Gamma-glutamyltransferase—friend or foe within?
Abstract
Gamma-glutamyltransferase (GGT) is a liver enzyme, which is located on the plasma membranes of most cells and organ tissues, but more commonly in hepatocytes, and is routinely used in clinical practice to help indicate liver injury and as a marker of excessive alcohol consumption. Among the liver enzymes, important advances have especially been made in understanding the physiological functions of GGT. The primary role of GGT is the extracellular catabolism of glutathione, the major thiol antioxidant in mammalian cells, which plays a relevant role in protecting cells against oxidants produced during normal metabolism; GGT, therefore, plays an important role in cellular defence. Beyond its physiological functions, circulating serum GGT has been linked to a remarkable array of chronic conditions and diseases, which include nonalcoholic fatty liver disease, vascular and nonvascular diseases and mortality outcomes. This review summarizes the available epidemiological and genetic evidence for the associations between GGT and these adverse outcomes, the postulated biologic mechanisms underlying these associations, outlines areas of outstanding uncertainty and the implications for clinical practice.
Key points
- Serum elevated GGT is associated with vascular disease outcomes, metabolic syndrome, diabetes, cancer, chronic kidney disease, fractures, dementia and total mortality.
- Biological mechanisms postulated for these relationships include oxidative stress, inflammation and underlying fatty liver.
- Limited data suggest that GGT is unlikely to improve disease risk prediction and data on the causal relevance of GGT to these outcomes are lacking.
- GGT assays may have the potential to aid in the identification of individuals who need further evaluation of risk factors for adverse outcomes.
- Further work is needed to understand the pathophysiological mechanisms that underlie the associations and implications for clinical practice.
Data Sources and Selection Criteria
Relevant prospective cohort studies (with at least 1 year of follow-up) conducted in general populations were sought from MEDLINE, EMBASE and Web of Science, with particular emphasis on systematic reviews and meta-analyses of these study designs. Search terms included “gamma-glutamyltransferase,” “nonalcoholic fatty liver disease,” “cardiovascular disease,” “coronary heart disease,” “diabetes,” “metabolic syndrome,” “hypertension,” “cancer,” “chronic kidney disease,” “fracture,” “dementia” and “mortality.” Studies were limited to those in adults and written in English.
The liver plays a major role in metabolism and has a number of functions including protein synthesis, glycogen storage, lipid metabolism and secretion of acute phase proteins in response to inflammation. Plasma liver biochemistry tests are groups of clinical laboratory blood assays widely used in the clinic to give information about the state of a patient's liver; assays for gamma-glutamyltransferase (GGT), alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALP) are the most commonly used for this purpose. Circulating levels of these enzymes are markers of liver injury, associated with cellular integrity, or with conditions linked to the biliary tract and can be used to measure the severity of hepatic inflammation, cellular injury or obstruction.1 They are commonly used to identify patients with liver diseases and monitor the course and severity of these diseases and the effect of therapies.2 There have been important advances in the understanding of the physiological functions of these liver enzymes, and several epidemiological associations have been reported. Among the liver enzymes, research has largely focused on GGT. Beyond its physiological functions, circulating serum GGT has been linked to several adverse outcomes, which include nonalcoholic fatty liver disease (NAFLD), vascular and nonvascular diseases and mortality outcomes. This review aims to summarize available information on the physiological role of GGT, evidence on the epidemiological and genetic associations between GGT and these outcomes, evidence on the postulated biologic mechanisms underlying these associations, outlines areas of outstanding uncertainty and implications for clinical practice.
