Iron and the liver
Abstract
Humans have evolved to retain iron in the body and are exposed to a high risk of iron overload and iron-related toxicity. Excess iron in the blood, in the absence of increased erythropoietic needs, can saturate the buffering capacity of serum transferrin and result in non-transferrin-bound highly reactive forms of iron that can cause damage, as well as promote fibrogenesis and carcinogenesis in the parenchymatous organs. A number of hereditary or acquired diseases are associated with systemic or local iron deposition or iron misdistribution in organs or cells. Two of these, the HFE- and non-HFE hemochromatosis syndromes represent the paradigms of genetic iron overload. They share common clinical features and the same pathogenic basis, in particular, a lack of synthesis or activity of hepcidin, the iron hormone. Before hepcidin was discovered, the liver was simply regarded as the main site of iron storage and, as such, the main target of iron toxicity. Now, as the main source of hepcidin, it appears that the loss of the hepcidin-producing liver mass or genetic and acquired factors that repress hepcidin synthesis in the liver may also lead to iron overload. Usually, there is low-grade excess iron which, through oxidative stress, is sufficient to worsen the course of the underlying liver disease or other chronic diseases that are apparently unrelated to iron, such as chronic metabolic and cardiovascular diseases. In the future, modulation of hepcidin synthesis and activity or hepcidin hormone-replacing strategies may become therapeutic options to cure iron-related disorders.
Abbreviations
-
- cAMP
-
- cyclic adenosine monophosphate
-
- DMT1
-
- divalent metal transporter 1
-
- ER
-
- endoplasmic reticulum
-
- FD
-
- ferroportin disease
-
- FPN
-
- ferroportin
-
- H2O2
-
- hydrogen peroxide
-
- HAMP
-
- hepcidin
-
- HCC
-
- hepatocellular carcinoma
-
- HC
-
- hemochromatosis
-
- HJV
-
- hemojuvelin
-
- HO
-
- hydroxyl radical
-
- LPI
-
- labile plasma iron
-
- NAFLD
-
- non-alcoholic fatty liver disease
-
- NH
-
- neonatal hemochromatosis
-
- PCT
-
- porphyria cutanea tarda
-
- PNPLA3
-
- patatin-like phospholipase domain containing-3 gene
-
- SF
-
- serum ferritin
-
- TfR2
-
- transferrin receptor 2
-
- TIBC
-
- total iron-binding capacity
-
- TS
-
- transferrin saturation
-
- UROD
-
- uroporphyrinogen decarboxylase
Key points
- The liver as the main source of hepcidin, the iron hormone is the central regulator of iron homeostasis and the main storage site of iron.
- Genetic loss of hepcidin or hepatic proteins involved in hepcidin expression, such as HFE, cause hereditary hemochromatosis, a paradigmatic multiorgan iron overload disease.
- Acquired loss of hepcidin-producing liver mass or disease factors that impair the capacity of the liver to produce hepcidin may cause iron overload and damage the liver itself or the vascular system and peripheral organs.
- Manipulating hepcidin synthesis or hepcidin hormone-replacing strategies may represent a future aetiologic cure of iron-related disorders.
Iron is a vital micronutrient. Its principal role in mammalian homeostasis is the oxygen transport system of haemoglobin. However, iron is also crucial for many biochemical reactions including DNA synthesis, oxidative phosphorylation and host defense. Total body iron content ranges from 3 to 5 g, mostly in haemoglobin or as iron storage in the liver. Iron stores vary widely: hepatic iron can range from 300 mg in a menstruated female to 1 g in an adult male, but can reach 25–30 g in a patient with hemochromatosis. This is because iron excretion is very limited, making it difficult for the organism to get rid of excess except by bleeding. The tendency to retain iron in the body exposes humans to a substantial risk of iron overload and iron-driven toxicity. As it is the main iron depot of the body, the liver is regarded as the main site of iron toxicity during iron overload 1. However, the recent discovery that the liver is the main source of the iron hormone, hepcidin, has shed new light on its central role both in the regulation of body iron homeostasis and in the pathogenesis of numerous human diseases apparently unrelated to iron.
