Epidemiology and Risk Determinants of Drug-Induced Liver Injury: Current Knowledge and Future Research Needs
Funding: This work was supported by Office of Women's Health.
Handling Editor: Luca Valenti
ABSTRACT
Aims
Drug-induced liver injury (DILI) is a major global health concern resulting from adverse reactions to medications, supplements or herbal medicines. The relevance of DILI has grown with an aging population, the rising prevalence of chronic diseases and the increased use of biologics, including checkpoint inhibitors. This article aims to summarise current knowledge on DILI epidemiology and risk factors.
Methods
This review critically appraises available evidence on DILI frequency, outcomes and risk determinants, focusing on drug properties and non-genetic host factors that may influence susceptibility.
Results
DILI incidence varies across populations, with hospitalised patients experiencing notably higher rates than outpatients or the general population. Increased medication use, particularly among older adults and women, may partly explain age- and sex-based disparities in DILI incidence and reporting. Physiological changes associated with aging likely increase susceptibility to DILI in older adults, though further exposure-based studies are needed for definitive conclusions. Current evidence does not strongly support that women are inherently more susceptible to DILI than men; rather, susceptibility appears to depend on specific drugs. However, once DILI occurs, older age and female sex are associated with greater severity and poorer outcomes. Other less-studied host-related risk factors are also discussed based on available evidence.
Conclusions
This article summarises existing data on DILI frequency, outcomes, drug properties affecting hepatotoxicity and non-genetic host risk factors while identifying critical knowledge gaps. Addressing these gaps through future research could enhance understanding and support preventive measures.
Summary
- DILI frequency is influenced by the at-risk population, specific drugs and the patient's status at the time of exposure (inpatient vs. outpatient).
- DILI occurs when a susceptible person receives a drug with hepatotoxic potential, typically associated with daily doses of 50 mg or greater, high lipophilicity and extensive hepatic metabolism.
- Current evidence does not strongly support that older age and female sex are universally associated with higher susceptibility to DILI; this may depend on specific drugs. However, once DILI occurs, older individuals and women tend to experience greater severity and worse outcomes.
- Robust data on clinical risk determinants remain lacking.
1 Introduction
Drug-induced liver injury (DILI) is an adverse reaction caused by medications, supplements or herbal medicines. In recent years, adverse liver events have been increasingly reported, reflecting the overall rise in adverse events reported in spontaneous reporting systems [43, 44]. The emergence of immunomodulating biologics, such as checkpoint inhibitors, has introduced a new category of DILI, drawing significant research attention.
This article aims to summarise our current knowledge of DILI epidemiology and risk factors. While several excellent review articles have covered the existing knowledge of DILI epidemiology [5, 45] and its genetic risk factors [38], this article analyses current data on epidemiology and discusses risk determinants, focusing on drug properties and non-genetic host factors that influence (or plausibly affect) DILI risk. We critically appraise the available evidence to identify gaps for future research. Liver injuries associated with herbal supplements and checkpoint inhibitors are covered separately in the special issue and are therefore not discussed in this article.
2 DILI Frequency and Outcomes
2.1 DILI: An Underdiagnosed Condition Requiring Evaluations in Diverse Study Settings
DILI is the most frequent cause of acute liver failure (ALF) in the United States [46]. It negatively impacts patients and health systems, with 0.1%–3% of hospital admissions associated with DILI [18, 47]. In prospective population-based studies, it is estimated that 14–19 per 100 000 inhabitants develop clinically significant DILI annually, requiring medical attention and leading to DILI diagnosis [15, 48]. Most patients with DILI present with asymptomatic liver enzyme elevations, which resolve with treatment cessation. However, about 10% of patients with clinically significant DILI potentially progress to ALF, liver transplantation or fatality [6, 14, 23, 49].
Despite being viewed as a rare diagnosis of exclusion, DILI is often undetected or misdiagnosed [2, 48, 50]. In a hospital setting, real-time monitoring of ALT elevation by hepatologists identified 12 times more DILI cases than standard care strategies (i.e., referral base) [50]. In a population-based study, special training of physicians in a community increasedly identified DILI cases by 16-fold compared to spontaneous adverse liver events reporting systems [48]. Thus, in existing medical practices, a substantial majority of DILI cases—11 out of 12 in hospitals and 15 out of 16 in community settings—remain undetected, underscoring that merely a fraction of DILI instances find their way to liver specialty services. Furthermore, several studies revealed the significant extent of misdiagnosis among the reported liver events, published DILI cases and DILI diagnosis identified in the electronic health records (EHR) [51, 52]. Additionally, cases with multiple comorbidities tend to receive lower causality assessment scores due to their complexity, affecting the confidence level of causality [53]. If DILI is undiagnosed and the offending drug continues, a fraction of patient may progress to ALF or chronic liver injury, while others may develop clinical adaptation where ALT elevation resolves despite continued treatment [5]. It is critical to recognise the significant underdiagnosis and misdiagnosis of DILI in current practice.
2.2 Medication Use Varies by Gender and Age
Medication use defines the population at risk for DILI. Survey studies consistently show that women use medications more often than men, regardless of whether they are prescription or non-prescription drugs. This female dominance in medication use is observed globally in Europe [36, 54-56], Asia [57, 58], Africa [8] and South America [12, 59].
