The complex role of bone morphogenetic protein 9 in liver damage and regeneration: New evidence from in vivo and in vitro studies
Funding information
Diego F. Calvisi was supported by a grant from the Italian Association Against Cancer (AIRC; grant number IG 12139).
Abstract
See Article on Page 1664
In chronically diseased liver, hepatocytes undergo a continuous series of insults and compensatory stimuli that trigger a complex response aimed at protecting the residual parenchyma and its functions. Depending on the stage and the extent of the liver disease, damaged cells are either eliminated or repaired by neighbouring cells.1, 2 In particular, when liver regeneration driven by hepatocytes is impaired, this function is mainly conducted by hepatic progenitor cells (HPCs), which are also commonly known as oval cells.1, 2 A large body of evidence has substantiated, both in animal models and in humans, that a robust activation, expansion, and differentiation of HPCs take place following persistent liver injury.1, 2 Despite these concordant findings, however, the effective contribution of HPCs on liver regeneration and repair has been intensively questioned and is still a matter of debate. Indeed, although HPCs are capable to restore the hepatic parenchyma in response to widespread damage, the efficiency of this process is somehow limited. Specifically, if the deceased cells cannot be adequately replaced, liver failure rapidly occurs, with severe implications for the survival of the affected individual.1, 2 As in many other liver diseases, the outcome of liver damage seems to be strongly influenced by the HPC cell microenvironment and the molecular mechanisms triggered by the latter following hepatic injury.3 Thus, a better and deeper understanding of the molecular players from the microenvironment that govern liver regeneration and repair is highly needed for the development of novel and effective therapies against this condition, besides liver transplantation.
In the present issue of Liver International, Addante et al investigated the role of bone morphogenetic protein 9 (BMP9), a member of the transforming growth factor beta (TGF-β) superfamily, in liver injury and regeneration, using in vivo and in vitro approaches.4 BMP9, also known as growth differentiation factor 2 (GDF2), is specifically expressed in the liver tissue and produced by hepatocytes.5, 6 At the biochemical level, immature and unprocessed human BMP9 consists of a precursor protein (pre–pro-BMP9) composed of 429 amino acids that include a 22 amino acid signal peptide, a 33 kDa prodomain, and a 12.5 kDa mature protein. Pre–pro-BMP9 is proteolitycally processed to generate each subunit of the disulphide-linked homodimer.6 Through binding to its interacting partners, BMP9 regulates a plethora of biological functions, including cartilage formation, angiogenesis, differentiation of neurons, as well as glucose and lipid metabolism.7 In the liver, previous observations have demonstrated that BMP9 induces proliferation, survival, and invasion of hepatocellular carcinoma (HCC) cells, thus acting as a bona fide oncogene in this tumour type.8, 9 In addition, recent data indicate that BMP9 possesses profibrogenic properties in the liver along carbon tetrachloride (CCl4)-driven chronic liver damage and interferes with hepatocyte-mediated liver regeneration in response to acute insults.10, 11
To further unravel the function of BMP9 in liver diseases, Addante et al employed the mouse model of cholestatic liver injury induced by 3,5-diethoxicarbonyl-1,4 dihydrocollidine (DDC). The DDC model has been proven to be a relevant system for the study of primary sclerosing cholangitis, a progressive cholangiopathy where biliary fibrosis and cholestasis finally result in end stage liver disease.12 Of note, since the cholestatic alterations are paralleled by a ductular reaction and expansion of HPCs, the DDC model is widely used as a chemical injury model for the study of HPCs-driven liver regeneration.12, 13 To pursue their goals, Addante and colleagues fed wild-type (WT) and BMP9 knockout (KO) mice a diet containing DDC for 2, 4, and 6 weeks and carefully evaluated the eventual differences in the two mouse genetic backgrounds. Importantly, the authors found that genetic elimination of BMP9 ameliorates DDC-induced liver damage, as indicated by better liver function tests conducted in the serum and a lower fibrogenic response. Unexpectedly, these parameters were paralleled by a marked ductular reaction and increased ductular proliferation in portal areas, thus suggesting that the enhanced expansion of HPCs plays a significant role in improving liver regeneration and restoring liver function in this model.4 These data are particularly intriguing, as HPCs have been found to play a contrasting role on liver regeneration, either favouring this event14, 15 or aggravating liver damage by driving fibrogenesis.16, 17 The present data by Addante et al seem to support an advantageous—rather than detrimental—function of HPCs on liver regeneration in the DDC model, and strengthen the hypothesis that BMP9 is a major profibrogenic factor in the damaged liver.
