Why and how to measure renal function in patients with liver disease
Abstract
Patients with advanced liver disease frequently have impaired renal function. Both acute kidney injury (AKI) and chronic kidney disease (CKD) are quite common in patients with cirrhosis and both are associated with a worse prognosis in these patients. A careful assessment of renal function is highly important in these patients to help physicians determine their diagnosis, prognosis and therapeutic management and to define transplantation strategies (liver transplantation alone vs simultaneous liver and kidney transplantation). Although they are still widely used in clinical practice, conventional biomarkers of renal function such as serum creatinine have several limitations in these patients. Recent progress has been made in the evaluation of renal function and new diagnostic criteria for AKI have been proposed. However, certain issues such as the noninvasive assessment of the glomerular filtration rate and/or improvement in the differential diagnosis between hepatorenal syndrome and acute tubular necrosis must still be addressed. The purposes of this paper are: (i) to highlight the importance of the evaluation of renal function in patients with cirrhosis; (ii) to review the state of the art in the assessment of renal function in these patients as well as advances that we expect will be made to improve the accuracy of available tools.
Abbreviations
-
- ACLF
-
- acute-on-chronic liver failure
-
- AKD
-
- acute kidney disease
-
- AKI
-
- acute kidney injury
-
- ATN
-
- acute tubular necrosis
-
- CKD
-
- chronic kidney disease
-
- DAA
-
- direct acting antivirals
-
- ESRD
-
- end-stage renal disease
-
- GFR
-
- glomerular filtration rate
-
- HRS
-
- hepatorenal syndrome
-
- HVPG
-
- hepatic venous pressure gradient
-
- ICA
-
- International Club of Ascites
-
- INR
-
- international normalized ratio
-
- KDIGO
-
- kidney disease improving global outcomes
-
- LT
-
- liver transplant
-
- MELD
-
- model of end-stage liver disease
-
- NGAL
-
- neutrophil gelatinase-associated lipocalin
-
- NUCs
-
- nucleoside/nucleotide analogues
-
- RRT
-
- renal replacement therapy
-
- RTA
-
- renal transplant alone
-
- sCr
-
- serum creatinine
-
- SLK
-
- simultaneous liver and kidney transplant
Key points
- Patients with cirrhosis frequently show an impairment of renal function.
- The evaluation of renal function guides diagnostic and therapeutic management, prognostic evaluation and indication to liver transplantation and/or simultaneous liver kidney transplantation.
- Serum creatinine and serum creatinine-based equations lead to an overestimation of GFR in patients with cirrhosis, although widely used for the definition of AKI and CKD.
- The differential diagnosis between hepatorenal syndrome-AKI and acute tubular necrosis/intrinsic-AKI is complex in these patients.
- New biomarkers of glomerular filtration rate and parenchymal kidney damage are promising tools in refining the evaluation of renal function in cirrhosis.
1 Introduction
Renal damage and/or an impaired renal function often occur in patients with liver disease. Three different clinical scenarios can be identified: (i) simultaneous involvement of both the liver and kidneys, (ii) primary liver disease with secondary renal dysfunction or (iii) liver disease secondary to a nephropathy.1 The latter is uncommon in clinical practice and is beyond the scope of this manuscript. In the former scenario, liver and kidney damage can share the same causative agent such as infection or metabolic diseases (Table 1). In these cases, renal dysfunction develops gradually with a few exceptions such as leptospirosis, certain viral haemorrhagic fevers and toxin-mediated injury, such as acetaminophen poisoning, causing acute failure of both organs. One of the most clinically relevant examples of the first scenario is renal involvement during acute or chronic HBV and HCV hepatitis.1 Both acute and chronic renal failure secondary to liver disease usually occurs in patients with cirrhosis, and it is often functional, initially occurring in the absence of significant changes in renal histology (pre-renal form and hepatorenal syndrome). However, renal structural alterations, in particular tubular necrosis, can complicate both acute and chronic liver disease, in particular when it is characterized by severe cholestasis (cholemic nephropathy).2
| Tubulo-interstitial involvement |
|
| Glomerular involvement |
|
| Vascular involvement |
|
2 Why Measure Renal Function in Patients With Liver Disease?
Based on these introductory remarks the evaluation of renal function in patients with liver disease has the following clinical purposes: (i) to define diagnostic assessment, (ii) to evaluate possible drug therapy, (iii) to assess the prognosis and (iv) to define a possible transplant strategy for one or both organs.
