Relative adrenal insufficiency in the non-critically ill patient with cirrhosis: A systematic review and meta-analysis
Abstract
Background & Aims
Characterization of relative adrenal insufficiency (RAI) in cirrhosis is heterogeneous with regard to studied patient populations and diagnostic methodology. We aimed to describe the prevalence and prognostic importance of RAI in non-critically ill patients with cirrhosis.
Methods
A systematic review and meta-analysis was performed using MeSH terms and Boolean operators to search five large databases (Ovid-MEDLINE, ScienceDirect, Web of Science, Cochrane Library and ClinicalTrials.gov). The population of interest was patients with cirrhosis and without critical illness. The primary outcome was the pooled prevalence of RAI as defined by a peak total cortisol level <18 μg/dl, delta total cortisol <9 μg/dl or composite of the two thresholds in response either a standard-dose or low-dose short synacthen test. Odds ratios and standardized mean differences from random-effects models estimated important clinical outcomes and patient characteristics by adrenal functional status.
Results
Twenty-two studies were included in final analysis, comprising 1991 patients with cirrhosis. The pooled prevalence of RAI was 37% (95% CI 33–42%). The prevalence of RAI varied by Child–Pugh classification, type of stimulation test used, specific diagnostic threshold and by severity of illness. Ninety-day mortality was significantly higher in patients with RAI (OR 2.88, 95% CI 1.69–4.92, I2 = 15%, p < 0.001).
Conclusions
Relative adrenal insufficiency is highly prevalent in non-critically ill patients with cirrhosis and associated with increased mortality. Despite the proposed multifactorial pathogenesis, no studies to date have investigated therapeutic interventions in this specific population.
Abbreviations
-
- ACTH
-
- adrenocorticotropic hormone
-
- CRP
-
- C-reactive protein
-
- Delta cortisol
-
- change in serum total cortisol
-
- ESR
-
- erythrocyte sedimentation rate
-
- HDL
-
- high-density lipoprotein
-
- HPA
-
- hypothalamic–pituitary–adrenal
-
- LD-SST
-
- low-dose short synacthen test
-
- MELD
-
- model for end-stage liver disease
-
- OR
-
- odds ratio
-
- PFC
-
- plasma-free cortisol
-
- PRA
-
- plasma renin activity
-
- RAI
-
- relative adrenal insufficiency
-
- SC
-
- salivary cortisol
-
- SD-SST
-
- standard-dose short synacthen test
-
- SMD
-
- standardized mean difference
-
- TC
-
- total cortisol
Key points
- Adrenal dysfunction is common in cirrhosis but its true prevalence is clouded by significant study heterogeneity
- We performed a comprehensive systematic review and meta-analysis to determine overall and pre-specified stratified prevalence based on important clinical and diagnostic variables
- Abnormal cortisol stimulating testing was present in 37% of patients overall and associated with increased 90-day mortality
- Current literature suggests that abnormal cholesterol metabolism may underlie adrenal dysfunction in cirrhosis but the relative contributions of inflammation and circulatory dysfunction remain underexplored
- Whether glucocorticoid replacement therapy provides benefit in the non-critically ill population remains unknown
1 INTRODUCTION
Relative adrenal insufficiency (RAI) in patients with cirrhosis is common and thought to have a complex pathophysiology.1, 2 While the literature has consistently demonstrated an association with severity of liver disease, the presence of acute illness may also alter adrenal responsiveness. There is a high prevalence of RAI in critically ill patients with cirrhosis and its presence is associated with increased mortality.3-9 In this population, corticosteroid replacement appears to reduce vasopressor requirements and improve in-hospital survival.3-6, 10 However, steroid supplementation is also associated with an increased risk of gastrointestinal bleeding and resistant bacterial infections, as well as a lack of reduction in 28-day mortality.4, 6
In contrast, prevalence rates, outcomes and the benefits of corticosteroid replacement are less well established in the non-critically ill population given significant study heterogeneity.2, 11 Numerous factors contribute to this heterogeneity, including severity of illness and/or liver impairment, selection of stimulation test, biological properties of the measured form of cortisol and thresholds utilized to define RAI. The lack of consensus around an optimal methodology to diagnose and treat RAI in cirrhosis (particularly in the non-critically ill population) is a source of confusion for clinicians. Current societal guidelines exclusively support total cortisol (TC) measurement to diagnose RAI.12 However, use of TC is controversial given that hypoalbuminemia and low cortisol-binding globulin levels may overestimate the presence of RAI.1, 13 Unfortunately, proposed alternatives such as plasma-free cortisol (PFC) or salivary cortisol (SC) levels carry practical limitations and uncertain prognostic significance, both of which have limited their widespread adoption.11
A prior meta-analysis by Kim et al. included 16 studies evaluating RAI in cirrhosis in both critically-ill and non-critically patients.14 In the 5-year interim since its publication, however, an additional 11 studies have been published on the topic.10, 15-24 Given the influx of this recent literature and uncertainty surrounding the concept of RAI in patients with cirrhosis but without critical illness, we undertook an updated systematic review and meta-analysis. We restricted our analysis to patients in either the outpatient or acute care (but not intensive care) setting to reduce the known suppressive effects of critical illness on hypothalamic–pituitary–adrenal (HPA) axis functionality.25-27 Our primary aim was to define the prevalence of RAI by controversial diagnostic variables (such as the type of stimulation test used, the form of cortisol studied, the specific threshold utilized to diagnose RAI and the clinical setting in which HPA axis testing was performed). A secondary aim was to assess differences in clinical variables or outcomes by adrenal functional status.
