Second-wave IFN-based triple therapy for HCV genotype 1 infection: simeprevir, faldaprevir and sofosbuvir
Abstract
With the approval of second-wave direct-acting antivirals simeprevir, sofosbuvir and faldaprevir in 2014–2015, for genotype 1 hepatitis C, patients and doctors will have more treatment options. During a first period, these treatments will still be used with peginterferon and ribavirin. The second wave of IFN-based triple therapy will have benefits and risk. These treatments have the following advantages: higher efficacy with more patient candidates for a shorten treatment duration (12–24 weeks, instead of 48 weeks). These new treatments appear to have a better safety profile than first generation, with no additional increase in anaemia over peginterferon/ribavirin. Then, these treatments are to take for patients with a decrease in pill burden (these three direct-acting antivirals are given orally one pill a day). Simeprevir and sofosbuvir may be approved in the US and Europe, in 2014, at the time this manuscript will be released. Approval of faldaprevir will follow. These direct-acting antivirals with many others will hopefully be combined in future interferon-free regimens. The goal of this review to summarize the results and safety of simeprevir, faldaprevir and sofosbuvir, to advise physicians and to inform patients on the benefits and risks of these second-wave IFN-based regimens for HCV genotype infection.
Abbreviations
-
- AEs
-
- adverse events
-
- DAA
-
- direct-acting antivirals
-
- IFN
-
- interferon
-
- PEG-IFN
-
- pegylated interferon
-
- RBV
-
- ribavirin
-
- RVR
-
- rapid virological response
-
- SVR
-
- sustained virological response
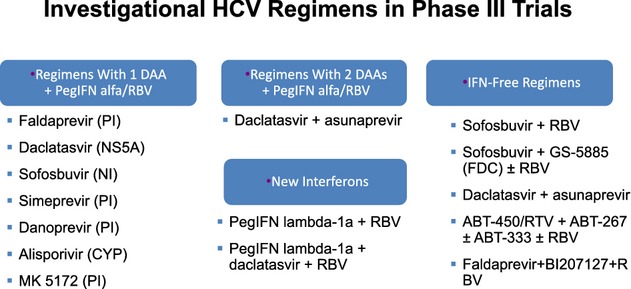
The goal of treatment of chronic hepatitis C is to obtain a sustained virological response (SVR) defined as undetectable HCV RNA in serum 24 weeks after the end of treatment. SVR results in the eradication of HCV infection and improvement of the histological outcome 1. Twelve-week post-treatment follow-up appears to be as relevant as 24 weeks to define SVR 2. Potentially, each step of the viral cycle is a target for drug development. All the HCV enzymes – NS2-3 and NS3-4A proteases, NS3 helicase, NS5A replication complex and NS5B RdRp – are essential for HCV replication, and are potential drug discovery targets. In 2011, two direct-acting antivirals (DAAs) were approved for HCV genotype 1 chronic infection, telaprevir and boceprevir and opened a new area for HCV therapy. These two first-generation NS3/4 protease inhibitors (PI), given in combination with pegylated interferon (PEG-IFN) and ribavirin (RBV), opened a window for the development of several DAAs. Since then, several DAA with different viral targets, including NS3 protease inhibitors, nucleoside/nucleotide analogue and non-nucleoside inhibitors of the RNA-dependent RNA polymerase, and NS5A inhibitors are under development (Table 1) 3.
During a first period, starting from early next year with the arrival of second-wave DAAs simeprevir, sofosbuvir and faldaprevir, we will continue to use PEG-IFN plus RBV. In a second period, treatment strategies that combine several drugs with different mechanisms of action could hopefully result in IFN- and/or RBV-sparing regimens. The goal of this review is to summaries the results and safety profile of the second wave of IFN-based triple therapy for HCV genotype 1 infection.
Simeprevir
Genotype 1 naïve patients (Quest-1 and Quest-2 studies)
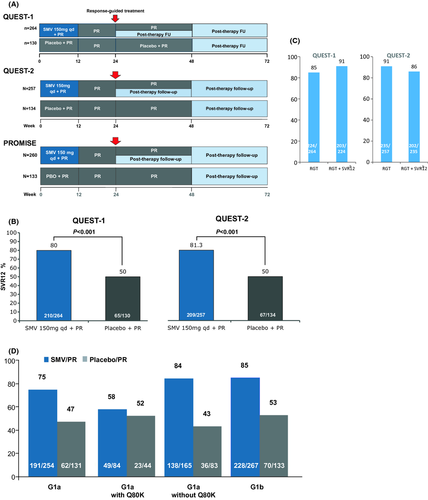
Two phase III randomized, double-blind, placebo-controlled clinical trials (Quest 1 and Quest 2) in GT1-naïve patients were reported 4-6. Patients in the treatment groups were given simeprevir 150 mg daily for 12 weeks plus peginterferon and ribavirin (PR) for 12 weeks, followed by PR only for either 12 or 36 weeks based on the individual's virological response to therapy (Fig. 1A). Patients in the control groups were given placebo for 12 weeks combined with PR for 48 weeks.
