2021 ISHEN guidelines on animal models of hepatic encephalopathy
Funding information
Financial support was provided by the Swiss National Science Foundation (project no 310030_173222).
Abstract
This working group of the International Society of Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) was commissioned to summarize and update current efforts in the development and characterization of animal models of hepatic encephalopathy (HE). As defined in humans, HE in animal models is based on the underlying degree and severity of liver pathology. Although hyperammonemia remains the key focus in the pathogenesis of HE, other factors associated with HE have been identified, together with recommended animal models, to help explore the pathogenesis and pathophysiological mechanisms of HE. While numerous methods to induce liver failure and disease exist, less have been characterized with neurological and neurobehavioural impairments. Moreover, there still remains a paucity of adequate animal models of Type C HE induced by alcohol, viruses and non-alcoholic fatty liver disease; the most common etiologies of chronic liver disease.
Abbreviations
-
- ALF
-
- acute liver failure
-
- ALT
-
- alanine transaminase
-
- AOM
-
- Azoxymethane
-
- APAP
-
- Acetaminophen
-
- AST
-
- aspartate transaminase
-
- BBB
-
- blood brain barrier
-
- BDL
-
- bile-duct ligation
-
- CCl4
-
- Carbon tetrachloride
-
- CLD
-
- chronic liver disease
-
- D-GAL
-
- Galactosamine
-
- HE
-
- hepatic encephalopathy
-
- ICP
-
- intracranial pressure
-
- ISHEN
-
- International Society of Hepatic Encephalopathy and Nitrogen Metabolism
-
- LPS
-
- Lipopolysaccharide
-
- NADP+/NADPH
-
- reduced and oxidized forms of nicotinamide adenine dinucleotide phosphate
-
- OP
-
- Ornithine-phenylacetate
-
- PCA
-
- portacaval anastomosis
-
- TAA
-
- Thioacetamide
-
- TIPS
-
- transjugular intrahepatic portosystemic shunt
Key points
- Valid animal models of liver failure/disease which manifest features/elements of HE currently exist.
- A suitable animal model of HE must have; (a) some degree of liver injury, failure and/or vascular impairment, (b) hyperammonemia and (c) some degree of neurological, behavioural or motor impairment.
- New animal models are warranted with a particular emphasis on the common etiologies associated with chronic liver disease (alcohol, hepatic viral infections and fatty liver disease) and their impact on the development of HE.
- An appropriate animal model should be selected once the interested feature(s) of HE have been identified.
1 INTRODUCTION
Hepatic encephalopathy (HE) is a debilitating neurological complication of liver disease/failure characterized by cognitive, psychiatric and motor disturbances. As a result of existing animal models of HE, many new insights into the pathogenesis and pathophysiology of this disorder have been realized. In addition, many animal models have been used for preclinical studies testing new therapeutic strategies for HE. Three types of HE have been defined based on degree and severity of liver failure, disease or impairment (including degree of portal-systemic shunting); (a) Type A HE: associated with acute liver failure (ALF) resulting from the rapid onset of hepatocyte necrosis and severe inflammation without pre-existing liver impairment; (b) Type B HE: associated with portal-systemic shunting in the absence of liver disease or failure; (c) Type C HE: associated with chronic liver disease (CLD; severe fibrosis or cirrhosis). A common feature of the three types of HE is that the overall capacity of the liver to remove ammonia is reduced and hyperammonemia ensues.
