Combination antiretroviral therapy improves recurrent primary biliary cholangitis following liver transplantation
Ellina Lytvyak and Mina Niazi shared first authorship.
Abstract
Recurrent primary biliary cholangitis (rPBC) is frequent following liver transplantation and associated with increased morbidity and mortality. It has been argued that rPBC behaves like an infectious disease because more potent immunosuppression with tacrolimus is associated with earlier and more severe recurrence. Prophylactic ursodeoxycholic acid is an established therapeutic option to prevent rPBC, whereas the role of second line therapies, such as obeticholic acid and bezafibrate in rPBC, remains largely unexplored. To address the hypothesis that a human betaretrovirus plays a role in the development of PBC, we have tested antiretroviral therapy in vitro and conducted randomised controlled trials showing improvements in hepatic biochemistry. Herein, we describe the utility of combination antiretroviral therapy to manage rPBC in two patients treated with open label tenofovir/emtricitabine-based regimens in combination with either lopinavir or raltegravir. Both patients experienced sustained biochemical and histological improvement with treatment, but the antiretroviral therapy was associated with side effects.
Abbreviations
-
- ART
-
- antiretroviral therapy
-
- EC50
-
- effective concentration of drug that provides half maximal inhibitory responses
-
- EC95
-
- effective concentration of drug that provides 95% inhibitory responses
-
- HBRV
-
- human betaretrovirus
-
- FTC
-
- Emtricitabine
-
- LPRr
-
- lopinavir boosted with ritonavir
-
- LT
-
- liver transplantation
-
- PBC
-
- primary biliary cholangitis
-
- PTLD
-
- posttransplant lymphoproliferative disorder
-
- RAL
-
- raltegravir
-
- TAF
-
- tenofovir alafenamide
-
- TDF
-
- Tenofovir disoproxil fumarate
1 INTRODUCTION
The management of primary biliary cholangitis (PBC) has advanced considerably over the last decade with establishment of second line therapies such as obeticholic acid and bezafibrate.1 The role of ursodeoxycholic acid (UDCA) in treating or preventing recurrent PBC (rPBC) following liver transplantation (LT) is understood,2 but the utility of obeticholic acid and bezafibrate in treating rPBC is unknown.1 Of utmost importance, we lack curative treatments and still have no functional symptomatic therapy to manage fatigue, which is directly related to our poor understanding of mechanisms that drive the disease process. The systemic nature of PBC related symptoms is underscored by reports that fatigue may persist following liver transplantation in up to 50% of individuals by 2 years even in the absence of histological rPBC in the allograft.1, 3
Observations from patients following LT suggest that rPBC may behave like an infectious disease process.4 For example, it is recognised that LT recipients on more potent immunosuppressive regimens with tacrolimus are at increased risk of earlier and more severe rPBC, whereas the less powerful cyclosporine (with broad antiviral effects) is protective.4, 5 Furthermore, the observation that rPBC is signalled by cholestatic liver changes within the first 6 months is more in keeping with an infectious disease process because an autoimmune process would be unlikely to surface in the first few months following LT when the immunosuppression levels are highest.4
Our laboratory has been working on the hypothesis that a human betaretrovirus (HBRV) closely related to mouse mammary tumour virus (MMTV) may trigger PBC in genetically susceptible individuals.6, 7 To determine the frequency of HBRV in PBC, we performed proviral integration studies considered the gold standard for documenting retroviral infection. More than 1500 unique HBRV insertions were detected within the human genome, and infection was localised to biliary epithelial cells in 84% of the PBC patients studied.6, 8 Two randomised controlled trials using antiretroviral therapy (ART) have shown significant but modest improvements in hepatic biochemistry but did not achieve that stated endpoints.9-11
1.1 Choice of repurposed HIV ART to treat HBRV infection
