Hepatorenal syndrome in the era of acute kidney injury
Abstract
Acute kidney injury (AKI) is a frequent complication of patients with advanced cirrhosis that it is associated with increased hospital admissions and decreased survival. The definition of AKI in cirrhosis has been recently modified and the new diagnostic criteria are based on small changes in serum creatinine with respect to previous values, occurring within a short period of time. The use of this new definition may lead to an earlier identification of renal impairment and better prognostic stratification. Hepatorenal syndrome (HRS) is a unique form of AKI developing in patients with end-stage liver disease. Systemic circulatory dysfunction and marked kidney vasoconstriction play a key role in the development of HRS. The modification of the definition of AKI has also led to a change in the diagnostic criteria of HRS. The new diagnostic criteria are based on AKI stages and there is no need to reach a specific serum creatinine threshold. According to these new criteria, treatment with vasoconstrictors and albumin for the management of HRS will be started at lower serum creatinine values, with expected higher response rates. Finally, there are consistent data showing that some urine biomarkers, particularly NGAL (neutrophil gelatinase-associated lipocalin), may be useful in daily clinical practice for the differential diagnosis of the cause of AKI in cirrhosis.
Abbreviations
-
- ACLF
-
- acute-on-chronic liver failure
-
- AKI
-
- acute kidney injury
-
- AKIN
-
- acute kidney injury network
-
- ATN
-
- acute tubular necrosis
-
- GFR
-
- glomerular filtration rate
-
- HRS
-
- hepatorenal syndrome
-
- ICA
-
- international club of ascites
-
- IL-18
-
- interleukin 18
-
- IL-6
-
- interleukin 6
-
- IL-8
-
- interleukin 8
-
- IV
-
- intravenous
-
- KIM-1
-
- kidney injury molecule 1
-
- L-FABP
-
- liver fatty acid-binding protein
-
- LT
-
- liver transplantation
-
- MELD
-
- model for end-stage liver disease
-
- NGAL
-
- neutrophil gelatinase-associated lipocalin
-
- RRT
-
- renal replacement therapy
-
- SBP
-
- spontaneous bacterial peritonitis
-
- SCr
-
- serum creatinine
-
- SIRS
-
- systemic inflammatory response syndrome
-
- TIPS
-
- transjugular intrahepatic portosystemic shunt
-
- TNF
-
- tumour necrosis factor
-
- uNGAL
-
- urinary neutrophil gelatinase-associated lipocalin
Key points
- The definition of acute kidney injury (AKI) in cirrhosis has been recently modified and is based on small changes in serum creatinine.
- Diagnostic criteria of HRS-AKI have also been modified. The fixed threshold of serum creatinine included in the “classical” diagnostic criteria diagnostic criteria of HRS has been removed and, therefore, this should lead to an earlier diagnosis of HRS-AKI.
- New urine biomarkers have shown to be useful in the differential diagnosis of the cause of AKI in cirrhosis; particularly in the differential diagnosis of acute tubular necrosis vs HRS-AKI.
- First-line pharmacological treatment for patients with HRS-AKI is the combination of vasoconstrictors and albumin.
1 INTRODUCTION
Acute kidney injury (AKI) is a common complication occurring in patients with cirrhosis, with reported frequencies of up to 20%-50% in patients with cirrhosis admitted to hospital for complications of the disease.1, 2 Hepatorenal syndrome (HRS) is a unique form of AKI that develops in patients with advanced cirrhosis and is mainly related to a marked renal vasoconstriction secondary to the systemic circulatory impairment characteristic of patients with advanced cirrhosis.3 Although HRS is a unique form of AKI typical of patients with cirrhosis, these patients may develop AKI due to other different aetiologies.3, 4 The development of AKI is associated with impaired prognosis; however, prognosis markedly differs according to the cause of AKI.4 Therefore, early diagnosis and identification of the cause of AKI followed by the early administration of correct treatment is of utmost importance to improve outcomes.
Recently, the definition of AKI in cirrhosis has been modified according to the Acute Kidney Injury Network (AKIN) criteria that were already widely used for the definition of AKI in the general population.5 According to the new definition, the diagnosis of AKI is based on small changes in serum creatinine (SCr) with respect to previous values, rather than relying on a fixed cut-off value of SCr. The use of the new definition may lead to an earlier identification of episodes of renal impairment, which may have a beneficial impact on prognosis. This review will give an update on the diagnosis and management of HRS in cirrhosis in 2018, in the setting of the changes in AKI definition in cirrhosis and the emergent data on the potential usefulness of urine kidney biomarkers in this scenario.
2 NEW DEFINITION OF ACUTE KIDNEY INJURY IN CIRRHOSIS
Traditionally, kidney failure in cirrhosis has been defined as SCr > 1.5 mg/dL.6 Despite the definition being widely used for many years, there was evidence that it had limitations. Particularly, that it led to a delayed diagnosis of kidney failure, as the cut-off level identified patients who already had a marked decrease in glomerular filtration rate (GFR) (SCr > 1.5 mg/dL corresponds to approximately GFR < 30 mL/min)7; and that the use of a fixed cut-off level did not consider changes in SCr with respect to previous values.