2 WHAT DEFINES A GOOD MODEL OF HE?
The fundamental basis for an animal model of HE is the presence of liver injury, failure and/or impairment that consequently leads to hyperammonemia. In addition, an animal model of HE should also exhibit some degree of neurological, behavioural or motor impairments. Other features believed to be implicated in HE include systemic inflammation1 and oxidative stress2 as well as neuroinflammation.3-5 However, their roles in the pathogenesis of HE in the absence of hyperammonemia as well whether they are contributory factors or consequential effects remain undetermined. The cardinal features of a Type A HE animal model is progression to overt neurological symptoms, such as loss of corneal reflex (coma) and loss of righting reflex (precoma), within hours or days upon induction of ALF. Moreover, intracranial hypertension is another HE landmark associated with ALF which depicts cerebral oedema, which is commonly observed in most animal models of Type A HE. In addition, various scoring scales have been developed for HE in ALF based on observed behavioural changes, impairment in reflexes and/or the presence of ataxia.6, 7 A commonly used methodology involves assigning the animals to a particular stage of HE based on their observed behaviours, with the data presented as the average stage of HE per experimental cohort at any time point.6 Recently, an improved categorical neurological scoring system was developed whereby five individual reflexes and presence of ataxia were each assessed and assigned a score from 0-2 (2 = reflexes intact, no ataxia; 1 = reflexes delayed, minor ataxia, 0 = reflexes absent, significant ataxia). The neurological score is the sum of scores for each reflex at any time point7 (Table 1). Type B HE, as well as Type C HE, both chronic models of HE, do not develop evident or overt neurological symptoms (including obvious behavioural changes, reflex impairments). As a result, HE is evaluated through different neurobehavioural tests8 such as Open Field, Morris Water Maze and Y-Maze tests which assess exploratory behaviour, locomotor activity, anxiety and memory (including spatial and working) respectively (Table 2). Furthermore, while an increase in intracranial pressure (ICP) is rare in Type C HE, brain oedema is still present. However, whether low-grade oedema or age-related brain atrophy prevents an increase in ICP in patients with CLD still remains unresolved.9
| Parameter | Methodology |
|---|---|
| Pinna reflex | Touching the auditory meatus with a cotton applicator and observing ear retraction or head movement |
| Corneal reflex | Gently touching the cornea with a cotton applicator and measuring the blink response |
| Tail flexion | Tail pinch with forceps and assessing tail flexion |
| Escape response | Tail pinch by forceps and a subsequent movement of mice away from stimuli |
| Righting reflex | Placing the mice on their backs and assessing the time for them to right themselves |
| Ataxia | Placing mice on wire cage lid and observing the number of times a foot falls through the lid |
Note
- A categorical neurological scoring system based on five individual reflexes and presence of ataxia in mice. Neurological score is determined by assigning a semi-quantitative evaluation to each of the above-mentioned parameters. 0 (no reflex evident/significant ataxia), 1 (weak or delayed reflex/minor ataxia) or 2 (intact reflex/no ataxia). The summation of these six reflexes and ataxia gives a neurological score between 0 and 12. This scoring system has been developed for the assessment of acute liver failure in mice. The applicability of this scale to rat models of acute liver failure are lacking
| Reference | Subjects | Type of test | Results |
|---|---|---|---|
| Leke, et al, 108 |
Adult female Wistar rats @ 6 weeks post-BDL |
Open Field Elevated Plus-Maze Foot-Fault |
Disturbed spontaneous locomotor and exploratory activities as a consequence of altered spatio-temporal organization of behavior |
| Leke, et al, 96 |
Adult male Wistar rats @ 6 weeks post-BDL |
Object Recognition Task | Impaired STM for recognition memory |
| Jover, et al, 155 |
Adult male Wistar rats @ 2 weeks post-BDL and +3 weeks HD diet |
Rotarod Beam Walking Locomotor Activity |
Mild impairment of motor coordination ↓ spontaneous activity |
|
Adult male SD rats @ 6 weeks post-BDL BDL +Allopurinol and BDL +AST-120 |
Locomotor Activity | ↓total distance traveled and normalized after AST-120 or allopurinol | |
| Braissant, et al, 105 |
Adult male Wistar rats @ 4, 6, 8 weeks post-BDL |
Open Field (Locomotor Activity) | ↓ total distance traveled |
| Dhanda, et al, 157 |
Adult male Wistar rats @ 4 weeks post-BDL |
Morris Water Maze Memory Retrieval Novel Object Recognition Task |
↑ in total time taken and distance travelled, no change in velocity ↓ no of entries, time spent and distance travelled in platform zone ↓ no of entries in known and novel object zone |
| Rodrigo, et al,113 |
Adult male Wistar rats @ 2 weeks post-BDL +10 and 21 days after ibuprofen |
Motor Activity Y-Maze Learning Test |
↓ Motor activity ↓ Learning ability Ibuprofen restored learning and motor activity in BDL rats |
| Cauli, et al, 180 |
Male Wistar rats @ 4 weeks post PCS surgery |
Automated actimeter (Motor activity) Rotarod and beam walking test (Motor coordination) Y maze test (Learning) Morris water maze (Spatial memory) |
↓ Spontaneous motor activity at 4 weeks post-PCS Motor coordination was impaired at 4 weeks post-PCS ↓ Learning ability at 4 weeks post-PCS ↓ Memory at 4 weeks post-PCS |
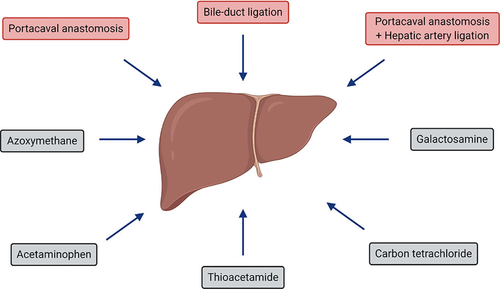
Various degrees of liver failure/injury and hepatic vascular impairment can be induced by (a) numerous liver toxins (including azoxymethane [AOM], acetaminophen [APAP], thioacetamide [TAA], carbon tetrachloride [CCl4] and galactosamine [D-GAL]), with the dose (± barbiturate pre-treatment to enhance the sensitivity to the toxin), and length of treatment dictating the type of HE model: Type A vs. Type C and (b) surgical interventions (bile-duct ligation [BDL], portacaval anastomosis [PCA] and PCA +hepatic artery ligation [HAL]) (Figure 1). Whereas the major etiologies of CLD in humans include alcohol, viral hepatitis and non-alcoholic fatty liver disease (NAFLD), for which appropriate animal models of severe fibrosis/cirrhosis currently do not exist. This is primarily because of the resistance of rodents to such etiological factors which do not develop severe fibrosis/cirrhosis. Consequently, this remains a major obstacle in the field which hinders the understanding of the independent role of each of the etiological factors on brain function.