The liver as target of iron toxicity
Excess iron is common to a number of human diseases (Table 1). Some are known to be hereditary, while in others the genetic and hereditary basis has not been confirmed. A large percentage reflect systemic iron overload (e.g. hemochromatosis, post-transfusion siderosis, etc.), while others result in regional or local iron accumulation because of necro-inflammatory or other disease processes (e.g. chronic liver diseases). Finally, an increasingly recognized class of iron disorders is because of disrupted intracellular (e.g. Friedreich ataxia) or body iron traffic (e.g. anaemia of chronic diseases) leading to iron misdistribution despite normal total body iron content (Table 1).
| Disorder/Cause | |
|---|---|
| Iron overload | Iron misdistribution |
| Hereditary | |
| Hereditary hemochromatosis (HFE-TfR2-, HJV-, HAMP-, FPN-related) | X-linked sideroblastic anaemias |
| Ferroportin disease | Friedreich ataxia |
| Aceruloplasminemia | Neuroferritinopathy |
| Atransferrinemia | |
| DMT-1 deficiency | |
| H-ferritin related iron overload | |
| Hereditary iron-loading anaemias with inefficient erythropoiesis | |
| Acquired | |
| Oral | |
| Parenteral | Anaemia of chronic diseases (ACD) |
| Post-transfusion | |
| Chronic liver diseases (viral- and alcohol-related; NASH) | |
| Neurodegenerative disorders | |
| Miscellaneous | |
| Porphyria cutanea tarda | |
| African siderosis | |
| Alloimmune (neonatal) hemochromatosis | |
Whatever the cause, an unregulated increase in iron in the blood, in the absence of increased erythropoietic needs, saturates the buffering capacity of serum transferrin so that non-transferrin-bound and highly reactive forms of iron appear (labile plasma iron, LPI). These pro-oxidant forms of iron are eventually diverted towards the parenchymal cells of the liver, where they may fuel oxidative damage 2. In fact excess iron in solution with oxygen can generate free radical formation via Fenton and Haber–Weiss chemistry, with hydrogen peroxide (H2O2), being changed into its noxious hydroxyl radical (HO.). This leads to consequent damage to DNA, proteins and membranes. The iron-driven damage of hepatocytes can result in paracrine induction of hepatic stellate cells and portal myofibroblasts through lipid-peroxidation by-products leading to increased collagen deposition, fibrosis and long-term micronodular cirrhosis and hepatocellular carcinoma HCC 3.
Hereditary hepatic iron overload
HFE and non-HFE hereditary hemochromatosis
Hemochromatosis (HC), or hereditary hemochromatosis was historically considered to be a unique clinico-pathological entity that was probably because of a single gene defect. In fact, in 1996, a single gene polymorphism (the 845G–A polymorphism in HFE that results in Cys282YTyr, C282Y, change in HFE protein) was found in patients with hemochromatosis 4. However, as genetic testing for HFE became more widespread, and nearly 20% of HC patients were found to lack the pathogenic C282Y change, it became clear that the situation was more complicated. Other iron genes were reported whose mutations were associated with hereditary iron overload syndromes with the phenotypic features of classic HC 5 (Table 1). Today, the term ‘hereditary hemochromatosis’ embraces all forms of hereditary hemochromatosis associated with the common HFE C282Y polymorphism and, thus far, with pathogenic mutations of the transferrin receptor 2 (TfR2), hemojuvelin (HJV), hepcidin (HAMP) and ferroportin (FPN). Despite their genetic diversity, all known hemochromatoses belong to the same clinicopathological entity, as they all originate from failure to prevent unneeded iron from entering the circulatory pool because of a hepcidin deficiency (see section on hepcidin). Depending on the gene involved and its role in hepcidin regulation, the phenotype of HC varies, ranging from the severe HJV- and HAMP juvenile forms, to relatively milder adult-onset TfR2- and FPN- phenotypes.
