A synthesis on troglobitic springtails in Europe
Contributing authors: Javier I. Arbea ([email protected]); Robert S. Vargovitsh ([email protected]); Shalva Barjadze ([email protected])
Abstract
enThis paper provides an overview on troglobitic springtails found in European caves, including a checklist at species level. The paper also reviews what is currently known about Collembola, which occur in caves of the most important mountain ranges in Europe. Only troglobitic species were included since many troglophiles were of uncertain ecological status. A total of 338 troglobitic species of Collembola is recorded from European caves, distributed across 12 families. Spain and France appear to host the highest richness of species, including endemics. From a biogeographic perspective, troglobitic species are unevenly distributed in Europe, especially in the most important mountain ranges, like the Alps, the Carpathians, the Pyrenees, the Caucasus, and other European mountains. Troglobitic springtails are far more abundant in temperate zones than in the tropics. Despite this, several genera of Collembola appear to be well represented, while some are poorly represented (or lacking) in European caves. Many advances in knowledge of subterranean springtails have been made, particularly in the description of new species. However, there are still major gaps in the knowledge of the biology, environmental requirements, and impacts on subterranean fauna. This paper highlights the need for further research and provides baseline data for such efforts.
Resumen
esEn este trabajo se proporciona una visión general de los colémbolos troglobios encontrados en las cuevas europeas, incluyendo un listado a nivel de especie. También se revisa lo que hasta ahora se conoce sobre los colémbolos que se encuentran en las cuevas de las cadenas montañosas más importantes de Europa. Se incluyen solamente las especies troglobias ya que el estado ecológico de muchos troglófilos es dudoso. Se registran 338 especies troglobias de Collembola en cuevas europeas, distribuidas en 12 familias. España y Francia parecen albergar la mayor riqueza de especies, incluidas las endémicas. Desde una perspectiva biogeográfica, las especies troglobias se distribuyen de forma desigual en Europa, especialmente en las cadenas montañosas más importantes, como los Alpes, los Cárpatos, los Pirineos, el Cáucaso y otras montañas europeas. Los colémbolos troglobios son mucho más abundantes en las zonas templadas que en los trópicos. A pesar de esto, varios géneros de Collembola parecen estar bien representados, mientras que otros están poco representados (o no existen) en las cuevas europeas. Últimamente se han realizado muchos avances en el conocimiento de los colémbolos subterráneos, particularmente en la descripción de nuevas especies. Sin embargo, aún existen importantes lagunas sobre su biología, los requisitos ambientales y los impactos sobre la fauna subterránea. Este trabajo destaca la necesidad de realizar más investigaciones y aporta datos de referencia en este sentido.
1 INTRODUCTION
Karst and caves are extremely valuable natural environments, hosting a wide variety of organisms (Pipan & Culver, 2013). Unlike surface habitats, deep caves are completely devoid of sunlight, with neither photosynthesis nor plant growth, and have constant temperatures and a limited food supply (Culver & Pipan, 2009). Biologists have long been fascinated by the peculiarities of typical subterranean organisms (e.g., Darwin, 1859; Jeannel, 1943; Racovitza, 1907), especially troglobite animals (terrestrial troglobites and aquatic stygobites), as they can serve as models for understanding endemism and its causes (Christman et al., 2005). Cave invertebrates are classically categorized as troglobites, troglophiles, and trogloxenes. We used the classification of Sket (2008): troglobitic are species or population, strictly bound to a hypogean habitat. Troglophiles are organisms which are able to maintain stable subterranean populations (eutroglophile) or are inclined to inhabit subterranean habitats, being, however, intimately associated with epigean habitats for some biological functions (subtroglophile). Trogloxene is an epigean species occurring accidentally underground. Our review is limited to troglobitic animals that are the most interesting in terms of evolution, geographic distribution, and endemism.