3 SAMPLING AND MEASURING AMMONIA
Since elevated blood ammonia levels are a cardinal feature of HE, measuring ammonia is imperative and various methods to quantify ammonia currently exist. The most commonly used method to measure ammonia involves an enzymatic kinetic assay in which ammonia reacts with α-ketoglutarate and nicotinamide adenine dinucleotide phosphate (reduced form NADPH) to form glutamate and NADP+. The amount of ammonia is related to the amount of NADPH oxidized, which is measured photometrically. Regardless of the method, the measurement of ammonia is extremely sensitive, with the stability of the molecule being an important issue which needs to be carefully controlled. As a result, anomalous plasma ammonia concentrations can be because of preanalytical events such as delayed sample processing, temperature, type of test matrices, hemolysis and storage time in freezer.10, 11 Accordingly, it is important to place the sample on ice and immediately remove the plasma from cells to reduce the reaction rate of ammonia metabolism. The delay between sampling and analysis should be kept within 2 hours.12 Heparin, EDTA and oxalate anticoagulated plasma have been tested with an EDTA-anticoagulated matrix found to be superior.13 Frozen samples have been shown to affect ammonia levels when extensively kept frozen as well as multiple freezing and thawing cycles having a profound effect. Measuring brain tissue ammonia is further challenging because of the multiple preanalytical steps including extraction of the brain and postmortem metabolic changes. Aside from using liquid nitrogen, brains can be fixed for analysis by using focused beam microwave irradiation. This is a technique which causes permanent inactivation of enzymes in less than 1s, thereby minimizing enzyme-dependent postmortem metabolic changes.14 Nessler's staining is a histological method to identify high levels of ammonia (corresponding plasma levels ≥ 150 µM) which has been demonstrated in liver.15 However, this technique has not been validated in brain. Alternatively, cerebrospinal fluid and microdialysate measurements of ammonia have been performed which are believed to better reflect brain ammonia concentrations.16
4 CURRENT ANIMAL MODELS OF HE
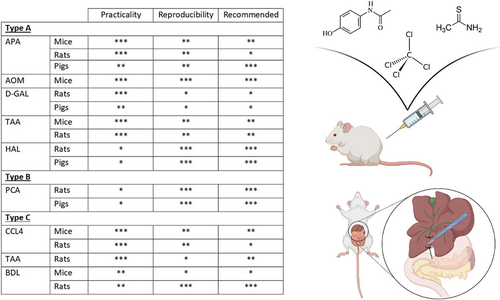
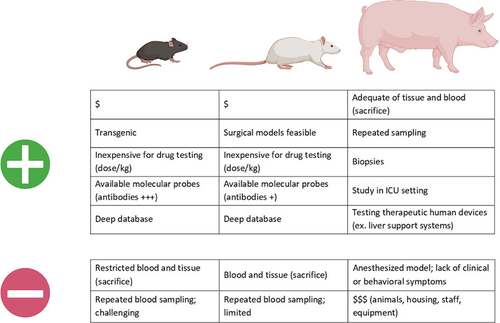
There are a number of advantages and disadvantages in using small animals vs large animals which may explain the choice of a particular animal model (Figure 2). Primarily mice, rats and pigs have been used in recent years for studies in HE and therefore we will focus on these species. Type A HE have been developed to induce ALF and subsequent overt, progressive neurological decline. In a majority of these models care must be taken to control associated features such as body temperature, blood glucose and potassium levels to prevent these confounders from interfering with outcomes and interpretation of data. Models of Type B and C HE mimic features of covert/minimal HE where overt symptoms do not develop and are not observed.