Medication use generally increases with age. An Italian study using outpatient prescription data from administrative databases showed that medication prevalence and prescription rates per 1000 treated patients per day increased with age, with higher rates in females except in the 0–14 and > 85 age groups [60]. However, disparities in medication use by age and gender likely vary by the therapeutic class and indication. For instance, the Italian study reported that women had a higher prevalence of use compared to men in most therapeutic classes, including systemic antibacterials, analgesics and antiepileptics but a slightly lower prevalence of antidiabetics use [60]. These findings underscore the need for more data on exposure-based DILI incidence to better evaluate risk determinants and susceptibility.
2.3 DILI Incidence Varies in Inpatients and Outpatients
DILI incidence significantly differs based on a patient's status at the time of exposure. In exposure-based studies using electronic medical records, the incidence is 32.8 per 100 000 adult patients (0.033%) who received one of the commonly implicated DILI-causing drugs in the United States, combining both outpatient and inpatient exposures [61]. The reported incidence of DILI is much higher among hospitalised patients, ranging from 0.7% to 3.1% [9, 62, 63]. However, methodological differences make direct comparisons of these incidence rates difficult.
A recent extensive EHR study on amoxicillin-/clavulanate-associated acute liver injury, including both inpatient and outpatient exposures, showed that inpatient exposure was associated with a 3.6-fold higher incidence of amoxicillin-/clavulanate-associated acute liver injury compared to outpatient exposure, even after adjusting for age, sex, race/ethnicity and pre-existing liver conditions [64]. These findings suggest that inpatient status is associated with a higher DILI incidence. In general, patients receive closer care and more frequent lab monitoring during hospitalisation [64]. It remains uncertain whether the increased incidence among inpatients is due to a greater opportunity to detect liver enzyme elevation or increased susceptibility due to underlying pathophysiological conditions.
2.4 DILI Incidence Varies Depending on Drug-Specific Risks
Among the many pharmaceuticals implicated in DILI, certain drug classes are more frequently identified: Anti-antimicrobials, central nervous system agents, analgesics/anti-inflammatory agents, immunomodulatory agents, lipid-lowering agents, anti-neoplastic agents and anti-viral agents [28]. Registry and case-series studies across various countries/regions highlight these therapeutic classes (summarised in ref [65]). Regional disparity also exists in implicated drugs, with herbal/dietary supplement (HDS) products predominating in Asian countries, accounting for one- to two-thirds of DILI cases in China, Korea and Singapore [1, 66-69]. The dominance of specific drug classes and regional disparity, reflecting the volume of specific medication use and the drug's hepatotoxic potential, informs in screening causal drugs in DILI cases based on location and patient race/ethnicity.
HDS-DILI cases have been increasingly identified outside Asian counties, including the United States, Spain and Iceland [7, 15, 70], requiring heightened attention in DILI assessments. In the United States, the expanding use of HDS has been associated with an increasing HDS-related DILI and ALF cases [70, 71] (reviewed in [41]) as well as liver transplant candidates [72]. HDS now comprises 16%–20% of agents associated with DILI in the United States and Iceland [15, 73]. In national US surveys, one in two to three respondents reported using HDS [10, 74, 75], with a higher prevalence among females [10, 75]. Among those aged 60 and over, 70% use HDS [75]. Notably, only one-third to half of HDS users disclose their use to the healthcare providers [29, 76], underscoring the importance of thorough medication histories.
DILI incidence, accounting for exposure size, varies based on drugs, reflecting their hepatotoxic potential. Few studies have reported exposure-based DILI incidence. In an Icelandic study [15] with a median age of 55 years and 56% women, the incidence of the top eight drugs implicated in DILI cases among outpatients ranged from 6 per 100 000 patients for doxycycline to 752 per 100 000 patients for azathioprine (Table 1). The Kaiser EHR analysis [61] also showed significant differences in drug-specific DILI incidence in a cohort with a median age of 45 years and 76% women without underlying liver conditions (Table 1).
| Population | Iceland study [15] | US Kaiser study [61] | ||
|---|---|---|---|---|
| General population | EHR | |||
| exposures | Nationwide pharmaceutical database on outpatient prescriptions | Inpatient and outpatient prescription records | ||
| Study period | 2 years | 6.5 years | ||
| N of exposures | Incidence per 100 000 | N of exposures | Incidencea per 100 000 | |
| Amoxicillin/Clavulanate | 35 252 | 43 | 75 333 | 34 |
| Aripiprazole | — | — | 3986 | 50 |
| Atorvastatin | 7385 | 27 | — | — |
| Azathioprine | 532 | 752 | — | — |
| Ciprofloxacin | — | — | 266 369 | 27 |
| Diclofenac | 54 889 | 11 | 3437 | 21 |
| Doxycycline | 32 677 | 6 | — | — |
| Duloxetine HCL | — | — | 2432 | 57 |
| Infliximab | 593 | 675 | — | — |
| Interferon-beta | 637 | 295 | ||
| Isoniazid | — | — | 6935 | 606 |
| Isotretinoin | 2169 | 138 | — | — |
| Lamotrigine | — | — | 3216 | 0 |
| Levofloxacin | — | — | 2729 | 0 |
| Nitrofurantoin | 5476 | 73 | 158 697 | 7 |
| Phenytoin | — | — | 3863 | 110 |
| Sulfamethoxazole-trimethoprim | — | — | 234 655 | 26 |
| Terbinafine HCL | — | — | 21 357 | 21 |
| Valproic acid | — | — | 7350 | 77 |
- a Adjusted for age and gender using the US Standard Population in 2000.