HFE-hemochromatosis
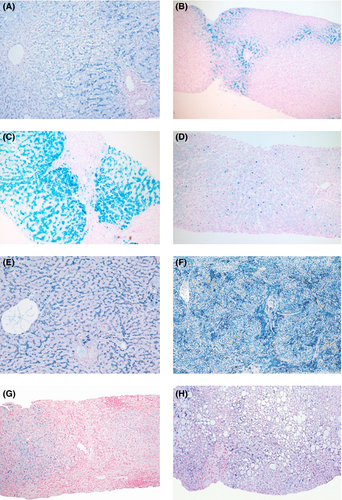
Excess hepatic iron has been reported in almost half the women and two-thirds of men with HFE C282Y homozygotes 6 (Fig. 1). Increased transaminases are present at diagnosis in 24–32% of C282Y homozygotes identified by screening, fibrosis in 30–42% of men and 2.7–4.0% of women, and cirrhosis in 4.4–11.8% of men and up to 2.7% of women 5. Clinical expression is low in HFE-HC 7 and the disease only progresses in a minority of untreated patients. As many as 38–50% of patients who are homozygous for HFE C282Y will develop iron overload and 10–33% will eventually develop hemochromatosis-associated morbidity 8, 9. Penetrance is usually higher in men than in women, probably because of menstruation/pregnancy and because of the inhibitory effect of androgens on hepcidin expression 10. Alcohol abuse is probably an important host ‘modifier’ associated with hemochromatosis-related cirrhosis, while combined mutations in HAMP, HJV and TfR2 have been associated with more severe phenotypes, but patients with these combinations are rare 5. Novel loci affecting iron homeostasis in individuals at risk of hemochromatosis have recently been reported, some including known iron-related genes and other novel genes 11, 12. An increased risk of advanced fibrosis has been associated with the patatin-like phospholipase domain containing- 3 gene (PNPLA3 13) I148M polymorphism and the rs236918 variant in the proprotein convertase subtilisin/kexin type 7 (PCSK7) gene 14.
Non-HFE-hemochromatosis
Patients with TfR2-HC mimic HFE-hereditary HC, but the age range is somewhat younger. Liver pathology includes early iron deposition in periportal hepatocytes, while progression to cirrhosis has been also reported (Fig. 1).
Most reported cases of FPN-HC include patients with clinical manifestations identical to HFE- HC such as high transferrin saturation (TS) and serum ferritin (SF) levels, predominant hepatic parenchymal iron overload and cirrhosis and organ failure in advanced cases.
Juvenile –HC (usually because of HJV mutations) is markedly different from HFE-HC for age, with an almost equal ratio between the sexes and a greater frequency of cardiac and endocrine disturbances. The hepatic complications of iron overload in juvenile HC may seem not as common as in hereditary HC but this may simply be because the clinical picture is dominated by endocrine and cardiac failure. In fact, liver disease may be profound, with histologically diagnosed cirrhosis in up to 40% of younger patients. However, the clinical diagnosis of J-HC is often incidental relating to investigation of endocrine or cardiac abnormalities including cardiac shock.
Hereditary non-hemochromatotic syndromes
The ferroportin disease
Unlike HFE- and non-HFE-HC, ferroportin disease (FD) is an autosomal dominant iron overload disorder due to a lack-of-function mutation of the hepcidin receptor, ferroportin. It was clinically identified in 1999 15, and associated with an FPN mutation in 2001 16. The disorder is due to a loss in iron export function of FPN: the resulting reduction in iron efflux causes a bottleneck in macrophages, which generate the largest iron flows, resulting in iron accumulation in Kupffer cells and macrophages with high ferritin levels and low-to-normal TS until late in the disease, when TS also increases. The low-to-normal TS despite high SF is the biochemical hallmark of the disease and, along with the early and preferential accumulation of iron in hepatic Kupffer cells (Fig. 1) is central in the diagnostic work-up of the disorder 17. Clinical expression is milder than in classic HC and the associated liver disease is usually not as severe, although late complications including liver cancer have been reported 18.