1 Occurrence, Physiology and Functions of Gamma-Glutamyltransferase
Gamma-glutamyltransferase, originally called gamma-glutamyl transpeptidase, was first adopted as a liver biochemistry test in the 1960s and 1970s.3 It is a glycoprotein with a molecular weight of 68 000 daltons and consist of two proteins—a larger and smaller chain with molecular weights of 46 000 and 22 000 daltons respectively.1 It is located on the plasma membranes of most cells and organ tissues, but more commonly hepatocytes.3 Gamma-glutamyltransferase is also found in the extracellular fluid attached to α and β lipoproteins4 and albumin carrier molecules.5 It has recently been reported that GGT is made up of four fractions namely big-GGT (b-GGT), medium-GGT (m-GGT), small-GGT (s-GGT) and free-GGT (f-GGT), with each having its own molecular weight and distinct physiochemical properties.6, 7 Human GGT genes are located on chromosome 22,8, 9 with related sequences that are nonfunctional or represent pseudogenes on chromosomes 18, 19 and 20.9 There are seven or more GGT genes in humans, but only one of these gives rise to a complete and functional protein.10 The active GGT enzyme is coded by GGT1 on chromosome 22.11 Gamma-glutamyltransferase activity is significantly genetically determined and its heritability has been estimated to range between 50% and 77% in adults.12-15 It has been suggested that half of the genetic variance in GGT is shared by ALT and AST. Although the same genes influence GGT activity across age and sex, their relative contribution to the variation in its activity differs in males and females and across age.15
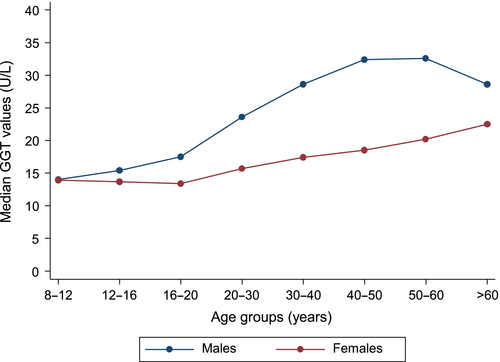
The primary role of GGT is the extracellular catabolism of glutathione, the major thiol antioxidant in mammalian cells, which enables precursor amino acids to be assimilated and re-utilized for intracellular synthesis of glutathione.3 Glutathione plays a relevant role in protecting cells against oxidants produced during normal metabolism. The reaction that GGT catalyzes is the transfer of a glutamyl residue (linked through glutamate's gamma carboxylic acid to an amine or to another amino acid) to an acceptor,3 therefore maintaining adequate levels of glutathione. Gamma-glutamyltransferase is also involved in the transfer of amino acids across cell membranes16 and metabolism of leukotriene.17 Liver injury or blockage of bile ducts can cause accumulation of GGT in the liver and secretion of excess GGT into the circulation. In clinical practice, raised circulating GGT values are routinely and widely used to help indicate potential hepatic or biliary disease and as a biologic marker of excessive alcohol intake.3 A number of demographic and physiological factors, which shade into risk factors for disease, affect GGT values, making the definition of a reference range very complicated.3 Common factors influencing the reference range include sex, pregnancy, childbirth, race, smoking, oral contraceptive use and exercise. The reference range for GGT activity is similar across ages, though there are significant gender differences, with males having higher values than females18 (Fig. 1). The gender difference in GGT activity is most likely physiologic and has been attributed to the effect of sex hormones. The recommended cut-off for the upper normal limit of GGT has been set at an average of 51 U/L for males and 33 U/L for females.19 Common causes of elevated GGT activity include liver disease, obesity, excessive alcohol consumption, medications (such as phenytoin, phenobarbital, furosemide and heparin), congestive cardiac failure and smoking.20, 21 Younger females and pregnancy are associated with decreased GGT activity, while postmenopausal women and those taking oral contraceptives have higher GGT activity closer to that of men.3 Black populations have higher values compared with Caucasian populations. Over the past two decades, significant progress has been made in understanding the physiological functions of GGT and evidence for several epidemiological associations has been uncovered.
2 Epidemiological Associations of Gamma-Glutamyltransferase with Risk Markers for Disease