Efficacy
Efficacy data from Quest-1 and Quest-2 are reported in Figure 1B. These two studies were pooled because they were nearly identical in design; pooled results showed an SVR 12 rate of 80% in the treatment group and 50% in the control group. SVR12 rates were significantly higher in the simeprevir arm compared with the placebo arm in all other subgroup analyses. SVR rates were lower in patients with bridging fibrosis and cirrhosis.
In Quest-1, 85% of patients treated with simeprevir met the RGT criteria and were eligible for 24 weeks of treatment, among whom 91% achieved SVR12. In Quest-2, 91.4% of patients met RGT criteria and were eligible for 24 weeks of treatment, among whom 86% achieved SVR12 (Fig. 1C).
In the pooled trials, the differences in SVR12 rates in GT1a patients with the Q80K polymorphism were not statistically significant between the treatment (58%) and control (55%) groups (Fig. 1D). In those without the Q80K polymorphism, the SVR12 rates were 84% in the treatment group vs 43% in the control group for the two pooled trials.
HCV G1 relapsers to prior PEG-IFN/RBV (Promise study)
In the Promise trial, patients had received 24 weeks or more of a PEG-IFN-based treatment and had relapsed within 1 year after the last medication dose (Fig. 1A) 7, 8.
Efficacy
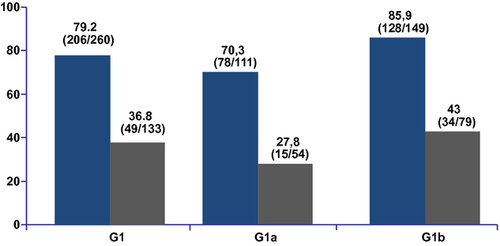
The SVR12 rate was 79% in the treatment group and 36% in the control group. Among patients treated with simeprevir, 92.7% were eligible to complete therapy at 24 weeks and achieved an SVR12 of 83% (Fig. 2). The SVR12 rates for those with the Q80K polymorphism were 47% in the treatment group and 30% in the control group. In those without the Q80K polymorphism, the SVR12 rates were 78% in the treatment group vs 24% in the control group for the relapser trial.
Safety
A total of four deaths occurred in the treatment groups, and they were judged to be unrelated to treatment. In the pooled analysis, 2% of those in the simeprevir group had serious adverse events, vs 3% of those in the control group during the initial 12 weeks (Table 2). A total of three patients (0.4%) in the simeprevir group had significant adverse events, which were determined to be related to simeprevir by the study investigator; one patient experienced major depression and two patients experienced photosensitivity reactions.
| QUEST-1 | QUEST-2 | PROMISE | ||||
|---|---|---|---|---|---|---|
| Placebo + PR, % (N = 130) | SMV + PR, % (N = 264) | Placebo + PR, % (N = 134) | SMV + PR, % (N = 257) | Placebo + PR, % (N = 130) | SMV + PR, % (N = 264) | |
| Serious AEs | 4 | 3 | 2 | 2 | 4 | 3 |
| Discontinuation because of AEs | 3 | 3 | 1 | 2 | 3 | 3 |
| Fatigue | 38 | 40 | 39 | 35 | ||
| Pruritus | 11 | 21 | 15 | 19 | 28 | 28 |
| Rash (any type) | 25 | 27 | 11 | 24 | 23 | 23 |
| Anaemia | 11 | 16 | 14 | 16 | 20 | 17 |
Other common adverse events were rash [218 (28%) treatment groups; 79 (20%) control groups], influenza-like illness [203 (26%) treatment groups; 84 (21%) control groups], pruritus [168 (22%) treatment groups; 58 (15%) control groups] and nausea [173 (22%) treatment groups; 70 (18%) control groups].
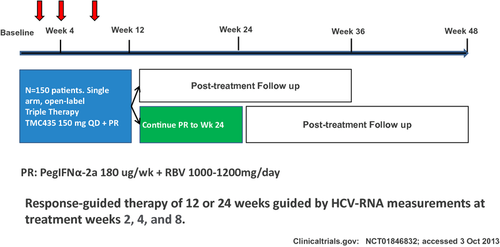
Interestingly, enrolment has been completed rapidly for an ongoing phase III, open-label, single-arm study to evaluate the safety and efficacy of simeprevir plus PEG-IFN alfa-2a and RBV administered for 12 weeks in treatment-naïve subjects with chronic genotype 1 HCV infection (EudraCT number: 2012-004905-29) (Fig. 3).