4.1 Type A HE
4.1.1 Liver toxin models
Acetaminophen
Rodents: Also known as paracetamol, APAP is commonly used clinically as an antipyretic and analgesic. APAP is metabolized by p450s leading to N-acetyl-p-benzoquinoneimine (NAPQI) when intracellular glutathione levels are saturated. Therefore, high doses of APAP lead to centrilobular hepatocyte necrosis. While the APAP-treated experimental model of Type A HE was not well-established prior to the 2009 ISHEN guidelines,17 there is substantial use of APAP to induce Type A HE both in rats and mice. APAP is injected at various dosages (from 300 mg/kg to 5 g/kg, ip) with mice being more susceptible than rats.18 APAP-induced ALF in mice leads to brain oedema and coma together with increased blood ammonia levels.19 Additionally, activation of intracellular signalling mechanisms, including elevated levels of cytokines, activation of brain transcription factors (increased levels of NF-kB, NRf2 etc), induction of oxidative stress, glial fibrillary acidic protein expression, as well as autophagy-related events have been observed in the brains of these animals.19-27
Pigs: Large animal models of ALF have been a long-standing topic of interest for testing of ‘human scale’ therapeutic devices. However, there was considerable difficulty in creating a stable, reproducible model that reflected the development of human toxicity.28 In 2011, work by Thiel et al demonstrated that a reproducible model of ALF can be achieved through careful monitoring of blood APAP levels within a narrow window (300-450 mg/dL),29 which was further refined by Lee et al to show that effective studies could be conducted in relatively few animals.30 This APAP-induced ALF model demonstrates core features of disease as a consequence of progressive hepatic necrosis and the consequent systemic inflammatory response. Hyperammonemia develops progressively once liver failure is established to levels in excess of 300 µM at exitus, concomitant with the occurrence of increased intracranial pressure (ICP) elevation (>30 mm Hg).29 The model is conducted in a terminally anaesthetized animal with continuous intensive care support, preventing testing of any behavioural or cognitive impairments. However, the availability of samples and the ability to use invasive tools to investigate specific mechanisms has allowed a better understanding of the pathophysiology of the condition.31-33
Azoxymethane
Rodents: AOM is an active metabolite of the cycad palm nut (found primarily on the island of Guam) which is hepatotoxic. The AOM model of ALF has been used to characterize the molecular changes associated with the development of HE in mice. It involves a single intraperitoneal (ip) injection of AOM at doses ranging from 50 mg/kg to 100 mg/kg.6, 7, 34, 35 Characterized by massive hepatocyte cell death, metabolism of AOM by cytochrome p450s generates toxic metabolites in the liver resulting in the formation of DNA adducts.36 Associated with acute severe liver injury is a progressive loss of reflexes, acquisition of cognitive dysfunction and ultimately hepatic coma6, 7, 34 within 24hr to 48hr. It is associated with hyperammonemia6, 34 cerebral edema,6, 34, 37 presence of neuromuscular deficits (manifested by ataxia), microglia activation and the upregulation of neuroinflammatory signals.6, 7, 34 Additionally, studies demonstrating AOM induces ALF and HE in rats are lacking.
Galactosamine
Rodents: Type A HE animal models using D-GAL have been reported since 1970. D-GAL is an amino sugar, which when metabolized in the liver consequently disturbs hepatocyte RNA metabolism, resulting in hepatocyte necrosis. The D-GAL model of Type A HE in rats replicates characteristic features of HE including the characteristic stages of HE, altered liver enzymes and histopathological changes similar to that observed in humans with Type A HE.38, 39 D-GAL given at a dose of up to 1.5g/kg (single dose, ip) leads to severe hepatic necrosis (diffuse degenerative changes with focal coagulative necrosis and the appearance of eosinophilic bodies), inflammatory infiltration of periportal areas and serum levels of liver enzymes are greatly increased while prothrombin is reduced.40 There is a vast array of studies demonstrating diverse effects with different doses of D-GAL with 2.5g/kg (ip) leading to progressive HE and death within 48hrs, accompanied with an increase in ICP.41 D-GAL induced HE studies in mice are few and therefore lacking. Furthermore, the limitations of this model include a lack of reproducibility since animals die unexpectedly and even brain oedema, increased ICP and coma are not consistently observed.