Apart from being very abundant in almost all terrestrial ecosystems, from polar regions to tropical rainforests and deserts, Collembola (springtails) are highly diversified in subterranean habitats across the world. They generally have small ranges (Gibert & Deharveng, 2002), and endemism at all scales is high. Small ranges are often related to limited dispersal abilities, due to morphological and physiological adaptations of species (Christiansen, 1961). Along with general troglomorphism widespread across cave-adapted taxa (larger body size, reduced eyes and pigment, elongated appendages, and claws), specific modifications appear in cave Collembola: basal shift and regression of inner and lateral teeth on claws; the elongation of claw; the regression of tibiotarsal tenent hair (being pointed instead of capitate); the elongation of the mucro and mucronal tooth; higher number of specialized chaetae (sensilla); and hypertrophy of some sensilla on antennae (Christiansen, 2012; Deharveng, 1988; Deharveng & Bedos, 2018). The isolation and distinctiveness of each individual cave ecosystem, in combination with the locally evolved endemic species, make caves important habitats for research in evolutionary adaptations (Barr, 1968; Christiansen & Bullion, 1978; Culver & Wilkens, 2000). Because this, caves are considered as a potential model system in ecological, biogeographical, and evolutionary research (Sánchez-Fernández et al., 2018).
The first checklist of European cave arthropods was prepared by Bedel and Simon (1875), followed by Hamann's “Europäische Höhlenfauna” (1896), who published an extensive monograph with descriptions of animal species from European caves. In the second half of the 19th century, Massoud and Thibaud (1977) published the first essay of European “cavernicolous” Collembola with 187 species as troglobitic from a total of 1,192 species found the Europe. The number of known Collembola species has increased rapidly since then. Recently, in a synthesis chapter, Lukić (2019) has given 583 troglobitic and cave-restricted guanobiotic species of Collembola, which are known worldwide. The number of species found in caves is often strongly correlated with the number of sampling occasions (Culver et al., 2004).
Sampling efforts in cave springtails continue to be considerably uneven in European countries compared to other regions. Troglobites are far more abundant in temperate zones than in the tropics (Barr, 1968; Deharveng & Bedos, 2012). In contrast, the tropics are known to have the highest biodiversity on Earth for surface ecosystems. Howarth (1973) was the first to challenge the view that cave-adapted species were absent or exceptional in the tropics after the discovery of a rich troglobitic fauna in the lava tube fauna of Hawaii. Later, Christiansen and Bellinger (1992) described the first highly troglomorphic Collembola, Coecobrya nupa from Hawaii. Troglobitic Collembola are increasingly reported from the tropics. In South-East Asia, a single species of Neanuridae (Coecoloba plumleyi Deharveng, 1983) is found, while all others are Entomobryidae from various genera: Coecobrya Yosii, 1956, Cyphoderopsis Carpenter, 1917, Lepidonella Yosii, 1960, Pseudosinella Schäffer, 1897, Sinella Brook, 1882, and Troglopedetes Absolon, 1907 (Deharveng, 1987; Deharveng & Bedos, 2000, 2012; Deharveng & Gers, 1993; Deharveng et al., 2018; Jantarit et al., 2013, 2016; Nilsai et al., 2017). The recent discovery of the most troglomorphic Collembola in South-East Asia (Jantarit et al., 2019) is new compelling evidence that morphological modifications linked to cave life are often as strong in the lowland tropics than in temperate regions.
There are unquestionably a number of widely scattered troglobites of Collembola in tropical and subtropical areas (Deharveng & Bedos, 2000; Gnaspini & Trajano, 1994; Lukić, 2019), but even here, the species diversity does not approach that of temperate areas. Jeannel (1965) attributed the high occurrence of terrestrial troglobites in temperate zones to the “anisothermic” conditions, where cold, wet periods alternate with hot, dry periods, and the progenitors of troglobites survived only in cave refugia as relicts (Christian & Spötl, 2010; Kováč et al., 2016). In contrast, in the tropics an "isothermic" regime (soils with mean annual soil temperatures of 15–22°C with no prevails freezing conditions) that has persisted there for 2.58 Ma of years during the Pleistocene glaciation. Nevertheless, many troglobitic species are relicts, with their presumed ancestors being extinct or surviving only in areas geographically remote from the cave region (Barr, 1968). Deharveng and Bedos (2012) pointed out that the extensive gaps in biogeographic and taxonomic knowledge still hamper our global understanding of tropical cave biodiversity.