2.1 Defining diagnostic assessment
To complete the diagnosis of potential kidney dysfunction it must first be determined whether the patient with liver disease has “acute kidney injury (AKI),” “acute kidney disease (AKD), “chronic kidney disease (CKD)” or an overlapping diagnosis (AKD/CKD, AKI/CKD or AKI/AKD).3 Although serum creatinine (sCr) and sCr-based equations have several limitations for the assessment of renal function in patients with cirrhosis, the diagnosis of renal dysfunction in liver disease is still based on the trend of sCr in the past months and/or the past week.4 When an increase in sCr is found in a patient with liver disease, it is first necessary to determine whether the patient has CKD, AKD, AKI or an overlapping diagnosis (Table 2). This step is crucial to define the treatment strategies that should be started immediately in case of AKD or AKI (or if there is an overlap with CKD) and less urgently in case of CKD.4
| Functional criteria | Structural criteria | |
|---|---|---|
| AKI | Increase in SCr by ≥ 50% within 7 days, ORIncrease in SCr by ≥ 0.3 mg/dL (26.5 μmol/L) within 2 days | No criteria |
| CKD | GFR<60 mL/min per 1.73 m2 for >3 months | Kidney damage for >3 months |
| AKD | GFR<60 mL/min per 1.73 m2 for <3 months, ORDecrease in GFR by ≥ 35% or increase in SCr by <50% for <3 months | Kidney damage for <3 months |
- AKI, acute kidney injury; sCr, serum creatinine; CKD, chronic kidney disease; GFR, glomerular filtration rate; AKD, acute kidney disease.
The next step in the management of CKD, AKD or AKI in patients with liver disease, is the differential diagnosis. The differential diagnosis is essential with AKI because the different types of AKI in these patients (mainly hypovolaemia, sepsis-induced AKI, hepatorenal syndrome and acute tubular necrosis)5 require different therapies and are associated with different risks of mortality.6 In most cases, hypovolaemia and sepsis-induced AKI can be managed by plasma volume expansion and antibiotic treatment. It is therefore most important to differentiate between hepatorenal syndrome (HRS)-AKI and acute tubular necrosis or intrinsic-(ATN)–AKI.4 We will discuss the current diagnostic criteria of AKI, CKD and AKD as well as the differential diagnosis of AKI in these patients.
2.2 Evaluation of drug therapy
Before administering pharmacological treatments to patients with liver disease, renal function should be assessed for two reasons: (i) to evaluate the adequate doses to be administered (if drugs require a renal dose adjustment); (ii) to evaluate the specific treatment of renal dysfunction correlated with liver disease. The discussion of the former is outside the scope of this paper. However, it should be noted that renal function should also be measured before prescribing antiviral treatment for HBV- or HCV-related hepatitis. Renal function must be assessed to titrate ribavirin or choose a direct acting antiviral agent (DAA) for HCV or nucleoside/nucleotide analogues(NUCs) for HBV infections.
The second case involves different scenarios, from the management of renal glomerular damage during HCV or HBV infection to that of HRS. Three different approaches can be used to treat glomerular nephropathies associated with HCV infection: (i) antiviral therapy, (ii) selective immunosuppressive therapy to break down the synthesis of cryoglobulins or (iii) nonspecific immunosuppressive therapy to inhibit glomerular inflammation.7 The widespread use of DAAs will increasingly reduce the indication for immunosuppressive therapy, in the same way as NUCs have in HBV-associated nephropathies.8 Pharmacological treatment of HRS-AKI involves the use of vasoconstrictors plus albumin. The rationale for this treatment is to counteract severe splanchnic arterial vasodilation, which is considered to be the main cause of the reduction in effective circulating volume in these patients.9, 10 A combination of terlipressin (1 mg every 4–6 hours with a maximum of 2 mg every 4 hours, or by a continuous infusion of 3 mg/24 hours with a maximum of 12 mg/24 hours) and albumin (20–40 g a day) resolves HRS-AKI in 35–60% of cases.11, 12 Noradrenaline (0.5 mg/hour with a maximum of 3 mg/hour) may be an alternative to terlipressin13 while a combination of midodrine and octreotide has been shown to be less effective than terlipressin.14 In patients with HRS-AKI who do not respond to medical therapy and in those with ATN-AKI, medical treatment should be based on the same criteria as those in patients without liver disease, including renal replacement therapy (RRT), when indicated. The different methods of RRT (haemodialysis, continuous veno-venous haemofiltration etc.) have not been formally compared in these patients.