Pigs: An alternative large animal of Type A model that has attracted attention in recent years is that of a bolus injection of D-GAL in pigs (0.75g/kg iv).42 This model has demonstrated the core symptoms of Type A HE within 48 hours, with blood biochemistry meeting the criteria for ALF, elevated plasma ammonia levels and raised ICP (>25 mm Hg) in combination with histological evidence of ALF obtained from sequential biopsies.42 Animals are supported throughout the study with dextrose and crystalloid solutions as required and without further intervention, survival is expected to be between 72-90 hours. There are several advantages in using this model including the ability to study awake animals and observe the development of the stages of HE progressing to precoma and eventually coma, at which time point the animals are euthanized. However, it should be noted that a high degree of variability has also been found in this model43 and there have been questions raised as to its clinical relevance.28
Thioacetamide
Rodents: TAA’s toxicity is induced by the production of thioacetamide-s-oxide (a reactive oxygen species) through flavin adenine dinucleotide monooxygenase.44 The TAA-treated rat model of Type A HE has been well-established relative to the clinical status, liver function and brain oedema development.45-52 In this model, rats are given TAA in doses ranging from 300 to 500 mg/kg by ip injection on consecutive days for 2-3 days. While not as well-characterized in mice, TAA-induced hepatotoxicity has shown to induce ALF and subsequent HE following the administration of TAA (100-300 mg/kg, ip) for 3 consecutive days.53-56 TAA treatment in both species results in severe hepatocellular and bridging necrosis without cholestasis.47, 49 Furthermore, increased blood and brain ammonia have been observed.49 Associated with the decline in neurological function and brain edema54 are enlarged, vacuolated nuclei and pale/expanded cytoplasm in the astrocytes of the cerebral cortex54 with the neuropil highly vacuolated (spongiotic), especially around blood vessels and neurons.54
4.2 Surgical models
4.2.1 Hepatic devascularization
Rodents: Hepatic devascularization in rats is an established model of Type A HE which has been commonly used since 2010.57-60 Investigators have typically studied models of ALF by employing anastomosis of the portal vein to the vena cava and ligating the hepatic artery, seizing all blood flow to the liver. This model manifests a reproducible progression of Type A HE. A consistent and progressive increase in blood and brain ammonia as well as astrocyte swelling and brain oedema have been characterized in this model.61 Altered gene expression and CNS inflammation have also been identified.62, 63 Hepatic devascularization in smaller rodents is technically challenging and therefore is yet to be developed or characterized in mice.
Pigs: There is no standardization in the literature on the use of hepatic devascularization models in large animals. Studies have reported the use of additional hepatic artery occlusion resulting in total devascularization with64 or without reversal of the procedure.65 Other investigators have combined portal shunting with varying degrees of hepatectomy66-69 to create Type A models. As such, these models display a degree of heterogeneity in their findings and a spectrum of severities70 from mild to intensively monitored terminal models limited to several hours post procedure to those with a recovery phase.71 Porcine models of hepatic devascularization demonstrate central features of Type A HE, including cerebral oedema, intracranial hypertension, hyperammonemia and blood brain barrier (BBB) breakdown,72-74 along with hyperlactatemia and significant hemodynamic perturbation.75, 76 Because of the fact that the majority of studies have been conducted in anaesthetized models, there is scarce information on cognitive impairment in these models other than the reported changes in ICP,73 and the lack of a standardized approach to experimental design makes direct comparison difficult.
4.3 Type B HE
4.3.1 Portacaval anastomosis
Portal-systemic shunting (when the normal flow from the portal vein is diverted, either partially or completely, to the systemic circulation, thus bypassing the liver) leads to a decrease in hepatic ammonia extraction and consequently the development of hyperammonemia, even in the absence of liver disease. Congenital shunts77 occur in humans but are commonly observed in dogs which present with hyperammonemia and psychomotor dysfunction78 and are often admitted to veterinarian clinics with various symptoms, including behavioural changes. Congenital shunts are believed to be strain specific as has been reported in C57BL/6J mice.77 In rats, an end-to-side portacaval anastomosis is a surgical procedure which leads to a rise in blood ammonia levels and neurological impairment.79 The diversion of blood during portal systemic shunting consequently leads to liver atrophy.80-82 These surgical procedures are also achievable in larger animals such as pigs83 whereas it is very difficult to realize this surgery in mice. Alternatively, graded portal-vein stenosis which leads to spontaneous portal-systemic shunts and various degrees of portal-systemic shunting, is a much easier model to develop but with greater variability and therefore less reproducible.84 In CLD, an increase in hepatic resistance can lead to acquired liver shunts where the degree of shunting increases the risk of developing severe HE.85 Portal-systemic shunting without liver impairment is evidently not a model of liver disease, nor is it a model of transjugular intrahepatic portosystemic shunt (TIPS). Ideally, performing a portal-systemic shunt in an animal model of CLD would be a valuable model for studying post-TIPS HE.