A general view of world subterranean invertebrates is found in the most comprehensive contribution of Deharveng and Bedos (2018). Moreover, while state-of-art synthesis for some invertebrate subterranean groups has been published in recent years (e.g., ants: Pape, 2016; nematodes: Du Preez et al., 2017 and spiders: Mammola et al., 2018), overviews on most subterranean animals are still missing.
Our review on the current state of cave Collembola revealed that Europe had been better studied compared with other continents. We provide a comprehensive checklist of European subterranean species of springtails, and aim to synthesize and review the scientific knowledge on the geographic distribution of Collembola living in subterranean habitats across Europe. The checklist will represent a useful baseline for advances in biogeographic studies, for application to environmental protection policy planning, and for conservation of cave biodiversity.
2 HISTORICAL BACKGROUND
The description of the first troglobitic springtail, Tritomurus scutellatus living in a cave of Slovenia was published by Frauenfeld (1854). Kolenati (1858a, 1858b), Müller (1859) and Wankel (1860) investigated cave fauna in Moravia, Czech Republic, while Absolon (1900, 1901) described genera Mesachorutes and Verhoeffiella from Moravian caves, as well as the other species from Slovenia (Absolon, 1907). His collection “Biospeleologica Balcanica” came from 193 localities and contains over 5,000 specimens of Collembola. Some of these species were described later (Absolon & Kseneman, 1932; Kseneman, 1938). The history of Belgian research on cave-dwelling Collembola began with Willem (1902), who described several species collected in the caves of Han and Rochefort. The first troglobitic springtail species described from the Crimean Peninsula (Syundyurlyu Cave) was Oncopodura hamata (Carl & Lebedinsky, 1905), while Plutomurus baschkiricus was the first species described from the Ural Mountains (Skorikow, 1900).
These early insights into the subterranean habits of springtails were followed by several descriptions of cave-dwelling species across Europe in the 20th century: Austria (Stach, 1946b; Strouhal, 1939, 1940); France (Delamare-Deboutteville, 1948; Denis, 1923, 1924a, 1924b, 1935a, 1935b, 1938); Portugal (Delamare-Deboutteville, 1944, 1946); Spain (Bonet, 1928, 1929; Bonet, 1931a); Italy (Bonet, 1931b; Stach, 1934, 1946a; Denis, 1938; Tarsia in Curio, 1941). Guéorguiev (1977) produced a modern census of the terrestrial, troglobitic fauna of the whole Balkan Peninsula, which also included an extensive biogeographical analysis. Some species of Collembola have been described from hypogenic caves, which are chemoautotrophic and, because of this energy source, harbor a very diverse fauna. Such caves include Peştera Movile in Romania (Gruia, 1989), one of the biodiversity richest caves in the world (Culver & Pipan, 2013) and Frasassi cave in Italy (Fanciulli, 1999).
Other important contributions on taxonomy of troglobitic Collembola species were made by Gruia (17 species in 1965–2003), Deharveng (22 species in 1978–2018), and Jordana (47 species in 1983–2020). A significant leap forward in the number of described European springtail species were due to the Swiss collembologist Gisin (1950–1972), who described numerous cave-adapted species (57 species), especially in the genus Pseudosinella and Deuteraphorura (see full list of references in the Table S1). A historical review of Hungarian cave springtails is given in Dányi (2011).
Intensive investigation of the cave-dwelling springtails from the Caucasus Mountains began in the 1970s. Nineteen troglobitic taxa were described from Georgian caves, two species from Azerbaijani caves and a single one from North Ossetia (Babenko, 1987; Barjadze et al., 2015, 2019; Djanaschvili, 1971; Vargovitsh, 2012, 2013, 2017, 2019). Information about cave-recorded Collembola from Georgia is provided in Barjadze et al. (2015), while for the Caucasian cave species, it is given in Turbanov et al. (2016). Subterranean biota of European Russia, including those of the northwestern Caucasus, southern Ural Mountains are summarized in Golovatch et al. (2018).
A very important contribution to the knowledge of world subterranean biodiversity, including Collembola is Encyclopaedia Biospeologica published in three volumes and coordinated by Juberthie and Decu (1994, 1998, 2001). Since then, a huge number of subterranean Collembola have been discovered. In the last decade, several new springtail species have been described in Europe (e.g., Fanciulli et al., 2010; Lukić et al., 2010; Vargovitsh, (2012, 2017, 2019), Jordana et al., 2017; Arbea, 2017; Papáč et al., 2019; Parimuchová et al., 2020; Barjadze et al., 2020).