In the last decade, the Acute Kidney Injury Network (AKIN) proposed new diagnostic criteria for kidney failure, currently named AKI, based on small changes in SCr.5 These criteria were validated in the general population and are now widely used in this setting. According to the AKIN criteria, AKI is defined as an increase in serum SCr ≥ 0.3 mg/dL occurring within a short period of time (48 hours).5 Moreover, AKI is classified into three different stages (AKI stage 1, 2 and 3) depending on the intensity of changes in SCr.
In recent years, several studies have investigated the usefulness of the AKIN criteria for the diagnosis of AKI in patients with cirrhosis.8-12 Results from these studies showed that the diagnosis of AKI, according to the new criteria and its stages, was good at prognostic stratification, as AKI was associated with increased in-hospital stay and mortality. On this background, in 2012 the International Club of Ascites (ICA) organized a consensus meeting to discuss a new definition of AKI in cirrhosis based on a modified version of the AKIN criteria. According to the new ICA-AKI criteria, AKI in cirrhosis is defined as an increase in SCr ≥ 0.3 mg/dL (≥26.5 μmol/L) within 48 hours; or a percentage increase in sCr ≥ 50% from baseline which is known, or presumed, to have occurred within 7 days.13 These criteria also stratify AKI into 3 different stages with respect to the intensity of the changes in SCr and provide definitions for the concepts of progression and regression of AKI, and response to treatment (Table 1).
| Definition of AKI | |
|---|---|
| Increase in sCr ≥ 0.3 mg/dL (≥26.5 μmol/L) within 48 hours; or, a percentage increase in sCr ≥ 50% from baseline which is known, or presumed, to have occurred within the prior 7 d | |
| AKI stages | |
| AKI 1 | Increase in sCr ≥ 0.3 mg/dL (26.5 μmol/L) or an increase in sCr ≥ 1.5-fold to 2-fold from baseline |
| AKI 1A | SCr at diagnosis < 1.5 mg/dL |
| AKI 1B | SCr at diagnosis ≥ 1.5 mg/dL |
| AKI 2 | Increase in sCr >2-fold to 3-fold from baseline |
| AKI 3 | Increase in sCr >3-fold from baseline or sCr ≥ 4.0 mg/dL (353.6 μmol/L) with an acute increase ≥ 0.3 mg/dL (26.5 μmol/L) or initiation of renal replacement therapy |
- AKI, acute kidney injury; ICA, International Club of Ascites; SCr, serum creatinine.
An important point derived from the new definition is that it is essential to have a baseline value of SCr to apply the new diagnostic criteria. For patients developing AKI during hospitalization, this situation is easy as the SCr value at admission may be used as a baseline SCr.13 However, a high percentage of patients with cirrhosis develop AKI before hospitalization and, therefore, present with an already high SCr value at admission.2 In this population, the diagnosis of AKI should be made using pre-admission values of SCr as baseline. Patients with cirrhosis may develop impairment of kidney function during the progression of the disease; thus, the closest the baseline SCr value from admission, the more accurate the diagnosis. The ICA-AKI criteria arbitrarily defined baseline SCr as a value of SCr within the previous 3 months; if more than 1 value is available, the value closest to hospital admission should be used. In those patients without a previous SCr value, the SCr on admission should be used as baseline. It is important to highlight that for this latter group of patients who already have a high SCr at hospital admission but no baseline SCr is available, the diagnosis of AKI could be missed. Therefore, special attention should be made to this group of patients, especially if SCr ≥ 1.5 mg/dL. If a baseline SCr is not available, a formal diagnosis of AKI according to the ICA-AKI criteria cannot be made; however, clinical experience would suggests that patients with such an impairment of kidney function, especially if there is an identifiable precipitating event, probably have AKI and should be treated accordingly.