2.3 The refinement of the prognostic evaluation
The prognosis in patients with cirrhosis and renal dysfunction is poor.2 The overall rate of survival is approximately 50% at 1 month and 20% at 6 months. The prognosis is largely dependent upon the type and nature of renal dysfunction (AKI or CKD).2 Thus, parenchymal nephropathy has the best prognosis and HRS the worst.6 The negative impact of renal dysfunction on survival in patients with advanced liver disease was the reason the model for end-stage liver disease (MELD) score was introduced as an alternative to the Child–Pugh score in the early 2000s based on three variables: serum bilirubin, sCr and international normalized ratio (INR).15 Kidney disease improving global outcome (KDIGO criteria) provide a highly accurate prognostic evaluation of patients with AKI.8 In particular, several prospective studies have shown that the AKI classification based on KDIGO criteria predicts short-term and mid-term mortality in a stage dependent-fashion in hospitalized patients with cirrhosis.16-19 More recently, the CANONIC study showed that renal dysfunction played a central role in defining acute-on-chronic liver failure (ACLF). Indeed, renal failure (sCr≥2 mg/dL) was the only single organ failure to meet ACLF criteria (28-day mortality>15%). Furthermore, mild renal dysfunction (sCr>1.5 mg and <2 mg/dL) associated with single organ failure was sufficient to define ACLF.20 These results have helped confirm that impaired kidney function has a marked negative impact on the prognosis of hospitalized patients with cirrhosis and has also shown that a more comprehensive prognostic classification including nonrenal organ failure is more accurate than KDIGO criteria.21
2.4 The definition of a possible transplantation strategy
In 2002, the MELD score was adopted in the USA to prioritize organ allocation in liver transplantation (LT) candidates22 and it is now used worldwide. Use of the MELD score has increased the number of patients with renal dysfunction who receive LT and has reduced mortality in patients on the waiting list, increasing the number of simultaneous liver–kidney transplants (SLK).23 It should be emphasized that the aetiology of renal failure in the latter has a significant impact on post-LT outcome, both for AKI and CKD. It has also been shown that patients with ATN-AKI have a significantly higher probability of developing CKD within 5 years after LT than patients with HRS-AKI (56 vs 16%, respectively). Post LT-CKD was associated with an increased risk of post-LT mortality in patients with ATN-AKI.24 Patients with CKD and type 2 HRS have a high probability of recovering renal function after LT. However, the risk of developing CKD during follow-up is still high. In some patients with CKD of unknown origins, renal biopsy results, such as the extent of global glomerulosclerosis, can identify patients at a high risk of developing CKD after LT.25 For this reason, it is worth considering SLK in patients in whom the outcome of SLK is better than LT alone. Table 3 summarizes the guidelines proposed by OPT/UNOS for SLK.23
| Chronic kidney disease |
|
| Sustained acute kidney injury |
|
| Metabolic diseases |
|
- CKD, chronic kidney disease; RRT, renal replacement therapy; GFR, glomerular filtration rate; aEstimated by MDRD-6 equation or measured by iothalamate/iohexol clearance; AKI, acute kidney injury.
In other cases, patients with liver disease and end-stage renal disease (ESRD) may need to receive a kidney transplant alone (KTA) or SLK. KTA can be considered in patients with a METAVIR fibrosis score of F1 or F2. KTA is still feasible in patients with F3 fibrosis, but liver disease must be closely followed up after renal transplantation, especially if the aetiology of the former cannot be adequately treated. There is little experience in KTA in patients with F4 fibrosis (initial cirrhosis). Even in patients without clinical and/or instrumental evidence of portal hypertension and with a normal hepatic venous pressure gradient (HVPG), the medium- and long-term outcome after KTA is still unknown. The debate in this field is further fuelled by the availability of DAAs for HCV infection, in patients with ESRD26 on the one hand, and the remaining risk of hepatocellular carcinoma in a patient with cirrhosis on the other hand.
3 How to Measure Renal Function in Patients with Chronic Liver Disease
Several biomarkers can be used to measure renal function in patients with cirrhosis.27 Biomarkers can be classified into biomarkers used to measure GFR and those used to assess parenchymal kidney damage. We will briefly describe the current classification of AKI and CKD in patients with chronic liver disease to better understand the role of these biomarkers in the diagnosis of renal impairment in cirrhosis. The epidemiology and clinical impact of AKD in patients with chronic liver disease will not be discussed in detail because of the limited information on this topic.