Lastly, not all drugs implicated in DILI are associated with ALF; some are more likely to cause ALF than others, while some drugs are known to cause DILI but have no reported cases of ALF (summarised in [77]). For example, halothane, disulfiram, acetaminophen, flutamide, isoniazid and valproic acid are more likely to cause ALF, whereas amoxicillin, amlodipine, valsartan and many others are known to cause DILI but rarely causes ALF. This observation indicates that the likelihood of causing severe DILI is at least partially determined by the drug's hepatotoxic potential or related to certain drug properties. Although patient factors also influence DILI severity, the drug's likelihood of causing ALF is an important consideration in DILI case assessment.
3 DILI Risk Determinants
Numerous drugs have been implicated in DILI [16]. Various drug attributes, such as pharmacological, toxicological and physicochemical properties, have been reportedly associated with increased DILI risk in humans. Section 3.1 covers several relevant drug properties (dose, hepatic metabolism/reactive metabolites, oxidative stress and mitochondrial dysfunction) to be considered in the clinical evaluation of DILI.
Only a fraction of patients receiving drugs with hepatotoxic potential develop liver injury, indicating that host susceptibility factors also significantly influence DILI risk. Certain host factors, such as age, sex, reproductive health, comorbidities, comedications, genetics and epigenetics may modulate hepatic drug concentration, metabolism/transport, cellular functions, immune regulation and liver regeneration. These factors consequently impact the likelihood of developing, progressing or recovering from DILI [27]. Although robust data are still lacking, the available evidence on a few clinically relevant host factors (age, sex, race/ethnicity and comorbidities) is discussed in Section 3.2.
3.1 Drug Properties Associated With Hepatotoxicity
3.1.1 Drug Dose: Daily Dose of 50 mg or Greater Is Associated With a Higher Risk of DILI
Idiosyncratic DILI has traditionally been considered dose-unrelated; however, accumulating evidence suggest otherwise. Drugs with a daily dose of 50 mg or greater carry a higher risk of DILI [78], indicating a potential threshold dose to initiate liver injury [5]. Supporting this threshold effect, a drug's maximal plasma concentration (Cmax) ≥ 1.1 μM has been identified as a marker for drugs associated with DILI, yielding high sensitivity and specificity [79]. Most of US drugs withdrawn due to hepatotoxicity [78] and 77%–88% DILI reports in Iceland [15], Sweden [13, 14, 78] and Spain [80] met the 50 mg threshold. Furthermore, previous studies consistently demonstrated that drugs with a dose of 50 mg/day or greater were significantly associated with poor outcomes such as death or liver transplantation and are disproportionately prevalent among drugs associated with drug-induced ALF [78].
Clinical case reports have shown that patients may tolerate drugs at a certain dose but develop DILI or drug-induced ALF after dose escalation [21, 34, 81, 82]. Conversely, dose reduction may lead to tolerance in some patients [83]. These reports suggest that certain drugs exhibit dose-dependent hepatotoxicity. Therefore, drug dose is a key factor to consider when assessing potential DILI cases and implementing preventive measures [31, 84-86].
3.1.2 Drug's Lipophilicity: Fat-Soluble Drugs Are Associated With Hepatotoxic Potential
Physicochemical properties of drugs influence their hepatotoxic potential. Fat-soluble (or lipophilic) drugs are associated with a higher likelihood of hepatotoxicity [26]. These drugs are frequently metabolised by the liver [87] to reactive metabolites [88] or inhibit biliary excretion [4]. Reactive metabolites are usually produced from oxidation catalysed by CYP enzymes or glucuronidation reactions by UDP-glucuronosyltransferases [89].
A drug or metabolite that inhibits the bile salt export pump (BSEP) can increase intracellular bile salts and drug concentrations, leading to mitochondrial damage [4]. Mitochondrial dysfunction can then increase reactive oxygen species, impair fatty acid oxidation and decrease cellular ATP production to result in cell necrosis [90]. Additionally, reactive metabolites can bind to intracellular proteins to create covalent adducts, disrupting protein homeostasis and cellular function, causing cellular stress and potentially leading to cellular damage (reviewed in [5, 27, 91]).
Cellular stress and oxidative injury activate protective anti-oxidant genes and proteins to enable adaptation to injury. However, when cellular defences are overwhelmed, cell death occurs, and drug–protein adducts can be released to activate an immune response (reviewed in refs [5, 27, 91].) Thus, lipophilic drugs, in general, pose a greater hepatotoxic potential, triggering diverse toxicological responses that lead to liver injury and inflammation. In fact, a high risk of DILI is associated with BDDC class 2 drugs, which are characterised by extensive metabolism and low solubility (i.e., high lipophilicity) [92].
3.1.3 Drug Metabolism: Hepatic Biotransformation Can Potentiate Drug's Hepatotoxic Potential
The liver metabolises drugs through varying phases of cytochrome P450 (CYP) oxidation, hydrolysis or reduction (Phase I) and/or glucuronidation, acetylation and sulfation reactions (Phase II). These generally beneficial reactions convert compounds to more water-soluble forms for safe excretion. However, this process can go awry when high doses of fat-soluble drugs are transformed to reactive or toxic metabolites to initiate liver injury. Four in ten frequently prescribed drugs are associated with DILI [78]. Most of these are fat-soluble (lipophilic) drugs with a high daily dose.