To overcome the problem of the absence of a baseline SCr in some patients, the proposal was made to use an estimated SCr calculated by the reverse application of the MDRD formula using a predefined value of GFR. However, MDRD underestimates GFR in patients with cirrhosis and, therefore, this method would be inaccurate and is not recommended in cirrhosis.13, 14
2.1 Categorization of patients with AKI stage 1 into two groups: 1A and 1B
AKI stages have been shown to stratify prognosis of patients with cirrhosis and AKI.2, 8-10 Patients with AKI stage 2 and 3 are those showing a worse prognosis (3-month probability of survival of 42% and 31%, respectively), compared to patients with AKI stage 1 (3-month probability of survival > 70%).8 However, the results of 2 studies performed in independent series of patients suggest that the population of patients included in AKI stage 1 is heterogeneous and that it should be divided into 2 subgroups with different prognosis. These studies showed that patients with stage 1 and SCr at diagnosis < 1.5 mg/dL named as AKI stage 1A had much better prognosis than those with stage 1 and SCr at diagnosis ≥ 1.5 mg/dL named as AKI stage 1B.8, 9 Interestingly, these results have recently been validated in a large prospective study including 547 patients admitted to hospital for acute decompensation of cirrhosis in 2 tertiary care hospitals in Padova and Barcelona. Results from this study confirmed that patients with AKI stage 1A had significantly higher 90-day survival compared to that of patients with AKI stage 1B (82% vs 55%, respectively; P = .001); and moreover, patients with AKI stage 1A showed survival rates similar to those of patients without AKI (82% vs 89%, respectively; P = ns). According to the results of this study, AKI stage 1 is not only heterogeneous because of prognosis but AKI stage 1A and stage 1B also showed differences regarding the cause of AKI, evolution of AKI and development of acute-on-chronic liver failure (ACLF). HRS and acute tubular necrosis (ATN) were significantly more frequent in patients with AKI stage 1B compared to 1A; by contrast, hypovolemia was the most frequent cause of AKI in patients with stage 1A. Moreover, progression of AKI and development of ACLF were significantly more common in patients with AKI stage 1B compared to stage 1A.2 In view of these results, it is currently recommended that in patients with cirrhosis AKI stage 1 should be divided into 2 groups for better prognosis stratification.15 The goal of this strategy is to highlight the higher risk of progression and complications of patients with AKI stage 1B; thus, recommending a closer monitoring of these patients similar to that recommended for patients with AKI stage 2 or 3.15
2.2 New diagnostic criteria of hepatorenal syndrome
The definition of the new ICA-AKI criteria also led to changes in the diagnostic criteria of HRS-AKI. Traditionally, HRS has been classified into 2 entities according to severity and progression of kidney failure: type-1 HRS and type-2 HRS.6 Type 1 HRS is characterized with rapidly progressive kidney failure and very low survival expectancy, with median survival time of only 1 month if untreated. According to the classical diagnostic criteria, the diagnosis of type-1 HRS was made when SCr value doubled from baseline to a final value ≥ 2.5 mg/dL.6 Studies investigating the efficacy of terlipressin and albumin for the management of type-1 HRS showed that a higher SCr value at the beginning of treatment was associated with lower probability of response to treatment and poor survival.16, 17 These data suggest that the approach of waiting until SCr has increased above 2.5 mg/dL may decrease the probability of response to treatment compared to an approach based on an earlier treatment.6 In contrast to type-1 HRS, type-2 HRS is characterized by stable kidney failure and has a better prognosis than type-1 HRS.6
The new diagnostic criteria of HRS-AKI are shown in Table 2. The only change that was made with respect to the classical diagnostic criteria of HRS was the removal of the cut-off value of SCr.6, 13, 15 Experts agreed that the cut-off value of SCr for diagnosis of HRS-AKI should be removed to allow an earlier identification of HRS-AKI. Therefore, the current definition of HRS-AKI includes patients who meet ICA-AKI criteria and fulfil all diagnostic criteria of HRS-AKI, irrespective of the SCr value at diagnosis.
| (A) Diagnostic criteria of HRS-AKI |
|---|
| Cirrhosis and ascites |
| Diagnosis of AKI according to ICA-AKI criteria: increase in sCr ≥ 0.3 mg/dL within 48 hours |
| Absence of shock |
| No response after 2 consecutive days of diuretic withdrawal and plasma volume expansion with albumin (1 g/kg of body weight) |
| No current or recent use of nephrotoxic drugs (NSAIDs, aminoglycosides, iodinated contrast media, etc.) |
| No macroscopic signs of structural kidney injury, defined as:- absence of proteinuria (>500 mg/d)- absence of microhaematuria (>50 RBCs per high power field),- normal findings on renal ultrasonography |
| (B) Treatment criteria of HRS-AKI |
|---|
| Meeting all the diagnostic criteria of HRS-AKI |
| AKI stage ≥ 1B (after plasma albumin expansion) |
| No contraindication to vasoconstrictor therapy |
| Criteria for treatment individualized |
- AKI Acute kidney injury; AKI Acute kidney injury; HRS-AKI, Hepatorenal syndrome-AKI; ICA, International Club of Ascites; NSAIDs, non-steroidal anti-inflammatory drugs; RBCs, red blood cells.
Importantly, the term type-2 HRS is not included in the current concept of AKI-HRS because it is not an acute impairment but rather a chronic impairment of kidney function. Therefore, type-2 HRS is currently considered a form of chronic kidney disease (HRS-CKD) that is characteristic of cirrhosis.13, 15, 18
3 DIFFERENTIAL DIAGNOSIS OF AKI: ROLE OF URINE BIOMARKERS IN 2018
As mentioned before, HRS-AKI is a characteristic form of AKI that develops in patients with advanced cirrhosis.3 However, as a result of the impairment of circulatory function, patients with cirrhosis have labile kidney function and are prone to develop AKI due to aetiologies other than HRS, such as hypovolemia, ATN or nephrotoxicity. Differential diagnosis between different causes of AKI is essential as treatment completely differs between HRS-AKI and other causes of AKI.15 Moreover, prognosis differs according to AKI aetiology, with patients with hypovolemic AKI showing the best prognosis, compared to patients with HRS-AKI or ATN who have the worst prognosis.4
Currently, the differential diagnosis of the cause of AKI is based on clinical criteria, that include some degree of subjectivity, and standard analytical data. Classical urinary parameters such as proteinuria, fractional excretion of sodium (FeNa) and urine osmolality, which are used to help in the differential diagnosis, have several limitations, as they may be influenced by the use of diuretic treatment and have not been validated in large studies. In some cases, the differential diagnosis of the cause of AKI may easily be made only on clinical data with no need for further investigation. Nevertheless, some other clinical scenarios may be challenging, particularly the differential diagnosis between HRS-AKI and ATN, as these are both entities that occur in critically ill cirrhotic patients in whom several other complications may be present making the diagnosis more intricate.