2 METHODS
- Change in serum TC (delta cortisol) of <9 μg/dl or a peak serum TC <18 μg/dl in response to administration of either a standard-dose short synacthen test (SD-SST; 250 μg of synacthen) or low-dose short synacthen test (LD-SST; 1 μg of synacthen) OR
- Peak PFC level of <1.2 μg/dl in response to administration of either a SD-SST or LD-SST OR
- A composite of baseline SC <0.2 μg/dl, peak SC <1.3 μg/dl or delta SC <0.3 μg/dl.
Utilizing a similar framework to Kim et al.14 prevalence of RAI, baseline demographic and disease aetiology/severity characteristics were recorded. Outcome variables of interest included mortality, portal-hypertension complications and infection rates.
A comprehensive literature search for the systematic review and meta-analysis was performed using Ovid-MEDLINE, ScienceDirect, Web of Science, Cochrane Library and ClinicalTrials.gov; all articles and studies through December 2021 were included in each database query. Search terms used for Ovid-MEDLINE included the following MeSH terms and Boolean operators: ‘liver cirrhosis’ and ‘adrenal insufficiency’. ScienceDirect, Web of Science, Cochrane Library and ClinicalTrials.gov were queried using the following search terms and Boolean operators: [(cirrhosis OR cirrhotic OR hepatic OR liver) and (adrenal insufficiency OR relative adrenal insufficiency OR RAI)].
- Published manuscript in the English language,
- Study design was cross-sectional, case–control, cohort or a randomized clinical trial,
- Population included patients with decompensated cirrhosis who were not receiving care in an intensive care unit and/or being treated with vasopressors,
- Diagnostic criteria for RAI were clearly defined, and
- Prevalence of RAI and/or clinical outcomes were reported.
Studies were excluded if they did not meet all of the above criteria, including abstracts and unpublished studies. Prior systemic reviews were cross-referenced to ensure completeness of study inclusion.1, 14, 28, 29
2.1 Data extraction and methodological quality assessment
Two experienced researchers (BJW and MS) independently performed database queries using the specified search strategy. Potentially relevant titles were compiled and duplicates were removed. Abstracts were independently reviewed (BJW and MS) for appropriateness to proceed with full-text analysis. Data of interest was extracted as follows: authors, study year, country, study design, duration of follow-up, patient characteristics, sample size, RAI diagnostic criteria, RAI prevalence and clinical outcomes. Methodological quality and internal/external validity of included studies was evaluated using the Newcastle–Ottawa Quality Assessment Scales for cohort and cross-sectional studies in independent fashion by two experienced researchers (BJW and MS). Disagreements were rectified through independent assessment by a third experienced researcher (CG) and consensus if needed.
2.2 Data synthesis and analysis
The primary outcome was the overall pooled prevalence of RAI defined by a peak TC level < 18 μg/dl, delta cortisol <9 μg/dl or composite of the two thresholds in response to either a SD-SST or LD-SST. Secondary outcomes of interest were differences between patients with and without RAI with respect to clinical variables reported in a majority of studies including the model for end-stage liver disease (MELD) score, Child–Pugh score, albumin levels, HDL levels, mean arterial pressure (MAP), as well as baseline TC, peak TC and delta cortisol. Ninety-day mortality was also assessed as a secondary outcome. Pre-planned stratified pooled prevalence by Child–Pugh classification, type of SST, RAI diagnostic threshold and illness acuity (stable outpatient vs hospitalized, non-critically ill) were performed.
Other variables of interest between patients with and without RAI related to proposed mechanistic underpinnings, including markers of inflammation (erythrocyte sedimentation rate, C-reactive protein and cytokine levels) and neurohormonal levels (plasma renin activity, aldosterone) were recorded (Table S1). However, meta-analysis was not performed given few studies and small sample size.