The multi-functional role of the liver in metabolism and inflammation suggests that complex relationships are likely to exist between the liver markers and several biochemical, metabolic, lipid or inflammatory factors. Although GGT has a high heritability,13-15 its activity is highly variable, with the variability significantly affected by various environmental factors, such as body mass index (BMI), alcohol consumption and age.14 Gamma-glutamyltransferase has been demonstrated to be associated with several lifestyle, biophysical and biochemical factors, majority of which are risk factors for vascular and nonvascular disease. Positive associations with age, BMI, waist circumference, alcohol consumption, smoking, heart rate, blood pressure, serum levels of glucose, ferritin, uric acid and lipids (triglycerides, total cholesterol and low-density lipoprotein [LDL] cholesterol) have been demonstrated,22, 23 whereas inverse associations have been observed with high-density lipoprotein cholesterol, physical activity and lung function.3 Gamma-glutamyltransferase is also known to correlate with several dietary factors, such as coffee consumption, fruit and meat intake, and vitamin status.24 Studies have consistently demonstrated that BMI has the strongest association with GGT compared with other risk markers, with GGT increasing progressively across all classes of BMI.22, 25-27 Indeed, GGT has been shown to have a causal association with BMI, as well as with fasting insulin.28, 29 The strong relationships between GGT and several risk markers have been attributed to common genetic loci that affect GGT activity and these risk markers.13
3 Relationship Between Gamma-Glutamyltransferase and Nonalcoholic Fatty Liver Disease
Nonalcoholic fatty liver disease is a common clinical condition characterized by hepatic steatosis with varying degrees of necroinflammation and fibrosis and which develops in the absence of substantial alcohol intake.30 It spans a range of conditions from simple hepatic steatosis to nonalcoholic steatohepatitis and cirrhosis30, 31 and is regarded as the hepatic component of the metabolic syndrome (MetS).32-34 Nonalcoholic fatty liver disease has reached epidemic proportions, and it is emerging as the most common cause of chronic liver disease in the developed world.30, 35, 36 The global prevalence of NAFLD has been estimated to be around one-third of the general population,37 with estimates varying between 70% and 90% in individuals who are obese or have type 2 diabetes mellitus (T2DM).38-40 Its diagnosis is based on (i) imaging techniques (i.e. ultrasonography, CT scan or MRI) confirming the presence of fat infiltration of the liver and/or liver biopsy showing steatosis in at least 5% of hepatocytes41 and (ii) exclusion of other liver diseases of other etiology such as significant alcohol consumption or drug-induced liver disease, autoimmune or viral hepatitis, and cholestatic or metabolic/genetic liver disease.42 It is the most common cause for unexplained elevated liver enzymes including the transaminases and GGT.43-46 The most commonly observed biochemical pattern in NAFLD is increased levels of transaminases, with ALT levels exceeding that of AST.47 Elevated ALT has frequently been used as a biochemical surrogate for NAFLD diagnosis; however, it is not uncommon to diagnose NAFLD in patients with normal ALT levels using ultrasonography or histology.36, 48 Elevated GGT activity has less frequently been used as a surrogate biomarker for NAFLD.
Obesity is one of the most important factors in the development of NAFLD. Fall et al.29 in their recent Mendelian randomization (MR) study provided novel evidence for a causal effect of adiposity (as measured by BMI) on GGT and ALT, with suggestions that elevated activity of these liver enzymes caused by an increased BMI is likely to be related to NAFLD. Nonalcoholic fatty liver disease is strongly associated with obesity and several cardiometabolic risk factors,38, 39 MetS, T2DM49 and mortality, with cardiovascular disease (CVD) being the most common cause of death among patients with NAFLD.46, 50 Independent associations have also been demonstrated for other adverse outcomes such as heart failure (HF),51 cardiac arrhythmias,52, 53 hepatocellular cancer (HCC),54 chronic kidney disease (CKD)55 and cognitive decline.56 For these reasons, it has been hypothesized that NAFLD may be the underlying cause for the associations of elevated GGT with several vascular and nonvascular outcomes.
4 Prospective Associations of Gamma-Glutamyltransferase with Vascular, Nonvascular and All-Cause Mortality Outcomes
4.1 Cardiovascular disease
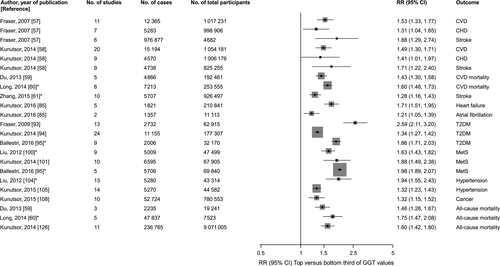