Urinary biomarkers of tubular damage previously described in the nephrology field have shown to be potentially useful in the differential diagnosis of AKI in cirrhosis. Particularly, they have been shown to be accurate in distinguishing HRS-AKI from ATN. Among all these biomarkers, the most investigated and promising one appears to be neutrophil gelatinase-associated lipocalin (NGAL), followed by interleukin-18 (IL-18) and albumin.
NGAL is a glycoprotein that is overexpressed in the kidney by injured kidney tubular epithelia. Urinary NGAL (uNGAL) levels rise exponentially during AKI, prior to serum creatinine elevation.19 Several studies have shown that uNGAL levels are useful in the differential diagnosis of AKI in cirrhosis.20-23 AKI studies consistently show that patients with ATN have significantly higher uNGAL levels compared to those in patients with HRS-AKI or hypovolemia-induced AKI. Patients with hypovolemia show the lowest uNGAL levels, similar to those of patients without AKI. Interestingly, patients with HRS show intermediate uNGAL levels, which are significantly lower than those of patients with ATN and slightly higher than those of patients with hypovolemia-induced AKI. Interestingly, results from these studies show that uNGAL has good predictive accuracy for the differential diagnosis of ATN vs other causes of AKI with areas under the receiver-operating characteristic curves (AUROC) ≥ 0.8.20-23 It should be noted that uNGAL may increase in the context of urinary tract infections, as it is also produced by leucocytes. Therefore, uNGAL values should be interpreted with caution in patients with urinary tract infections.
Besides NGAL, other urine biomarkers, including IL-18, albumin and liver fatty acid binding protein (L-FABP), have also been investigated for the differential diagnosis between ATN and HRS.22, 23 Interestingly, the performance of all 3 biomarkers is similar, showing the highest values in patients with ATN, compared to patients with HRS-AKI or hypovolemia-induced AKI. Patients with HRS-AKI consistently show intermediate values between the other 2 groups. Among all these biomarkers, IL-18 and albuminuria have been the ones showing the best diagnostic accuracy in 2 studies.22, 23
Recently a meta-analysis investigated the role of uNGAL and urine IL-18 for the differential diagnosis and prognosis of AKI in cirrhosis.24 Results from this meta-analysis, including 8 prospective studies, showed that urinary levels of NGAL and IL-18 were able to discriminate between ATN and other types of AKI with high accuracy. The AUROC for the diagnosis of ATN was 0.86 (95%CI 0.68-0.94) for NGAL and 0.88 (95%CI 0.82-0.93) for IL18.24 Results from this meta-analysis and also from other individual studies show that biomarkers are not only useful for the differential diagnosis of AKI but can also be useful to predict short-term mortality. There are data showing that urinary levels of NGAL and IL-18 are significantly higher in patients who eventually died compared to those who were alive at the end of follow up.24
Overall, currently there is accumulating evidence showing that urine biomarkers and, particularly NGAL and IL-18, are useful for the differential diagnosis and to predict prognosis in patients with cirrhosis and AKI. In fact, the new EASL clinical practice guidelines on the management of decompensated cirrhosis recommend to implement the use of urine biomarkers, particularly NGAL, in the differential diagnosis between ATN and HRS-AKI.15 Nonetheless, the performance of these biomarkers in the setting of the new algorithm for the diagnosis of HRS-AKI in cirrhosis will require investigation in future studies.