Pooled prevalence ratios and 95% confidence intervals (overall and by strata) were calculated using the PROC GENDMOD procedure within the SAS Statistical Package (SAS Version 9.4, Cary, NC) using a binomial distribution with unstructured covariance matrix and using each individual study as the link function. The metafor meta-analysis package30 within the open-source RStudio (RStudio Team 2022, R Studio: Integrated Development for R, RStudio, PBC, Boston, MA) was then utilized to develop DerSimonian and Laird mixed random effects given the significant clinical heterogeneity between studies to ensure conservative estimates were attained. Statistical heterogeneity within the data was assessed using the I2 statistic. Degree of heterogeneity was defined as low (≤25%), moderate (26–74%) and high (≥75%). Estimation of study variable means and standard deviations from medians and ranges or interquartile ranges was performed using previously published methods.31-33 Odds ratios (ORs) and standard mean differences (SMDs) between groups were calculated. Visual representation of this analysis utilized forest plots to display individual studies and pooled results. Funnel plots were created to assess for publication bias.
3 RESULTS
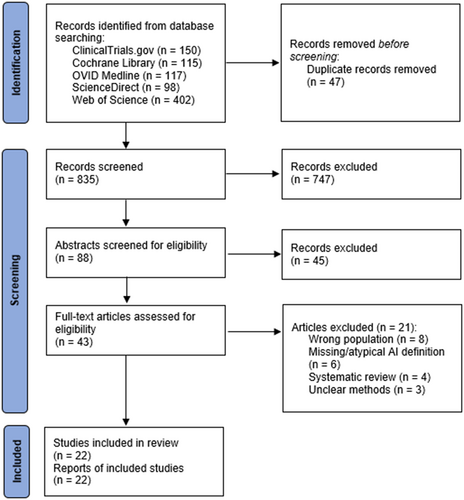
Based on the search strategy defined in the Methods section, 882 records were initially identified. After removal of duplicates, 835 records were screened, producing 88 records which underwent abstract screening for eligibility. Of this group, 43 were deemed potentially relevant and underwent full-text review. After excluding 21 studies for selection of a non-target population (n = 8), unclear or unusual AI definition (n = 6), being a systemic review (n = 4) or unclear study methodology (n = 3), 22 studies were included in the final analysis (see Figure 1).7, 9, 13, 15-18, 20, 21, 23, 34-45
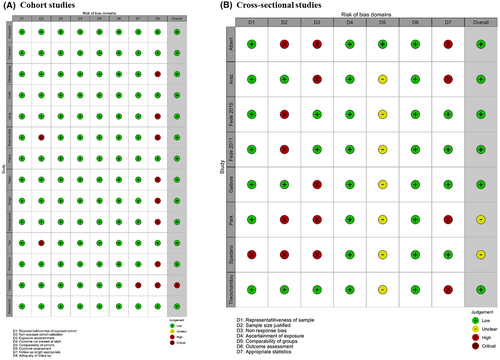
3.1 Quality assessment
Studies were stratified by the two principal design types and assessed for methodological quality (Figure 2). All but one cohort study had low overall risk of bias; many studies though did not report if any patients were lost to follow-up. The cross-sectional studies had higher rates of bias, including many not justifying the sample size or lack of description about eligible patients who did not enrol (non-response bias), although these were not deemed to be fatal flaws.
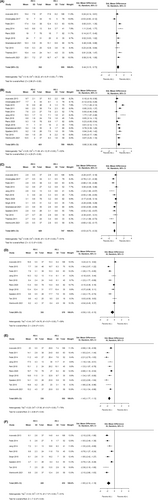
There was no significant publication bias related to the primary outcome or secondary outcomes of interest as identified through multiple funnel plots, with the ORs or SMDs plotted on the x-axis and the standard error plotted on the y-axis (Figure S1A-I).
3.2 General characteristics of selected studies
The 22 studies included in final analysis comprised 1991 patients (65% male, 45% with alcohol-associated cirrhosis). Males accounted for 65% (n = 1303) of the population and alcohol was the most common aetiology of cirrhosis (n = 904, 45%). Child–Pugh classification was reported in 17 studies (n = 1334) with following breakdown: A – 22%, B – 42% and C – 36%. Four studies evaluated outpatients with stable cirrhosis,13, 17, 21, 34 compared to 18 studies investigating inpatients without critical illness.7, 9, 15, 16, 18, 20, 23, 35-45 All studies were observational and single centre, including 14 prospective cohort studies7, 9, 13, 15-18, 35, 36, 39, 41-43, 45 and eight cross-sectional studies.20, 21, 23, 34, 37, 38, 40, 44 There was strong international and multi-continent representation with 11 unique countries of origin.
