Glecaprevir/pibrentasvir for 8 weeks in patients with compensated cirrhosis: Safety and effectiveness data from the German Hepatitis C-Registry
Funding information
The design, study conduct, analysis and financial support of the study were provided by AbbVie. AbbVie participated in the interpretation of data, review and approval of the content. All authors had access to all relevant data and participated in writing, review and approval of this manuscript. No honoraria or payments were made for authorship.
Abstract
Glecaprevir/pibrentasvir, a pangenotypic, direct-acting antiviral combination approved for chronic hepatitis C virus treatment, has limited real-world evidence supporting 8-week therapy in compensated cirrhosis. We investigated effectiveness and safety of 187 hepatitis C virus-infected, treatment-naïve, patients with compensated cirrhosis receiving 8-week glecaprevir/pibrentasvir therapy in the German Hepatitis C-Registry between 2 August 2017 and 1 January 2020. Sustained virologic response was 98.4% (127/129) in the per-protocol analysis (excluding patients lost to follow-up or who discontinued treatment due to compliance) and was 85.8% (127/148) in patients with data available in an intention-to-treat analysis. Nineteen patients were lost to follow-up; nine genotype 3 patients, nine nongenotype 3 patients and one mixed genotype patient. One patient relapsed, and one died, unrelated to treatment. Adverse events (>5%) were fatigue and headache. Two serious adverse events occurred; no adverse events resulted in drug discontinuation. An 8-week glecaprevir/pibrentasvir therapy was effective and well-tolerated in this real-world analysis.
Abbreviations
-
- AE
-
- adverse event
-
- ALT
-
- alanine aminotransferase
-
- APRI
-
- aspartate aminotransferase to platelet ratio index
-
- AST
-
- aspartate aminotransferase
-
- DAA
-
- direct-acting antiviral
-
- DHC-R
-
- German Hepatitis C-Registry
-
- EoT
-
- end-of-treatment
-
- FIB-4
-
- Fibrosis-4
-
- G/P
-
- glecaprevir/pibrentasvir
-
- GT
-
- genotype
-
- HCC
-
- hepatocellular carcinoma
-
- HCV
-
- hepatitis C virus
-
- ITT
-
- intention-to-treat
-
- LSM
-
- liver stiffness measurement
-
- LTFU
-
- lost-to-follow-up
-
- mITT
-
- modified intention-to-treat
-
- PP
-
- per-protocol
-
- PTW
-
- post-treatment week
-
- RAS
-
- resistance-associated substitutions
-
- SVR12
-
- sustained virologic response at post-treatment week 12
-
- TN
-
- treatment-naïve
-
- ULN
-
- upper limit of normal
-
- WHO
-
- World Health Organization
Key points
This real-world study demonstrated high effectiveness and safety of 8 weeks of G/P in TN patients genotypes 1-6 with compensated cirrhosis in the German Hepatitis C-Registry and similar effectiveness in genotype 3-infected vs non-genotype 3-infected patients based on a modified intention-to-treat analysis.
1 INTRODUCTION
Approximately 71 million individuals are infected with hepatitis C virus (HCV) globally, with over 200 000 HCV cases in Germany.1, 2 About 15%–30% of chronic HCV patients develop cirrhosis within 20 years; untreated cirrhosis could lead to complications of portal hypertension, hepatic decompensation, hepatocellular carcinoma (HCC) and liver-related death.2, 3 Glecaprevir/pibrentasvir (G/P) is a pangenotypic, interferon-free, ribavirin-free, fixed-dose direct-acting antiviral (DAA) drug combination approved for chronic HCV treatment in patients with compensated cirrhosis for 8-16 weeks based on prior treatment experience and cirrhosis status. In the EXPEDITION-8 study, the per-protocol (PP) sustained virologic response at posttreatment week (PTW) 12 (SVR12) was 99.7% (334/335) in treatment-naïve (TN), chronic HCV genotype (GT) 1-6 patients with compensated cirrhosis after 8-week G/P therapy, leading to approval in Europe and the United States, where previously 12 weeks were recommended; current EASL guidelines continue to recommend 8-12 weeks of G/P in TN, GT3-infected patients with compensated cirrhosis due to limited evidence.4-7 One real-world study demonstrated 99% SVR12 in the PP population of GT1-6 TN, compensated cirrhotics; another demonstrated 99.2% SVR12 in the 8-week duration and 97.1% in GT3 patients, irrespective of treatment duration, in cirrhotic and noncirrhotic patients receiving G/P for 8, 12 or 16 weeks.8, 9 To date, real-world evidence for this shorter 8-week treatment duration remains limited across all genotypes, notably in HCV GT3 patients. We aimed to investigate real-world effectiveness and safety of 8 weeks of G/P in compensated cirrhosis patients and effectiveness in GT3- vs non-GT3-infected patients in the German Hepatitis C-Registry (DHC-R).
