Secondary Sclerosing Cholangitis due to Drugs With a Special Emphasis on Checkpoint Inhibitors
Handling Editor: Luca Valenti
Funding: The authors received no specific funding for this work.
ABSTRACT
Background
Secondary sclerosing cholangitis (SSC), is one of the phenotypes of DILI first described in the 1980s. Check point inhibitors (CPIs) are currently the most frequent cause of SCC. Aims: To describe the epidemiology, clinical and biochemical features at presentation, differential diagnoses, pathophysiology, imaging, histological characteristics and management associated with SSC.
Materials and Methods
A language and date-unrestricted Medline literature search was conducted to identify case reports and clinical series on SSC with special emphasis on CPIs (2007-2023).
Results
We identified 19 different drugs that have been shown to induce SSC. A total of 64 cases with SSC due to CPIs are presented. This was mostly seen in patients treated with anti-Programmed cell death (PD)-1/PD-L1 inhibitors. The most frequent presenting signs and symptoms were abdominal pain and jaundice. Large-duct cholangitis induced by CPIs is a very rare condition while small-duct cholangitis is more common. Nivolumab and pembrolizumab were the most commonly implicated agents. Biopsies have revealed predominant CD8+ T cell infiltration in biliary strictures. Corticosteroids is linked to a low frequency of success and is the only agent recommended to begin the treatment.
Conclusions
CPIs-induced SSC seems to affect the entire biliary system. Clinicians should consider and suspect SSC when a probable CPIs-induced hepatitis does not respond to corticosteroids. Additionally, further randomized, controlled trials should prospectively investigate alternative therapies for treatment.
Abbreviations
-
- Biochemical resolution
-
- is that observed after suspension of offending agent, or after response to treatment leading to absolute normality of the biochemical parameters.
-
- Cholangiopathy
-
- a damage or injury to the bile ducts
-
- Cholestatic pattern of DILI
-
- increase of 2 or more times the ALP alone or when alanine aminotransferase (ALT)/ALP serum activity is two or less.
-
- DILI
-
- drug-induced liver injury. Liver injury associated with drug therapy after exclusion of competing etiologies.
-
- Hepatocellular pattern of DILI
-
- ALT ≥ 5 ULN and R ≥ 5.
-
- Large duct PSC
-
- typical findingas of sclerosing cholangitis on high-quality cholangiography, after exclusion of secondary sclerosing cholangitis.
-
- Mixed pattern of DILI
-
- when the ALT/ALP ratio is between 2 and 5.
-
- Secondary Sclerosing cholangitis (SSC)
-
- biliary strictures and/or biliary dilatations associated with recent drug use, in patients with cholestatic/mixed injury pattern, with exclusion of other causes of SSC than DILI.
-
- Small duct PSC
-
- compatible histology in a patient with a biochemical and clinical suspicion of PSC and with a normal cholangiography.
Summary
- One emerging clinical phenotype of drug-induced liver injury (DILI) is secondary sclerosing cholangitis (SSC) due to drugs.
- SSC has been found to develop in a significant proportion of unselected patients with DILI.
- SSC has been shown to lead to jaundice in most cases with a slow recovery of DILI compared to those without signs of SSC.
- Checkpoint inhibitors have been found to have the highest number of reports of SSC with more than 60 case reports published.
- The current review aimed at describing the epidemiology, clinical and biochemical features at presentation, differential diagnoses, pathophysiology, imaging and histological characteristics and management.
1 Background
Drug-induced liver injury (DILI) is very important to keep in mind when patients present with jaundice and/or elevated liver tests without obvious aetiology and a normal hepatobiliary imaging. It has though been increasingly recognised in recent years that DILI can also cause structural changes in the liver [1]. One of the type of changes that can be seen on imaging is abnormalities in the biliary tree such as biliary strictures, dilatations and other changes described in patients with primary sclerosing cholangitis (PSC). Apart from previous reports on secondary sclerosing cholangitis (SSC) from different kinds of drugs, this has recently been demonstrated in patients on treatment with checkpoint inhibitors (CPIs). SSC was reported in the 1980s after local treatment of liver metastases from colon cancer with hepatic intraarterial infusion of floxuridine, a cytotoxic agent [2]. Ludwig et al. from the Mayo Clinic, reported sclerosing cholangitis following hepatic intrarterial infusion of floxuridine in a 43-year-old male with hepatic metastasis [3]. Since the early reports, many different drugs have been associated with liver injury with imaging features mimicking sclerosing cholangitis [4-13]. Drugs leading to liver injury with features of SSC described in case reports or case series are shown in Table 1. Many drugs have only been published as single case report on this association but other drugs such as ketamine has a well-documented capicity to cause SSC [8-11, 13, 14, 19]. Most of the reports were originally described in young males who abused ketamine but this has recently been reported in critically ill patients in the intensive care unit (ITU), where ketamine has been used as long-term sedation [13, 19]. A convincing report of a 54-year-old woman with COVID-19 and ketamine-induced sclerosing cholangitis was recently published [13]. The patient received first oral ketamine and developed a sharp rise in alkaline phosphatase (ALP), which decreased after cessation of ketamine but increased considerably again after ketamine infusion. MR cholangiography demonstrated dilated common bile duct with distal narrowing and intrahepatic dilatations. Liver histology showed biliary ductular reaction with lobular inflammation and one small non-necrotizing lobular granuloma [13]. Long-term liver-related outcome was not reported [13]. In another case report, a 78-year-old male received docetaxel as a chemotherapy developed sclerosing cholangitis changes [12]. Imaging demonstrated diffuse intrahepatic biliary dilatation with multifocal narrowing and dilatations of the intrahepatic bile ducts [12]. Docetaxel was discontinued and liver biopsy showed subacute bile duct obstructive lesions, canalicular cholestasis, eosinophilic infiltration and bile duct stricturing. Thus, the patient had both features of large and small biliary duct changes. Imaging performed 5 months later, still revealed features of SSC [12]. Interestingly, a drug belonging to the same Taxane chemotherapy class of drugs, nab-docetaxel was found to induce SSC, with multiple stenoses of the biliary tracts found on imaging [15]. Recently, pacitaxel was implicated in another patient with SSC [16]. Since 2015, SSC associated with drug therapy has been observed with a number of agents (Table 1) [17, 18, 20].