As seen in Table 1, most studies (n = 14) utilized the SD-SST for diagnosis of RAI,9, 13, 15, 16, 18, 20, 23, 34-36, 40-43 with a minority (n = 5) using the LD-SST.21, 37-39, 44 Three studies utilized both a SD-SST and LD-SST to evaluate for RAI7, 17, 45; data for each SST type were included in pooled subgroup analyses. The most common criteria to define RAI was delta cortisol (n = 15),9, 13, 15-18, 20, 21, 36, 38, 40-42, 45 with 13 studies using peak TC instead or as an alternative criterion.7, 13, 17, 20, 21, 23, 37-40, 42, 44, 45 Peak levels of PFC were utilized in five studies,13, 21, 34, 37, 39 whereas four studies described use of SC to define RAI.17, 20, 36, 40 The measurement methodology for TC, PFC and/or SC was variable and is described for each study in the (Table S2).
| First author | Year, country | Study design | N (AI/non-AI) | Population | Summary MELD score | Summary CP score or class (A/B/C) | ACTH stim test | AI defa | AI prev |
|---|---|---|---|---|---|---|---|---|---|
| Acevedo | 2013, Spain | PC | 143 (37/106) | Hospitalized – ADC | 18 ± 7 | 9 ± 2 | SD-SST | Δ TC | 26% |
| Albert | 2019, Spain | CS | 39 (19/20) | Hospitalized – ADC | n/a | 9 ± 2 (6/18/15) | SD-SST | Δ TC, Peak TC, SC | 26%, 23%, 31% |
| Araz | 2016, Turkey | CS | 110 (23/87) | Outpatient – stable cirrhosis | n/a | 7 ± 2 (56/32/22) | SD-SST | Peak FC | 15% |
| Chawlani | 2015, India | PC | 120 (69/51) | Hospitalized – non-septic cirrhosis | 20 (6–40) | 10 (6, 13) | SD-SST | Combo: Δ TC or Peak TC | 58% |
| Cholongitas | 2017, Greece | PC | 113 (34/79) | Hospitalized – liver transplant evaluation | 14 ± 5 | 8 ± 2 | SD-SST | Δ TC, SC | 30%, 22% |
| Fede | 2015, UK/Italy | CS | 121 (46/75) | Hospitalized – non-septic ADC | 14 (6–25) | 9 (5, 21) | LD-SST | Peak TC, Peak FC | 38%, 6% |
| Fede | 2011, UK/Italy | CS | 101 (38/63) | Hospitalized – non-septic cirrhosis | 14 (11, 18) |
8 (6, 10) (29/38/34) |
LD-SST | Δ TC, Peak TC | 59%, 38% |
| Fede | 2014, UK | PC | 79 (27/52) | Hospitalized – liver transplant evaluation or non-septic ADC | 15 (13, 20) |
8 (7, 9) (19/43/17) |
LD-SST | Δ TC, Peak TC, Peak FC | 71%, 34%, 28% |
| Galbois | 2010, France | CS | 88 (29/59) | Hospitalized – non-septic ADC | 16 ± 8 |
10 ± 2 (4/24/60) |
SD-SST | Δ TC, Peak TC, SC | 11%, 11%, 9% |
| Jang | 2014, South Korea | PC | 54 (13/41) | Hospitalized –non-septic cirrhosis | 11 ± 5 |
8 ± 2 (18/22/14) |
SD-SST | Δ TC | 24% |
| Kalambokis | 2021, Greece | PC | 95 (27/68) | Outpatient – stable cirrhosis | 14 ± 4 |
9 ± 2 (31/38/26) |
SD-SST, LD-SST | Δ TC, Peak TC, SC | 24%, 9%, 20% |
| Park | 2018, South Korea | CS | 69 (24/45) | Hospitalized – non-septic cirrhosis | n/a |
8 ± 2 (19/37/13) |
SD-SST | Peak TC | 35% |
| Piano | 2020, Italy | PC | 160 (78/82) | Hospitalized–ADC | 18 ± 7 | 9 ± 2 | SD-SST | Δ TC | 49% |
| Risso | 2015, Italy | PC | 93 (30/63) | Hospitalized – non-septic cirrhosis with ascites | 19 (15, 23) |
9 (8, 10) (0/50/43) |
SD-SST | Δ TC, Peak TC | 15%, 32% |
| Singh | 2018, India | PC | 66 (31/35) | Hospitalized – non-septic cirrhosis with ascites | n/a | 11 ± 2 (1/15/50) | SD-SST | Combo: Δ TC or Peak TC | 47% |
| Siramolpiwat | 2021, Thailand | PC | 115 (35/80) | Hospitalized – non-septic ADC | 16 ± 7 | 8 ± 2 (18/62/35) | SD-SST | Δ TC | 30% |
| Spadaro | 2015, Italy | CS | 81 (26/55) | Hospitalized – non-septic cirrhosis without ADC | n/a |