Even with more than a century and a half of description and cataloging of the European subterranean springtail fauna, species descriptions and inventories are far from complete, and it is expected that new species will be discovered in future in the most karst regions of European countries (Spain, France, Romania, Slovenia, Bosnia and Herzegovina, Macedonia, Montenegro, Albania, Greece, or Croatia).
3 THE CHECKLIST OF EUROPEAN TROGLOBITIC SPRINGTAILS
We extracted the information on troglobitic species of Collembola maintained in a European database by Fiera C. (updated version found in Fiera et al. (2017)). We also used additional catalogues available for some countries—France (Thibaud, 2017), Iberian Peninsula (Arbea et al., 2021), and recent species descriptions and records from European caves. We included all European countries as defined in Fauna Europaea (Deharveng, 2013). The big islands (the Azores, Madeira, and the Canary Islands) were also included. From a biogeographical point of view, we have taken into account also the species, which occur in the Caucasus Mountain (European Russia, Transcaucasian countries: Azerbaijan and Georgia) to highlight that troglobitic Collembola are unevenly distributed in the most important mountain ranges. We classified species into families and orders sensu Bellinger et al. (1996–2021).
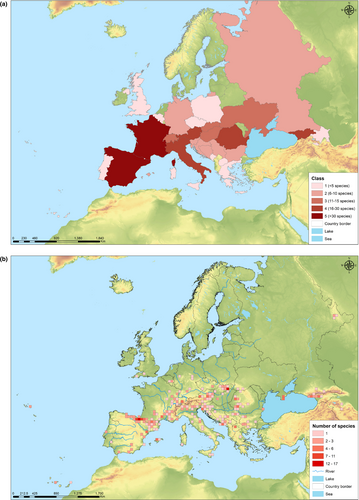
The comprehensive checklist is reported in the Table 1. We compiled a table containing 338 troglobitic species of Collembola (Figure 1) living in European caves, comprising 50 genera across 12 families (Table S1). A number of these species are poorly described, given that many descriptions (more than 50%) are based on a single sex and/or lack diagnostic details and illustrations. About 90% of European cave springtails show a restricted distribution, being found exclusively in one country. The remaining 10% have a more widespread distribution, ranging across multiple countries.
| Order | Family | Genus | Number of species | Distribution in countries with species number |
|---|---|---|---|---|
| Poduromorpha (127) | ||||
| Hypogastruridae (19) | Acherontides | 1 | Romania 1 | |
| Acherontiella | 4 | Azerbaijan 1, France 1, Italy 1, Sicily 1 | ||
| Bonetogastrura | 3 | France 2, Italy 1 | ||
| Ongulogastrura | 1 | France 1 | ||
| Pseudacherontides | 2 | Azerbaijan 1, Bulgaria 1, Georgia 1, Greece 1, Romania 1, Spain 1 | ||
| Schaefferia | 2 | Georgia 1, Russia 1 | ||
| Taurogastrura | 1 | Ukraine 1 | ||
| Typhlogastrura | 5 | Spain 2, Croatia 1, Georgia 1, Russia 1 | ||
| Neanuridae (4) | Deutonura | 2 | Austria 1, France 1 | |
| Philotella | 1 | Russia 1 | ||
| Anurida | 1 | Georgia 1 | ||
| Onychiuridae (102) | Absolonia | 1 | Slovenia 1 Croatia 1 | |
| Allonychiurus | 1 | Bulgaria 1 | ||
| Argonychiurus | 4 | Georgia 1, Italy 1, Romania 1, Switzerland 1 | ||
| Cribrochiurus | 2 | Switzerland 2, Austria 1 | ||
| Deharvengiurus | 5 | Belgium 1, Czech Republic 1, France 1, Hungary 1, Romania 1 | ||