3.1 Diagnosis and classification of AKI in patients with liver disease
The diagnosis of AKI in cirrhosis is currently based on variations in sCr. KDIGO criteria3 were slightly modified by the International Club of Ascites (ICA)28 to correspond better to the characteristics of patients with cirrhosis. Thus, at present AKI is defined in patients with liver disease by: (i) an increase in sCr≥0.3 mg/dL (≥26.5 μmol/L) within 48 hours; or, (ii) a percentage increase in sCr≥50% from baseline which is known, or presumed, to have occurred within the past 7 days28 (Table 4). It is worth noting that approximately 30% of AKI episodes occur before the patient is hospitalized, thus baseline SCr before hospital admission is crucial for a timely diagnosis of “community-acquired AKI”.5, 17 According to ICA criteria, the most recent sCr value available from the past 3 months should be considered to be baseline.28 This time frame is also necessary to clearly differentiate AKI from CKD (see below). Other approaches suggested by KDIGO criteria in patients without baseline SCR values, such as a reverse calculation of sCr from sCr-formulas using a standard GFR value of 75 mL/min, should be discouraged because they are highly inaccurate in these patients.29
| Subject | Definition |
|---|---|
| Baseline sCr | A value of sCr obtained in the previous 3 months, when available, can be used as baseline sCr. In patients with more than one value within the previous 3 months, the value closest to the admission time to the hospital should be used |
| In patients without a previous sCr value, the sCr on admission should be used as baseline | |
| Definition of AKI | Increase in sCr ≥0.3 mg/dL (≥26.5 mmol/L) within 48 h; or a percentage increase sCr≥ 50% from baseline which is known, or presumed, to have occurred within the prior 7 days |
| Staging of AKI | Stage 1: increase in sCr ≥0.3 mg/dL (26.5 mmol/L) or an increase in sCr ≥1.5-fold to twofold from baseline |
| Stage 2: increase in sCr >2 to 3-fold from baseline | |
| Stage 3: increase of sCr >3-fold from baseline or sCr ≥4.0 mg/dL (353.6 mmol/L) with an acute increase ≥0.3 mg/dL (26.5 mmol/L) or initiation of renal replacement therapy |
- sCr, serum creatinine; AKI, acute kidney injury.
Kidney disease improving global outcomes criteria for the diagnosis of AKI based on urinary output were not taken into consideration by the recent ICA consensus meeting because: (i) these patients are frequently oliguric with significant sodium retention despite relatively normal GFR, (ii) these patients may have increased urine output because of diuretics, and (iii) urine collection is often inaccurate on a regular hospital wards and is always untimely.28 More recently, another group of experts has stated that since urine output has been found to be a sensitive and early marker for AKI in intensive care unit patients, worsening oliguria or the development of anuria should be considered to be AKI in critically ill patients with cirrhosis.30 Until there is proof of the contrary, we agree with these criteria as long as all of these patients have a bladder catheter.
The severity of AKI according to the percentage increase in sCr is the following:
- Stage 1: an increase in sCr≥0.3 mg/dL or an increase in sCr ≥1.5-fold to 2-fold from baseline;
- Stage 2: an increase in sCr>2-fold to 3-fold from baseline;
- Stage 3: an increase in sCr>3-fold from baseline or sCr≥4.0 mg/dL with an acute increase in at least 0.5 mg/dL
Several studies have validated these criteria in patients with cirrhosis and showed that AKI is associated with mortality5, 16-19, 21 and that there is a stepwise increase in mortality as the stage of AKI increases.5, 16-18, 21 Furthermore, the progression of AKI from a lower to a higher stage has been found to be associated with worsening survival.5, 16, 17 These studies have been used to develop an algorithm for the management and differential diagnosis of AKI in these patients28 (Figure 1).