8 (5–12) (20/39/22) |
LD-SST | Peak TC | 32% |
| Tan | 2010, Australia | PC | 43 (25/18) | Outpatient – stable cirrhosis | 13 (n/a) |
9 (5–13) (9/13/21) |
SD-SST | Δ TC, Peak TC, Peak FC | 47%, 42%, 12% |
| Theocharidou | 2019, Greece | CS | 61 (27/34) | Outpatient – stable cirrhosis | 15 ± 7 |
n/a (17/28/16) |
LD-SST | Δ TC, Peak TC, Peak FC | 44%, 18%, 0% |
| Thevenot | 2012, France | PC | 95 (47/48) | Hospitalized – non-septic cirrhosis | n/a |
n/a (34/29/32) |
SD-SST, LD-SST | Δ TC, Peak TC | 49%, 27% |
| Triantos | 2011, Greece | PC | 50 (24/26) | Hospitalized – non-septic cirrhosis | 16 (6–29) |
9 (5–15) (10/18/22) |
SD-SST, LD-SST | Peak TC | 48% |
| Wentworth | 2021, USA | PC | 95 (37/58) | Hospitalized – non-septic cirrhosis | 17 (13, 21) |
9 (8, 11) (0/51/44) |
SD-SST | Δ TC | 39% |
- Abbreviations: ACTH, adrenocorticotropic hormone; ADC, acute decompensated cirrhosis; Combo, combination; CP, Child-Pugh; CS, cross-sectional; Def, definition; FC, free cortisol; LD-SST, low-dose short synacthen test; MELD, Model for End-Stage Liver Disease; PC, prospective cohort; Prev, prevalence; SC, salivary cortisol SD-SST, standard-dose short synacthen test; TC, total cortisol.
- a AI defined as: ΔTC <9 μg/dL, Peak TC < 18 μg/dl, Peak FC <1.2 μg/dl or composite baseline SC <0.2 μg/dl, peak salivary cortisol <1.3 μg/dl or delta salivary cortisol <0.3 μg/dl.
3.3 Prevalence of AI: Overall and by Subgroup
The overall pooled prevalence of RAI was 37% (95% CI 33–42%). When comparing prevalence between Child–Pugh classifications, RAI prevalence was highest in Child–Pugh C patients (64%, 95% CI 58–71%) as compared to Child–Pugh B (39%, 95% CI 28–50%) and Child–Pugh A patients (27%, 95% CI 16–38%). The prevalence of RAI was slightly higher in hospitalized, non-critically ill patients (38%, 95% CI 34–43%) as compared to stable outpatients with cirrhosis (33%, 95% CI 21–45%).
The prevalence was also assessed by two levels of diagnostic test characteristics—the type of SST utilized as well as the clinical threshold used to diagnose AI (Table 2). Use of the LD-SST resulted in a RAI prevalence of 44% (95% CI 36–52%) compared to a prevalence of 34% (95% CI 28–41%) when the SD-SST was administered. When comparing various clinical thresholds, use of delta cortisol yielded the highest RAI prevalence (36%, 95% CI 28–45%). Peak TC was associated with a 29% prevalence (95% CI 23–35%). Salivary cortisol produced a pooled prevalence of 19% (95% CI 13–25%) and peak PFC had the lowest RAI prevalence at 12% (95% CI 4–20%).
| Analysis | Pooled prevalence (95% CI) |
|---|---|
| Overall | 37% (33–42%) |
| By Child–Pugh classification | |
| Child–Pugh A | 27% (16–38%) |
| Child–Pugh B | 39% (28–50%) |
| Child–Pugh C | 64% (58–71%) |
| By type of ACTH stimulation test | |
| SD-SST | 34% (28–41%) |
| LD-SST | 44% (36–52%) |
| By diagnostic threshold | |
| Peak total cortisol <18 μg/dl | 29% (23–35%) |
| Delta total cortisol <9 μg/dl | 36% (28–45%) |
| Peak free cortisol <1.2 μg/dl | 12% (4–20%) |
| Salivary cortisol (composite)a | 19% (13–25%) |
| By illness acuity | |
| Stable outpatient | 33% (21–45%) |
| Hospitalized, non-critically ill | 38% (34–43%) |
- Abbreviations: ACTH, adrenocorticotropic hormone; SD-SST, standard-dose short synacthen test; LD-SST, low-dose short synacthen test.