4 PATHOPHYSIOLOGY OF HEPATORENAL SYNDROME-AKI
Great amount of evidence indicates that circulatory dysfunction plays a key role in the pathophysiology of HRS-AKI.3, 25 On this context, HRS-AKI has been traditionally defined as a characteristic cause of AKI of functional origin that develops in patients with cirrhosis, in the absence of histological kidney abnormalities. In recent years, there are data suggesting that the marked systemic inflammatory response present in patients with advanced cirrhosis may also be involved at some degree in the development of HRS-AKI.26
4.1 Systemic circulatory dysfunction
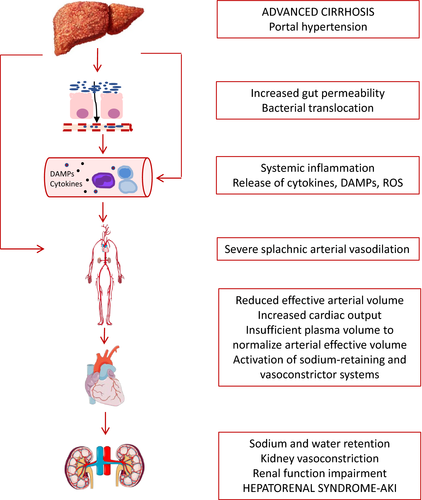
It is accepted that HRS-AKI occurs as a consequence of a marked reduction in renal blood flow and GFR secondary to impairment of effective arterial blood volume caused by splanchnic arterial vasodilation.3, 6, 25 The vasodilation of the splanchnic circulation is likely caused by the release of vasodilator factors, such as nitric oxide, carbon monoxide or endocannabinoids, as a consequence of portal hypertension.27-29
In early stages of cirrhosis, the increase in portal pressure is moderate and, therefore, there is slight reduction in systemic vascular resistance due to moderate splanchnic arterial vasodilation. At this stage of the disease, the reduction in systemic vascular resistance is compensated by an increase in cardiac output, which helps maintaining arterial pressure within normal values.3, 6, 25 In advanced stages of the disease, when patients have already developed decompensations, there is a marked reduction in systemic vascular resistance, as a result of a marked splanchnic vasodilation, that cannot be compensated by the increase in cardiac output.3 Moreover, there are data showing that at advanced disease stages, there is also a decrease in cardiac output that contributes to arterial underfilling.30 Overall, at this stage, patients develop a marked impairment of effective arterial blood volume. In this context and in order to maintain arterial pressure within normal limits, there is activation of endogenous vasoconstrictor systems, including renin-angiotensin system, sympathetic nervous system and, at later stages, a non-osmotic hypersecretion of vasopressin. These vasoconstrictor systems help maintain effective arterial blood volume and arterial pressure within normal limits, but have also important detrimental effects on kidney function. The effect of vasoconstrictor systems is associated with sodium and solute-free water retention, with the accumulation of ascites and oedema. If the activation of these systems is extreme, they lead to a marked renal vasoconstriction with the consequent reduction in GFR and development of HRS-AKI3, 6, 25 (Figure 1).
4.2 Systemic inflammation
There is growing evidence showing that decompensated cirrhosis is associated with marked inflammatory response that increases with the progression of the disease and may be involved in the development of complications, such as HRS-AKI.26
Bacterial translocation, typical of patients with decompensated cirrhosis, may trigger an inflammatory response with the release of proinflammatory cytokines in the splanchnic area, which may in turn lead to further arterial vasodilatation.31, 32 Bacterial infections are the main triggers of HRS.4
Moreover, independently of the presence of bacterial infections, patients with advanced cirrhosis show increased systemic levels of proinflammatory cytokines, such as interleukin 6 (IL-6), interleukin 8 (IL-8) or tumour necrosis factor (TNF).26, 33 Levels of these inflammatory markers increase in parallel with the severity of the disease, showing highest values in patients with acute-on-chronic liver failure (ACLF), a syndrome in which AKI has been shown to be one of the most frequent associated organ failures.34, 35
A study that investigated the relationship between inflammation and AKI showed that a high proportion of patients with HRS-AKI (78%) had either a documented bacterial infection or systemic inflammatory response syndrome (SIRS), compared to only 14% of patients with hypovolemia-induced AKI. Moreover, results from this study showed that about 30% of patients with HRS-AKI had SIRS without bacterial infection, suggesting that inflammation may be involved in the development of HRS-AKI regardless of the presence of bacterial infections.36
Although any bacterial infection may trigger the development of HRS-AKI, spontaneous bacterial peritonitis (SBP) has been shown to be one of the most common precipitating factors. Among patients with SBP, those developing HRS-AKI showed significantly higher levels of IL-6 and TNF, compared to patients with SBP who did not develop HRS-AKI.37 These findings suggest that in the context of the same infectious precipitating factor, a higher inflammatory state could be associated with the development of HRS-AKI.
A recent study evaluating a large number of cytokines showed that patients with HRS-AKI had higher levels of proinflammatory cytokines, particularly TNF-α, IL-6 and VCAM, compared to those patients without AKI or with hypovolemic AKI, independently of the presence of bacterial infections. This study therefore supports the existence of a marked systemic inflammation in the setting of HRS-AKI.38
Overall, it appears that systemic inflammation plays a role in the pathophysiology of HRS (Figure 1). Nonetheless, whether inflammatory mediators further impair circulatory function or if they are involved in direct kidney tissue damage, will have to be investigated in future studies.