4.4 Type C HE
4.4.1 Liver toxin models
Carbon tetrachloride
Rodents: CCl4 is mainly metabolized by centrilobular hepatocytes producing the toxic metabolite trichloromethyl (CCl3-) via cytochrome p-450s, causing centrilobular liver damage. CCl4 administration in rodents varies in terms of route of administration (injections [ip, sc], gavage), dosage, frequency of dosing and duration. Long-term treatment of rats or mice with CCl4 leads to repeated insults to the liver causing hepatocyte damage, ductular reaction and myofibroblast activation, hepatic stellate cell activation, imbalance between extra cellular matrix production and degradation and development of progressive liver fibrosis.86, 87 In mice, doses used range from 0.5 mL/kg to 1 mL/kg, administered either via oral gavage or ip injection, twice per week for up to 16 weeks.88-91 These treatments result in hyperammonemia,89, 91 hyperpermeability of the BBB,91 increased neuroinflammatory signals,88-90 microglia/glial cell activation88, 89 and increased GABA signaling.88 In rats, CCl4 administration (0.1-0.2 mL/kg twice per week; ip for up to 5 months)87, 92, 93 results in hyperammonemia,87, 92 impaired memory acquisition as determined using the Morris Water Maze87, 93 and a decrease in hippocampal neurogenesis.87
Thioacetamide
Rodents: Similar to CCl4, TAA primarily causes centrilobular hepatocyte damage.44 A TAA-treated rat model of Type C HE has recently been established relative to the clinical status, liver function and behavioural and cognitive deficits.94, 95 In this model, rats were given TAA (100 mg/kg, ip) for 10 consecutive days. Hepatic damage (ballooning degeneration, hydropic changes and the presence of eosinophilic bodies, affecting approximately 60%-70% of the liver parenchyma) as well as increased serum ALT and AST levels were observed in this model. The presence of Alzheimer type II astrocytosis (predominantly in the cerebral cortex), behavioural abnormalities associated with cognitive dysfunction are observed, including drowsiness, decreased wakefulness, impaired attentiveness, decreased grooming and exploratory behaviour96 together with increased blood and brain ammonia.94, 95 In addition, many studies have administered 12 weeks of TAA by its addition to the drinking water.97-102 TAA-induced type C HE models in mice are lacking.
4.4.2 Surgical models
Bile-duct ligation
Rodents: The BDL rat model is the most widely used model of type C HE and has been shown to be a reproducible model of biliary CLD, simulating metabolic aspects of cirrhosis. In this model, the common bile duct is ligated (2 or 3 points) and then resected to avoid reversibility. BDL rats survive to 6 to 8 weeks post ligation, develop liver failure, jaundice, portal hypertension, portal-systemic shunting, bacterial translocation and immune system dysfunction.17, 103-105 The signs of CLD in these rats include increased levels of plasma bilirubin, liver enzymes (AST and ALT) and histological changes in the liver architecture (i.e. bile duct epithelial cell proliferation, disturbed cytoarchitecture, formation of septae between portal areas and a noticeable increase in collagen fibres).106 BDL rats also develop hyperammonemia and show motor and learning deficits.105-108 In vivo neuro-imaging studies performed on BDL rats have reproduced the changes observed in humans (i.e. increased brain glutamine and decreased brain osmolytes as an osmotic response and other more subtle changes including a decrease in antioxidants and creatine).105, 107, 109-111 The changes in water content (i.e. brain oedema) measured in BDL rats are subtle107, 111 (details on brain water measurements and brain oedema can be found in the following reviews8, 9, 112). Systemic oxidative stress, as a result of primary liver injury, combined with hyperammonemia was shown to stimulate an increase in brain water content in BDL rats.79 Inflammation (systemic and central) has not been thoroughly characterized in these rats and results to date remain controversial.113-115
In contrast, BDL mice have been primary used to study cholestatic liver injury and liver inflammation. The survival rate of BDL mice is much less than BDL rats, with studies lasting between 5 days post ligation till 2 weeks.116, 117 It is possible to prolong survival in BDL mice, but this requires intensive care (i.e. cage warming, wet food placed on the cage floor and potentially dextrose supplementation subcutaneously).118 The BDL mice are characterized by decreased social investigative behaviour and increased immobility together with increased serum bilirubin, changes in liver enzymes (ALT) and the presence of portal based inflammatory cells in stained liver sections.117 Beyond these observations, no features of HE have been observed, likely because of the short survival time of the mice.
4.5 Models of overt HE in chronic liver disease
In order to study the precipitating factors of overt HE and hence the pathogenic factors involved in the acute neurological impairment in CLD, insults are used to induce overt HE in animals. Ammonia, a driver of a number of precipitating factors, has been administered to rats to induce an overt episode of HE. Exacerbating hyperammonemia through diet in BDL rats has also elucidated overt symptoms of HE.113 The use of PCA to shunt blood away from the liver to establish hepatic insufficiency prior to the administration of a secondary insult has similarly been extensively studied.79, 119 Although early studies sought to investigate impaired hepatic clearance of bacteria120 post PCA, more recent studies have investigated episodic HE induced by repeated administration of endotoxin or ammonia which demonstrated neuronal loss and cerebral inflammation.121, 122 Other studies in this model have explored the effects of simulated esophageal bleeding123 as a source of hyperammonemia, and the effects of superadded ammonia on other body systems.124, 125 Lipopolysaccharide (LPS) injected into BDL rats is used to mimic severe inflammation and infection; a precipitating factor of overt HE. This model is the creation of a single inflammatory event on a background of established liver injury, after which the animals are euthanized for sample collection. The BDL with a secondary insult of bacterial endotoxin (BDL +LPS) was originally described in 1999 by Harry et al126 in which the heightened response to the inflammatory insult was observed. Subsequently this BDL +LPS model was shown to demonstrate the key features of HE with evidence of cerebral inflammation.114 However, the key point was that there was significant worsening of most measured parameters following the administration of endotoxin in a relatively short time frame (3 hours), indicating the possibility of a priming effect during CLD development leading to a hyper-responsiveness to the secondary insult.