- a Composite of baseline salivary cortisol <0.2 μg/dL, peak salivary cortisol <1.3 μg/dL or delta salivary cortisol <0.3 μg/dL.
3.4 Patient characteristics stratified by presence of AI
Not all studies reported stratified analysis of clinical variables by adrenal functional status. Figure 3 displays the SMD between patients with and without RAI with respect to six different domains. The MELD score was reported in 10 studies with a high degree of heterogeneity (I2 = 76%).7, 9, 13, 15, 16, 18, 36, 38, 41, 43 The SMD in patients with RAI was significantly higher at 0.44 (95% CI 0.15–0.73, Z = 2.96, p < 0.001). Similar results were obtained for the Child–Pugh score (SMD 0.66, 95% CI 0.36–0.96, Z = 4.29, p < 0.001) over 13 studies with high heterogeneity (I2 = 83%).9, 13, 15, 16, 18, 23, 36-39, 41, 43, 44 Patients with RAI had lower levels of serum albumin (SMD −0.53, 95% CI −0.73 – (−0.33), Z = −5.13, p < 0.001) across 13 studies with moderate heterogeneity (I2 = 61%).7, 9, 13, 15, 16, 18, 23, 36, 38, 39, 41, 43, 44
Measurements of TC were also significantly lower in patients with RAI and displayed high heterogeneity (I2 = 76–89%). Ten studies reported baseline TC levels, with a SMD of −0.59 (95% CI −1.03 – (−0.15), Z = −2.64, p = 0.01) in the RAI group.9, 13, 15, 18, 23, 37, 38, 41, 43, 44 Ten studies described peak TC levels, with the RAI group having a lower SMD of −1.45 (95% CI −1.77 – (−1.13), Z = −8.89, p < 0.001).9, 13, 15, 18, 23, 38, 39, 41, 43, 44 Differences in delta cortisol level were even more pronounced in the RAI group, with a SMD of −1.65 (95% CI −2.10 – (−1.19), Z = −7.12, p < 0.001) over eight studies.9, 13, 15, 23, 39, 41, 43, 44
Potential pathophysiological variables driving the development of RAI in cirrhosis were also compared with respect to adrenal functional status (Figure S2A,B). Lower levels of serum HDL were seen in patients with RAI (SMD −0.28, 95% CI −0.56 – (−0.01), Z = −2.03, p = 0.04) over 11 studies with high heterogeneity (I2 = 76%).9, 13, 15, 16, 18, 23, 36, 39, 41, 43, 44 Patients with RAI also had lower MAPs (SMD −0.18, 95% CI −0.32 – (−0.04), Z = −2.60, p = 0.01) across nine studies with negligible heterogeneity (I2 = 0%).9, 15, 18, 23, 37-39, 43, 44 Meta-analysis was unable to be performed on other biomarkers indicative of circulatory dysfunction and inflammation. Higher levels of aldosterone (two studies) and plasma renin activity (PRA; three studies) were present in patients with RAI.9, 36, 41 There was a clear trend towards elevated C-reactive protein (CRP) levels in patients with RAI across three studies.9, 16, 18 In contrast, erythrocyte sedimentation rate (ESR) and pro-inflammatory cytokine (TNFα, IL-1β and IL-6) levels were similar in patients with and without RAI in the few studies that investigated these specifically.9, 16, 36
True subgroup analysis by clinical setting (i.e. inpatient vs outpatient) was not feasible given all included studies with available stratified data by adrenal functional status were conducted in the inpatient setting except one (Tan et al.13). However, when the aforementioned study was excluded, there was no significant change in the relative strengths of variable association with RAI (Table S3).
3.5 Odds of 90-day mortality stratified by the presence of AI
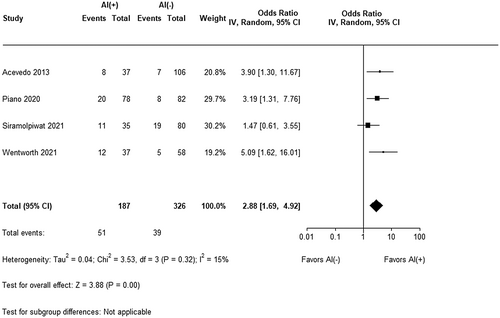
Given the significant heterogeneity between cohort studies with respect to length of follow-up and outcomes of interest, only 90-day mortality was appropriate for meta-analysis. As seen in Figure 4, the presence of RAI was associated with increased mortality (OR 2.88, 95% CI 1.69–4.92, Z = 3.88, p < 0.001) across the four included studies.9, 15, 16, 18 There was low heterogeneity (I2 = 15%) amongst the studies. All studies except Siramolpiwat et al.16 explicitly described the degree of loss to follow-up and thus there was an overall low risk of selection bias.