| Reference | Cases (n) | Drug | IMC pattern | Clinic | Location of abnormal imaging | Imaging | Treatment | Biochemical response | Time for recovery | Imaging response | Death |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dikengil et al. [2] | 1 | Floxuridina intra-arterial | SD | Jaundice | IBD | Intrahepatic biliary dilatation and strictures | No | — | — | — | — |
| Ludwig et al. [3] | 1 | Floxuridina intra-arterial | LD | Jaundice, pruritus | EBD | Sclerosis of the proximal common hepatic duct | Prednisone (not available), liver transplantation | No | — | — | No |
| Schwab et al. [4] | 1 | Methimazole | SD | Pruritus, coluria | IBD | Strongly rarefied intrahepatic branches | Methimazole suspension | Yes | 3 months | Yes | No |
| Aldrighetti et al. [5] | 3 | Floxuridina intra-arterial | Mixed | Juandice | IEBD | Common hepatic duct stenosis, intrahepatic bile ducts dilation | Percutaneous biliary drainage, balloon dilation, stent in bile duct | Yes | — | Yes | No |
| Phongkitkarun et al. [6] | 34 | Floxuridine intra-arterial, 5-fluorouracil | SD/LD | Jaundice | 20 IEBD, 5 EBD, 9 IBD. | Extrahepatic duct abnormalities (periductal edema, enhanced and thickened wall and strictures), intrahepatic duct dilatation | No | — | — | — | — |
| Sandrasegaran et al. [7] | 11 | 9 Patients Floxuridine, 2 patients 5-fluorouracil. Both intra-arterial | SD/LD | Jaundice, pruritus | Bile Duct dilatation, bile duct enhancement, periportal edema. | Stent in bile duct | — | — | — | Eight of the patients yes for metastatic disease. Three no. | |
| Seto et al. [8] | 1 | Ketamine | Mixed | Abdominal pain | IEBD | Multiple long-segment strictures and narrowing in the intrahepatic ducts. Mildly dilated common bile duct | No | — | — | — | No |
| Lo et al. [9] | 1 | Ketamine | LD | Abdominal pain | EBD | Dilated common bile duct | Stent in bile duct | Yes | < 3 months | — | No |
| Turkish et al. [10] | 1 | Ketamine | SD | Fever, abdominal pain | IBD | Normal TAC | Ketamine suspension | Yes | — | Yes | No |
| Lui et al. [11] | 1 | Ketamine | Mixed | — | EBD | Dilated common bile duct, stricture at common bile duct | Stent in bile duct | Yes | — | No | No |
| Horsley-Silva et al. [12] | 1 | Docetaxel | SD | — | IBD |
Diffuse intrahepatic biliary dilatation with periductal enhancement |
Docetaxel suspension | No | — | No | No |
| Knooihuizen et al. [13] | 1 | Ketamine | Mixed | — | IEBD | Intrahepatic dilatation with a beaded appearance, dilated common bile duct with distal narrowing | Ketamine suspension | Yes | < 3 months | No | No |
| Teymouri et al. [14] | 11 | Ketamine | LD | Abdominal pain | EBD | Wall thickening of the gallbladder and dilated common bile duct | Papillotomy/ketamine suspension | Yes | — | — | No |
| Ketamine | LD | Abdominal pain | EBD | Intra and extrahepatic biliary dilatation | Papillotomy/ketamine suspension | Yes | — | — | No | ||
| Ketamine | LD | Abdominal pain | EBD | Extrahepatic biliary dilatation | Biliary drainage | Yes | — | Yes | No | ||
| Ketamine | LD | Abdominal pain | EBD | Extrahepatic biliary dilatation | Biliary stent | — | — | Yes | No | ||
| Ketamine | LD | Abdominal pain | EBD | Extrahepatic biliary dilatation | Ketamine suspension | — | — | — | No | ||
| Ketamine | LD | Abdominal pain, nausea | EBD | Extrahepatic biliary dilatation | Papillotomy/ketamine suspension | Yes | — | Yes | No | ||
| Ketamine | LD | Abdominal pain, nausea | EBD | Extrahepatic biliary dilatation | Papillotomy/ketamine suspension | Yes | < 3 months | Yes | No | ||
| Ketamine | LD | Abdominal pain, nausea, fever | EBD | Extrahepatic biliary dilatation and ultra-short narrowing | Sphincterotomy/ketamine suspension | Yes | — | — | No | ||
| Ketamine | LD | Abdominal pain, nausea | EBD | Extrahepatic biliary dilatation | Ketamine suspension | Yes | — | — | No | ||
| Ketamine | Mixed | Abdominal pain | IEBD | Moderate intrahepatic biliary dilatation and strictures, with thickening of the common duct wall and a stricture of the inferior common duct | Ketamine suspension | Yes | — | Yes | No | ||
| Ketamine | LD | Jaundice, abdominal pain | EBD | Extrahepatic biliary dilatation | Biliary stent/ketamine suspension | Yes | — | — | No | ||
| Matsuo et al. [15] | 1 | Nab-paclitaxel | Mixed | Jaundice | IEBD | Intrahepatic dilatation, right hepatic duct dilation | UDCA (600 mg) + PRED (40 mg) + Bezafibrate (400 mg) | Yes | — | No | No |
| Antón Rodriguez et al. [16] | 1 | Paclitaxel | Mixed | Jaundice, pruritus | IEBD | Multiple strictures and dilatations involving the intra and extrahepatic bile ducts | UDCA (not available)/stent in bile duct | — | — | — | Yes |
| Gudnason et al. [17] | 10 | Green tea extract | LD | — | EBD | Extrahepatic biliary dilation and strictures | — | — | > 6 months | — | — |
| Amoxicillin/clav. Acid | LD | — | EBD | Extrahepatic biliary dilation and strictures | — | — | > 6 months | — | — | ||
| Amoxicillin/clav. Acid | Mixed | — | IEBD | Intra and extrahepatic biliary dilation and strictures | — | — | > 6 months | — | — | ||
| Atorvastatin | LD | — | EBD | Extrahepatic biliary dilation and strictures | — | — | > 3 months | — | — | ||
| Amoxicillin/clav. Acid | Mixed | — | IEBD | Intra and extrahepatic biliary dilation and strictures | — | — | > 3 months | — | — | ||
| Amiodarone | LD | — | EBD | Extrahepatic biliary dilation and strictures | — | — | > 6 months | — | — | ||
| Sevoflurane | Mixed | — | IEBD | Intra and extrahepatic biliary dilation and strictures | — | — | < 3 months | — | — | ||
| Infliximab | Mixed | — | IEBD | Intra and extrahepatic biliary dilation and strictures | — | No | — | — | — | ||
| Sevoflurane | Mixed | — | IEBD | Intra and extrahepatic biliary dilation and strictures | — | — | > 3 months | — | — | ||
| Venlafaxine | Mixed | — | IEBD | Intra and extrahepatic biliary dilation and strictures | — | — | < 3 months | — | — | ||
| Zhang et al. [18] | 1 | Lapatinib | Mixed | — | IEBD | Intra and extrahepatic biliary dilation and strictures | UDCA + reduced glutathione, polyene phosphatidylcholine, compound glycyrrhizin. | No | — | No | No |
- Abbreviations: EBD, extrahepatic bile duct; IBD, intrahepatic bile duct; IEBD, intra- and extrahepatic bile duct; IMC, immune-mediated cholangitis; LD, large-ducts type; PRED, prednisone; SD, small-ducts type; UDCA, ursodeoxycholic acid.
1.1 SSC Due to CPIs
SSC has also been frequently observed as adverse hepatic reactions in patients treated with CPIs. CPIs have revolutionised treatment of many different types of malignancies but at the cost of various immune-mediated adverse effects, including liver injury [21]. In early reports, hepatocellular type of injury with histological features of hepatitis was the dominating phenotype of liver injury due to CPIs [22, 23]. However, in 2017, four publications with case series and case reports were reported with the clinical picture of cholangitis associated with the use of CPIs [24-27]. These were small duct cholangitis [24, 25, 27], also vanishing bile duct syndrome [24] and large duct cholangitis with biliary strictures seen on imaging [26]. Since then a large number of patients have been reported presenting with clinical, biochemical and histological features of SSC [28-69]. Patients typically have cholestatic or mixed liver injury pattern, thickening of the bile ducts and diffuse biliary duct dilatation.
2 Epidemiology
Before 2015, information about SSC consisted of case reports or small case series of various drugs leading to biliary strictures and other changes in the biliary tree [2-11]. A study from Iceland of unselected patients with DILI analysed the proportion of patients with bile duct abnormalities [17]. The study was undertaken after the authors had the experience of a 40-year-old woman who developed strictures in the hilar region of the liver and multiple intrahepatic biliary strictures which were considered to be due DILI.