Gamma-glutamyltransferase has been implicated in CVD development with epidemiological evidence suggesting greater CVD risk with higher serum GGT activity. Although interest in GGT as an emerging risk factor for CVD goes back at least several decades, further research was stimulated with the publication of the first systematic review and meta-analysis by Fraser et al.57 (Table 1 and Fig. 2). The study authors reported an independent and positive association between GGT and risk of CVD. However, despite the evidence and the publication of additional studies, there were still uncertainties regarding the shape and nature of the association between GGT and CVD risk. In 2014, our group published an updated meta-analysis comprising 20 studies (1 054 181 participants and 15 194 events) and which included a dose–response analysis. The findings confirmed a positive and independent association between GGT and CVD, which was consistent with a log-linear relationship (Table 1 and Fig. 2).58 Positive and independent associations between GGT and CVD mortality have also been demonstrated.59, 60 Given the somewhat diverse aetiology of different vascular events such as stroke and coronary heart disease (CHD), it was uncertain whether the effect of GGT on these cause-specific vascular outcomes may differ. However, several studies have shown GGT to be positively and independently associated with both stroke and CHD outcomes, with similar magnitudes of effect.57, 58, 61 Given the independent association of higher GGT activity with increased cardiovascular risk, there has been an evolving debate on whether adding information on GGT to current CVD risk prediction algorithms might be associated with improvements in the ability to predict CVD beyond established cardiovascular risk factors.1, 62, 63 Whiles some studies have reported a marginal improvement in CVD risk prediction,64, 65 a recent large-population-based cohort analysis by our group has shown that addition of GGT to conventional CVD risk factors is unlikely to improve prediction of first-ever cardiovascular events.66
| Author, year of publication (reference) | No. of studies | Outcome | No. of participants | No. of cases | Combined risk (95% CI) | Risk comparison reported |
|---|---|---|---|---|---|---|
| Fraser, 2007 57 | 11 | CVD | 1 017 231 | 12 365 | 1.34 (1.22–1.48) | Per 1 U/L change |
| 7 | CHD | 998 906 | 5283 | 1.20 (1.02–1.40) | Per 1 U/L change | |
| 6 | Stroke | 976 877 | 4682 | 1.54 (1.19–1.99) | Per 1 U/L change | |
| Kunutsor, 2014 58 | 20 | CVD | 1 054 181 | 15 194 | 1.23 (1.16–1.29) | Per 1 SD change |
| 9 | CHD | 1 006 176 | 4570 | 1.17 (1.00–1.36) | Per 1 SD change | |
| 9 | Stroke | 825 255 | 4738 | 1.28 (1.10–1.50) | Per 1 SD change | |
| Du, 2013 59 | 5 | CVD mortality | 192 461 | 4866 | 1.52 (1.36–1.70) | Top vs bottom fourth |
| Long, 2014 60 | CVD mortality | 253 555 | 7213 | 1.60 (1.48–1.73) | Highest vs lowest category | |
| Zhang, 2015 61 | 10 | Stroke | 926 497 | 5707 | 1.28 (1.16–1.43) | Highest vs lowest category |
| Kunutsor, 2016 85 | 5 | Heart failure | 210 841 | 1821 | 1.28 (1.20–1.35) | Per 1 SD change in baseline values |
| 1.43 (1.31–1.56) | Per 1 SD change in usual vales | |||||
| Kunutsor, 2016 85 | 2 | Atrial fibrillation | 11 113 | 1357 | 1.09 (1.02–1.16) | Per 1 SD change in baseline values |
| 1.14 (1.03–1.25) | Per 1 SD change in usual values | |||||
| Fraser, 2009 93 | 13 | T2DM | 62 915 | 2732 | 1.92 (1.66–2.21) | Per 1 U/L change |
| Kunutsor, 2014 94 | 24 | T2DM | 177 307 | 11 155 | 1.34 (1.27–1.42) | Top vs bottom third |
| Ballestri, 2016 95 | 9 | T2DM | 2006 | 32 170 | 1.86 (1.71–2.03) | Highest vs lowest category |
| Liu, 2012 100 | 9 | MetS | 47 499 | 5009 | 1.63 (1.43–1.82) | Highest vs lowest category |
| Kunutsor, 2014 101 | 10 | MetS | 67 905 | 6595 | 1.88 (1.49–2.38) | Top vs bottom third |
| Ballestri, 2016 95 | 9 | MetS | 5706 | 69 840 | 1.98 (1.89–2.07) | Highest vs lowest category |
| Liu, 2012 104 | 13 | Hypertension | 43 314 | 5280 | 1.94 (1.55–2.43) | Highest vs lowest category |
| Kunutsor, 2015 105 | 14 | Hypertension | 44 582 | 5270 | 1.32 (1.23–1.43) | Top vs bottom third |
| Kunutsor, 2015 108 | 10 | Cancer | 780 553 | 52 724 | 1.32 (1.15–1.52) | Top vs bottom third |
| Du, 2013 59 | 3 | All-cause mortality | 19 241 | 2235 | 1.56 (1.34–1.83) | Top vs bottom fourth |
| Long, 2014 60 | All-cause mortality | 47 837 | 7523 | 1.75 (1.47–2.08) | Highest vs lowest category | |
| Kunutsor, 2014 126 | 11 | All-cause mortality | 9 071 005 | 236 765 | 1.60 (1.42–1.80) | Top vs bottom third |
- CHD, coronary heart disease; CVD, cardiovascular disease; MetS, metabolic syndrome; SD, standard deviation; T2DM, type 2 diabetes mellitus.