Faldaprevir
Genotype 1-naïve patients (STARTVerso™1&2)
Faldaprevir is a protease inhibitor in association with PEG-IFN plus RBV 9, 10, but also being investigated in an IFN-free regimen 11.
Two multicentre, randomized, double-blind, placebo-controlled phase III studies (N = 1314) had a similar design (summarized in Fig. 4A) 12, 13. In arms 2 and 3, patients achieving early treatment success (ETS) stopped all treatment at week 24. FDV plus PEG-IFN/RBv increased SVR12 compared with PR alone (Fig. 4B and C). SVR12 rates were lower in patients from North America than in patients from other regions (Fig. 4D).
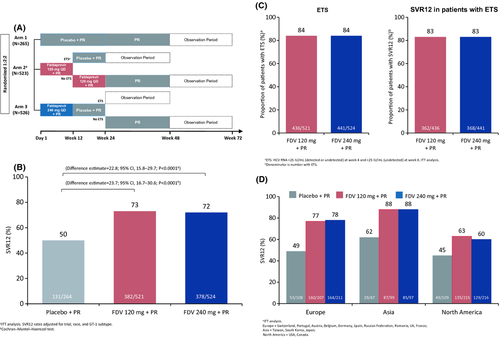
Most differences in SVR in the STARTVerso1 and STARTVerso2 trials are explained by baseline characteristics: reasons for virological failure are similar when adjusting for different factors impacting response (race, HCV genotype, IL28B genotype, HCV RNA level, GGT level and presence of cirrhosis). However, there was a higher discontinuation rate for reasons other than virological failure in North America, indicating different AE management and treatment discontinuation, which impacted the overall response. FDV efficacy was similar at 120 and 240 mg doses and with 12 or 24 weeks of treatment.
Among FDV-treated patients, 84% achieved ETS and were eligible to stop all treatments at week 24. In patients with an ETS, SVR12 was achieved by: 83% overall; 88% of patients who received 12 weeks of FDV (120 or 240 mg) and a total of 24 weeks of PR., FDV safety profile is shown in Table 3. Finally, the addition of FDV to PR was efficacious in treatment-naïve patients infected with HCV GT-1. FDV plus PR showed increases in SVR regardless of GT1 sub-type, IL28B genotype, liver disease stage and other factors associated with response to PR. The treatment regimen has the potential to improve tolerability and convenience compared with first-generation protease inhibitors.
| Placebo + PR (N = 264) | FDV 120 mg + PR (N = 521) | FDV 240 mg + PR (N = 524) | |
|---|---|---|---|
| Any AE, n (%) | 255 (97) | 511 (98) | 514 (98) |
| AEs leading to discontinuation of all medication, n (%) | 10 (4) | 27 (5) | 40 (8) |
| AEs leading to discontinuation of FDV or placebo only, n (%) | 1 (<1) | 6 (1) | 14 (3) |
| Serious AEs, n (%) | 16 (6) | 39 (7) | 43 (8) |
| AEs of at least moderate intensity (any)a, n (%) | 156 (59) | 302 (58) | 332 (63) |
| Rash | 11 (4) | 39 (7) | 50 (10) |
| Photosensitivity | 0 | 0 | 3 (1) |
| GI | 19 (7) | 58 (11) | 96 (18) |
| Anaemia | 38 (14) | 73 (14) | 70 (13) |
| Bilirubin associated | 2 (1) | 18 (3) | 47 (9) |
- One patient with cirrhosis at baseline developed acute-on-chronic liver failure after 16 days of FDV (24 0 mg) and PR, discontinued all treatment and died 12 days later. The event was considered not related to FDV, but to pegylated interferon by the investigator.
- a DAIDS grades 2–4; protocol-defined AEs of special interest.
- DAIDS, Division of AIDS table for grading the severity of adult and paediatric adverse events; DC, discontinuation; GI, gastrointestinal.