4 DISCUSSION
This is the first meta-analysis that exclusively focused on describing RAI in patients with cirrhosis who are not critically ill. The ‘hepatoadrenal syndrome’ was initially described in an ICU population in 2005 by Marik et al. but later noted in patients across the spectrum of liver disease.3 Many studies suggest that the presence of RAI in cirrhosis is fundamentally different from traditional aetiologies in the general population. In the majority of cases, basal production of cortisol is adequate to meet homeostatic needs. However, cirrhotic and/or acute illness-induced physiological changes are theorized to induce a ‘relative’ deficiency of cortisol whereby an individual patient may either produce an inadequate amount of cortisol or be unable to overcome tissue-level glucocorticoid resistance. Longitudinal studies measuring adrenal function over time are needed to test this hypothesis. Exhaustive literature search revealed only a very small subgroup from Acevedo et al. suggestive of potential reversibility of the adrenal dysfunction.9
Interestingly, the prevalence of RAI was only marginally lower in outpatients as compared to hospitalized, non-critically ill patients by measurement of total cortisol. We acknowledge that the outpatient estimate is less precise than the hospitalized estimate given the imbalance in number of included studies (four vs 18 respectively). Despite this, the similar prevalence may suggest that the development of RAI is related more to the intrinsic liver disease rather than the effect of acute illness. It is well established that acute illness leads to decreased hepatic synthesis of albumin and cortisol-binding globulin,46 thus, there is a concern that use of TC in low protein states may lead to over-diagnosis of RAI.1, 13
Our results support the latter notion that the prevalence of RAI is highly dependent on diagnostic methodology. Most studies utilized TC; whether a peak <18 μg/dl or delta <9 μg/dl threshold was selected led to similar prevalence (29% vs 36% respectively). Contrastingly, the five studies that included a peak PFC <1.2 μg/dl cutoff described a much lower prevalence at 12%. This large difference and the fact that PFC levels are not dependent on binding globulin availability has led some authors to prefer its use in cirrhosis. However, its potential benefits are balanced by several issues including a lack of validated reference range in cirrhosis, typical dependence on the analytical sample being sent to a reference laboratory (result reporting delays of up to 1–2 weeks), and unclear significance with regard to clinical outcomes.
Only one clinical outcome was reliably captured for inclusion in this meta-analysis: 90-day mortality. All four studies had similar patient populations (hospitalized, non-critically ill with comparable MELD and Child–Pugh scores) and used the combination of a SD-SST and delta total cortisol to diagnose RAI. Given the low degree of heterogeneity, the presence of RAI was found to be associated with a nearly threefold increased risk of mortality at 90 days. Whether this mortality association extends to RAI diagnosed by LD-SST and/or binding-globulin independent cortisol (i.e. PFC or SC) measurements is unknown and warrants further investigation. Additionally, an emerging concept is that this ‘hepatoadrenal syndrome’ may represent a previously poorly captured organ failure along the spectrum of acute-on-chronic liver failure.18 However, whether RAI as defined by an inadequate delta cortisol response is independently predictive of mortality (i.e. independent of underlying liver disease severity) is unsettled.9, 15, 16, 18
Further expanding upon the previous point, the development of RAI appears to be at least partially dependent upon the degree of hepatic impairment. We confirmed prior findings that RAI prevalence increases across Child–Pugh classification; a non-linear (exponential) trend was noted and nearly two-thirds of Child–Pugh C patients were diagnosed with RAI by TC criteria. Interestingly, while mean albumin and baseline TC levels were lower in patients with RAI, the SMDs were small. Therefore, other mechanisms may be involved in the development of adrenal dysfunction in patients with cirrhosis besides simply representing a relative deficiency of cortisol-binding globulins.
Some authors have proposed abnormalities in cholesterol metabolism as a pathophysiological contributor to RAI.1, 15, 18 The present meta-analysis did find HDL levels to be significantly lower in patients with RAI, albeit only to a modest degree. It is possible that isolated measurement of HDL levels may not represent an adequate surrogate biomarker to capture the complexity of the dyslipidemia of cirrhosis and its effect on adrenal steroidogenesis. Another explanation is that the high heterogeneity between studies diluted the effect; further study into whether the dyslipidaemia of cirrhosis is related to RAI is warranted.