Several mechanistic pathways for the increased cardiovascular risk associated with elevated activity of GGT have been postulated. Though at normal values, GGT counteracts oxidative stress by making cysteine available for regeneration of intracellular glutathione, recent evidence has also demonstrated that serum GGT values within the normal reference range are associated with promotion of atherosclerosis. Pro-oxidant and pro-inflammatory activities have been proposed to underlie this process.67-69 At normal values, serum GGT catalyzes LDL oxidation in vitro; it initially catalyzes the degradation of extracellular glutathione, the major thiol intracellular antioxidant in the body. Glutathione is hydrolyzed by GGT into glutamate and a cysteinyl-glycine dipeptide. Glutamate is subsequently recycled inside the cell, producing additional glutathione. The cysteinyl-glycine dipeptide acts as a strong reducing agent of iron on the cellular membrane and in the extracellular space, resulting in the development of free radical species1 which may cause oxidation of LDL, which is believed to participate in other processes such as cell proliferation and development of atheroma within the vascular endothelial wall.1, 67 Gamma-glutamyltransferase also mediates interconversion of the glutathione-containing inflammatory mediator leukotriene C4 into leukotriene D4.70 It has also been reported that GGT may be directly involved in atheromatous plaque formation.68 Indeed, GGT activity has been found within cerebral, carotid and coronary plaques, co-localizing with oxidized lipids and foam cells.71, 72 Of the four GGT fractions identified, only b-GGT is found within atherosclerotic plaques and has been suggested to be the fraction involved in the pathogenesis of CVD.7 In addition, GGT may be linked to CVD risk via underlying NAFLD, which is a major risk factor for CVD73, 74 and commonly associated with increased serum GGT activity.
The consistent findings of a linear and independent association between GGT and CVD risk are suggestive of causality, but this requires robust evidence from randomized controlled trials. However, trials to enable causal inferences may be unlikely, as several pharmacological agents (such as insulin sensitizers and antioxidants) that modify GGT activity also influence levels of other liver enzymes and lipid factors.75 Mendelian randomization studies of genetic variants specifically related to GGT may provide another route to assess causality.76 There is evidence to suggest that the GGT1 locus, which is the main protein-coding gene for GGT, may be specific for GGT activity,77, 78 and therefore, variants within this locus might be valid instrumental variables for MR studies. Till date, the causal relevance of GGT to CVD has been difficult to assess as these variants explain only a small fraction of the variability in levels of GGT, in addition to their pleiotropic effects on other phenotypic traits.79 Larger Genome Wide Association Studies (GWASs) may help uncover new variants to explain the missing heritability in GGT activity. Next-generation DNA sequencing may also provide an opportunity to identify rare genetic variants which have large effects on GGT activity.
4.2 Heart failure
The first population-based prospective study of GGT activity and HF risk was reported in 2005 by Ruttman et al.20 This study comprising of 163 944 participants and 162 HF cases, reported a positive and independent association between GGT and HF risk. Three more prospective studies80-82 were published after this study. To provide a better indication of the relevance of GGT to HF, given the small number of HF cases included in previous studies, our group has recently conducted a detailed assessment of the association of GGT activity with risk of HF using a prospective cohort of 1780 men and also performed a pooled analysis of available published prospective evidence on the association in one comprehensive analysis. Given that GGT exhibits high within-person variability which has been recently reported,83 we also corrected for “regression dilution bias.”84 Our meta-analysis of five studies indicated that a two-fold increase in usual GGT values was associated with approximately 40% higher risk of HF (Table 1 and Fig. 2).85 Whether GGT has the potential to be used in the identification of individuals at high risk of HF is yet to be elucidated. Dhingra et al.80 reported a marginal improvement in HF risk prediction on addition of GGT to a standard risk engine.
Postulated pathways underlying the relationship between elevated GGT values and increased HF risk include the pro-oxidant and pro-inflammatory properties of GGT,67 as well as its direct involvement in atheromatous plaque formation.68 Other pathways implicated include underlying fatty liver67 (which is associated with low-grade inflammation, insulin resistance and oxidative stress86, 87), endothelial dysfunction and exposure to environmental pollutants.88, 89
4.3 Cardiac arrhythmias
Until recently, there was uncertainty as to whether GGT was associated with cardiac arrhythmias. Alonso et al.90 in the Atherosclerosis Risk in Communities (ARIC) study reported a positive, linear and independent association between GGT activity and risk of atrial fibrillation (AF). Our group has also recently shown a positive log-linear association of GGT with risk of AF in age-adjusted analysis, but which was attenuated on further adjustment for conventional risk factors.85 In the same study, we also demonstrated a positive log-linear association between GGT and ventricular arrhythmias in analyses adjusted for established vascular risk factors, but the association was attenuated on further adjustment for other potential confounders. Putative biological mechanisms accounting for the associations include oxidative and inflammatory pathways,67 direct involvement of GGT in atheromatous plaque formation,68 fatty liver,67 endothelial dysfunction and exposure to environmental pollutants.88, 89 Given the limited evidence available, further large-scale prospective studies are warranted to assess the associations and to evaluate whether measurement of GGT activity can usefully contribute to risk prediction algorithms for cardiac arrhythmias.