She had normal liver tests at the time of a scheduled surgery for a benign oesophageal tumour; and was discharged within a few days, without any complications However, she developed cholestatic jaundice and thorough diagnostic work-up revealed only the SSC as the cause of jaundice. The only drugs she received at the time of the surgery were sevoflurane and cefazolin. The latter one was considered a more likely cause as the latency and the biochemical features were similar to a previous report from the Drug-induced liver injury network (DILIN study) [70]. Cholangiographies are usually not undertaken in patients with suspected DILI. However, in a total of 102 patients who had been diagnosed with DILI, overall 25 had magnetic resonance cholangiopancreatographies (MRCPs) performed as a part of the diagnostic work-up [16]. In those who had MRCP available for analysis, 10/25 (40%) had cholangiographic changes [16]. Overall 9/10 (90%) had biliary strictures and in eight patients dilatations were demonstrated in the intra-and/or extrahepatic biliary tract [16]. A validation study of these findings was undertaken in patients in the DILIN study [20]. In that study, secondary sclerosing-like changes were observed in 7% of those with available imaging but only 24% of magnetic resonance imagings (MRIs) performed were available for review. Reports of some unavailable images suggested cholangiographic abnormalities and the proportion of patients with cholangiographic abnormalities might have been higher and only a small minority of patients were investigated with MRCP [20].
3 Clinical Features and Biochemical Patterns at Presentation
A recent meta-analysis of the features of biliary tract diseases in ketamine abusers showed that the most common presenting symptoms were right upper quadrant abdominal pain and/or epigastric pain, together with elevated liver tests [14]. In the Icelandic study on secondary sclerosing changes in DILI patients, all had cholestatic liver injury and had more frequent jaundice and liver injury was significantly slower to resolve compared with those who did not have evidence of biliary changes [17]. The involvement of the common bile duct was similar to those found in ketamine-induced sclerosing cholangitis [8-11, 13]. Liver histology was only available in four patients with concomitant demonstrated mostly as portal hepatitis with acute and chronic hepatitis as well as canalicular cholestasis [16]. The patients with SSC in the DILIN study were in line with the results of the Icelandic study, as most patients had jaundice and were hospitalised and were more likely to have cholestatic liver injury and prolonged liver injury than the overall DILIN cohort [17]. Overall 3/10 (30%) had SSC due to amoxicillin-clavulanate [17]. Two had both intra-and extrahepatic biliary strictures, whereas one had only extrahepatic strictures. The biochemical resolution was very slow and occurred after 4–14 months, but they recovered finally and had normal liver tests [17].
3.1 SSC Due to CPIs
In a study from three tertiary hospitals in London from 2018 to 2020, 10 patients reported cholangiopathy due to CPIs [71]. Pembrolizumab was the most commonly implicated agent, 8/10, with clinical jaundice in 6/10, and median alanine aminotransferase (ALT) and ALP, 225 and 1549 U/L, respectively [71]. The clinical features of SSC are very variable and include asymptomatic biochemical parameters of cholestasis, pruritus, upper abdominal discomfort and jaundice [46, 51]. Occasionally, it may debut with fatigue and fever and much more rarely present with delayed symptoms or asymptomatic alteration of liver biochemical parameters days after drug discontinuation [46, 63, 66]. According to our analysis of 64 cases of CPIs-induced SSC, the most frequent presenting signs and symptoms were abdominal pain and jaundice, while pruritus was described in only two cases (Table 3).
A study from Japan analysed 56 patients with all-grade liver injury and only 11 (19.6%) showed hepatocellular-type liver injury whereas 34 patients (61%) developed cholestatic or mixed-type liver injury, although only one patient showed abnormal image findings in the bile duct [36]. Cholangiopathy was a rare phenotype (0.7% of all ICIs treated patients), according to Berry et al., in contrast to the more often described hepatitis pattern (15% in this cohort) [71]. When a cholestatic pattern of liver injury is documented in clinical practice after ICIs therapy, oncologists and clinicians should be aware of the possibility of a secondary cholangiopathy and consider MRCP studies and sometimes a liver biopsy as well.
4 Differential Diagnoses Focusing on PSC
In a patient with drug-induced cholangitis, there is a long list of other causes that can be taken into consideration. Diagnosis of large duct PSC is based on typical findings of sclerosing cholangitis on high-quality cholangiography, after exclusion of SSC. Diagnosis of small duct PSC is based on compatible histology in a patient with normal cholangiography (most of the time with concomitant inflammatory bowel disease [IBD]), with a biochemical and clinical suspicion of PSC [72].
Some of these have only been reported in a few case reports and are extremely rare and the number of clinical and laboratory testing is dependent on the clinical context. The most common types of SSC and examples of causes of SSC are shown in Table 2.
| Type of SSC | Causes (examples) |
|---|---|
| Infectious | Recurrent pyogenic cholangitis, Cytomegalovirus, AIDS-related cholangiopathy, Cryptosporidiosis |
| Ischemic | Sclerosing cholangitis of the critically ill, hepatic artery thrombosis after liver transplantation, transarterial chemotherapy therapy |
| Chronic obstructive | Surgical trauma, post-cholecystectomy, strictures after liver transplantation, Portal hypertensive biliopathy, chronic pancreatitis |
| Immune-mediated | IgG4-associated cholangitis, eosinophilic cholangitis, sarcoidosis |
| DILI | See drugs in Table 1 |
If the patient is asymptomatic and has a history of IBD, the most likely diagnosis is PSC [72]. If the patient has been in the ITU for a long time, SSC due to critical illness is a likely cause of biliary strictures and other abnormalities in the biliary tree [73]. The cholangiography is most often motivated by jaundice and/or cholestatic liver injury. IgG4-associated cholangitis is a differential diagnosis to PSC and can occur concomitantly with a known PSC or separately [74, 75]. Measuring IgG4 in serum in patients with cholangiographic abnormalities is of importance as these patients usually respond to corticosteroids. The predominant aetiology of SSC has been divided into chronic obstructive such as post-surgical trauma, immune-mediated such as IgG4-related cholangitis, infectious, i.e., cytomegalovirus, ischemic, for example, hepatic artery thrombosis post liver transplant [76]. The detailed diagnostic work-up of patients with SSC is beyond the scope of this review article. In a patient with cholestatic DILI with jaundice, who has a very slow resolving of the liver injury, the results of evaluation of SSC among DILI patients [17, 20] suggest that MRCP should be considered in patients with suspected DILI and cholestatic liver injury.
5 Potential Mechanistic Pathways of Liver Injury
Many different drugs have been shown to induce SSC and SSC due to drugs is probably not a single entity. Thus, the pathophysiological mechanisms behind injury to the biliary tract leading to biliary strictures and dilatations of the biliary probably show considerable variation. Regarding ketamine-induced SSC, it has been shown to be able to affect N-methyl-D-aspartate (NMDA) receptors on smooth muscle cells which may lead to biliary tract dilatation [77]. Studies in cats have shown that ketamine was able to stimulate opiate receptors and lead to increased activity in the sphincter of Oddi, which might lead to resistance to bile flow and biliary strictures [78]. Several convincing reports of SSC have been described with chemotherapeutic drugs from the taxane family, i.e., docetaxel, nab-paclitaxel in paclitaxel [12, 15, 16]. Docetaxel is heavily metabolised in the liver and transported from the blood into hepatocytes by organic anion-transporting peptides and excreted into the bile via ABCB1- and ABCC2-mediated transport. It was also suggested that drug–drug interactions might have increased concentrations of the drug and therefore predisposed the patient to develop SSC [12].