Other rare hereditary iron-loading disorders
Severe hepatic iron overload can also be caused by the hereditary disorders aceruloplasminemia 19 and hypo- or atransferrinemia 20. Both are extremely rare and can easily be distinguished from hemochromatosis by their clinical features: aceruloplasminemia causes neuro-logical manifestations (progressively severe extrapyramidal signs, cerebellar ataxia, dementia) and hypo- or atransferrinemia causes life-threatening anaemia. Despite massive iron overload, fibrosis or cirrhosis is uncommon.
Autosomal recessive mutations of divalent metal transporter 1 (DMT1), the protein at the apical membrane of the duodenal enterocyte that transports iron, have recently been reported 21. Most patients with DMT1 deficiency present with severe hypochromic microcytic anaemia at birth, increased TS with normal total iron-binding capacity (TIBC), slightly elevated SF and marked hepatic iron overload.
Within the spectrum of hereditary anaemias, variable iron overload in the liver may be identified not only due to transfusional iron but also to primary iron metabolism abnormalities (Fig. 1). In pre-transfusional thalassaemias and other hereditary anaemias, ineffective erythropoietic induces excess iron absorption via inhibition of hepcidin synthesis and leads to hepatic iron overload similar to HC. In X-linked sideroblastic anaemia the primary pathogenic event is excess mitochondrial iron deposition due to mutations of delta-aminolevulinic acid synthetase 2. In Friedreich ataxia, 22 an autosomal recessive, degenerative disease that involves the central and peripheral nervous systems and the heart, a defect in iron-sulphur cluster assembly seemingly interferes with iron export from the mitochondria.
Acquired hepatic iron overload
Enteral and parenteral iron overload
Iron overload be a result of excessive iron introduction through the enteral or parenteral routes (Table 1). As discussed, long-term blood transfusions for hereditary anaemia or various causes of bone marrow failure (e.g. aplastic anaemia, myelodysplastic syndrome etc.) may cause a clinically apparent iron-loading disorder. The excess iron in all these cases is derived from senescent red blood cells. Therefore, it will mainly accumulate in Kupffer cells and macrophages (Fig. 1) and it is usually associated with some architectural disturbance in the liver, whereas endocrine glands and the heart are the most frequent targets of toxicity and failure. Unlike HC, iron chelators are the mainstay of treatment in post-transfusion iron overload.
Chronic liver diseases and liver failure
Common chronic liver diseases including viral hepatitis, alcoholic liver disease and non-alcoholic fatty liver disease (NAFLD) may be associated with hepatocellular, sinusoidal or mixed pattern iron-loading. This is usually low grade, but may contribute to the progression of the underlying disease to cirrhosis and HCC through iron-driven oxidative stress 23, 24 (Fig. 1). The cause of iron deposition is multifactorial and is influenced by age, diet, race, HFE status and environmental factors. Interestingly, recent studies have shown that a number of pathogenic factors responsible for the underlying liver disease may directly affect hepcidin expression and, consequently, modify iron distribution and accumulation in the liver (see below) 25. This is particularly true when considering the loss of the hepcidin-producing liver mass associated with end-stage liver disease or acute/subacute liver failure.
Miscellaneous causes of hepatic iron overload
A number of hereditary (clearly identified or suspected) and acquired human diseases are characterized by iron accumulation in the liver.
Porphyria cutanea tarda
Porphyria cutanea tarda (PCT), is caused by a deficiency in uroporphyrinogen decarboxylase activity (UROD): in the sporadic subtype (75% of cases) UROD activity is only deficient in the liver, while the family subtype (25% of cases), an autosomal dominant, early onset disorder affecting both sexes, the defect leads to a constitutive 50% UROD deficiency in erythrocytes as well 26. The risk factors that contribute to inactivation or inhibition of this enzyme and enhance clinical expression are mainly alcohol abuse, estrogens, hepatitis C virus and to a lesser extent human immunodeficiency virus infection and inheritance of one or more HFE genotypes. Symptoms develop when residual, hepatic UROD decreases below a threshold of about 25%. Clinical features include photosensitive skin lesions, hepatic accumulation and urinary excretion of uroporphyrins, altered iron indices and hepatic iron overload 27.