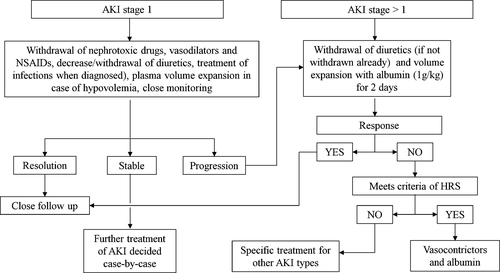
Several types of AKI have been described in patients with cirrhosis in particular hypovolaemia, sepsis-induced AKI, hepatorenal syndrome (HRS), acute tubular necrosis (ATN) and other less common miscellaneous causes (IgA nephropathy, nephrotoxicity, glomerulonephritis etc.).5, 6 As reported above, the differential diagnosis of the types of AKI is highly important because it determines the therapeutic approach to AKI. At present, the differential diagnosis of AKI is mainly based on the response to withdrawal of diuretics and plasma volume expansion (hypovolaemia and to some extent AKI induced by bacterial infections; Figure 1). The differential diagnosis of HRS-AKI and ATN-AKI can be difficult because the sensitivity and specificity of existing criteria to exclude signs of parenchymal damage in HRS-AKI (proteinuria >500 mg/24 hours, microhaematuria >50 red blood cells for high power field and abnormal renal ultrasonography)9, 10 are low. The potential use of new tools for this purpose will be discussed in detail below.
3.2 Diagnosis and classification of CKD in patients with liver disease
The diagnosis of CKD in patients with cirrhosis should be based on KDIGO criteria.31 CKD is defined as a GFR<60 mL/min/1.73 m2 or an increase in the markers of renal damage (albuminuria, abnormal urine sediment, renal histology and/or renal ultrasonography) for more than 3 months. A classification of CKD has been provided according to the level of GFR (Table 5). Although GFR should be measured according to gold standard methods (reported below), in clinical practice, equations based on sCr or cystatin C (CysC) have been used to estimate the GFR in patients with cirrhosis.27
| GFR categoryb | GFR (mL/min/1.73 m2) | Terms |
|---|---|---|
| Grade 1 | ≥90 | Normal or high |
| Grade 2 | 60–89 | Mildly decreasedb |
| Grade 3a | 45–59 | Mildly to moderately decreased |
| Grade 3b | 30–44 | Moderately to severely decreased |
| Grade 4 | 15–29 | Severely decreased |
| Grade 5 | <15 | Kidney failure |
- a In the absence of evidence of parenchymal kidney damage, neither GFR category G1 nor G2 fulfill the criteria for CKD.
- b Relative to young adult level.
3.3 Assessment of glomerular filtration rate
The gold standard for assessment of GFR in patients with cirrhosis is the clearance of exogenous markers that are freely filtered by the glomeruli and are neither reabsorbed nor secreted by the tubules. These markers may be or not be radioactive such as (125)I-iothalamate, (99 m)Tc-diethylene triamine pentaacetic or (51)Cr-ethylene diamine tetracetic acid and inulin or iohexol, respectively. However, these tools have several limitations in clinical practice because they are expensive, time-consuming and not feasible for dynamic assessment of renal function. For these reasons, several surrogate biomarkers of GFR have been developed. The most widely used is sCr. The use of sCr in assessing renal function has several limitations, for example, it is influenced by gender, race, age and body weight.27, 29 Several equations have been suggested to overcome these limitations, in particular the Modification for Diet in Renal Disease (MDRD) and the Chronic Kidney Disease-Epidemiology (CKD-EPI) equations, which are quite accurate to assess renal function in the general population. However, SCr has several further limitations in patients with cirrhosis. Indeed, creatinine formation from creatine is reduced due to muscle wasting, tubular secretion of creatinine is increased and there is potential interference with sCr assays from elevated bilirubin.28 Therefore, it is not surprising that sCr-based equations have been shown to overestimate the GFR in patients with cirrhosis and thus be inaccurate biomarkers of kidney dysfunction.29, 32 Despite these disadvantages, sCr is still the most frequently used biomarker for GFR in patients with cirrhosis both for the diagnosis of AKI28 and CKD. Furthermore, the MDRD-6 equation, based on sCr, blood urea nitrogen (BUN), age, gender, body weight, race and albumin has been suggested to estimate the GFR when assessing indications for simultaneous liver–kidney transplantation.33
Cystatin C is a protein secreted by all nucleated cells that passes freely through the glomeruli due to its low molecular weight and has been shown to be a good biomarker of GFR. CysC has potential advantages compared to SCr, because it is not influenced by gender, muscle mass and/or bilirubin levels. Studies performed in patients with cirrhosis found that CysC was a good biomarker of GFR in this group. Furthermore, an equation combining SCr and CysC was found to be more accurate than SCr-based equations to estimate GFR in patients with cirrhosis34, 35, although it is the diagnostic accuracy markedly worse than in subjects without cirrhosis.