2 METHODS
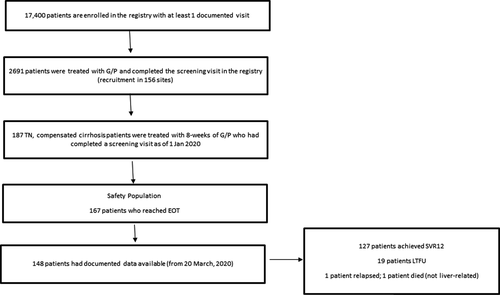
The DHC-R is an ongoing, noninterventional, multicentre, prospective, observational cohort study. Approximately, 17 400 patients are in the DHC-R with at least one documented visit; as of 1 January 2020, 2691 patients were documented with G/P treatment that completed a screening visit across 156 sites. Preliminary real-world effectiveness data were collected from 187 enrolled patients between 2 August 2017 and 1 January 2020 with an SVR12 update on 20 March 2020. The study protocol was approved by the Institutional Review Board (Ethics Committee of Ärztekammer Westfalen-Lippe; 2014-395-f-S). This study was registered with the German Clinical Trials Register (DRKS; ID: DRKS00009717). Patients gave written informed consent.
2.1 Study population
Chronic HCV-infected TN patients who were ≥18 years of age with compensated cirrhosis, treated for 8 weeks with G/P, were included in this analysis. Compensated cirrhosis was defined as sonographic, histological or clinical findings of cirrhosis, a liver stiffness measurement (LSM) > 12.5 kPa, an aspartate aminotransferase to platelet ratio index (APRI) > 2 or Fibrosis-4 (FIB-4) > 3.25 or a combination of these findings. Baseline demographics were assessed in all patients receiving G/P during the study period including GT3 and non-GT3 patients. Effectiveness was assessed as patients who achieved SVR12 on a PP analysis (excluding patients who were lost-to-follow-up (LTFU) or discontinued treatment due to compliance) and on an intention-to-treat (ITT) analysis. SVR12 was defined as an HCV RNA concentration ≤25 IU/mL at PTW12. A separate effectiveness analysis was performed on GT3 and non-GT3 patients; this subgroup excluded patients with mixed or unknown genotypes.
Safety parameters were assessed by monitoring adverse events (AEs) and laboratory abnormalities in patients from the baseline population who reached planned or premature end-of-treatment (EoT).
2.2 Statistical analyses
Data were summarised by medians and ranges, frequencies and percentages. The ITT analysis included all patients with validated SVR12 data by the specified study cut-off dates. Statistical comparison of baseline characteristics in GT3 vs non-GT3 patients was performed using a two-sided Fisher's exact test.
3 RESULTS
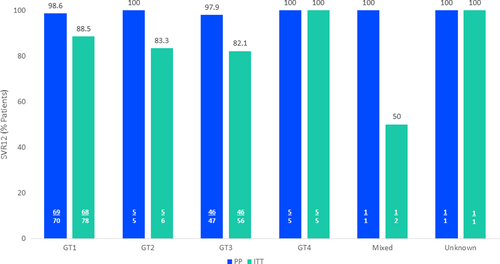
The DHC-R is an ongoing registry; patients are enrolled at various time points and within various stages of treatment. This interim analysis included 187 patients receiving 8-week G/P therapy (Figure 1). The majority of patients were male (62.6%); 97 patients (51.9%) had HCV GT1 infection and 68 patients (36.4%) had HCV GT3 infection. Supporting Information Table S1 lists additional baseline characteristics of the overall group. In GT3- vs non-GT3-infected patients, 41.2% vs 24.3% (P = .020) were on drug substitution therapy and APRI-Score >2 was seen in 59.7% vs 44.2% (P = .048), respectively. Supporting Information Table S2 lists baseline characteristics of GT3 patients compared to non-GT3 patients. In the overall group, 142 patients (75.9%) had a FIB-4 > 3.25 and 93 patients had an APRI >2 (49.7%); cirrhosis was diagnosed based on a singular criteria in 56.1% (105/187) of patients whilst 36.9% (69/187) were diagnosed based on 2 criteria. Sixty-nine patients (36.9%) had confirmed cirrhosis findings via FIB-4 alone, whilst 27 patients (14.4%) had a confirmed cirrhosis diagnosis through only APRI; 55 (29.4%) patients had a cirrhosis diagnosis through a combination of APRI and FIB-4 scores. Three patients had a LSM >14.6 kPa and documented SVR12 data available, with two achieving SVR12 (66.7%) and one being LTFU. Supporting Information Table S3 lists full diagnostic methods