5.1 SSC Due to CPIs
The underlying pathophysiology of SSC associated with the use of CPIs is probably totally different. The pathophysiology has been considered to be due to loss of self-tolerance. How this occurs is not clear but various mechanisms for breakdown of self-tolerance have been proposed [79]. The large number of patients reported with SSC due to CPIs has increased the knowledge on SSC considerably. Biopsies from injured bile ducts have shown predominantly CD8+ T-cytotoxic lymphocytes [24, 26, 29, 31, 59]. The reason for the dominance of these cells is unclear but it is conceivable that is related to the imbalance between regulatory T cells and effector T cells.
Recently, Japanese researchers who originally identified indoleamine 2,3-dioxygenase 1(IDO-1) as a histologic biomarker for the cholangiopathy of primary biliary cholangitis (PBC), investigated this biomarker in patients with SSC due to CPIs [79]. IDO-1, an intracellular enzyme degrading L-tryptophan along the L-kynurenine pathway, is an immune modulator and is associated with immunotolerance and immune inhibition [78]. Interestingly, expression of IDO-1–positive biliary epithelial cells was found in both intrahepatic and extrahepatic bile ducts, SSC induced by CPIs. Thus, it seems that IDO-1 might be a novel pathological marker for the diagnosis of SSC due to CPIs.
6 Imaging Characteristics
Imaging examination plays a key role in both diagnosing and identifying the different lesional patterns of the biliary tract induced by drugs. Image findings usually reveal large-duct cholangitis, involving both intrahepatic and extrahepatic ducts. Additionally, diffusely or segmentally non-obstructive biliary dilatation or stenosis are among the most relevant features (Figures 1 and 2). Anatomical changes, such as hypertrophy and enhancements of the bile duct walls can also be observed, along with gallbladder wall thickening, Gleason's sheath edema, and gallbladder edema [80].
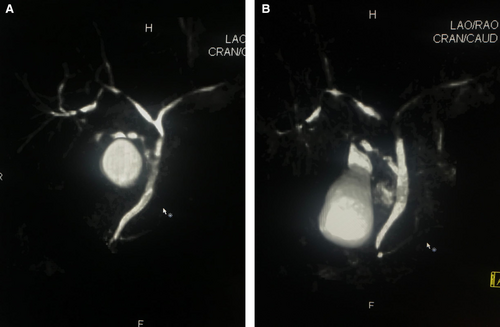
MRCP and endoscopic retrograde cholangiography are valuable imaging techniques for visualising the biliary tree and detecting morphological changes, such as luminal dilatation, strictures, and irregularities [81]. Contrast-enhanced MRI and computed tomography (CT) scans are also useful for evaluating bile duct walls, peribiliary or periportal masses, and liver and pancreas diseases. However, MRI offers superior soft tissue contrast resolution compared to CT, making MRCP with contrast-enhanced MRI the preferred method for evaluating biliary strictures [81].
Positron-emission tomography-computed tomography (PET-CT) and peroral cholangioscopy serve as valuable complementary methods in clinical practice. PET-CT aids in the early detection of cholangitis by identifying elevated fluorodeoxyglucose uptake in both gallbladder and bile ducts [54]. In turn, peroral cholangioscopy offers enhanced visualisation of various biliary duct abnormalities, including narrowing and diverticulum-like outpouching [39].
6.1 SSC Due to CPIs
Large-duct cholangitis induced by CPIs is a very rare condition while small-duct cholangitis is more common, and accounts for a significant number of cases previously diagnosed as “hepatitis” [82].
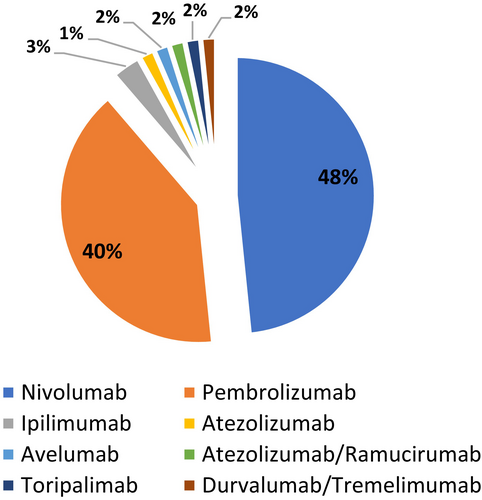
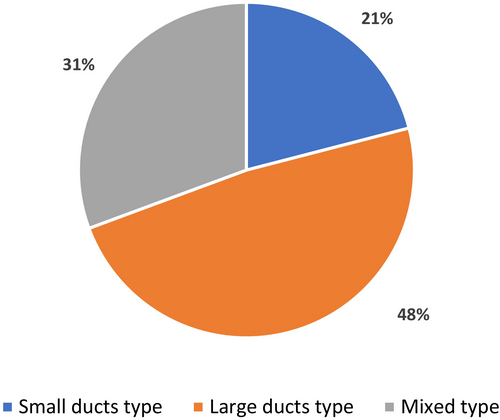
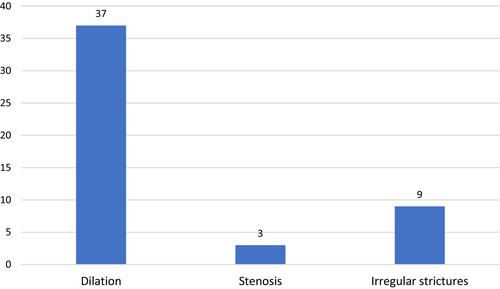
We carried out a comprehensive literature review from 2007 to 2023 and identified 64 published cases of SSC induced by immunotherapy (Table 3). Nivolumab and pembrolizumab were the most commonly implicated agents (Figure 3). We also analysed the imaging features and the frequency of biliary involvement reported according to the topography of the affected area. According to the type of involved area, we found that large ducts were compromised in 30/64 (48%) of cases, while small ducts showed biliary injury in 13/64 (21%) cases (Figure 4). The mixed subtype, which is a frequent finding in PSC, occurred only in 21/64 (31%) of cases. Bile duct dilatation was the most common feature of biliary damage in our analysis (Figure 5).