| Deuteraphorura | 38 | Spain 10, Italy 8, Romania 6, Austria 5, France 4, Slovakia 3, Switzerland 3, Hungary 1, Germany 1 | ||
| Hymenaphorura | 2 | Austria 1, Serbia 1 | ||
| Micronychiurus | 7 | Spain 5, France 2 | ||
| Oligaphorura | 4 | Austria 3, Ukraine 1 | ||
| Ongulonychiurus | 1 | Spain 1 | ||
| Onychiuroides | 14 | Bulgaria 6, Austria 3, Slovenia 3, Italy 2, Romania 2, Greece 1, Serbia 1 | ||
| Onychiurus | 7 | Italy 2, France 1, Macedonia 1, Portugal 1, Romania 1, Spain 1 | ||
| Orthonychiurus | 2 | Romania 1, Serbia 1 | ||
| Protaphorura | 12 | Serbia 3, Macedonia 2, Slovakia 2, Germany 1, Hungary 1, Italy 1, Poland 1, Romania 1, Spain 1, Ukraine 1 | ||
| Troglaphorura | 1 | Georgia 1 | ||
| Yosiiphorura | 1 | Spain 1 | ||
| Entomobryomorpha (164) | ||||
| Entomobryidae (103) | Pseudosinella | 103 | France 42, Spain 36, Austria 8, Italy 5, Switzerland 4, Romania 3, Azores 2, Balearic Islands 2, Bulgaria 2, Canary Is 2, Croatia 2, Germany 2, Great Britain 2, Hungary 2, Slovakia 2, Andorra 1, Belgium 1, Serbia 1 | |
| Isotomidae (7) | Folsomia | 1 | Hungary 1 | |
| Gnathofolsomia | 1 | Austria 1 | ||
| Isotomiella | 3 | France 2, Spain 1 | ||
| Isotomurus | 1 | Italy 1 | ||
| Sericeotoma | 1 | Russia 1 | ||
| Oncopoduridae (16) | Oncopodura | 16 | France 5, Balearic Islands 4, Spain 4, Romania 2, Austria 1, Belgium 1, Bosnia and Herzegovina 1, Hungary 1, Slovenia 1, Ukraine 1 | |
| Orchesellidae (16) | Bessoniella | 1 | France 1 | |
| Heteromurus | 1 | Romania 1 | ||
| Verhoeffiella | 14 | Bosnia and Herzegovina 5, Montenegro 4, Croatia 2, Spain 2, Italy 1, Macedonia 1 | ||
| Paronellidae (9) | Cyphoderus | 1 | Canary Is 1 | |
| Serroderus | 1 | Sicily 1 | ||
| Troglopedetes | 7 | Portugal 2, Spain 2, Canary Is 1, Italy 1, Slovenia 1 | ||
| Tomoceridae (18) | Plutomurus | 11 | Georgia 8 Russia 4 | |
| Tomocerus | 3 | France 1, Slovenia 1, Spain 1 | ||
| Tritomurus | 4 | France 2, Croatia 1, Slovenia 1, Spain 1 | ||
| Neelipleona (10) | ||||
| Neelidae (10) | Megalothorax | 6 | Slovakia 3, France 2, Romania 1, Spain 1 | |
| Neelus | 4 | Croatia 2, Serbia 1, Slovakia 1 | ||
| Symphypleona (33) | ||||
| Arrhopalitidae (32) | Arrhopalites | 6 | Georgia 2, Ukraine 2, Hungary 1, Germany 1 | |
| Pygmarrhopalites | 25 | Spain 7, Ukraine 6, Hungary 4, Slovakia 4, Georgia 2, Germany 2, Austria 1, Czech Republic 1, France 1, Italy 1, Romania 1, Sardinia 1 | ||
| Troglopalites | 1 | Georgia 1 | ||
| Sminthuridae (2) | Disparrhopalites | 1 | Italy 1 | |
| Galeriella | 1 | Bosnia and Herzegovina 1 |
The species that colonized underground habitats do not contribute to cave diversity in proportion to their diversity in surface habitats, as clearly illustrated by Deharveng and Bedos (2018). Collembola that are often linked to soil (i.e., euedaphic life forms) have a higher proportion of cave-related species (e.g., Pseudosinella, many genera of Onychiuridae). The interface between subterranean and soil habitats is perhaps more of a “filter” rather than a major evolutionary pathway for cave colonization (Gibert & Deharveng, 2002; Fernandes et al., 2016).