5 MANAGEMENT OF HEPATORENAL SYNDROME-AKI
The goal of the management of HRS-AKI, particularly in those patients awaiting liver transplantation (LT), is the normalization of kidney function in order to provide successful bridge to LT.39-42 Once diagnosed, HRS-AKI should be treated as soon as possible. Ideally, patients should be managed in an intensive or intermediate care unit, especially those awaiting LT. Patients should be closely monitored to assess kidney function evolution and for early detection of possible associated complications, particularly bacterial infections, and start treatment as soon as possible. Intravenous (IV) fluids should be administered with caution to prevent pulmonary oedema and the development/or further impairment of hypervolemic hyponatremia. The use of a central venous catheter is recommended to monitor central venous pressure in patients who are going to receive pharmacological treatment because such treatment involves the administration of albumin. The use of a bladder catheter is not necessary in all cases because it is associated with high risk of urinary tract infections. Therefore, its use is recommended only in patients with marked oliguria.41, 43
5.1 Specific management
5.1.1 Vasoconstrictors and albumin
Vasoconstrictors and albumin is considered the pharmacological treatment of choice for the management of HRS-AKI. Vasoconstrictor drugs used for the management of HRS-AKI are vasopressin analogues, such as terlipressin, and alpha-adrenergic agonists, such as noradrenaline and midodrine.15, 41, 42, 44 Most of the existing evidence and published data are related to the use of terlipressin and albumin. It should be highlighted that all evidence available so far is derived from studies including patients with type-1 HRS defined according to the classical definition. To date, there are still no studies reported assessing the efficacy of vasoconstrictors and albumin in patients with HRS-AKI who do not meet the classical criteria of type-1 HRS (ie those in whom the sCr is lower than 2.5 mg/dL). Moreover, it should be acknowledged that terlipressin is approved in management of patients with type-1 HRS.
Terlipressin
Results from randomized, controlled trials and systematic reviews indicate that treatment with terlipressin and albumin is associated with significant improvement of kidney function in approximately 40%-50% of patients with type-1 HRS.3, 44-46 Systematic reviews also showed that this treatment was associated with improved survival.47, 48
Traditionally, the scheme of treatment with terlipressin is based on repeated IV boluses. The classical scheme recommended to start terlipressin at a dose of 1 mg/4-6 h. If after 3 days of treatment there is no improvement of kidney function, defined as a reduction in serum creatinine of more than 25% from pre-treatment value, the dose should be increased up to 2 mg/4-6 h.41 Treatment should be maintained until there is complete response, defined as a reduction in sCr to less than 1.5 mg/dL, or for a maximum of 14 days.41, 49
Recently, a randomized, controlled trial compared the safety and efficacy of terlipressin given by continuous IV infusion vs iv boluses. This study showed that the percentage of response to treatment was not significantly different between patients treated with continuous iv infusion vs patients treated with iv boluses (76% vs 65%, respectively; P = NS). Interestingly, the mean daily effective dose of terlipressin was significantly lower in the group treated with continuous infusion compared to the group treated with iv boluses. More importantly, the rate of adverse events was significantly lower in the group of patients treated with continuous IV infusion, compared to the group of patients treated with iv boluses (35% vs 62%, respectively; P < .025). Therefore, these results suggest that terlipressin given by continuous IV infusion is better tolerated and is effective at lower doses than terlipressin given by iv boluses.50 Nonetheless, patients receiving treatment with terlipressin should be monitored closely for early detection of potential side effects, as terlipressin is a very intense vasoconstrictor that may lead to ischaemic or cardiovascular effects. The frequency of adverse events leading to treatment withdrawal is of approximately 20%.50 The most common side effects of terlipressin include abdominal pain, diarrhoea, cardiovascular ischaemic complications and circulatory overload, with frequencies of up to 40% when terlipressin is administered as iv bolus. In patients presenting serious adverse events, treatment should be discontinued. If adverse events are not severe, the dose of terlipressin could be decreased and treatment may be continued, but close monitoring of these patients should be performed.
Finally, a comment on the recently published REVERSE trial, which is the largest randomized, placebo-controlled, double-blind study aimed at assessing the efficacy of terlipressin in the reversal of type-1 HRS seems pertinent.51 It included 196 patients with cirrhosis and type-1 HRS from North America who were randomized to receive terlipressin or placebo, both with IV albumin. In contrast to previous randomized trials that clearly showed the efficacy of terlipressin in the reversal of HRS, the results of the REVERSE trial were negative. The incidence of confirmed HRS reversal (defined as 2 sCr values ≤ 1.5 mg/dlL, at least 48 hours apart, on treatment without renal replacement therapy (RRT) or liver transplantation) was higher in the terlipressin group compared to placebo group, but this was not statistically significant (P = .22). Moreover, the main secondary endpoints (overall and transplant-free survival) were also negative. Nevertheless, the study described some positive findings, particularly a greater improvement of kidney function in patients treated with terlipressin, and survival was highly correlated with changes in SCr levels.51
There are some reasons that could explain the negative results of this study in contrast to previous trials. First, the duration of treatment with terlipressin was relatively short in this study as up to one-third of patients received ≤ 3 days of treatment and only 6% completed the 14 days of therapy. Moreover, there was a high use of competitive treatments such as RRT and liver transplantation.51, 52 Particularly, RRT was used as a rescue therapy in a high proportion of patients in the early stages of treatment, being considered one of the main reasons for treatment failure.