| Type of ICI | Reference | Cases (n) | ICI | IMC pattern | Clinica | Location of abnormal imaging | Imaging | Treatment | Biochemical response | Time to recovery | Imaging response | Death | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Small ducts types | Programmed cell death (PD)-1 | Doherty et al. [24] | 3 | Pembrolizumab | SD | Jaundice | — | — (Without image) | PRED (1 mg/kg) + MMF (2 g) + UDCA | Poor | — | — | Yes CRD |
| Nivolumab | SD | — | — | — (without image) | PRED (1 mg/kg) + UDCA | Partial | > 6 months | — | No | ||||
| Pembrolizumab | SD | Jaundice, hepatomegaly | — | — (Without image) | MPRED (2 mg/kg) + MMF (1 g) + UDCA | Partial | > 3 months | — | Yes | ||||
| Gelsomino et al. [25] | 1 | Nivolumab | SD |
Jaundice, pruritus |
— | Normal CT scan, diagnosis by biopsy | PRED (1 mg/kg) + UDCA (15 mg/kg) | Partial | > 3 months | — | Yes CRD | ||
| Oda et al. [33] | 1 | Nivolumab | SD | Fever, tachycardia, hyporexia | — | Capsule Glisson edema | PRED (1 mg/kg) + MMF (2 g) | Poor | — | — | Yes CRD | ||
| Kurokawa et al. [37] | 1 | Pembrolizumab | SD | Fatigue, fever, jaundice | — | Normal CT scan, diagnosis by biopsy | PRED (1 mg/kg) + UDCA (900 mg) | Partial | > 3 months | — | Yes CRD | ||
| Thorsteinsdottir et al. [52] | 1 | Pembrolizumab | SD | Jaundice, poor general condition | — | Normal CT scan, diagnosis by biopsy | PRED (120 mg) + MMF (2 g) + plasmapheresis | Poor | — | — | Yes DRD | ||
| Zhang et al. [58] | 1 | Nivolumab | SD | Fever, abdominal pain | — | — (Without image) | Glucocorticoids (not available) | Partial | — | — | Yes CRD | ||
| Moi et al. [60] | 2 | Pembrolizumab | SD | — | — | — (Without image) | MPRED (125 mg) + TCZ (500 mg) | Complete | < 3 months | — | — | ||
| Pembrolizumab | SD | — | — | Normal RMI | MPRED (125 mg) + TCZ (500 mg) | Partial | — | — | — | ||||
| Kynaston et al. [65] | 1 | Nivolumab | SD | Jaundice, nausea | — | Normal ultrasound | MPRED/PRED | Complete | > 3 months | — | No | ||
| PDL-1 | Nabeshima et al. [59] | 1 | Atezolizumab | SD | Nausea | — | Normal CT scan, diagnosis by biopsy | PRED (0.5 mg/kg) + UDCA | Partial | > 6 months | — | Yes CRD | |
| CTLA-4 | Yildirim et al. [27] | 1 | Ipilimumab | SD | Jaundice | — | Normal CT scan, diagnosis by biopsy | Partial | — | — | No | ||
| Mixed/ large duct types | PD-1 | Kawakami et al. [26] | 3 | Nivolumab | LD | Fever, abdominal pain | EBD | Dilation and hypertrophy EBD and gallbladder | PRED (0.5 mg/kg) | Partial | > 3 months | — | No |
| Nivolumab | LD | Fever, abdominal pain, diarrhoea | EBD | EBD dilation | PRED (0.5 mg/kg) + biliary drainage | Partial | > 6 months | — | No | ||||
| Nivolumab | LD | Fever, general fatigue | IEBD | EBD dilation | Biliary drainage | — | — | — | No | ||||
| Hamoir et al. [29] | 1 | Nivolumab | Mixed | — | IBD | Irregular walls and stenosis in IBD | MPRED (0.5 mg/kg) + UDCA | Complete | 3 months | — | No | ||
| Kashima et al. [30] | 1 | Nivolumab | LD | Abdominal pain, diarrhoea | EBD | EBD dilation and stenosis | PRED (2 mg/kg) + biliary drainage | Partial | > 3 months | Improve | No | ||
| Kuraoka et al. [31] | 1 | Nivolumab | LD | Pruritus | EBD | Dilation and thickening of EBD | PRED (60 mg)/MPRED (0.5 g) | Poor | — | — | Yes DRD | ||
| Le Tallec et al. [32] | 1 | Nivolumab | LD | Myalgia and a diffuse sclerodermiform skin thickening | IEBD | IEBD dilation | Glucocorticoids (not available) | — | — | — | No | ||
| Koya et al. [34] | 1 | Pembrolizumab | Mixed | Abdominal pain | IEBD | Dilation and thickening of IEBD | MPRED (0.5 g)/PRED (1 mg/kg) + UDCA (900 mg) + biliary drainage | Poor | — | — | No | ||
| Imoto et al. [36] | 1 | Nivolumab | Mixed | Abdominal pain | IEBD | IEBD dilation and multiple stenoses | MPRED (1 g) /PRED (0.6 mg/kg) + UDCA (600 mg) | Partial | — | — | No | ||
| Reddy et al. [38] | 1 | Nivolumab | LD | — | IEBD | Dilation and thickening of IEBD | PRED (0.85 mg/kg) + MMF (2 g) + TACRO (2 mg) + TCZ (4 mg/kg) + UDCA (1500 mg) | Poor | — | Worsen | No | ||
| Onoyama et al. [39] | 3 | Pembrolizumab | LD | Fever | EBD | Symmetric thickening of EBD | PRED (1 mg/kg) + UDCA (600 mg) | Partial | — | — | No | ||
| Pembrolizumab | LD | — | EBD | Symmetric thickening of EBD | UDCA (600 mg) | — | — | — | No | ||||
| Pembrolizumab | LD | — | EBD | Symmetric thickening of EBD | PRED (1 mg/kg) + UDCA (600 mg) | Partial | — | — | No | ||||
| Cheung et al. [40] | 1 | Pembrolizumab | LD | — | IBD | IBD stenosis | PRED (40 mg) + UDCA (75 mg) | Partial | > 3 months | — | No | ||
| Izumi et al. [41] | 3 | Nivolumab | LD | Abdominal pain, hyporexia | EBD | Dilation and thickening of EBD | PRED (2 mg/kg) + MMF (2 g) | Partial | — | — | No | ||
| Nivolumab | LD | Abdominal pain, hyporexia | EBD | Dilation and thickening of EBD | PRED (2 mg/kg) + MMF (2 g) | Partial | — | — | No | ||||
| Nivolumab | LD | Abdominal pain, hyporexia | EBD | Dilation and thickening of EBD | PRED (2 mg/kg) | Partial | — | — | No | ||||
| Cǎlugǎreanu et al. [42] | 1 | Nivolumab | Mixed | Nausea, abdominal pain | IEBD | Multiple IBD stenosis, IEBD dilation | PRED (1 mg/kg) | Partial | > 6 months | — | No | ||
| Anderson et al. [43] | 1 | Nivolumab | Mixed | Abdominal pain | IEBD | IBD dilation, EBD thickening | PRED (50 mg) + MMF + TAC | Poor | — | — | Yes CRD | ||
| Fouchard et al. [44] | 2 | Nivolumab | LD | Abdominal pain | — | EBD medium size without obstruction | PRED (0.5 mg/kg) + UDCA + cholecystectomy | Partial | > 6 months | — | No | ||
| Pembrolizumab | LD | — | — | Bile duct dilation and thickening | PRED (1 mg/kg) | Complete | > 6 months | — | No | ||||