Another unexpected observation of the study was the finding that DDC-fed BMP9-KO mice displayed an augmented hepatic inflammatory response. Indeed, BMP9-KO mice subjected to DDC feeding exhibited a more pronounced intralobular inflammation as well as a more elevated production of proinflammatory cytokines (interleukin 6 and tumour necrosis factor alpha) and related downstream targets (signal transducer and activator of transcription 3 or Stat3).4 These data suggest that the increased inflammation resulting from BMP9 genetic depletion could exert a beneficial effect on HPCs (but not limited to) along DDC-driven regeneration.
At the molecular level, the increased liver regeneration observed in BMP9-KO mice fed a DDC diet was paralleled by the activation of the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) and extracellular signal-regulated kinase (ERK) pathways,4 two of the major signalling cascades governing hepatocyte- and HPCs-mediated proliferation and regeneration.18, 19 Furthermore, DDC-treated BMP9-KO mice exhibited a remarkable induction of the hepatocyte growth factor (HGF)/c-Met pathway, whose absence in fact has been previously shown to severely impair DDC-induced liver regeneration.20, 21 The authors speculate that overactivation of the HGF/c-Met pathway might in turn induce the PI3K/AKT and ERK cascades and promote HPCs expansion. The employment of KO models targeting members of the PI3K/AKT and ERK signalling would be highly useful to address this important issue.
The inhibitory role played by BMP9 over HPC expansion has been further substantiated by the authors in an in vitro model. Specifically, they showed that BMP9 significantly reduces the number of HPCs in culture by restraining cell proliferation and inducing apoptosis through the activation of small mother against decapentaplegic (SMAD) 1, 5, and 8 proteins (Figure 1). Through additional, elegant experiments, the authors also demonstrated that activin receptor-like kinase 2 (ALK2)—but not ALK1 mediates the suppressive function of BMP9 over the growth and expansion of HPCs.4
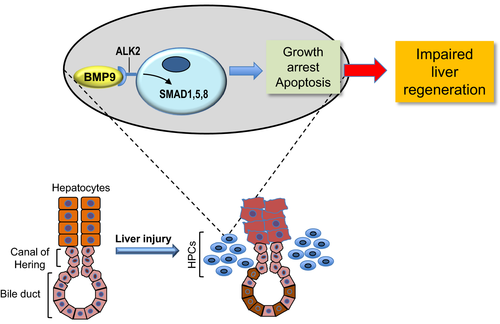
Altogether, the excellent study by Annante et al provides substantive in vivo and in vitro evidence that BMP9 plays a critical role on HPCs biology. Thus, together with previous findings showing that BMP9 influences the fate and growth of various liver cell types, including hepatocytes, HCC cells, and stellate cells, the present data strongly support the hypothesis that BMP9 is a central regulator of liver pathophysiology. However, although the present investigation has considerably improved our knowledge on the function and activity of BMP9 in the diseased liver, further investigation is imperative on this topic. In particular, it remains poorly understood the reason(s) why BMP9 exerts different effects in the liver depending on the cell type and/or the context. This is presumably because of the interaction with specific receptors and pathways (and their functional cross-talks), resulting in apparently opposite outcomes. In-depth characterization of cellular and molecular interactors of BMP9 in the liver will presumably address this issue. Among them, it will be crucial to understand the effect of BMP9 on the inflammatory response following liver damage. Finally, a comprehensive understanding of the consequences of BMP9 modulation in the liver might be highly useful for a better definition of the hepatic pathophysiology as well as for the development of innovative therapeutic strategies targeting BMP9 in chronic liver diseases and HCC.
CONFLICT OF INTEREST
The authors have no conflict of interests to disclose.