4.4 Sudden cardiac death
In analyses of the Kuopio Ischemic Heart Disease (KIHD) prospective cohort study of 1780 men aged 42–61 years that recorded 136 sudden cardiac deaths (SCDs) during 22 years of follow-up, we have shown for the first time that GGT is positively, log-linearly and independently associated with future risk of SCD.83 The association was not importantly modified under different circumstances (such as age, smoking status or different levels of established vascular risk factors). As postulated previously, the pro-inflammatory activities of GGT67 and its direct involvement in atheromatous plaque formation68, 72 may underlie the association. Nonalcoholic fatty liver disease, which is associated with cardiac autonomic dysfunction (a risk factor for SCD),91 may also be mediating the association. Whether GGT has any clinical use in improving SCD risk prediction is yet to be investigated.
4.5 Type 2 diabetes mellitus
The first prospective study to examine the association between GGT and incident T2DM was reported in 1998.92 Since then, several studies have evaluated the associations between GGT and T2DM risk, but reported apparently conflicting results. To put the data into context and provide a better indication of the relevance of GGT to T2DM risk, Fraser et al.93 in a meta-analysis of 13 prospective studies showed a positive and independent association between GGT and T2DM risk (Table 1 and Fig. 2). Given the uncertainty regarding the shape of the GGT-T2DM association, our group conducted an updated meta-analysis which included 24 prospective cohort studies and showed that GGT contributes to an increased risk of T2DM in a nonlinear dose–response pattern.94 A graded increase in T2DM risk was evident at GGT levels 9–35 U/L, with the effects of GGT seeming to level off beyond 35 U/L. Ballestri et al.95 in a meta-analysis of nine studies evaluating the prospective association between NAFLD (as diagnosed by elevated GGT activity) and T2DM demonstrated an almost two-fold increase in the risk of incident T2DM. Gamma-glutamyltransferase has been postulated to be linked to the development of T2DM via oxidative stress, increased inflammation and underlying fatty liver (NAFLD), which are major pathways in the pathophysiology of T2DM.95-97 The causal relevance of GGT to T2DM is still yet to be investigated, and current evidence suggests that serum GGT provides little incremental benefit for prediction of T2DM risk.98, 99
4.6 Metabolic syndrome
In a review of nine prospective cohort studies, Liu et al.100 reported a positive and independent association between GGT and the MetS (Table 1 and Fig. 2). In an updated meta-analysis of 10 studies, we showed a nonlinear relationship (albeit using limited published data) to the positive association which was evident within normal reference values of GGT.101 In pooled analysis of five studies, Ballestri et al.95 reported NAFLD to be associated with a two-fold increase in the risk of incident T2DM, when elevated GGT activity was used as an indicator of NAFLD. Mechanistic pathways underlying the relationship between GGT and the MetS have been linked to similar processes suggested for GGT and T2DM; which include oxidative stress, increased inflammation and excessive deposition of fat in the liver, all of which are implicated in impaired insulin signalling and insulin resistance.32, 102 The relationships between GGT activity and the MetS as well as its components have been attributed to genetic origins. Loomba et al.103 using a twin study design reported genetic covariance between GGT and MetS traits, such as insulin resistance, increased triglycerides and blood pressure. The adrenergic locus ADRB2 was also shown to have pleiotropic effects on both circulating GGT and triglycerides.
4.7 Hypertension
In a meta-analysis of 13 prospective cohort studies, Liu et al.104 reported a positive association between GGT activity and hypertension risk (Table 1 and Fig. 2). In an updated meta-analysis of 14 studies, we showed an approximately 30% increased risk of future hypertension when comparing individuals in the top vs bottom thirds of circulating GGT values, and this was consistent with a linear dose–response relationship.105 Elevated GGT activity has been suggested to signify states of oxidative stress, increased inflammation and fatty liver, consequently leading to impaired insulin secretion and insulin resistance, all of which have been implicated in the development of hypertension.106, 107
4.8 Cancer
Gamma-glutamyltransferase has also been linked to the risk of cancer. Long et al.60 in a review of available prospective epidemiological data suggested a positive association between GGT and cancer-related mortality. However, a pooled analysis was not conducted which precluded assessment of the magnitude of the association. In a meta-analyses of 10 cohort studies involving 780 553 participants and 52 724 cancer events, we have shown a positive association between GGT and overall cancer outcomes and which was also consistent with a log-linear relationship108 (Table 1 and Fig. 2). Significant positive associations for site-specific cancers, such as breast cancer, cancers of male genital organs, cancers of digestive organs and liver cancer, were also reported in this study. In a subgroup analyses, the associations were consistent across several study characteristics including incident cancers and cancer mortality. In a pooled analysis of two population-based cohorts comprising a total of 107 058 participants, Preyer et al.109 reported a significantly higher risk of breast cancer comparing the