| Ogawa et al. [45] | 1 | Pembrolizumab | LD | — | IEBD | Multiple stenosis and dilations of IEBD | — | — | < 3 months | Improve | No | ||
| Noda-Narita et al. [46] | 1 | Nivolumab | LD | Abdominal pain | IEBD | Diffuse narrowing IBD, IEBD dilation | UDCA (300 mg) | — | — | Improve | No | ||
| Kono et al. [47] | 1 | Nivolumab | LD | Jaundice | EBD | Diffuse intrahepatic cholestasis + gallbladder wall thickening | Biliary drainage | — | — | — | No | ||
| Sawada et al. [48] | 1 | Nivolumab | Mixed | — | EBD | EBD dilation | PRED (0.5 mg/kg) + UDCA | Partial | > 3 months | — | No | ||
| Zen et al. [49] | 2 | Pembrolizumab | Mixed | Abdominal pain, vomiting | IEBD | Irregular calibre changes and wall thickening IEBD | PRED (50 mg) | Partial | — | — | No | ||
| Pembrolizumab | Mixed | Fever, malaise | IEBD | Irregular calibre changes and wall thickening IEBD | PRED (40 mg) | Poor | — | — | No | ||||
| McClure et al. [50] | 1 | Nivolumab | Mixed | Abdominal pain | IBD | IBD dilation | PRED (2 mg/kg) | Partial | > 6 months | — | No | ||
| Onoyama et al. [51] | 3 | Nivolumab | LD | Backache | IEBD | IBD stenosis, EBD dilation | MPRED (2 mg/kg) + MMF (2 g) | Partial | — | — | No | ||
| Nivolumab | LD | Abdominal pain | EBD | EBD dilation | MPRED (2 mg/kg) + MMF (2 g) + biliary drainage | Partial | — | — | No | ||||
| Nivolumab | LD | — | EBD | EBD dilation | MPRED (1.6 mg/kg) | Partial | — | — | No | ||||
| Matsumoto et al. [54] | 1 | Pembrolizumab | LD | Abdominal pain | IEBD | Irregular strictures IEBD, gallbladder wall thickening | PRED (80 mg)/MPRED (1 g) + AZA (50 mg) | Partial | — | Improve | No | ||
| Sato et al. [55] | 1 | Pembrolizumab | LD | Abdominal pain | IEBD | Irregular strictures + thickening IEBD | MPRED (30 mg) + UDCA (300 mg) | Partial | > 6 months | Improve | No | ||
| Ooi et al. [56] | 1 | Pembrolizumab | Mixed | — | IBD | Irregular walls and discontinuous narrowings | PRED (40 mg)/ MPRED (1 g) + AZA (50 mg) | Partial | > 6 months | — | No | ||
| Moi et al. [60] | 1 | Pembrolizumab | Mixed | — | IBD | Dilation and thickening of IBD | MPRED (125 mg) + TCZ (500 mg) | Partial | — | — | — | ||
| Yoshikawa et al. [61] | 1 | Nivolumab | Mixed | — | IEBD | EBD dilation, multiple IBD stenoses | PRED (1.5 mg/kg) + MMF (2 g) + UDCA (600 mg) | Poor | — | — | Yes DRD | ||
| Takinami et al. [62] | 4 | Pembrolizumab | Mixed | — | EBD | Dilation and thickening of EBD | PRED | — | — | — | No | ||
| Pembrolizumab | LD | — | EBD | Dilation and thickening of EBD | — | — | — | — | No | ||||
| Nivolumab | Mixed | — | EBD | Dilation and thickening of EBD | — | — | — | — | No | ||||
| Nivolumab | LD | — | EBD | Dilation and thickening of EBD | — | — | — | — | No | ||||
| Qu et al. [63] | 1 | Toripalimab | Mixed | Abdominal pain, vomiting | IEBD | IEBD dilation, Vater ampulla thickening | None | Complete | — | Improve | No | ||
| Tanaka et al. [66] | 1 | Pembrolizumab | Mixed | Abdominal pain, fever | EBD | Common bile duct dilation and gallbladder wall thickening | MPRED 0.5 mg/kg | Poor | — | — | Yes CRD | ||
| Suzuki et al. [67] | 1 | Pembrolizumab | Mixed | — | EBD | Dilation and thickening of EBD | PRED + UDCA/MPRED + AZA | Poor | — | — | Yes DRD | ||
| Liang et al. [68] | 1 | Pembrolizumab | Mixed | — | EBD | Dilation and thickening of EBD | PRED (50 mg) + AZA + UDCA | Partial | > 3 months | — | No | ||
| Tsukaguchi et al. [69] | 1 | Nivolumab | Mixed | Abdominal pain, anorexia | EBD | EBD dilation and thickening, gallbladder wall thickening | PRED 1 mg/kg | Partial | > 3 months | Stable | No | ||
| PDL-1 | Cho et al. [28] | 1 | Avelumab | LD | Abdominal pain | EBD | Dilation and thickening of the wall of the common bile duct and gallbladder | MPRED 1 mg/kg | Partial | — | — | No | |
| Fujii et al. [57] | 1 | Atezolizumab/Ramucirumab | Mixed | Fatigue, abdominal pain | IEBD | Dilation and thickening EBD and gallbladder wall | PRED (30 mg) | Partial | > 3 months | Improve | No | ||
| CTLA-4 | Mizuno et al. [53] | 1 | Ipilimumab | LD | — | IEBD | IEBD dilation, gallbladder wall thickening | PRED (40 mg) | Poor | — | Worsen | Yes DRD | |
| CTLA-4 + PDL-1 | Fouchard et al. [44] | 1 | Durvalumab/Tremelimumab | LD | Abdominal pain | — | Bile duct dilation | PRED (120 mg) + UDCA + cholecystectomy | Partial | > 6 months | — | No |
- Abbreviations: AZA, azathioprine; EBD, extrahepatic bile duct; IBD, intrahepatic bile duct; ICI, immune checkpoint inhibitors; IEBD, intra- and extrahepatic bile duct; IMC, immune-mediated cholangitis; LD, large-ducts type; MMF, mycophenolate mofetil; MPRED, methylprednisolone; PRED, prednisone; SD, small-ducts type; TAC, tacrolimus; TCZ, tocilizumab; UDCA, ursodeoxycholic acid.
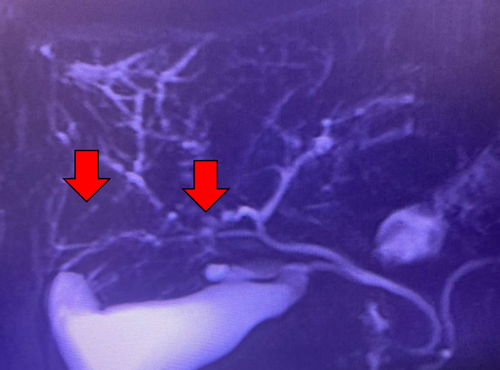
Pi et al. conducted a systematic review analysing 53 patients with SSC after ICI treatment [83]. Twenty-nine cases of intrahepatic cholangitis with predominant large-duct subtype and 12 cases with mixed subtype were identified using imaging techniques. Key features included dilation, stenosis and irregular thickening of the bile ducts, with either segmented or diffuse patterns. Except for three cases lacking comprehensive data, the remaining 38 cases exhibited abnormalities across various anatomical bile duct segments. Specifically, five cases presented abnormalities in the intrahepatic bile ducts, 15 in the extrahepatic bile ducts, and 18 in both intra- and extrahepatic bile ducts. Notably, patients with the large-duct subtype demonstrated a significantly higher incidence of extrahepatic bile duct abnormalities (53.8% vs. 8.3%), and a lower incidence of intrahepatic ones (3.8% vs. 33.3%), compared to those with mixed cholangitis.