4.6 Ammonia supplementation to induce hyperammonemia
In order to study the effect of neurotoxicity from elevated blood ammonia levels without the impact of liver disease, hyperammonemia is induced in naïve rats through an ammonia supplemented standard diet (ammonia acetate; 20% w/w)127 to study the effects of hyperammonemia on brain function and metabolism in absence of liver dysfunction. Hyperammonemia is induced within 10 days and studies have demonstrated that rats can tolerate this ammonia-supplemented diet for up to 100 days. Ammonium acetate is much more effective in the diet as oppose to in the drinking water. Also, other ammonia salts, such as ammonium carbonate are not well-tolerated. In these diet-induced models, pair-fed animals are highly recommended as controls. Cognitive impairment has been demonstrated after 7 days of ammonia-supplemented diet.113 This is a simple, reproducible animal model of chronic hyperammonemia.
4.7 Preclinical models for HE treatments
Preclinical models of HE are imperative for testing novel treatments for HE. Even though treating the liver disease itself may resolve HE, it is very difficult to improve the liver at end-stage liver disease. Therefore, novel therapeutic strategies for HE primarily target a pathogenic factor which is precipitating the observed neurological impairment. In the CLD setting, the BDL rat is an excellent model to assess the efficacy of ammonia-lowering strategies. AST-120 carbon microspheres, when administered by gavage, were shown to lower blood ammonia in BDL rats, was associated with a reduction in brain oedema and HE symptomatic behaviour.104 Ornithine-phenylacetate (OP) and liposome-supported peritoneal dialysis have also shown to be beneficial in lowering blood ammonia levels in BDL rats.128, 129 The probiotic VIVOMIXX has been recently shown to attenuate hyperammonemia and improve both the performance in behavioural tests and the neurometabolic profile of BDL treated rats.130 OP has also been shown to lower blood ammonia in rats with portal-caval anastomosis.123 Interestingly, GABA-A receptor antagonists have shown to be beneficial in PCA rats as well as rats administered ammonia in the diet.131 In ALF, L-ornithine-L-aspartate, minocycline, N-acetylcysteine and hypothermia have all shown to be beneficial in liver devascularized rats.60, 132-134 Similarly, OP was beneficial in lowering ammonia and ICP in liver devascularized pigs.16 In the AOM mouse model, strategies to inhibit cerebral inflammation, such as targeting proinflammatory cytokine signalling, or inducing mild hypothermia were shown to attenuate parameters of HE.3-5, 135-137 Furthermore, emerging evidence suggesting that aberrant bile acid signalling in the brain may contribute to the pathogenesis of HE in the AOM mouse model and that inhibition of bile acid receptors such as farnesoid X receptor or sphingosine-1-phosphate receptor 2 was recently shown to be beneficial.138-140
4.8 Concerns when using animal models of HE
Several limitations exist in using toxin models of both Type A and C HE. Firstly, there is controversy surrounding the reported off-target effects of the toxins that may confound the interpretation of the data. For example, direct effects of AOM on BBB hyperpermeability have been suggested using a monolayer of mouse brain microvascular endothelia cells,141 which has not been confirmed in other studies.37, 142 However, in vivo, the hyperpermeability of the BBB is either not observed143 or if it is, it is not until the later stages of HE37, 142-144 and requires the presence of systemic proinflammatory signals,37, 145 suggesting that direct effects of AOM on the permeability of the BBB is negligible in vivo. APAP has been demonstrated to have a direct toxic effect on the brain in rats22, 25 and issues may be present in other toxin models as well and therefore experiments should be designed to consider these possible off target effects. Secondly, issues with reproducibility of these models, both between laboratories and between experiments within a single laboratory are often observed. These variations may arise because of a multitude of reasons including animal strains. It has been clearly demonstrated that BDL Wistar rats can be studied at longer time points (8 weeks) than BDL Sprague-Dawley rats (6 weeks).104, 105, 109 Other factors include variations in lot quality of the toxin, environmental differences, male vs female, as well as alterations in standard animal husbandry and nature of the supportive care provided. Variations between experiments within one lab may be somewhat mitigated by careful experimental design, and strict adherence to the supportive care regime (e.g. monitoring body temperature, dextrose supplementation to prevent hypoglycemia etc.143). Differences in outcomes between laboratories are a little more difficult to control for, although a thorough description of the experimental conditions, to include strain details, analgesics, and other supportive care strategies used should be included in publications to aide in the reproducibility of the experimental model.