The hepatotoxic potential also depends on activities of drug metabolising enzymes (i.e., the rates of hepatic drug transformation), including both CYP [93] and non-CYP enzymes [94]. Most (70%–80%) drugs are metabolised by CYP enzymes [95]. Factors such as sex, age, hormones, genetic polymorphisms and inhibition or induction by concomitant medications affect CYP enzyme activities. Compared to major effects of CYP enzymes on DILI, non-CYP enzymes affect DILI to a lesser extent [94]. Although generally detoxifying drugs, UDP-glucuronosyltransferases (UGT) can generate toxic metabolites. For example, in addition to multiple CYP, UGT metabolises diclofenac to a toxic acyl glucuronide which binds covalently to hepatocellular proteins [39]. Assessing the combined effects of Phase I and II drug metabolism on DILI may improve its prediction.
Women generally metabolise drugs more rapidly than men—particularly for CYP3A4 in which drugs can exhibit ≈20%–50% faster pharmacokinetics in women than men [95]. CYP3A4/5 metabolises ≈50% of medications [95]. Its activity is stable in normal aging and higher in women than men [95-97]. Among premenopausal women, oestrogen and progesterone pills are the most commonly prescribed oral contraceptives [35]. Oestrogen inhibits drug metabolism of the ‘workhorse’ CYP3A4, increasing the concentration of affected drugs. For example, oestrogen inhibits midazolam, triazolam and nifedipine clearance up to 30% in clinical studies, all of which are metabolised through CYP3A4, although these drugs do no exhibit hepatotoxic potential [98]. Oestrogen can also inhibit CYP1A2, 2C8, 2C9/19, 2D6 to contribute to clinical drug–drug interactions [98] which can amplify the risk for DILI [99]. Therefore, it is important to consider drug–drug interactions when assessing potential DILI cases with multiple medications involved. The drug–drug interaction information can be found in the drug's label [100] and in drug interaction tables [99, 101].
In addition, disease states such as inflammation, infection or cirrhosis generally decrease drug metabolism by reducing the activity of CYP3A4/5, CYP2D6, CYP2C9/19 and CYP1A2 (reviewed in ref [95]). In inflammation or infection, inflammatory cytokines result in the downregulation of many CYP enzymes [3, 102]. CYP downregulation also occurs in alcoholic or metabolic dysfunction-associated steatotic liver disease (MASLD, formerly non-alcoholic fatty liver disease) (reviewed in [95]). In these diseases, CYP2E1, CYP4A and fatty acid metabolising enzymes increase activities to produce free radicals, lipid peroxidation, mitochondrial and hepatocellular damage (reviewed in ref [95]). In summary, drugs undergoing extensive hepatic metabolism are associated with hepatotoxic potential, which varies with individuals' drug-metabolising enzyme activities. These activities are influenced by age, sex, concomitant medications, comorbidities and pharmacogenomics. For a more comprehensive discussion on age effects, refer to Section 3.2.1.
3.1.4 Drug-Associated ALT Elevations in Clinical Trials Predict Post-Market DILI
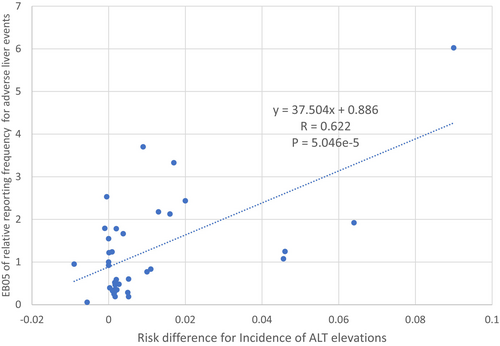
In clinical trials of new drugs, most (71%) drugs exhibiting a higher incidence of ALT elevations (defined as an ALT ≥ 3× upper limits normal and more than 1.2% higher in the treated vs. placebo group) or ‘ALT signal’ were associated with post-marketing liver safety signals (i.e., disproportionately high liver event reporting systems) [103]. Figure 1 shows a direct correlation between the risk difference for incident elevated liver enzymes observed in premarketing clinical trials and the post-marketing liver safety signal computed using the FDA Adverse Event Reporting System (FAERS) database [103]. For drugs with a daily dose > 50 mg, nearly nine in ten drugs associated with post-marketing liver safety signals exhibited an ALT signal in clinical trials. Conversely, drugs without an ALT signal in clinical trials and with a daily dose under 50 mg had a very low (2%) probability of DILI. US drug labels [100] may provide ALT incidence data within adverse reactions, which is helpful for assessing potential DILI related to newly approved drugs. The LiverTox website [104] provides a broad evidence base of published liver injury reports associated with medications, herbals and supplements for products in longer use, which is also a valuable source in evaluating potential DILI cases.