Takinami et al. [62] compared the features between hepatitis and cholangitis in a large number of patients who developed CPIs-induced hepatic abnormalities in a retrospective study of 530 consecutive patients undergoing immunotherapy. They differentiated immune-related sclerosing cholangitis (irSC) and immune-related hepatitis (irHepatitis), according to the presence and absence of radiological features, such as bile duct dilation and wall thickness, respectively. Forty-one (7.7%) patients developed immune-related DILI. Among them, 12 patients underwent a CT scan, with 11 out of 12 presenting grade 3 or higher elevations of aminotransferase levels. IrSC was diagnosed in four patients (0.8%), all of whom had received anti-PD-1 treatment. Conversely, irHepatitis was more prevalent among patients treated with anti-CTLA-4 compared to those treated with anti-PD-1/PD-L1 inhibitors (14% vs. 0.2%). All irSC patients exhibited a a cholestatic pattern. Among these patients, 3 out of 4 (75%) experienced grade 3 or higher elevations of aminotransferases. The most important message of this study was that if patients had at least two times ALP showing a cholestatic pattern, radiological examination should be considered for the diagnosis of irSC.
Imaging showed biliary dilation without obstruction (77%), diffuse extrahepatic biliary tract hypertrophy (91%), and multiple intrahepatic biliary tract strictures (30%). Onoyama et al. [51] conducted a systematic review that analysed the clinical and pathological features of PD-1 inhibitor-related SSC. Thirty-one cases of PD-1 inhibitor-related SSC were evaluated, with non–small-cell lung cancer as the main indication requiring PD-1 inhibitor treatment. Nivolumab (n = 19), pembrolizumab (n = 10), avelumab (n = 1) and durvalumab (n = 1) were the culprit drugs triggering the biliary disease. The median onset of PD-1 inhibitor-related SC occurred after 5.5 cycles.
Although some features of SSC may mimic PSC in clinical presentation and in imaging features, SSC is usually more severe than PSC, although the susceptibility for SSC progression depends on the underlying aetiology. Unlike PSC, SSC often has well-identified and sometimes reversible causes [80]. On the other hand, PSC is a disease of unknown origin, often challenging to manage and associated with both a progressive clinical course and no established medications with confirmed efficacy [84].
Like PSC, SSC is a long-term condition characterised by segmental stenosis and dilatation of bile ducts (Figure 2). Despite many specific causes having been identified over time, clinical outcomes are generally less favourable for SSC if not promptly identified [85].
7 Histological Features and Role of Liver Biopsy
7.1 SSC Due to CPIs
CPIs-associated hepatitis often manifests with lobular inflammation characterised by histiocytes, vague to well-formed granulomas, fibrin ring granulomas and endothelialitis [86]. There is a tendency to describe cases of CPIs-associated cholangitis with the use of anti-PD-1/PD-L1 while those who received anti-CTLA-4 have more frequently been linked to hepatitis [62]. However, to our knowledge, there are no data in the literature that demonstrate distinctive histological features in CPIs-induced SSC according to whether PD1/PDL1 or CTRL4 were used.
CPIs-induced cholangitis may exhibit bile duct dilatation or obstructive changes on imaging, along with portal-based inflammation and bile-duct injury [87]. Additionally, predominant CD8+ T cell infiltration is a characteristic feature of ICI-induced cholangitis [49].
Zen et al. [88] compared the CD8+/CD4+ T cell ratio among cases of immune-mediated hepatitis, immune-mediated cholangitis, autoimmune hepatitis, and cases of DILI. A significantly higher CD8+/CD4+ T cell ratio was observed in immune-mediated hepatitis/immune-mediated cholangitis group (12.2 ± 5.1), compared to the autoimmune hepatitis group (2.7 ± 1.1) and the DILI group (5.0 ± 1.1). Additionally, they also noted various types of small bile duct injury, including irregularity of the bile duct epithelium, cytoplasmic vacuolization, duct degeneration, intraepithelial lymphocyte infiltration, periductal lymphocyte infiltration and periductal fibrosis. Features mimicking PBC and PSC as florid lesión of interlobular ducts and concentric periductal fibrosis were observed in the histological examination. In addition, the authors suggest that CPI-related cholangitis may be less responsive to steroid treatment than the hepatitis phenotype [88].
A retrospective review conducted by Cohen et al. included 60 ICI-treated patients who underwent liver biopsy due to elevated liver tests suggestive of immune-related hepatic toxicity [82]. Data revealed a cholangitic pattern of injury in 16 cases (26%). These cases exhibited focal or mild lobular injury but were characterised by mild to marked duct injury, often accompanied by mild portal edema. Portal inflammation was typically mild to moderate, with 69% of cases showing conspicuous neutrophils around injured ducts (pericholangitis). Granulomas adjacent to portal tracts were rare, as was the case of endothelialitis. Steatosis was present in some cases but not confined to areas of lobular injury. The cholangitic pattern was associated with bile duct dilatation or narrowing on liver imaging, and two patients were diagnosed with bile-duct obstruction due to tumour. Surprisingly, the pattern of inflammation, presence of granulomas, or endothelialitis on liver biopsy did not predict the response to steroids or the need for secondary immunosuppression. The authors concluded that liver biopsy findings in patients on immune checkpoint inhibitors may not reliably predict the necessity or duration of steroid therapy or the need for additional immunosuppression [82].
The histopathological features of large-duct cholangitis often include inflammatory infiltration of epithelial lining and diffuse fibrosis of the extrahepatic bile duct [82]. Zen et al. [49] reported a very interesting case of immuno-cholangitis linked to a mixed pattern induced by pembrolizumab. The intrahepatic duct injury resembled PBC, characterised by dense infiltration of inflammatory cells around the septal bile duct associated with granulomatous changes. In contrast, microscopic examination of extrahepatic duct resembled IgG4-related cholangitis, with extensive inflammatory infiltrate in the fibrotic duct wall [49]. However, the number of IgG4-positive plasma cells was less than 10 per high-power field. The authors stated that while ICI cholangitis may present with nonspecific inflammatory features, ductal damage and florid ductal injury can guide diagnosis [49].
Hountondji et al. [89], carried out a retrospective study, involving 117 European patients with CPIs-induced DILI. Forty-three out of 117 (39%) cases presented with a cholestatic pattern. Liver biopsy was carried out in 42% of patients, revealing granulomatous lesions, endothelialitis or lymphocytic cholangitis [89]. Biliary stenosis occurred in eight patients (6.8%), and was significantly more frequently associated with the cholestatic clinical pattern. The authors suggested that liver biopsy may play an important role in differentiating cholangitis and hepatitis for predicting steroid responsiveness. However, the response rate for steroid therapy in SSC is reportedly only 11.5% [84].
Coukos et al. reported 14 patients who underwent a liver biopsy where 4 (16%) of them showed a cholangitic pattern while 6 (24%) presented a mixed pattern linked to florid bile duct injury [90]. These histological findings were associated with an important increase of cholestatic liver enzymes and higher occurrence of jaundice. These authors propose that liver biopsy played an important role in diagnosing and managing liver adverse events induced by immunotherapy and sometimes in avoiding potentially deleterious immunosuppressive therapy in an oncologic patient.