We evaluated HIV ART for activity against HBRV and found that reverse transcriptase inhibitors, such as tenofovir (TDF), and the HIV protease inhibitor lopinavir (LPR), can inhibit betaretrovirus activity in vitro.5 Others have reported that HIV integrase inhibitor raltegravir (RAL) exhibits broad spectrum activity against gammaretroviruses and betaretroviruses.12 Accordingly, we evaluated the ability of RAL to inhibit pseudotyped particles containing either the HIV or HBRV integrase from expressing green fluorescent protein in vitro.13 We found that RAL inhibited HBRV reporter expression by 94%-97% at the EC50 and EC95 concentrations used for HIV (effective concentration of drug providing half-maximal or 95% inhibitory responses), suggesting that RAL should provide sufficient antiviral HBRV activity in patients at the 1200 mg daily dosing.12
In vivo studies using the NOD.c3c4 mouse model of PBC with MMTV cholangitis showed that combination of emtricitabine/tenofovir (FTC/TDF) and lopinavir boosted with ritonavir (LPRr) resulted in biochemical and histological improvement with amelioration of cholangitis.14 Others have reported that the FTC/TDF/LPRr regimen normalised hepatic biochemistry in a PBC patient with both HIV and HBRV co-infection.15 Accordingly, we used the same regimen in a randomised controlled for PBC patients unresponsive to UDCA but enrolment was discontinued prematurely because two thirds of subjects developed gastrointestinal intolerance to LPRr.9 Nevertheless, patients who continued to take FTC/TDF/LPRr in the open label extension study demonstrated significant and sustained biochemical responses to treatment.
Herein, we describe the clinical course of two liver transplant recipients with rPBC who were treated with different combination ART regimens. As we had not established the utility of prophylactic UDCA at the time, both patients received UDCA once rPBC was diagnosed.2 Also, the combination ART regimens was instituted prior to the licencing of obeticholic acid in Canada, and therefore, other second line therapies were not employed. Written informed consent was obtained from each patient, and their management was discussed by all members of the Liver Transplant committee prior to starting therapy.
1.2 Case 1
At the age of 15, the female patient was diagnosed with PBC by liver biopsy, an AMA titer of 1:800 and cholestatic liver tests, and then was commenced on UDCA. She reported sensory neuropathy in a glove-and-stocking distribution and was subsequently diagnosed with rheumatoid arthritis and Sjogren's disease. Three years later, her mother required LT as a result of end-stage PBC and then the proband required LT at the age of 21. The initial immunosuppression regimen included mycophenolate mofetil (MMF) and tacrolimus, which was reduced to tacrolimus monotherapy for the second year as her liver tests stabilised. A flare in her liver enzymes occurred 2 years following LT, and the liver histology (biopsy 1) confirmed rPBC with mild cholangitis and F1 fibrosis (Figure 1). On reintroduction of UDCA and restarting the MMF 500-mg BID, her ALP level returned to near baseline by year 3. But the cholestatic liver disease worsened considerably, and into year 5 she developed abnormal bilirubin with histological evidence of cholangitis and ductopenia (biopsy 3). The tacrolimus immunosuppression was changed to cyclosporin A in an effort to improve the rPBC, but cyclosporin A was not tolerated and the tacrolimus was restarted.
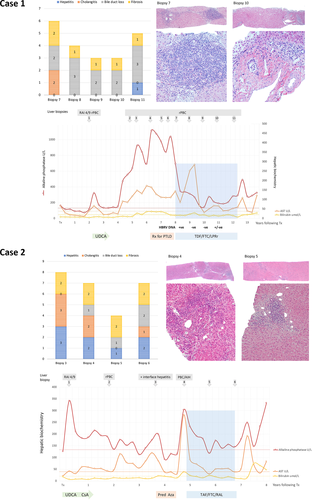
At year 7 following LT, the patient developed EBV DNA in peripheral blood (105-105 copies/ug DNA), which was initially managed with valganciclovir and decreased dosing of immunosuppression. A diagnosis of posttransplant lymphoproliferative disorder (PTLD) was established by bone marrow biopsy showing a diffuse aggressive large B cell lymphoma. The MMF was discontinued, and methotrexate, Ara-C, hydrocortisone and Rituximab treatment was instituted that led to undetectable EBV DNA levels within the month. However, the biochemical disease had progressed significantly, and the liver biopsy (biopsy 7) revealed evidence of progressive ductopenia and bridging fibrosis.