top vs bottom quartile of GGT values. Whether GGT has a direct aetiological role in carcinogenesis or just a risk marker of an underlying aetiology is uncertain. It has, however, been postulated that the persistent production of reactive oxygen species (ROS) from GGT-mediated metabolism may contribute to tumour progression, as low levels of ROS have been suggested to modulate a range of biological responses involved in cellular growth, proliferation and apoptosis.110, 111 Gamma-glutamyltransferase activity has also been considered to confer rapid turnover and survival advantages for cancer cells.112 A number of experimental studies have also suggested a direct causative role of GGT in carcinogenesis.110, 113 The association between GGT and liver cancers may also reflect underlying NAFLD, which itself is an important risk factor for HCC.54
4.9 Chronic kidney disease
In the first prospective evaluation of GGT and CKD, Ryu et al. employed a large cohort of 10 337 nonhypertensive and nondiabetic Korean male workers with normal kidney function at baseline, and demonstrated increased GGT activity to be significantly associated with an increased risk of future CKD in a nonlinear fashion.114 The association remained consistent on adjustment for a comprehensive panel of confounders and mediators. The authors speculated several mechanistic pathways to underlie the association between GGT and CKD; some of which include alcohol consumption, liver disease, obesity, insulin resistance and low-grade inflammation. However, since all these factors were accounted for in their multivariate analyses, the authors reported that it was highly unlikely that these pathways were involved in the pathophysiology. Based on the broad body of evidence demonstrating GGT as a marker of oxidative stress, it was suggested that the association of GGT with risk of CKD might be due to mechanisms related to oxidative stress.69 It has been reported that renal ROS cause vasoconstriction of renal vasculature, leading to sodium retention and subsequently renal damage.115, 116 Targher et al.55 in a recent study reviewed evidence on the link between NAFLD and CKD. It was postulated that the origins for this relationship were via pathways such as atherogenic dyslipidemia, insulin resistance, dysglycemia, and the release of pro-inflammatory, pro-coagulant and pro-fibrogenic factors, which cause kidney damage. Given the strong relationship between NAFLD and GGT activity, NAFLD may mediate the association between GGT and CKD. Because of the limited evidence on the GGT–CKD relationship, further studies are needed to replicate this association especially among female populations.
4.10 Fractures
Gamma-glutamyltransferase has been demonstrated to have harmful effects on bone metabolism in in vitro studies and animal models.117, 118 In the first epidemiological data involving humans, a large prospective study involving 16 036 Korean men with an average follow-up of 3 years demonstrated a higher serum GGT activity to be independently associated with an increased risk of osteoporotic fractures. Etiopathogenic pathways suggested to underlie the association between GGT and risk of fractures include oxidative stress, inflammation and a direct pathogenic role of GGT in metabolic bone diseases. Oxidative stress has been shown to have adverse effects on bone metabolism.119, 120 An in vitro study has shown GGT to induce the formation of osteoclasts, independent of its enzymatic activity, via stimulation of the receptor activator of nuclear factor-kappaB ligand expression.117 An animal study has also shown that GGT overexpression accelerates bone resorption and causes osteoporosis.118
4.11 Dementia
GGT has recently been shown to be positively, log-linearly and independently associated with future risk of dementia in a population-based cohort of 2415 apparently healthy men with good cognitive function at baseline from eastern Finland.121 This association remained robust in several sensitivity analyses. Since mechanistic research provides strong support for inflammatory and oxidative processes in the etiogenesis of dementia,122-124 it was proposed that GGT might contribute to the development of dementia via its pro-inflammatory and pro-oxidant properties.67 In addition, since NAFLD has been linked to the pathogenesis of cognitive impairment via insulin resistance,125 there is a possibility that underlying NAFLD may be a link between the observed association. Being the only longitudinal study so far to report on this association, further research is needed to replicate these findings and help unravel the mechanistic pathways of GGT in the pathogenesis of dementia.
4.12 All-cause mortality
Finally, GGT has also been linked to the risk of all-cause mortality, an outcome which has been suggested to be a more ultimate indicator of health than cause-specific outcomes.126 In a meta-analysis of three prospective cohort studies, Du et al.59 showed a positive association of GGT with all-cause mortality comparing the highest vs lowest GGT quartile (Table 1 and Fig. 2). Long et al.60 in their review also reported a positive association between GGT and all-cause mortality. In the most recent review, pooled analysis of 11 prospective cohorts (comprising over 9 million participants and 236 765 all-cause mortality outcomes) also showed a positive association between GGT and all-cause mortality which was consistent with a linear dose–response relationship.127 Increased mortality risk associated with GGT has been suggested to be mediated by increased cardiovascular risk via pathways reported above.