8 Management
8.1 SSC Due to CPIs
Discontinuing CPIs and initiating glucocorticosteroid therapy are recommended according to clinical guidelines for treating high-grade immune-mediated liver disease induced by CPIs [91, 92]. Fifty percent of patients who develop hepatitis induced by CPIs regress without the need for treatment while a high percentage of those who have an indication for therapy respond favourably to corticosteroids, with clinical and biochemical improvement within 6–12 weeks [87]. However, corticosteroids appear to be less effective in cholangitis induced by immunotherapy. In a study from London [71], patients with SSC due to CPIs responded poorly to corticosteroids, which is in line with results from a recent systematic review, which reported a mean response to corticosteroids rate in only 11.5% [51]. Berry et al. [71] showed that after stopping CPI, all 10 patients received first-line treatment with prednisolone (1–2 mg/kg oral /IV). Three patients who did not initially respond with corticosteroids received mycophenolate mofetil as a second-line therapy. As a third-line treatment, tacrolimus was prescribed in one patient. Ursodeoxycholic acid was indicated in five patients after CPI therapy was stopped. Four patients had a clinical response but radiological signs of cholangiopathy persisted. They had a mean follow-up of 27 weeks where five patients passed away and the primary cause of death was the advancement of the cancer [71]. Like most of the reports, the therapy time was not stated in this analysis. Based on our literature review of 64 patients, only 11% of cases were reported to have a complete response, while 66% showed partial biochemical resolution. Pi et al. highlighted two interesting features regarding biochemical response after corticosteroid therapy [83]. Firstly, treatment was associated with decrease in cholestatic enzymes to a varying degree in most individuals, but total normalisation was uncommon. Secondly, it often took a long period of time for cholestatic enzymes to recover. For example, a case report showed that cholestatic enzymes did not return to normality even after 18 months of follow-up [50]. These findings are in agreement with those reported by Gudnason et al. [17], who confirmed 10 cases of SSC by biliary imaging (all females), among 102 patients with DILI. Five of them resolved their liver tests in 3–6 months, while 3 patients required 6 months for resolution. It was found that these patients were more likely to present with jaundice and longer recovery of liver parameters than other patients with cholestatic/mixed type of DILI [17].
In the context of CPIs and because the underlying cancer is frequently advanced in this scenario, patients suffering from immuno-cholangitis usually have a short survival. While corticosteroid therapy should always be initiated when SSC is suspected, it is important to take into account its high failure rate. Unfortunately, we still do not have guidelines, consensus or recommendations that clearly state the dose and time of therapy that should be used for corticosteroid treatment [83, 91-93]. However, most authors start with doses similar to those used in suspected hepatitis induced by checkpoint inhibitors (1–2 mg/kg oral or IV) [91, 92]. Cunningham et al. reinforce the concept that ursodeoxycholic acid addition has been shown to aid in biochemical resolution in certain patients that were unresponsive to corticosteroids or immune system therapy [93]. These authors also draw attention to reports of cases associated with vanishing bile duct-like syndrome following pembrolizumab treatment, which has been linked to a cholestatic presentation and lack of immunosuppression response. In addition, it should be taken into account that patients diagnosed with CPIs-induced hepatitis that fail to respond to corticosteroid treatment, should be considered SSC induced by ICIs as an alternative diagnosis.
Ursodeoxycholic acid (UDCA) may be a therapeutic option in CPIs-induced SSC, due to its cytoprotective and anti-apoptotic properties [93]. By replacing human hydrophobic biliary acids by itself in the bile acid pool, which is reflected in a less toxic biliary bile acid composition, it can mitigate bile acid-induced cytotoxicity of the retained bile on cholangiocytes. Additionally, UDCA enhances phospholipid and bicarbonate biliary excretion, which further reduces bile acid toxicity by keeping harmful, monomeric bile acids in micellar and anionic forms, respectively, thus preventing interaction with cholangiocytes [94]. Finally, it can suppress the overexpression of MHC antigens and inhibit the release of proinflammatory cytokines, due to its immunomodulatory properties [37, 95].
Three patients reported by Fouchard et al. exhibited similar features of immune-related cholangitis without other immune-related adverse events [44]. ALP levels continued to decline even after corticosteroid discontinuation and further UDCA administration for an extended period [44].
Although data on the actual efficacy of UDCA in SSC are limited, this agent seems to be able to improve cholestasis in some cases, similar to patients with PSC. In the study by Houtondii et al., 17 patients improved without any treatment [89]. Corticosteroids were mainly administered to patients with a hepatocellular clinical pattern (27%), whereas UDCA was more frequently used in the cholestatic clinical pattern in around 20% than in the hepatocellular or mixed ones (p < 0.001).
Three patients with cholangiohepatitis were reported by Moi et al. [37] induced by immunotherapy refractory to corticosteroids therapy. They were treated with a second-line therapy using targeted interleukin (IL)-6 receptor blockade by tocilizumab showing favourable outcomes. T-cell-targeted therapy with the IL-6 receptor-neutralising antibody tocilizumab was shown to inhibit signalling downstream of interferon-gamma and several other Janus kinase-dependent cytokines [60]. The authors pointed out that patients with both cholangitic and cholangiohepatitis pattern of liver injury may have a refractory response to corticosteroids and need a individualised tailored therapy.
In summary, despite both the lack of clear statements on how to clearly approach therapy and the reported low frequency of success with the use of corticosteroids, this is the only agent recommended to begin the treatment of this disorder. Because of its safety and therapeutic effectiveness in several cholestasis-predominant settings, UDCA should be more widely recommended initially in combination with corticosteroids in patients suffering from SCC.
9 Outcome
9.1 SSC Due to CPIs
Unfortunately, because the underlying cancer is often advanced in these patients, SSC is usually associated with poor survival. Persistent alterations in liver tests likely lead to a limitation of antitumor therapy alternatives, thereby accelerating the cancer spread.
Response to treatment with corticosteroids is disappointing and a long-term follow-up after therapy is lacking in most of the patients reported who developed CPIs-induced SSC so far (Table 3). The majority of deaths in our analysis of 64 cases were non-liver related. Only 5 cases died due to cholangitis disease–related causes (Table 3). More reliable and robust data are needed to confidently establish the real morbidity and mortality rates of this disease.
10 Conclusions and New Horizons
Although biliary damage seems to be immune-mediated, the underlying molecular mechanisms causing bile duct injury remain unclear. Clinicians should consider and suspect SSC in all patients with cholestatic/mixed DILI who resolve slowly after discontinuation of the implicated agent. The preferred imaging modality is MRCP.
10.1 SSC Due to CPIs
CPIs-induced SSC seems to affect the entire biliary system. Patients with large-duct damage usually have a later onset and higher ALP levels than those with small-duct damage. Unfortunately, based on the available data, the response to immunosuppressive therapy is often absent or only partial.
Physicians should keep in mind that patients with CPIs-induced SSC showing large-duct damage usually have radiological signs of cholangiopathy, which included diffuse intra- and extrahepatic biliary strictures in addition to common bile duct thickening. On the other hand, small duct cholangiopathy is characterised by microscopic findings of pericholangitis on liver histology which can be focally severe. In addition, degenerative duct injury with ductular reaction, focal periductal fibrosis has been linked to severe cholestasis, and duct injury can be also observed.
Clinicians should consider and suspect SSC when probable CPIs-induced hepatitis does not respond to corticosteroids, particularly if patients develop a mixed or cholestatic pattern associated with high ALP levels. For non-invasive diagnostic tools, cholangio-MRI is the preferred option to confirm an SSC diagnosis, although liver biopsy may be helpful in cases involving small ducts. There are no reliable data on patient's outcome, but the few clinical cases and small patient series show poor outcomes. Future studies should be conducted to confirm the benefits of UDCA alone or in combination with corticosteroids, especially in CPIs-induced cholangitis. Additionally, further randomised, controlled trials should prospectively investigate alternative therapies for the treatment of SSC, given the poor response to corticosteroid therapy. Finally, a more detailed mapping of environmental and genetic risk factors in selected ethnic groups involving SSC due to CPIs needs to be carried out.
Author Contributions
The authors contributed equally to the paper.
Conflicts of Interest
The authors declare no conflicts of interest.