3.2 Host Risk Factors
3.2.1 Older Age Shows an Inconsistent Association With DILI Frequency but Increases the Risk of Adverse Outcomes
A general population study in Iceland demonstrated that the incidence of all-cause DILI increases with age: 9 per 100 000 among 15–29-year-olds compared to 41 per 100 000 among those over 70 years old [15]. DILI incidence increased markedly with age regardless of gender, coinciding with increased prescription numbers. However, these frequency estimates are not based on the population at risk (exposure-based); thus, higher medication use among older individuals may partly explain the higher DILI frequency. Otherwise, current epidemiological evidence does not consistently support age > 65 as a general risk factor for DILI [32]. Table 2 summarises methods, populations and age-related DILI frequencies of selected studies. Generally, the number of DILI cases in referral-based studies does not directly increase with age; rather, they peak between 40 and 70 years.
| Study | Country | Population | N of cases | Case adj. | Exposure-based | Study drugs | Age effects | |
|---|---|---|---|---|---|---|---|---|
| Population-based studies | ||||||||
| Sgro, 2002 [48] | Pros | France | General population, including adolescents and adults | 34 | Yes | No | Not specified | Age↑, DILI incidence ↑ |
| Björnsson, 2013 [15] | Pros | Iceland | General population, including adolescents and adults | 96 | Yes | No | Not specified | More than a half of identified cases: Age 50 or older |
| Referral-based studies | ||||||||
| Stephens, 2021 [111] | Pros | Spain | Referral centres | 843 | Yes | No | Not specified | Peak DILI cases: Age 60–69 years Higher frequency of jaundice in the oldest age category hepatocellular patients aged ≥ 65 years had the highest proportion of liver-related fatalities |
| Chalasani, 2015 [22] | Pros | USA | Referral centres | 899 | Yes | No | Not specified |
No data on dominated age group Higher cholestatic injury among the elderly, with higher ALP The frequency of chronic DILI was lower in the elderly No association between the elderly group and higher frequency of liver transplantation or death |
| Takikawa, 2006 [134] | Retro | Japan | Referral centres | 1676 | Yes | No | Not specified | Peak DILI cases: Age 50–69 years |
| Suk, 2012 [66] | Pros | South Korea | 17 referral centres | 371 | Yes | No | Not specified | Peak DILI cases: Age 40–49 years |
| EHR/medical record-based studies | ||||||||
| Shen 2019 [68] | Retro | China | Inpatients at medical centres, including children, adolescents and adults | 25 927 | Yes | No | Not specified |
Peak DILI cases: Age 40–59 years Cases w jaundice and fatal cases were older than cases w/o jaundice |
| Suzuki 2022 [64] | Retro | USA | Inpatient and outpatient, EHR at VA | 2249 | No | Yes | Amoxicillin/Clavulanate | Age↑, AMX/ CLAV-associated acute liver injury incidence ↑ |
Older age poses plausible risks for increased DILI. In normal aging, hepatic blood flow, liver mass and the rate of liver regeneration decline [105, 106]. Reduced hepatic blood flow can lower the clearance of drugs with a first-pass effect. While CYP3A4 activity remains stable in healthy elderly individuals, it can decrease in comorbid conditions like inflammation or cirrhosis [95]. Increased adipose tissue with aging expands the volume of distribution and prolongs a half-life elimination of fat-soluble drugs. Bile acid secretion and bile flow moderately decline with age [105]. Aging-associated mitochondrial dysfunction, due to accumulated mitochondrial DNA (mtDNA) mutations and decreased mtDNA copy numbers, impairs cellular energy production [107]. Renal excretion also decreases in most elderly patients. These cumulative physiological changes may affect DILI susceptibility to specific drugs. A previous data-mining analysis using spontaneous adverse event reporting systems and Bayesian signal detection identified [108] ten drugs associated with higher reporting frequencies among the elderly compared to children and adults, including amoxicillin/clavulanate, flucloxacillin, carbimazole, chlorpromazine, the isoniazid/pyrazinamide/rifampicin combination and cyproterone. These drugs exhibit a higher prevalence of certain drug properties: High lipophilicity, biliary excretion, BSEP inhibition, reactive metabolites and glutathione adducts [108]. Further studies are needed to address age-specific DILI susceptibility and underlying mechanisms.
Despite inconclusive evidence on age as a factor for DILI incidence, older age is associated with a higher risk of adverse outcomes. Elderly DILI patients exhibited significantly more prevalent jaundice and hospitalisation than those younger [109] and have a greater risk of progression to chronic liver injury (i.e., lack of biochemical resolution) [110]. Additionally, older individuals with hepatocellular injury exhibit increased risk of Hy's law cases compared to younger age groups among patients with amoxicillin/clavulanate-associated acute liver injury [64]. These adverse outcomes may be related to diminished hepatic reserve and liver regeneration in older subjects [105], as seen in acute hepatitis A, where severity, measured by hospitalisation rates, steadily increases with age over 40 [19]. A recent study in a large DILI registry also reported worse outcomes among older patients with hepatocellular injury [111]. In summary, whether older age increases susceptibility to DILI is inconclusive and may depend on specific drugs. However, accumulating data suggest that once DILI occurs, older subjects face a heightened risk of developing adverse outcomes.