However, a continued analysis of patients included in the REEVRSE trial as well as in a previous trial in North America (OT-0401) demonstrated that treatment with terlipressin plus albumin in patients with HRS-1 resulted in a significantly higher rate of HRS reversal compared to that of patients who received albumin alone, confirming that terlipressin treatment is associated with improved renal function.53
During treatment with terlipressin patients must receive concomitant treatment with IV albumin at a dose of 1 g/kg body weight the first day followed by 20-40 g/d.41, 54 As mentioned above, it is recommended to monitor central venous pressure during treatment. If patients have high central venous pressure levels during treatment, with values above 15 mmHg, IV albumin should be temporarily discontinued.43
As described above, treatment with terlipressin and albumin should be started as soon as possible after the diagnosis of HRS-AKI. Several studies show that the besides SCr value at the moment of starting treatment is an independent predictive factor of treatment response, with higher SCr values associated with lower probability of response.16 Recent data show that beside SCr values, the presence and the severity of ACLF have also an important impact on treatment response. Patients with grade 3 ACLF (the most severe stage of ACLF) have significantly lower probability of response to treatment compared to patients with ACLF grade 1 or 2 (29% in ACLF-3, compared to 60% and 48% in ACLF-1 and ACLF-2 respectively; P < .001).55
Other vasoconstrictors
Vasoconstrictors other than terlipressin represent an alternative pharmacological treatment in countries where terlipressin is not available. These include IV noradrenaline and oral midodrine plus subcutaneous (sc) octreotide, in both cases associated with IV albumin at the same dose recommended for treatment with terlipressin.15, 44
Although information is limited, noradrenaline appears to be effective for the management of type-1 HRS.56, 57 A randomized-controlled trial that compared the efficacy and safety of treatment with terlipressin vs noradrenaline for patients with HRS showed that approximately 40% of patients presented response to treatment in both groups and the adverse event profile was also similar.57 In a recent meta-analysis, noradrenaline appeared to be as effective and safe as terlipressin for the management of type-1 HRS and represents a good alternative treatment.58 However, the number of patients treated with noradrenaline is small to confirm the effectiveness, and a more systematic review and network meta-analysis showed a low-quality evidence supporting the use of noradrenaline to reduce mortality and reverse the HRS.48
The combination of oral midrodrine plus octreotide together with albumin has also been shown to improve kidney function in patients with type-1 HRS. Two proof-of-concept studies investigated the effects of treatment with midodrine plus octreotide on kidney function and survival in patients with type-1 HRS.59, 60 Both studies showed that kidney function significantly improved in patients treated with midodrine plus octreotide compared to controls. However, a randomized-controlled trial that compared the safety and efficacy of midodrine and octreotide vs terlipressin, showed that response to treatment was significantly higher in those patients receiving terlipressin compared to the group receiving midodrine and octreotide (70.4% vs 28.6%, P = .01).61 Therefore, this combination therapy appears to be of limited efficacy.
5.1.2 Liver transplantation
Liver transplantation (LT) is the treatment of choice for patients with HRS-AKI as it represents the definitive treatment of the underlying liver disease. Patients with HRS-AKI have a very poor prognosis, particularly those with classical type-1 HRS and, therefore, should be considered candidates for LT and referred to hospitals with liver transplant programmes. As HRS-AKI is reversible after LT, liver transplant alone is preferred to combined liver-kidney transplant.15, 39, 41 Combined liver-kidney transplant should only be considered in patients who have either a CKD in the following conditions: (i) estimated GFR (using MDRD6 equation) ≤ 40 mL/min or measured GFR using iothalamate clearance ≤ 30 mL/min, (ii) proteinuria ≥ 2 g/d, (iii) kidney biopsy showing >30% global glomerulosclerosis or >30% interstitial fibrosis, or (iv) inherited metabolic disease, or an HRS-AKI refractory to drug therapy, which has required renal replacement therapy for more than 4 weeks or with GFR ≤ 35 mL/min or measured GFR ≤25 mL/min ≥ 4 weeks, and thus who have a low probability of recovery of kidney function.15, 62
Patients with type-1 HRS have high mortality on the waiting list; thus, these patients should be given high priority for transplant. The use of the model for end-stage liver disease (MELD) score as the system for organ allocation allows giving high priority to these patients. It is important to remark that in order to avoid a reduction in MELD score in patients who respond to pharmacological treatment with vasoconstrictors and albumin, leading to a delay in LT allocation, it has been suggested to maintain the MELD score calculated with the sCr value before treatment while these patients are on the waiting list.39, 41
5.1.3 Other therapeutic options
Transjugular intrahepatic portosystemic shunt (TIPS) has been proposed as an alternative therapy for the management of HRS-AKI because it reduces portal pressure leading to an improvement of circulatory dysfunction. However, the applicability of TIPS in patients with type-1 HRS, who have very advanced liver disease, is limited because many patients have contraindications for the insertion of TIPS. Two studies with small number of patients indicate that TIPS decreases the activity of endogenous vasoconstrictor systems and, in consequence, improves kidney function in approximately 60% of patients with HRS.63, 64 However, these studies excluded patients with Child-Pugh score ≥ 12, serum bilirubin >5 mg/dL and patients with previous hepatic encephalopathy. Therefore, TIPS is not recommended in the setting of type-1 HRS as existing data are limited and the applicability of TIPS in these patients is very low.