African siderosis
African iron overload, formerly called ‘Bantu siderosis’ is still an important disease in rural areas where up to 15% of adult males may be affected 28 (Fig. 1). Originally, iron overload was attributed to the consumption of large quantities of traditional beer prepared in iron pots. Later, a genetic modifier was postulated and a polymorphism of the ferroportin gene (p.Gln248His) restricted to Africans and African-Americans have been considered a candidate 29. Whatever the predisposing genetic trait, alcoholic beverages are main factors leading to iron overload and liver disease in the South African rural adult population.
Alloimmune (neonatal) hemochromatosis
Neonatal hemochromatosis (NH) is a severe neonatal disease characterized by stillbirth or neonatal liver failure in the antenatal or early neonatal period, usually within hours to days after birth 30. Intrauterine growth retardation and, often, associated placental oedema and either oligohydramnios or polyhydramnios are common. The prevailing presentation is jaundice with coagulopathy, hypoglycaemia and hypoalbuminemia and high SF. Iron overload typically involves the liver, pancreas and heart but spares the spleen. Various theories have been suggested to explain the aetiology of NH, but recently an alloimmune mechanism for the disease has been proposed 31. In fact, high-dose intravenous immunoglobulin therapy (IVIG) to the mother appears to significantly increase survival of newborns.
The liver as cause of iron retention or iron overload through hepcidin
Hepcidin the iron hormone
Hepcidin, the iron hormone, acts by binding ferroportin, the sole iron importer in mammals and triggering its internalization and degradation in lysosomes 32. This leads to cessation of iron transfer from the intestines and storing macrophages towards the bloodstream: therefore, induction of hepcidin leads to hypoferremia due iron retention/trapping in macrophages, while loss of hepcidin causes high serum iron and tissue iron overload.
Hepatic iron trapping/retention because of hepcidin induction
During chronic infectious and inflammatory states and cancer, prolonged induction of hepcidin can result in iron accumulation in hepatic and spleen macrophages, iron-restricted erythropoiesis and anaemia, a syndrome known for years as inflammation anaemia or chronic disease anaemia 33. Seemingly, chronic systemic and intrahepatic inflammation accompanying the course of liver disease can induce hepcidin and favour iron accumulation in sinusoidal cells. Endoplasmic reticulum (ER) stress involved in a number of pathophysiological states, including the inflammatory response, nutrient disorders and viral infection, can induce hepcidin through the cyclic adenosine monophosphate (cAMP) response element binding protein 3–like 3, CREB3L3 (also known as CREBH), and cause iron retention in vivo 34. Hepcidin is also regulated by PPARGC1A, a transcriptional co-activator that controls genes involved in energy metabolism, including gluconeogenic genes 35. In human disorders associated with food excess and storage, such as type 2 diabetes, obesity and the metabolic syndrome, where different iron abnormalities have been reported and iron removal has been found to be beneficial, persistently activated gluconeogenesis may result in overstimulation of hepcidin, iron accumulation and potential damage. Seemingly, in NAFLD with unexplained hepatic iron excess, characterized by high serum ferritin levels with normal or subnormal transferrin saturation may be encountered 36, hyperhepcidinemia because of insulin resistance and activated gluconeogenesis may represent a concomitant cause for iron abnormalities.