for cirrhosis assessment in all patients. At 12 weeks after EoT, 148 patients had documented data available; in the PP analysis, 98.4% (127/129) of patients achieved SVR12. In the ITT analysis, 85.8% (127/148) achieved SVR12. In GT3 vs non-GT3 patients, 97.9% (46/47) vs 98.8% (79/80) achieved SVR12 in the PP analysis, respectively; in the ITT analysis, 82.1% (46/56) vs 88.8% (79/89) achieved SVR12 in GT3- vs non-GT3 patients, respectively. The percentage of all patients achieving SVR12 based on PP and ITT analysis, broken down by GT, is as follows: GT1: 98.6% (88.5%), GT2: 100% (83.3%), GT3: 97.9% (82.1%), GT4: 100% (100%), mixed/unknown GT: 100%/100% (50%/100%) (Figure 2). One HCV GT3-infected, 54-year-old male patient with a comorbidity of hypertension relapsed, and one patient died, unrelated to liver injury (prior to PTW12 and therefore not included in the SVR12 analysis). Nineteen patients were LTFU: 9 patients in the GT3 population and 9 patients in the non-GT3 population. One patient with mixed genotype was LTFU and excluded in the non-GT3 subgroup but included in the ITT analysis. Of the 19 patients LTFU, 13 (68.4%) had comorbid psychosis, depression, drug abuse or were on drug substitution therapy. Safety was assessed in 167 patients who reached planned or premature EoT. Fifty-five patients (32.9%) reported adverse events; 2 AEs were serious but unrelated to G/P (Supporting Information Table S4). No AEs resulted in death or G/P discontinuation. The most frequent AEs were fatigue (n = 17, 10.2%) and headache (n = 13; 7.8%). Of patients with documented laboratory values during treatment, 1 patient (n = 138) had a bilirubin value >3 × upper limit of normal (ULN) that did not return to baseline after EoT; alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were 240 and 228 U/L at baseline, 66 and 98 U/L at EoT and 49 and 111 U/L at PTW4, respectively. No patients had ALT values >3 × ULN (n = 147).
4 DISCUSSION
To date, limited real-world evidence evaluates the safety and effectiveness of 8-week G/P treatment in compensated cirrhosis patients across all genotypes. In our analysis of 148 patients with documented data available, 98.4% and 85.8% achieved SVR12 in the PP and ITT analysis, respectively. The EXPEDITION-8 study, which led to the approval of 8-week G/P treatment for patients with compensated cirrhosis, had an overall 97.7% SVR12 for GT1-6 patients in the ITT population, which was higher than this analysis where more patients were LTFU; real-world evidence demonstrated SVR12 rates of ≥99%.4, 8, 9 Cirrhosis diagnostic criteria varied between studies: whilst in EXPEDITION-8, cirrhosis was defined in 83% of patients by LSM ≥14.6 kPa, one real-world study defined cirrhosis as METAVIR F4 or LSM ≥13 kPa, whilst another defined cirrhosis by FIB-4 > 5.2 or was physician reported.4, 8, 9 In this analysis, most patients were diagnosed based on noninvasive markers such as FIB-4 > 3.25 or APRI alone or a combination of both; whilst LSM was not primarily used as a diagnostic criteria in the majority of patients, the cut-off varied slightly from EXPEDITION-8 (>12.5 vs ≥14.6 kPa, respectively).4 The predictive specificity of these less-invasive markers at cut-offs specified in this study is lower than other markers of cirrhosis; however, LSM may not be available in office-based settings in which case APRI and FIB-4 may be used instead.10 Utilisation of these noninvasive markers in such clinical settings may aid in achieving HCV elimination targets.1, 2 This real-world cohort may be more reflective of early-stage, compensated cirrhosis populations diagnosed in office-based settings using similar criteria; the cut-off criteria may not reflect patients with later stages of cirrhosis.