Renal replacement therapy is not recommended as a first-line treatment in patients with type-1 HRS, as there are no studies specifically investigating its efficacy in this setting. RRT can be used as a rescue treatment in patients with type-1 HRS who do not respond to treatment with vasoconstrictors and who develop criteria for emergent RRT (ie hypervolemia, hyperkalaemia and metabolic acidosis).15, 41 Moreover, it should be noted that clinical experience indicates that the development of criteria leading to the indication of RRT is not common in patients with type 1, at least when treatment is started at early stages and soon after diagnosis. Nevertheless, it is important to emphasize that RRT is commonly used in countries in which terlipressin is not available due to the lower efficacy of another pharmacological therapies.
Alternative dialysis methods such as the use of the molecular readsorbent recirculating system (MARS®),65 or fractionated plasma separation and adsorption (Prometheus®),66 have been proposed as alternative methods for the management of type-1 HRS. These methods are based on the clearance of several substances from the circulation, including endogenous vasodilator, and appear to have some potential beneficial effects but data are still limited and results are not conclusive. Thus, further studies are needed to define their role as therapeutic alternatives for HRS.
5.2 New algorithm for the management of AKI-HRS and implications for therapy
It is important to remark that according to the new diagnostic criteria of HRS-AKI a new algorithm for the management of AKI in patients with cirrhosis has been proposed (Figure 2, Table 2B). When AKI is diagnosed, the cause of AKI should be investigated as soon as possible, to prevent AKI progression. However, even in the absence of a definitive recognized cause, management of AKI should be immediately started according to the initial stage. Irrespective of the stage, diuretics and beta-blockers should be discontinued or tapered. Other precipitating factors of AKI should be identified and treated, including screening and treatment of infections, volume expansion when appropriate and discontinuation of all nephrotoxic drugs, such as vasodilators, beta-blockers or NSAIDs. Volume replacement should be used in accordance with the cause and the severity of fluid loss. Patients with diarrhoea, excessive diuresis or acute gastrointestinal bleeding should be treated with crystalloids or packed red blood cells respectively. In contrast, in all patients with AKI stage ≥ 1B, 20% albumin solution at a dose of 1 g of albumin/kg of body weight (with a maximum of 100 g of albumin) for 2 consecutive days should be administered. If patients do not respond to volume expansion with albumin and meet all HRS-AKI criteria, at this point treatment with vasoconstrictors and albumin should be started, if there are no contraindications for therapy.13, 15
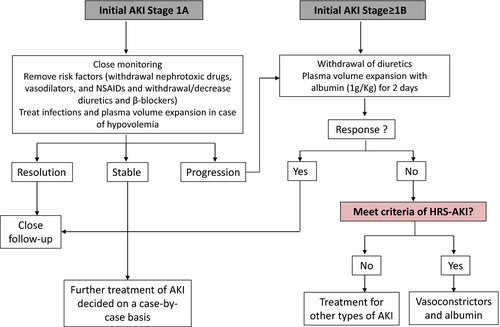
It should be highlighted that using this algorithm the differential diagnosis and management of HRS will be made earlier as compared to when using classical type-1 HRS diagnostic criteria because the need to reach a cut-off level of SCr of 2.5 mg/dL has been removed.13 In this context, treatment with vasoconstrictors and albumin will be initiated with lower SCr values. Considering that the baseline SCr at the initiation of pharmacological therapy is a predictive factor of treatment response, this new diagnostic and treatment strategy should likely lead to a higher treatment response rate. However, to date there are no studies investigating the efficacy and the safety profile of the use of vasoconstrictors and albumin in patients with HRS using the new ICA-AKI criteria. Therefore, treatment should be ideally performed in the setting of prospective studies to assess efficacy and safety of such approach.
ACKNOWLEDGMENTS
Some of the work mentioned in this review has been sponsored by the Instituto de Salud Carlos III through the Plan Estatal de Investigación Cientifica y Técnica y de Innovación 2013-2016, project reference PI 12/00330 and PI 16/0043 (this grant was co-funded by the European Regional Development Fund (ERDF), Agencia de Gestió d'Ajuts Universitaris I de Recerca (AGAUR) 2014/SGR 708, CERCA programme/Generalitat de Catalunya, EU Horizon 20/20 Programme H2020-SC1-2016-RTD, LIVERHOPE Project Number: 731875). PG is a recipient of an ICREA Academia Award. ES is a recipient of a grant M-BAE 2017 from the Instituto de Salud Carlos III.
CONFLICT OF INTEREST
CS, EP and ES declare that they have no conflicts of interests. PG declares consultancy advisory boards for: Ferring Pharmaceuticals, Grifols, Novartis and Promethera. He has received research funding from Grifols S.A.