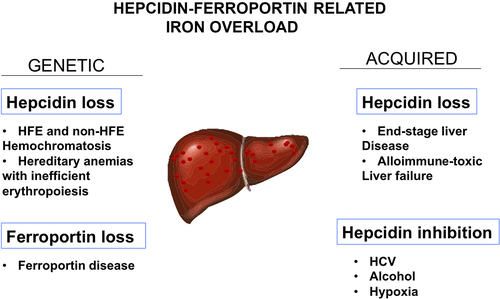
Hepatic iron overload because of hepcidin loss
Genetic partial or total loss of hepcidin is responsible for various forms of hereditary hemochromatosis (Fig. 2). Interestingly, a number of factors and mediators in chronic liver diseases have recently been found to inhibit hepcidin transcription. It was recently reported that hypoxia results in a significant decrease in hepcidin serum levels and elevated concentrations of platelet derived growth factor (PDGF)-BB by downregulating the CREBH expression 37. Hepatic oxidative stress can suppress hepcidin production after alcohol abuse 38, 39 or in chronic viral hepatitis 40, 41. Epidermal and hepatocyte growth factors (EGF and HGF), which contribute to liver regeneration after injury, also suppress hepcidin, apparently through a direct effect of HGF and EGF on the BMP/SMAD signalling pathway 42 (Fig. 2). The contribution of these pathways to physiological or disease-associated regulation of hepcidin has not yet been clarified. Finally, gonadal hormones have been recently reported to affect hepcidin transcription. In particular, testosterone can suppress serum hepcidin 10, most likely through inhibition of the BMP pathway 43. Finally, liver-specific miRNA, miR-122 44 and miRNA miR-130a 45 modify iron status by targeting genes that are central for iron homeostasis, including hepcidin.
Systemic iron overload due to hepcidin loss
Based on the model for the genetic causes of hepcidin loss, it is also clear that any non-genetic cause that chronically and consistently prevents hepcidin synthesis/activity will also lead to hemochromatosis 5. This is true for the massive liver iron overload associated with end-stage liver disease which resembles hemochromatosis and which is due to the loss of the hepcidin-producing liver mass 46 (Fig. 2). Seemingly, it is anticipated that circulatory iron overload associated with acute or subacute liver failure because of toxic or immune mediated insults may also result in hepcidin insufficiency and iron overload (UNCLEAR NO VERB IN THIS SENTENCE). One example is the dramatic neonatal hemochromatosis syndrome associated with alloimmune gestational liver disease 31 in which massive iron overload may be due to a loss of hepcidin function. In all these acquired conditions, the uncontrolled increase in NTBI (Non-transferrin-bound iron) in the vascular system with a strong tendency to induce reactive oxygen species and oxidant damage hits cells and organs, in particular those with a high rate of reactive oxygen species production, because of robust mitochondrial energy activities, and fewer antioxidants (endocrine glands and myocardium). Interestingly, in a recent study, serum iron and ferritin levels were markedly elevated and hepcidin levels were lower in patients with acute-on-chronic liver failure and multiorgan failure: TS was higher and correlated with poor outcome 47.
The selection of genetic traits in the general population that predispose to a high-iron phenotype (low hepcidin expressers) may directly determine the tendency for increased serum iron and enhanced redox-state within the vascular compartment (or in peripheral organs) 25. This may be especially important in the course of chronic metabolic or cardiovascular diseases associated with low-grade oxidant stress and inflammation. In fact, in the general population, body iron stores have been positively associated with the risk of type-2 diabetes and related mortality 48, 49. Serum iron status is strongly associated with low plasma adiponectin levels and adipocyte insulin resistance in subjects who are at an increased risk of type-2 diabetes and cardiovascular diseases 50. Iron stores seem to be associated with disease progression, plaque formation/instability and atherosclerosis in patients with cardiovascular diseases 51.
As the main producer of hepcidin, the iron hormone and the main iron depot in the body, the liver plays a central role in the regulation of human iron homeostasis. Yet, a number of liver diseases may be primarily or co-factorially linked to iron-driven toxicity when the buffer iron-storing and antioxidant capacity of the organ is overcome. In addition, when genetic and non-genetic factors impair the capacity of the liver to produce hepcidin, excess iron may be present in the liver itself or in the vascular system and peripheral organs. In the long run, this negatively influences the course of underlying liver disease through oxidative stress or other chronic diseases such as metabolic and cardiovascular disorders apparently unrelated to iron. We do not have tools to directly decrease excess serum iron or to block pro-oxidant forms of iron in the bloodstream or cells 25. Manipulating hepcidin synthesis activity or hepcidin hormone-replacing strategies may be an effective future option to cure iron-related disorders.
Acknowledgements
Financial support: This study was supported by Programma RARER-Regione Università 2010–2012.
Conflict of interest: The authors do not have any disclosures to report.