular mechanisms and regulatory networks underlying PANoptosis is necessary to identify new therapeutic targets and develop effective treatment strategies. Currently, our grasp of PANoptosis is limited, making it essential to explore its specific functions across various liver disorders.
Moreover, there are notable difficulties in selectively targeting PANoptosis without triggering unwanted side effects, as these pathways are essential for normal cellular processes and immune regulation. Bridging the gap between laboratory research and clinical practice is another significant obstacle, necessitating extensive clinical trials to ensure the safety and efficacy of therapies centred on PANoptosis. Ethical issues, including informed consent and potential treatment risks, also require careful consideration.
In addition, the mechanisms of PANoptosis in different liver diseases, such as hepatitis, cirrhosis, and HCC are not yet fully understood. Identifying the effects of PANoptosis and potential therapeutic targets in these contexts is crucial, as this knowledge will inform the design of effective treatment strategies and minimise side effects.
Ultimately, it is vital to convert foundational research into actionable clinical practices. This includes the development of innovative therapeutic strategies that target PANoptosis using approaches, such as gene therapy, drug modulation, and cellular immunotherapies to fine-tune this process. A cohesive evaluation system must also be devised to assess the safety and effectiveness of these new treatment modalities.
In conclusion, while PANoptosis holds considerable promise for liver disease management, addressing these challenges is critical. Advancing our understanding of PANoptosis, investigating its specific functions in liver diseases, and crafting effective clinical application strategies will be key to improving treatment outcomes and providing hope to patients. Future research will continue to validate the potential of PANoptosis as a key target, driving the development of the next generation of therapeutic approaches.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.