Circulatory dysfunction is theorized to contribute to RAI.9, 11 While this meta-analysis found a significantly lower MAP in patients with RAI, the SMD was small. However, MAP alone may not detect more subtle neurohormonal contributions. Unfortunately, vasoactive substances (i.e. nitric oxide and norepinephrine) were reported in only one study9; aldosterone levels and PRA were each reported in only two and three studies respectively.9, 36, 41 Aldosterone levels and PRA were elevated in patients with RAI but its causality in driving the development of RAI remains unclear. The aforementioned studies do not describe the prevalence of concurrent aldosterone antagonist use in patients with ascites; in addition, these biomarkers did not associate with increased mortality. Thus, the available literature to support circulatory dysfunction as a cause of adrenal dysfunction in cirrhosis is murky and requires further study outside the context of acute illness to better isolate liver-specific (rather than acute illness-related) contributions.
Similarly, some authors suggest systemic inflammation in cirrhosis may partially underlie RAI.1, 11 Validation is limited by only a few studies measuring a heterogeneous mixture of biomarkers (i.e. ESR, CRP and/or cytokine levels).9, 16, 18, 36 While CRP levels were numerically higher in patients with RAI, they did not reach statistical significance in any of the three studies.9, 16, 18 Certain pro-inflammatory cytokine levels (TNFα, IL-6) were higher on average in RAI were not statistically significant given large standard deviations. This uncertainty about the true population mean underscores the likely contribution of the acute illness. For example, the study by Acevedo et al. showed strong trends towards an increased prevalence of the systemic inflammatory response syndrome and Type 1 hepatorenal syndrome in patients with RAI.9 Additionally, the RAI groups in Piano et al. and Siramolpiwat et al. trended towards having higher rates of concurrent bacterial infection.16, 18 Thus, more studies and increased granularity are required before high-quality meta-analysis can more definitely assess the relative importance of circulatory dysfunction and/or systemic inflammation in the development of RAI.
Similar to the prior meta-analysis from Kim et al.14 we found that the prevalence of RAI to be higher when the LD-SST is administered rather than the SD-SST. The majority of included studies utilized the SD-SST exclusively, which aligns with current guidelines provided by the Endocrine Society.12 Some proponents of LD-SST note improved diagnostic sensitivity for RAI compared to the SD-SST21, 38; both represent supraphysiological adrenocorticotropic hormone (ACTH) dosages but the standard dose is excessively so. However, use of the LD-SST has not been validated in acute illness (such as hospitalized patients) or in patients with abnormal circadian rhythms, as can be seen in patients with hepatic encephalopathy.47 Debate remains about the optimal diagnostic methodology but clinicians should be aware that the association between RAI and increased mortality has been principally seen in studies utilizing the SD-SST.9, 15, 16, 18, 41
We acknowledge several limitations to this meta-analysis. While this study expanded the number of included studies and increased the pooled sample size as compared to Kim et al.14 there was still significant heterogeneity between studies. We attempted to minimize this by using random-effects models. Although sub-analysis by various methodological characteristics was important to perform on clinical grounds, the validity of certain estimates may be uncertain if only a limited number of studies were available. Additionally, the admitting diagnosis or comorbidities of hospitalized, non-critically ill patients was not consistently recorded and thus extraneous effects of illness or prescribed therapies could result in residual confounding. To counter this, most studies had well-described inclusion and exclusion criteria that attempted to mitigate potential sources of bias. Another limitation was that reporting of clinical outcomes was non-standardized and thus pooled estimates were unavailable outside of 90-day mortality. Finally, only observational studies were included as no published randomized clinical trials met inclusion criteria. Therefore, outcome data are only associative and cannot imply causality.
Overall, this study confirms that RAI is common in patients with cirrhosis and non-critical illness. While there is a lack of distinctive phenotype predictive of RAI based on available literature, it may be context dependent related to the severity of underlying liver disease and potentially the burden of acute illness. Methodological differences in HPA axis assessment resulted in varied prevalence estimates, but only RAI diagnosed through use of a SD-SST and delta cortisol threshold of <9 μg/dl is associated with increased short-term mortality. Prospective studies are needed to assess the HPA axis in longitudinal fashion, understand the prognostic implications of TC alternatives and determine the efficacy and safety of corticosteroid supplementation in this population.
FUNDING INFORMATION
B.J.W. has received research support through the American Association for the Study of Liver Diseases Foundation Clinical, Translational and Outcomes Research Award (50031). H.M.S has received research support through the National Institutes of Health R01 DK114875 and R01 HL091535. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
CONFLICT OF INTEREST
The authors have none to disclose.
ETHICS APPROVAL STATEMENT
Ethics approval was not required for this systematic review and meta-analysis.
PATIENT CONSENT STATEMENT
Patient consent was not required for this systematic review and meta-analysis as this was secondary data capture from previously published literature.
PERMISSION TO REPRODUCE MATERIAL FROM OTHER SOURCES
Not applicable.
CLINICAL TRIAL REGISTRATION
All authors have reviewed the final version of this manuscript and approve its submission.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analysed in this study.