4.9 Future directions
- There remains a distinct lack of CLD models that progress to HE and mirror the common etiologies of HE in humans (e.g. alcoholic liver disease, viral hepatitis and NAFLD). These etiological factors may exhibit features of mild fibrosis but fail to exhibit features of severe liver fibrosis or cirrhosis in animal models. The impact of etiological factors on HE merits to be investigated.
- Many existing models of liver fibrosis, in particular genetic models such as the MDR2 knockout mouse or the PDGF-B transgenic mouse, have not yet been fully characterized for features of HE.146-148
- Cholestatic liver disease affects both children and adults. In children, there is emerging evidence that chronic cholestasis early in life may be associated with long-term neurocognitive and neuromotor impairment,149-151 but studies examining the effect of liver disease during brain development are missing. In this context, ligation of the bile-duct can also be performed in young rats (i.e. 15 or 21 days after birth) leading to a model of type C HE in the developing brain.152
- Differences between gender in children with biliary atresia have been observed,153 with more women presenting with cholestatic liver disease than men. In this context, sex differences in animal models of HE warrants further investigation.
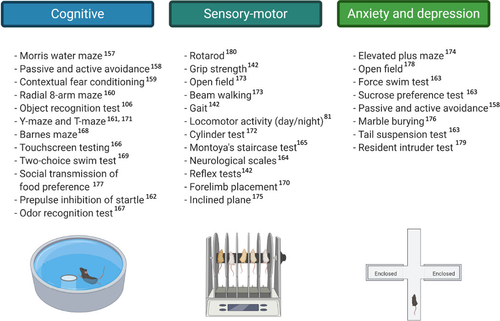
- Cognitive tests including those with increased sensitivity (touchscreen cognitive testing) merit to be implicated in the evaluation of HE in rodents (Figure 3). Electroencephalography (EEG) in rodents also warrants further studies.154
- Monitoring the brain using magnetic resonance imaging, magnetic resonance spectroscopy, microdialysis, positron emission tomography are excellent tools to elucidate the relationship between liver function, brain metabolic alterations, cellular changes, cell swelling/oedema and neurological manifestations in HE. Further longitudinal, multiparametric and multimodal studies are warranted.
4.10 Recommendations of the ISHEN working group
Numerous different animal models of HE currently exist and have been well-characterized. Based on practicality and reproducibility, certain models are recommended (Figure 4). These recommendations were based on the consensus agreement of our working group and are based on ease and reproducibility of the liver disease, the development of HE-associated neurological deficits and hyperammonemia. We have not based these recommendations on other features that may be associated with HE pathogenesis (e.g. biochemical analyses or imaging studies) as these have not been consistently studied in the majority of models. However, an animal model that exactly mirrors the etiology and subsequent effects on the brain in humans does not exist, highlighting the need for further refinement of existing models and the development of novel animal models for HE. New models should be developed with a particular emphasis on both the etiology (for example a rodent model of end-stage alcoholic liver disease resulting in HE), the underlying liver pathology and the pathogenic features that are associated with the development of each type of HE. To this end, key features that should be characterized when developing and classifying a novel animal model of HE include: (a) type of underlying liver pathology, (b) hyperammonemia and (c) cognitive and neurological deficits.
In conclusion, limitations are found of each of the current HE models just as no animal model exists that replicates the full human condition. However, animal models are critical for answering specific pathophysiological questions. They are also valuable in allowing to initially investigate, understand and test novel therapeutic strategies which could not be conducted in humans. It is therefore vital to choose the appropriate model when considering key features of HE to be studied.
ACKNOWLEDGEMENTS
Parts of this review were prepared with resources and the use of facilities at the Central Texas Veterans Health Care System, Temple, Texas. The content is the responsibility of the author(s) alone and does not necessarily reflect the views or policies of the Department of Veterans Affairs or the United States Government. Parts of this work was also made possible thanks to the Center for Biomedical Imaging (CIBM), founded and supported by Lausanne University Hospital (CHUV), University of Lausanne (UNIL), Ecole Polytechnique Federale de Lausanne (EPFL), University of Geneva (UNIGE) and Geneva University Hospitals (HUG)
CONFLICT OF INTEREST
SD: nothing to disclose. CC: nothing to disclose. ND: nothing to disclose. ARJ: nothing to disclose. CFR: Christopher F. Rose has research collaborations with Mallinckrodt Pharma and Neuractas and is an advisor for Axcella, Horizon Therapeutics, Lupin Pharma, Morphocell Technologies, and Neuractas.