Blood urea nitrogen (BUN) is extensively used as a biomarker of renal function in the general population. However, in patients with cirrhosis, BUN may increase or decrease independently of the GFR, due to GI bleeding or impaired liver function and poor nutritional status respectively.27
3.4 Assessment of parenchymal kidney damage
Assessment of parenchymal kidney damage is very important in both patients with CKD and AKI. In the former, it helps differentiate the type of CKD. In the latter, it provides the differential diagnosis between HRS-AKI and ATN-AKI, requiring completely different therapeutic approaches.
Following renal damage, molecules that are usually not filtered by glomeruli may appear in the urine. The most common marker of these alterations is urinary protein concentrations that should normally be below 500 mg/day. The assessment of proteinuria is quite simple and inexpensive, however, a 24-hour collection is needed and the sensitivity of this test is low for the detection of parenchymal renal damage. Microalbuminuria is widely used in the general population especially in those with hypertension and diabetes.31 Although microalbuminuria is a sensitive marker of CKD, there are very few data on its use in patients with cirrhosis in whom poor hepatic synthesis can reduce serum albumin concentrations. However recent data have shown that albumin is increased in the urine of patients with cirrhosis and AKI, it has been shown to be increased in patients with cirrhosis and ATN vs other types of AKI36, 37 and to be a good predictor of mortality in patients with cirrhosis.37
Conventional markers of ATN such as urine sodium or fractional excretion of sodium and urine osmolality can rarely be used in patients with chronic liver disease, mainly because most of these patients are chronically treated with diuretics.
Although the examination of urine sediment has been found to be useful in the general population,38 it has not been validated in patients with cirrhosis.
As mentioned above, the current diagnostic criteria to differentiate HRS-AKI from ATN-AKI in patients who do not respond to the withdrawal of diuretics and albumin volume expansion (Figure 1) are: (i) no recent use of nephrotoxic drugs, (ii) an absence of haematuria, significant proteinuria or shock and (iii) no changes in the renal ultrasound.28, 10 However, evidence has shown that these criteria cannot exclude parenchymal renal damage in patients with cirrhosis and AKI. First, a histopathological study on renal biopsies in patients with AKI on CKD but without haematuria or significant proteinuria, showed inflammation of the arterial wall and tubular interstitial damage in most patients.39 Second, the use of new urinary biomarkers of renal tubular damage has shown that they are increased in patients with HRS-AKI compared to patients with pre-renal AKI or without AKI.36, 40
Indeed, several new biomarkers have been discovered in past few years that have been shown to be accurate in the differential diagnosis of AKI. Urinary neutrophil gelatinase-associated lipocalin (NGAL) is the most extensively studied and promising biomarker in this field. NGAL is a small protein produced by several cells and organs such as renal tubular cells, leucocytes and hepatocytes. Urinary NGAL was found to be increased in patients with cirrhosis and ATN compared to those with HRS.36, 37, 40. Interestingly, NGAL has been found to be a strong predictor of mortality in patients with acute decompensation of cirrhosis with or without AKI.41 NGAL was found to be increased in the urine of patients with ACLF.41 However, because it is produced by leucocytes, NGAL may be increased in urine following urinary tract infections, thus, urinary NGAL values should be interpreted with caution in these patients.40 Furthermore, a significant overlap in urinary NGAL values was found between patients with ATN-AKI and those with HRS-AKI.36, 40
Other urinary biomarkers such as kidney injury molecule 1, liver fatty acid binding protein and interleukin-18 were found to be significantly increased in patients with ATN compared to those with HRS, however, these were found to be less accurate than NGAL.36, 37 Finally, a combination of several urinary biomarkers significantly increased the diagnostic accuracy.36 Currently, an important subject of investigation in this field is to validate these findings. In the near future, we expect the use of multiple biomarkers to be included in an algorithm for the differential diagnosis of AKI and to help in the differential diagnosis between HRS-AKI and ATN-AKI in patients with cirrhosis.
4 Conclusion
Assessment of renal function is crucial in the evaluation of patients with cirrhosis. It guides diagnostic and therapeutic management as well as helping determine the prognosis of these patients. Evaluation of renal function also optimizes the selection of candidates for SLK transplantation. Conventional tools to measure renal function have several limitations in patients with cirrhosis, both for the measurement of GFR and for the evaluation of parenchymal kidney damage. Promising new biomarkers are good candidates to become part of the standard protocol for the evaluation of renal function following validation in future studies.
Conflicts of Interest
The authors declare they have no conflicts of interest regarding the content of this manuscript.