This study included a higher number of GT3-infected patients with 64.7% having subtype 3a compared to other clinical and real-world studies.4, 8 Rates of SVR12 were similar in the PP analysis of GT3- vs non-GT3-infected patients (97.9% vs 98.8%, respectively); GT3 patients had lower SVR12 in the ITT analysis (82.1% vs 88.8%) with equal patients LTFU. Another real-world study evaluating 8-week G/P therapy showed similar results in GT3 cirrhotics with 71.5% of F4 patients achieving SVR12% vs 98.5% of non-GT3 infected patients; in F0-F3 patients, 96.3% of GT3-infected patients achieved SVR12% vs 98.6% of non-GT3-infected patients.11 However, only seven GT3-infected, F4 patients were included in the modified intention-to-treat (mITT) analysis vs 65 non-GT3 patients. This study did not break down patients by fibrosis, limiting the extrapolation of this data. Additionally, resistance-associated substitutions (RAS) were not studied, limiting the generalizability of results to other regions where different subtypes or higher GT3 RAS may be more prevalent.12
Despite the high effectiveness of G/P, potential limitations included 12.8% of patients having unavailable SVR12 data. Notably, a high number of LTFU patients had co-morbidities of drug abuse, drug substitution therapy and psychiatric disorders. In other real-world DAA studies, similar SVR12 differences in PP and ITT populations were observed in patients who use drugs and patients on opioid substitution therapy, due to a high number of patients LTFU.13-16 Whilst clinical trial data demonstrated high SVR12 rates in these vulnerable patients, failure to report for SVR results continues to remain a barrier for HCV care in real-world settings.13-15, 17, 18 Whilst this does not indicate withholding treatment from these patient populations, they may require additional support measures to prevent LTFU, achieve SVR, and reach World Health Organization (WHO) elimination targets.
Overall, G/P was safe and well-tolerated in this real-world study. The most common AEs were fatigue, headache and pruritis, similar to other G/P real-world and clinical trials.4, 9, 19 Laboratory abnormalities were rare, with only one patient experiencing a total bilirubin level >3 × ULN.
As countries work to achieve WHO HCV elimination targets, effective and tolerable DAA treatment options can greatly aid providers in reaching those goals. Simplified HCV algorithms allowing TN patients to receive 8 weeks of therapy, regardless of cirrhosis status, can potentially increase the number of patients providers are able to treat, expand access to care and minimise potential toxicity.10, 20
5 CONCLUSION
A shorter, pangenotypic, 8-week G/P treatment option was well-tolerated and effective in this real-world analysis of chronic HCV-infected TN patients with compensated cirrhosis, including GT3-infected patients. Additional multidisciplinary models are needed to assist reaching LTFU patients to achieve WHO elimination targets.
ACKNOWLEDGEMENTS
The authors would like to express their gratitude to the patients who participated in this study, and their families, as well as the study investigators and coordinators of the study. Glecaprevir was identified by AbbVie and Enanta. Medical writing support was provided by Sneh Mody, PharmD, MBA, BCCCP of AbbVie and funded by AbbVie.
DISCLOSURES
Hartwig Klinker: Advisory Committee or Review Panel: AbbVie, Gilead, Hexal, Janssen, MSD, Shionogi, ViiV, Grant/Research Support: Arrowhead, BMS, MSD, Hector foundation, Speaking and Teaching: AbbVie, BMS, Janssen, MSD, Pfizer Uwe Naumann: Speaker/Advisory board: AbbVie, Camurus, Gilead Sciences, Intercept, MSD, ViiV healthcare Martin Rössle: Consultation fees: Angiomed GmbH & Co. Medizintechnik KG, Bentley Innomed GmbH Thomas Berg: Grants/research support, honoraria or consultation fees, and speakers’ bureau: AbbVie, Bristol-Myers Squibb, Gilead, Janssen, Merck/MSD, Novartis Mark Bondin: Employee of AbbVie, Inc and may hold stock or stock options. Kristina Lohmann: Employee of AbbVie and may hold stock/share options Bettina Koenig: Employee of AbbVie and may hold stock/share options Stefan Zeuzem: Consultancies for AbbVie, BMS, Gilead, Janssen, Merck Markus Cornberg: Honoraria for lectures and advisory boards for AbbVie, Gilead, GSK, Janssen-Cilag, SOBI, Novartis, Spring Bank and Merck (MSD).
ETHICS AND CONSENT STATEMENT
The study protocol was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines and approved by the Institutional Review Board (Ethics Committee of Ärztekammer Westfalen-Lippe; 2014-395-f-S). All participants were required to provide written informed consent prior to enrollment in the registry.
Open Research
DATA AVAILABILITY STATEMENT
Data were derived from the German Hepatitis C-Registry (Deutsches Hepatitis C-Register, DHC-R) a project of the German Liver Foundation (Deutsche Leberstiftung), managed by Leberstiftungs-GmbH Deutschland in cooperation with the Association of German gastroenterologists in private practise (bng). The German Hepatitis C-Registry is financially supported by AbbVie Deutschland GmbH & Co. KG, Gilead Sciences GmbH, MSD Sharp & Dohme GmbH as well as Bristol-Myers Squibb GmbH & Co. KGaA and Janssen-Cilag GmbH (each until 2020-07-14) and Roche Pharma AG (until 2017-07-14).