5 Management of Asymptomatic Individuals with Isolated Raised Gamma-Glutamyltransferase Values
As there are established published clinical guidelines for the management of elevated circulating levels of liver enzymes including GGT, this section is not intended as a comprehensive management guide for elevated GGT. In light of the current overall evidence, there is a possibility that GGT may only be a risk marker for these adverse outcomes. It, therefore, appears that the clinical implications for these findings are elusive. However, despite the limited role of GGT measurements in disease risk prediction and absence of data showing any causal relevance of GGT to these adverse outcomes, the preceding observations may be translated into clinical improvements. Assays for GGT may have the potential to aid in the identification of individuals at moderate to high risk of these adverse outcomes. Gamma-glutamyltransferase assays are sensitive, well standardized, simple, inexpensive and commonly measured as part of routine liver biochemistry panels. Though GGT is not very specific for the liver, mild and subtle elevations in GGT values below the upper limits of normal are very common in the general population and may indicate the presence of subclinical liver disease. Patients with isolated elevated values (even within normal reference ranges) of GGT should be considered for further evaluation. Factors that are associated with elevated GGT (such as increased BMI, alcohol consumption, smoking and medication use) as discussed in a previous section, should be assessed for in the individual. Nonalcoholic fatty liver disease should also be suspected and screened for, especially in those with increased body weight and associated factors, though NAFLD may uncommonly be a cause of isolated raised GGT activity. Patients diagnosed with or suspected of having NAFLD should be screened for T2DM, given their close inter-relationship. Many patients with increased GGT activity will also have risk factors for vascular disease and other chronic diseases, which should be assessed, and such patients should have their disease risk assessed using established risk engines. Patients identified to have factors (e.g. NAFLD, excessive smoking and alcohol consumption, obesity) accounting for elevated GGT values should be provided with lifestyle advice on healthy eating, physical activity, weight loss, smoking cessation and reduction in alcohol intake. Several studies75, 128, 129 have found evidence to support a substantial lowering effect of a variety of interventions (including lifestyle-related factors, such as sustained weight loss,129 physical activity and dietary factors128) on circulating GGT activity. On implementation of these lifestyle changes, patients should have repeat liver biochemical tests in a few months for reassessment by the clinician. Patients with persistently elevated values not amenable to lifestyle advice should be considered for specialist referral.
6 Conclusion
Gamma-glutamyltransferase, a liver enzyme, which plays an important role in cellular defence and protection of cells against further oxidative stress, is linked to a remarkable array of chronic diseases as well as all-cause mortality outcomes. It has been consistently demonstrated that increased GGT activity (sometimes within normal reference values) is positively associated with each of the outcomes reviewed, with majority of these associations consistent with graded relationships. Plentiful putative mechanistic pathways underlying these associations have been proposed, but many of these are hypothetical and are not well understood. Undoubtedly, increased GGT activity is associated with adverse levels of classical vascular risk factors. However, although a large and broadly consistent body of evidence has established GGT as moderately to strongly linked to the development of these vascular and nonvascular outcomes, its role in the causal pathways for these outcomes is uncertain and current evidence (albeit limited) suggests that serum GGT is unlikely to improve disease risk prediction beyond established risk factors. Further work, however, is crucially needed to understand the pathophysiological mechanisms that underlie the associations between GGT and these adverse outcomes and to establish any causal relevance to the associations and whether these could be translated into clinical benefits.
Financial Support
None.
Conflicts of Interest
The author does not have any disclosures to report.
Abbreviations
-
- AF
-
- atrial fibrillation
-
- ALP
-
- alkaline phosphatase
-
- ALT
-
- alanine aminotransferase
-
- ARIC
-
- Atherosclerosis Risk in Communities
-
- AST
-
- aspartate aminotransferase
-
- BMI
-
- body mass index
-
- CHD
-
- coronary heart disease
-
- CI
-
- confidence interval
-
- CKD
-
- chronic kidney disease
-
- CVD
-
- cardiovascular disease
-
- GGT
-
- gamma-glutamyltransferase
-
- GWAS
-
- Genome Wide Association Study
-
- HCC
-
- hepatocellular carcinoma
-
- HF
-
- heart failure
-
- KIHD
-
- Kuopio Ischemic Heart Disease
-
- LDL
-
- low-density lipoprotein
-
- MetS
-
- metabolic syndrome
-
- MR
-
- Mendelian randomization
-
- NAFLD
-
- nonalcoholic fatty liver disease
-
- SCD
-
- sudden cardiac death
-
- SD
-
- standard deviation
-
- T2DM
-
- type 2 diabetes mellitus