Treatment-experienced patients with G1 HCV chronic infection (START-Verso3)
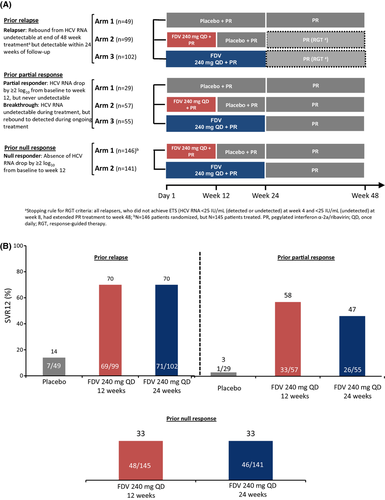
Design of STARTVerso3, a phase III trial assessing the efficacy and safety of FDV (240 mg QD) plus PR in treatment-experienced patients with chronic HCV GT-1 infection is presented in Figure 5A 14. It is a multicentre, randomized, double-blind, placebo-controlled phase III trial (N = 678). FDV 240 mg plus PR was effective in treatment-experienced patients with HCV GT-1 infection (Fig. 5B). The majority (87%) of prior relapsers receiving FDV achieved ETS and were eligible to stop treatment at week 24. The low SVR12 rates in the placebo groups (14% prior relapsers; 3% prior partial responders) reflect the difficult-to-treat population enrolled. Comparison with other DAA studies is limited by the early futility rule (>2 log10 decrease in HCV RNA at week 4). The low SVR12 rate in patients on placebo who met the futility criteria indicates that even in its absence, the SVR12 rates in the placebo arms would have been lower than previously reported rates. No additional benefit was observed by treating patients with FDV 240 mg for 24 weeks vs 12 weeks. Virological breakthrough was higher in prior null responders with HCV GT-1a compared with GT-1b. Q80K polymorphism did not affect GT-1a SVR12 following FDV treatment. FDV 240 mg + PR was well tolerated with a safety profile similar to PR alone. Lower rates of hyperbilirubinaemia were observed with a lower dose of 120 mg FDV in the STARTVerso1 and two studies. Finally, FDV plus PR demonstrated a significant and clinically meaningful improvement in SVR12 rates over PR. These results suggest that FDV plus PR provides an effective and well-tolerated treatment regimen in previously difficult-to-treat, treatment-experienced patients infected with HCV GT-1.
HIV-HCV co-infected patients (STARTVerso 4)
FDV was highly efficacious and well tolerated in difficult-to-treat patients co-infected with HIV and HCV GT-1 15. FDV resulted in a total SVR4 rate of 74% in all patients. A high proportion of patients (80%) achieved ETS and 88% of these patients achieved SVR4. Response rates were comparable across FDV doses and durations and among patients who received either 24 or 48 weeks of PR. The safety profile of FDV in HIV and HCV GT-1 co-infected patients was similar to that observed in HCV GT-1 mono-infected patients.
Sofosbuvir
Sofosbuvir with PEG-IFN/RBV for HCV G1 infection
For G1, two strategies have been developed: (i) 12 weeks sofosbuvir, PEG-IFN/RBV and (ii) new IFN-free regimens with sofosbuvir and ledipasvir (NS5A inhibitor).
The NEUTRINO trial was a single-group, open-label phase III study of sofosbuvir plus PEG-IFN/RBV in 327 naïve patients infected with HCV genotypes 1, 4, 5 or 6 16. Most of the patients who were included in the study had HCV genotype 1 (89%); 9% had genotype 4 and 2% had genotypes 5 or 6. All patients received sofosbuvir, PEG-IFN/RBV for 12 weeks. The trial design is represented in Figure 6A and the results in Figure 6B. Sofosbuvir was given orally at a dose of 400 mg, once a day, along with RBV, also given orally in a dose based on weight. Patients who weighed less than 75 kg received 1000 mg/d, and heavier patients received 1200 mg/d. Patients received PEG-IFN alfa-2a subcutaneously (180 μg/week).
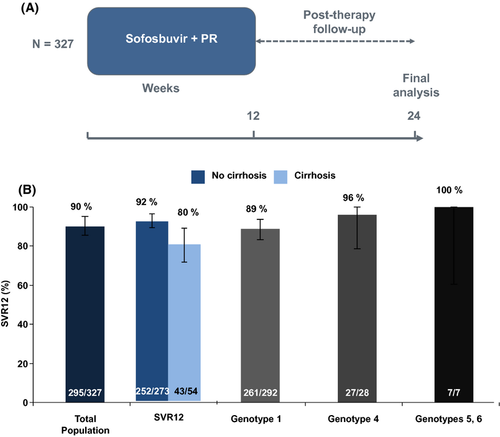
A total of 295 of the 327 patients (90%) had an SVR12. According to the HCV genotype: 89% for patients with HCV genotype 1 (92% for G1a and 82% for G1b) and 96% (27/28) for those with G4 had SVR. The single patient with G5 and all six patients with G6 in this trial had an SVR.