3.2.2 Women May Not Have Higher DILI Susceptibility but Face Greater Risk of ALF
Gender differences in reported adverse liver events are notable, with women exhibiting a higher prevalence, especially in severe cases [112]. Epidemiological data on gender differences in DILI incidence, however, are limited. Table 3 summarised methods, populations and gender-related DILI frequencies of selected studies. A French population-based study revealed that after the age of 50, the standardised incidence of DILI was more than twice as high in women compared to men, with no gender disparity noted before 50 years of age [48]. Similarly, an Icelandic population-based study observed a similar age-dependent trend in gender differences, although no statistically significant gender differences were noted [15]. The underlying cause of this gender difference in DILI incidence, whether it is due to higher medication usage among women or greater biological susceptibility, remains unresolved. Among referral-based studies, no consistent tendency was observed in gender distribution among the overall DILI cases. However, drug-specific DILI cases showed male dominance in amoxicillin/clavulanate-related cases [64, 111], while female dominance was observed among herb-related cases [66].
| Study | Country | Population | N of cases | Case adj. | Exposure-based | Study drugs | % Female | Gender/Sex difference | |
|---|---|---|---|---|---|---|---|---|---|
| Population-based studies | |||||||||
| Sgro, 2002 [48] | Pros | France | General population, including adolescents and adults | 34 | Yes | No | Not specified | 64.7% | After the age of 50, the standardised incidence of DILI was more than twice as high in women compared to men, with no gender disparity noted before age 50 |
| Björnsson, 2013 [15] | Pros | Iceland | General population, including adolescents and adults | 96 | Yes | No | Not specified | 56.3% | No significant gender difference in DILI incidence |
| Referral-based studies | |||||||||
| Stephens, 2021 [111] | Pros | Spain | Referral centres | 843 | Yes | No | Not specified | 48% |
Men had a higher likelihood of AMX/CLAV-induced cholestatic liver injury, with adjusted odds ratio of 2.2; 95% CI 1.3–3.8; p = 0.002 Female sex is independent predictor of liver-related mortality and liver transplantation. (OR 2.7; 95% CI 1.2–6.4; p = 0.019) |
| Chalasani, 2015 [22] | Pros | USA | Referral centres | 899 | Yes | No | Not specified | 59% | Females had higher prevalence of hepatocellular injury |
| Takikawa, 2006 [134] | Retro | Japan | Referral centres | 1676 | Yes | No | Not specified | 57% | — |
| Suk, 2012 [66] | Pros | South Korea | 17 referral centres | 371 | Yes | No | Not specified | 63% | Female sex was significantly dominant among herb-associated DILI |
| EHR/medical record-based studies | |||||||||
| Shen 2019 [68] | Retro | China | Inpatients at medical centres, including children, adolescents and adults | 25 927 | Yes | No | Not specified | 49% |
Females experienced longer latency and higher T-bill level and liver enzymes Female sex was more prevalent among cases with jaundice and life-threatening cases |
| Suzuki 2022 [64] | Retro | USA | Inpatient and outpatient, EHR at VA | 2249 | No | Yes | Amoxicillin/Clavulanate | 3.3% | Sex-specific incidence (M:F) = 0.16%: 0.06%. Adjusted OR of 0.65 for females |
No robust epidemiological data exist to address sex disparities in susceptibility to DILI, but a study using spontaneous adverse event reporting data suggest it may depend on specific drugs [112]. Women are more frequently reported than men for liver events involving diclofenac, macrolides, flucloxacillin, nitrofurantoin, halothane, ibuprofen and interferon Beta-1a [112]. In contrast, men predominate in reported liver events related to azathioprine and amoxicillin/clavulanate [112]. Notably, drugs known to form reactive metabolites, exhibit mitochondrial liability or have a higher potential for BSEP inhibition are more prevalent among those associated with a higher reporting frequency of liver events in women [112]. This suggests a potential interplay between these drug properties and female-biased biological factors (e.g., differences in CYP enzyme activity). Sex differences in DILI incidence may be drug-specific and can be assessed in drug-specific exposure-based studies.
Adult ALF or life-threatening DILI cases appear to disproportionately affect younger women. Among all-cause DILI cases, female sex was more prevalent among cases with jaundice and significantly more common among life-threatening or liver transplant cases [68, 111], with adjusted odds ratio of 2.2; 95% CI 1.3–3.8; p = 0.002 [111]. DILI is the most common cause of ALF in adults in the West [11]. In the United States, young females predominate (i.e., 69% females of median age 39 years) in the ALF Study Group cohort, with nearly half experiencing acetaminophen toxicity [113], of which 70% recover without transplant after N-acetylcysteine treatment. Another US study of liver transplantation after DILI-associated ALF found that 76% of patients were females (mean age 33) [114], with half having acetaminophen toxicity and others affected by isoniazid, propylthiouracil, phenytoin, valproate and HDS.
In summary, DILI incidence in the general population exhibits female dominance after 50. Whether this is due to higher medication use among women or greater biological susceptibility remains uncertain. While it remains inconclusive whether female sex or gender specifically increases susceptibility to DILI, female patients are at a higher risk of developing jaundice and experiencing life-threatening outcomes. Further investigation is needed to determine whether these poorer outcomes are primarily due to female-biased causal agents, specific DILI types (hepatocellular vs. cholestatic) or if they are universal across different drugs.
3.2.3 Women Predominate in Acetaminophen-Induced ALF but May Not Have a Heightened Risk of Developing Acetaminophen-Induced Liver Injury
Women also outnumber men in acetaminophen-induced ALF and are associated with worse clinical outcomes [115]. However, whether this gender difference is due to increased susceptibility in women is debatable, as registry data lack information on the population at risk.
Few acetaminophen-overdose studies have been conducted in the general population. In an Icelandic study, the dose of acetaminophen ingestion was 15 g (IQR: 7–20) for men and 13 g (IQR: 9–20) for women, with a median age of 35 years (IQR: 24–49) for men and 26 years (18–45) for women [116]. Women outnumbered men in overdose cases, but the prevalence of hepatotoxicity (liver enzyme elevation) was higher among men versus women (31% vs. 17%). No gender differences in ALF were observed, but older age was associated with increased ALF risk [116].