Following discussion with the patient and the liver transplant committee, the patient was commenced on daily FTC/TDF 200/300 mg and LPRr 800/200 mg. Maintaining stable tacrolimus levels became challenging as a result of LPRr blocking CYP450 3A activity. Eventually, a dose of 0.5-mg tacrolimus per week resulted in achievement of therapeutic levels. Within the first month of combination ART, she experienced a biochemical hepatitis consistent with LPRr toxicity.9 Although serum aminotransferases remained elevated, the ALP levels had fallen by >350 U/L and bilirubin by 4 μmol/ml by the end of the first month, and the combination ART was continued. In the ensuing years, her bilirubin and AST stabilised close to the ULN and the ALP subsided and normalised on one occasion. The serial HBRV DNA was measured by digital droplet PCR in whole blood DNA with the initiation of therapy.9 The viral load was initially positive and subsequently became negative with one borderline measurement (Figure 1).
To determine the efficacy of combination ART, coded liver biopsies were assessed using the cholangitis, hepatitis, ductopenia and fibrosis parameters from the Nakanuma scoring system.16 After 3 years of antiviral therapy, there was a reduction in fibrosis and the cholangitis score remained at baseline in all the biopsies derived whist on ART (biopsies 8-11). However, 4 years after commencing antiviral treatment (year 12), the patient found it difficult to swallow large tablets (LPRr and UDCA) because of the sensory neuropathy. Smaller UDCA and LPRr tablets were provided, but she subsequently discontinued all combination ART, which resulted in a degree of rebound in her hepatic biochemistry.
1.3 Case 2
A 45-year-old female, First Nations patient presented with a 3-year history of intermittent right upper quadrant pain, early satiety, weight loss and abnormal cholestatic liver tests. The family history revealed that her mother died aged 60 with cirrhosis and jaundice. A laparoscopic cholecystectomy was performed for chronic cholecystitis related to cholelithiasis, and a wedge liver biopsy revealed PBC with ductopenia and bridging fibrosis. The serology revealed a weakly positive antismooth muscle antibody 1:50, negative AMA and ANA and markedly raised serum IgM of 11.7 g/L. Despite UDCA treatment, her clinical disease rapidly progressed over the next 6 years and she subsequently became AMA positive. A LT was performed with a split-liver allograft at the age of 51.
MMF and tacrolimus were initially used for immunosuppression, but the latter was changed to sirolimus due to delirium in the postoperative period. Four months after LT, the patient developed increased ALP and the liver biopsy revealed evidence of cholangitis with a rejection activity index score of 4/9 (biopsy 1). The sirolimus was changed to cyclosporin A with MMF, and ursodiol was commenced. Her liver biochemistry improved but failed to normalise, and a second biopsy performed at 2 years was consistent with rPBC. Her low dose MMF was discontinued at this point. Following a rise in her AST and ALP three and a half years after LT, a third liver biopsy was obtained, which revealed moderate interface hepatitis accompanying the rPBC (biopsy 3). Her immunoglobulin IgG levels were within normal limits 12 g/L, whereas her IgM was elevated at 9.0 g/L. A decision was made to treat her with 40-mg prednisone, and the patient's liver tests subsequently normalised with a very slow taper and the institution of azathioprine as a steroid sparing agent.
After discontinuing the prednisone, she developed rebound biochemical hepatitis. Her azathioprine was also discontinued because of neutropenia. The liver biopsy showed histological features consistent with rPBC with focal lymphocytic cholangitis and moderate interface activity (biopsy 4).