Treatment discontinuation because of adverse events was uncommon among patients receiving sofosbuvir regimens, with rates of 2%. The most common adverse events in all study groups were fatigue, headache, nausea and insomnia (Table 4).
| Sofosbuvir + PR, % (N = 327) | |
|---|---|
| SAEs | 1 |
| Discontinuation because of AEs | 2 |
| Fatigue | 59 |
| Anaemia | 21 |
| Rash (any) | 18 |
| Pruritus | 17 |
| Neutropenia | 17 |
Other PEG-IFN-based regimens
Danoprevir, daclatasvir, asunaprevir, MK-5172
The combination of danoprevir, PEG-IFN and RBV leads to high rates of SVR in patients with HCV genotype 1 infection, but high doses of danoprevir can lead to grade 4 increases in alanine aminotransferase 17. Studies of lower doses of danoprevir with ritonavir, to reduce overall danoprevir exposure while maintaining potent antiviral activity, are underway. Daclatasvir (a potent NS5A replication complex inhibitor) seems to increase the antiviral potency of peginterferon and ribavirin 18. Asunaprevir (PI) has also been studied in association with PEG-IFN and RBV 19. Forthermore, high SVR were observed with MK-5172 with PEG-IFN and ribovirin (20).
Conclusion
With the approval of second-wave direct-acting antivirals simeprevir, sofosbuvir and faldaprevir in 2014-15 for genotype 1 hepatitis C, patients and doctors will have more treatment options. During a first period, the treatments will still be used with PEG-IFN and RBV. The second wave of IFN-based triple therapy will have benefits and risk. These treatments have the following advantages: higher efficacy with more patient candidates for a shortened treatment duration (12–24 weeks instead of 48 weeks). These new treatments appear to have a better safety profile than the first generation, with no additional increase in anaemia over peginterferon/ribavirine. An advantages is also a decrease in pill burden (these three direct-acting antivirals are given orally one pill a day).
Access to therapy is not equal worldwide, and we will still need PEG-IFN and RBV, and first-generation PI, until these second-wave IFN-based regimens become available. Unfortunately, we have to recall that a major medical need is HCV genotype 4-infected patients, and that the current standard of care is PEG-IFN and RBV for 48 weeks with an SVR near 50% 21. Mechanisms associated with fibrosis progression and IFN-response remain major issues 22.
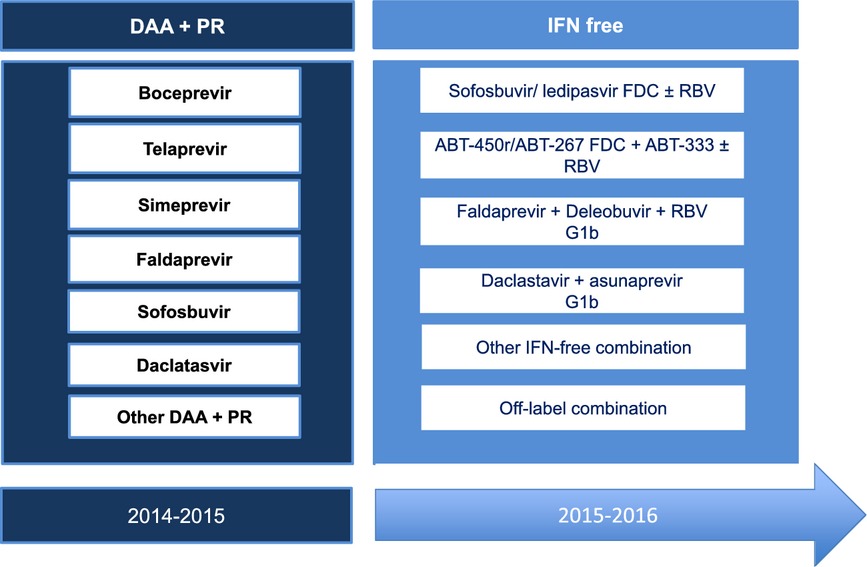
Fortunately, in the near future, we will benefit from an IFN-free regimen with several ongoing regimens (Table 5) 3, 11, 23, 24. Off-label combination may be useful for difficult-to-cure patients (cirrhosis null responders), and promising data have been already reported as the Cosmos study with the combination of simeprevir and sofosbuvir 25.
Disclosure
Tarik Asselah is a speaker and investigator for BMS, Boehringer-Ingelheim, Janssen, Gilead, Roche and MSD. Patrick Marcellin is a speaker and investigator for BMS, Boehringer-Ingelheim, Janssen, Gilead, Roche and MSD.