In a Malaysian study, the dose of acetaminophen ingestion was 10 g (IQR: 5–15 g), with a median age of 23 years (IQR: 20–28 years) [117]. Age or gender was not significantly associated with the risk of hepatotoxicity in this study, and no ALF cases were reported [117]. Acetaminophen induces more severe liver damage in male mice [37, 118, 119]. These discrepancies suggest that sex disparities in acetaminophen-induced acute liver injury may depend on disease stage and mechanisms.
3.2.4 Plausible Racial/Ethnic Disparities in DILI Risk Suggested by Limited Data
Robust data to assess racial/ethnic disparities in DILI susceptibility are currently unavailable. A DILI registry study reported that the prevalence of causal drugs significantly differs between African-American versus Caucasian, with higher prevalence among African-American for trimethoprim/sulfamethoxazole, methyldopa and phenytoin and a lower prevalence for amoxicillin/clavulanate [24] The study also reported higher prevalence in severe cutaneous reactions, hospitalisation, liver transplantation or liver-related death by 6 months and chronic DILI among African-Americans [24]. Etiological mechanisms of these disparities remain to be investigated.
Interestingly, a study using biopsy samples from patients with non-ALF DILI in the U.S. Drug-Induced Liver Injury Network (DILIN) found that young women (< 50 years) had predominantly hepatocellular injury with more severe interface hepatitis, plasma cell infiltration, lobular disarray and hepatocyte rosettes than men or older women [120]. Black race (vs. White race) was also associated with significantly increased plasma cell infiltration [120]. This is intriguing as black race is associated with an enhanced humoral immunological response following vaccination compared to other races [121]. Furthermore, previous studies reported that black women were overrepresented among non-acetaminophen-induced ALF [114, 122]. These findings suggest biological disparity in immune response may partially explain racial differences observed in clinical phenotypes of DILI.
A large EHR-based amoxicillin/clavulanate cohort study demonstrated that American Indian or Alaska Native showed an 80% higher likelihood of developing amoxicillin-/clavulanate-associated acute liver injury compared to non-Hispanic White, after adjusting for other significant confounders [64]. In the study, no difference was noted between non-Hispanic Black and non-Hispanic White.
A recent international collaboration conducted a comparative characterisation of leflunomide-induced liver injury, revealing distinct clinical phenotypes and varying severity between cases in India and the United States. Indian cases more frequently exhibited severe cutaneous adverse reactions, had shorter latency periods and showed higher mortality rates, with a predominance of female patients compared to US cases [42]. The observed differences may suggest possible racial variations in immune responses.
In summary, limited data suggest possible racial/ethnic disparities in DILI risk, with some disparities potentially related to differences in immune responses.
3.2.5 Chronic Liver Disease and Alcohol Use May Increase the Risk of DILI
In an Indiana University Health database, rates of suspect drug-associated liver injury were examined in patients with suspected non-alcoholic fatty liver disease (NAFLD) and compared to controls without NAFLD [123]. Those with potential NAFLD exhibited a fourfold higher rate of DILI than the controls (0.8% vs. 0.2%, respectively). These results suggest an increased risk for DILI with NAFLD and merit further study.
The effect of chronic alcohol use on liver injury outcomes was examined in the US ALF Study Group. Over 1000 enrolled patients were asked to categorise their alcohol use in the prior 6 months as none/minimal (< 3 alcoholic drinks/week) or at least moderate (≥ 3 drinks/week) [124]. In patients with acetaminophen overdose, non-acetaminophen ALF or other etiologies, moderate alcohol use increased the odds of presenting with ALF rather than acute liver injury by 75% or higher and significantly increased the risk of mortality by 45%. This study suggests that chronic alcohol use affects ALF severity and outcomes.
Chronic alcohol use can increase the risk of isoniazid-associated DILI as alcohol increases CYP2E1 activity [95]. In a prospective nested case–control study of patients receiving anti-tuberculosis drugs, chronic alcohol use was associated with DILI [125].
Chronic inflammation from viral infections can suppress the activity of CYP3A4 (and other CYPs) while increasing CYP2E1 activity [95]. As a result, patients with HIV may be more susceptible to DILI from highly active antiretroviral therapy and anti-tuberculosis therapy. Similarly, patients with chronic hepatitis B or C receiving anti-tuberculosis therapy experienced a two- to threefold increased risk of DILI [25, 30]. When patients with chronic hepatitis B received hepatitis B antiviral therapy while on anti-tuberculosis therapy, their risk for a DILI-related hospitalisation decreased more than 50% compared with those untreated for hepatitis B [30]. This suggests that decreased liver inflammation with antiviral treatment may have improved DILI outcomes. In summary, underlying liver disease and chronic alcohol use may increase the risk of DILI and affect its outcomes.
3.2.6 History of Drug Allergies May Be Associated With an Increased Risk of DILI Mortality
Older patients exhibited a higher risk of prior drug allergy [126]. Among the overall 6.7% with prior drug allergies, those with hepatocellular damage, jaundice and low platelet counts exhibited a significantly higher liver-related mortality than those without drug allergy (11% vs. 1.6%, respectively). Females exhibited somewhat higher rates of baseline drug allergies and allergy-associated fatalities [126] and predominated for drug-induced autoimmune hepatitis [111] (as also reported in the Prospective European DILI Registry) [17].