Following discussion with the patient and the liver transplant committee, a decision was made to commence combination ART. By then, we had established that RAL had an EC95 in the nM range against HBRV suitable for inhibition at doses used for HIV, whereas LPRr was associated with GI intolerance.9 As the patient already suffered from chronic kidney injury, the combination ART treatment was commenced with RAL and Tenofovir alafenamide (TAF)/FTC instead of than TDF/FTC. After 1 year, her ALP, AST and bilirubin had returned to normal values. A liver biopsy to monitor the combination ART showed reduction in the severity of interface hepatitis and curtailment of cholangitis (biopsy 5). A year later, she was admitted to hospital with confusion and acute on chronic kidney injury. A biopsy was performed to monitor therapy (biopsy 6), which showed increased inflammation with deterioration of the hepatitis and cholangitis scores. The combination ART was discontinued, and she developed a biochemical rebound with increased ALP, AST and bilirubin.
2 DISCUSSION
Herein, we report two LT patients with rPBC, who developed biochemical and histological improvement in cholangitis after commencement of combination ART and rebound on discontinuation of therapy. Both patients developed rPBC unresponsive to UDCA, and both experienced deterioration in disease following the discontinuation of corticosteroids, possibly as a result of immune reconstitution. For case 1, the initial worsening of liver tests on initiation of combination ART with LPRr mirrored the experience of a recent randomised controlled trial, where hepatotoxicity occurred after initiation of treatment, probably as a result of LPRr.9 LPRr proved difficult to use in the LT setting due to interaction with the metabolism of tacrolimus as well. In the recent combination ART trial, two thirds of the PBC patients were unable to tolerate LPRr treatment because of the gastrointestinal side effects, which was considerably more than that reported for patients with HIV.9, 11 However, those who stayed on therapy for 24 months experienced sustained biochemical responses that were comparable to the LT subjects with rPBC reported herein. 9, 11
For the two LT patients, it is notable that the biochemical improvement occurred over a protracted period, suggesting that the repurposed combination ART may take longer to inhibit HBRV as compared to HIV. Whilst were able to show that improvement in cholestasis occurred with a reduction in HBRV levels, it is a weakness of this report that we were unable to collect samples for measuring change in HBRV in Case 2. Following the recent report that patients with rPBC have diminished survival following LT,4 this case series serves as a proof of principle that sustained biochemical stabilisation and improvement in histological features may occur in patients with rPBC whilst on combination ART. However, the gastrointestinal tolerability as well as the hepatic and renal toxicity may limit the use of combination ART. Furthermore, the role of established second line therapies with obeticholic acid and bezafibrate requires further evaluation for utility in the transplant setting. Whilst this report does not provide evidence of causality, it does suggest a link of HBRV infection with disease. A second randomised controlled trial is now underway using RAL-based combination ART for patients with PBC in the nontransplant setting (clinicaltrials.gov NCT03954327).
ACKNOWLEDGEMENTS
We thank Dr S. Indik for proving the modified reporter constructs, Alberta Health Services for paying for off label combination antiretroviral therapy and the liver transplant coordinators who participated in the patient's care.
CONFLICT OF INTEREST
ALM has worked on an advisory committee and has research support from Intercept Pharma and has received research support from Abbvie, Gilead and Merck that includes the provision of medications for clinical trials.
AUTHOR CONTRIBUTIONS
Guarantor of article: Andrew L. Mason. Drafting and critical review of manuscript: Ellina Lytvyak, Daniel He, Stefan G. Hubscher, Mina Niazi, Rohit Pai and Guangzhi Zhang. Histological analyses: Stefan G. Hubscher. Data acquisition and expression of laboratory values and data: Daniel He, Stefan G. Hubscher, Ellina Lytvyak, Mina Niazi, Rohit Pai, Guangzhi Zhang. Critical revision of the manuscript: Andrew L. Mason and Stefan G. Hubscher.
STATEMENT OF ETHICS
This research was approved by the human ethics research board at the University of Alberta [Pro00005105, Pro00085859]. Both patients signed an informed consent for this study.