3.2.7 Polypharmacy and Its Potential Impacts on DILI Risk
In the United States, a survey conducted in 2017 found that nearly one in three adults had taken two or more drugs in the prior month, with the use of combination products on the rise [127]. In Italy, outpatient drug prescriptions in 3.8 million patients showed an increasing prevalence and prescription rates with age, regardless of sex [60]. Additionally, the prevalence of polypharmacy among the elderly has been rising consistently across different countries [128]. The global aging population is growing rapidly and is projected to double by 2050 [129]. Furthermore, the prevalence of chronic diseases has steadily increased, with 42% of US adults having two or more chronic conditions and 12% having at least five [20, 33]. Given these trends, polypharmacy is expected to become even more prevalent in the future, raising concerns over potential drug–drug interactions.
Drugs interact and influence more than just drug-metabolising enzymes. A previous epidemiological study demonstrated that the simultaneous use of drugs with hepatotoxic potential (e.g., diclofenac, amoxicillin/clavulanic acid, flucloxacillin, tetracyclines, macrolides, chlorpromazine, sulpiride, antiepileptics, metoclopramide, chlorpheniramine, betahistine, sulfasalazine and azathioprine) significantly increases the risk of acute liver injury, which is unexplained by non-drug causes (i.e., drug-associated) [40]. The risk of developing acute liver injury is 6 times higher when two or more drugs are used simultaneously compared to a single drug, suggesting a toxicological interplay among drugs [40]. Furthermore, analyses of spontaneous adverse events’ data showed that co-reported medications known to enhance liver injury or impair regeneration such as sympathetic stimulants are associated with a higher fatality rate of acetaminophen-associated liver events [130]. Conversely, certain co-reported medications (e.g., statins, beta-blockers, ACE-inhibitors, angiotensin II receptor blockers and NSAIDs) showed potential protective associations with the fatality of acetaminophen-associated liver events. These findings suggest biophysiological interplay among drugs.
In summary, no robust evidence exists to conclude the roles of polypharmacy in idiosyncratic DILI. However, as discussed above, in cases of polypharmacy, drugs likely interact in complex, multiphasic ways, exerting pharmacological, toxicological and biophysiological interplays that affect DILI risk.
4 Future Research Considerations for DILI
It is important to acknowledge that currently available data do not provide a comprehensive overview of DILI; rather, they offer detailed yet fragmented views. To capture a complete picture, we need to employ more inclusive approaches utilising diverse data sources (e.g., spontaneous adverse event reporting systems, EHR data) while recognising their inherent limitations. It is encouraging to see more studies on DILI using large EHR data [131], as the sheer size of these data sources enables the investigation of research questions that are often impossible to address in standard clinical cohorts (e.g., drug-specific incidence, the impact of comedications, polypharmacy, risk disparities in DILI and interactions among risk factors). By diversifying data sources, we can piece together a more accurate and comprehensive understanding of DILI, which will aid in future drug safety efforts.
Historically, conventional medicine has often overlooked gender- and sex-specific health concerns. However, age and sex are pivotal biological factors in human health and disease [132], and their consideration is essential for personalised medicine, including drug safety. Unfortunately, most current medical research still assumes males as the standard reference population [133], leading to missed opportunities to explore key disparities related to sex and sex hormones. Similarly, age significantly influences biological variance. Formulating a more effective analytical approach, incorporating our understanding of biological and pathophysiological variations, requires closer collaboration with statisticians and data scientists.
The liver is biologically distinct between sexes, exhibiting sex-specific gene expression patterns influenced by sex chromosomes, growth hormones and hormone receptor variations. These factors contribute to the sex-specific regulation of liver diseases. Considering sex and age in preclinical and genetic analyses will enhance our mechanistic understanding of DILI and these biological disparities, informing future clinical and epidemiological studies.
Lastly, racial and ethnic disparities in DILI remain understudied, partly due to the lack of large, diverse data sources that enable direct comparisons of risk and phenotypes among different racial and ethnic groups. Recent collaborative efforts to characterise drug-specific DILI across different DILI registries have uncovered thought-provoking disparities between countries [42]. To address these gaps, future international collaborations should be encouraged, with a focus on standardised methodologies, to generate more comprehensive data on racial and ethnic disparities in DILI.
5 Conclusions
This article analysed the available data on DILI frequency, outcomes and risk determinants, focusing on drug properties and non-genetic host factors influencing DILI risk. Our analysis identified critical gaps in understanding and highlighted future research directions to advance knowledge and implement preventive measures. We also discussed practical considerations for DILI prevention, such as drug dose adjustment, review of hepatotoxic potential, drug–drug interactions and assessment of vulnerabilities in older populations, providing valuable resources.
To develop effective and personalised drug safety measures, it is essential to gain deeper insights into how age, sex, reproductive status and pre-existing conditions contribute to DILI susceptibility and interact with drug properties and genetic risk factors. Multidisciplinary collaborations involving advanced statistics and data science are encouraged to develop novel approaches and methodologies to address these complex gaps in DILI research.
Acknowledgements
This work was supported by the FDA Office of Women's Health.
Disclaimer
The views presented in this article do not necessarily reflect those of the U.S. Food and Drug Administration. Any mention of commercial products is for clarification and is not intended as an endorsement.
Conflicts of Interest
The authors declare no conflicts of interest.