4 TAXONOMIC ACCOUNT
The classis Collembola includes 338 troglobitic species living in European caves (Table S1). In the order Poduromorpha, 125 cave-restricted species are known from four families, with the families Onychiuridae and Hypogastruridae having the largest number of troglobitic species, with 102 and 19 troglobitic species, respectively. The family Onychiuridae includes numerous species of meso- or oligotrophic habitats (Deharveng & Bedos, 2018), mostly belonging to the genera Deuteraphorura, Protaphorura, Onychiuroides, Onychiurus, and Micronychiurus. Other genera of this family are relict, phyletically isolated and with spectacular troglomorphic characters. These include Ongulonychiurus colpus from Cantabric range in Spain and Troglaphorura gladiator from the Caucasus Mountain, which has the slenderest and the longest claw among Poduromorpha, and Absolonia gigantea from the Dinarides of unusually large size. Most of the troglobitic Hypogastruridae are guanophilous species of the genera Acherontides, Acherontiella, Bonetogastrura, Pseudacherontides, Schaefferia, Taurogastrura, and Typhlogastrura with reduced eyes and pigment and sometimes elongated claw. Only the relictual Ongulogastrura longisensilla from the Oriental Pyrenees has an extremely slender claw. The family Neanuridae includes a few cave-restricted species that are white, blind, or with reduced eyes but without morphological modifications.
The Order Entomobryomorpha is the most diversified order in caves with 169 troglobitic species from six families: Entomobryidae, Isotomidae, Oncopoduridae, Orchesellidae, Paronellidae, and Tomoceridae. Entomobryidae and Orchesellidae are the most diverse of troglobitic springtail families (103 and 16 species, respectively), with the genera Pseudosinella and Verhoeffiella being particularly diversified in caves. In both genera, several species exhibit spectacular troglomorphic characters, such as claw and antennae elongation (Christiansen, 1988; Lukić et al., 2018). Few troglobitic species are known in other genera: Lepidocyrtus, Heteromurus, Orchesella, and the relict monospecific genus Bessoniella with the highly troglomorphic B. procera from the Oriental Pyrenees, which has the slenderest and the longest claw among Entomobryomorpha. Oncopoduridae and Paronellidae are diversified in Mediterranean regions with cave-restricted, blind, and completely depigmented species in the genera Oncopodura (16 troglobitic species) and Troglopedetes (seven species). The family Tomoceridae includes some troglobitic species without significant differences to their outside relatives in eyes, pigment, or appendage length, like Tomocerus (three species) and Plutomurus (11 species). Only two relict species Tritomurus falcifer (Pyrenees) and T. veles (Dinarides) have claws that are extremely elongated. Isotomidae has only seven endemic troglobitic species.
Neelipleona comprises a single family Neelidae with two genera and 10 species of small, blind, and globular Collembola: Megalothorax and Neelus with six and four troglobitic species, respectively. Most of the troglobitic Megalothorax species have weak troglomorphic characters (Papáč & Kováč, 2013), but the four species of Neelus show specific morphological adaptations to cave environments; increase in size, elongation of antennae and appendages, elongation of unguis, regression of tenent hairs, and modification of sensilla on antennae (Papáč et al., 2016).
Cave Symphypleona belong to the families Arrhopalitidae and Sminthuridae with 32 and two troglobitic species, respectively. All cave Symphypleona are microphthalmic or blind, weakly or not pigmented, but their appendages and claws are usually moderately or not elongated. Only Troglopalites stygios and Arrhopalites macronyx (Arrhopalitidae) from the Caucasus and Galeriella liciniana (Sminthuridae) from Bosnia and Herzegovina have highly troglomorphic characters, like very elongated antennae (Ćurčić et al., 2007).
5 BIOGEOGRAPHY OF SUBTERRANEAN SPRINGTAILS
Collembola are very informative for biogeographical studies as numerous species are restricted to small distribution ranges owing to their fragmented habitat and limited dispersal ability. Few contributions on general species-distribution patterns in Europe have been published (Christiansen & Bullion, 1978; Christian, 2002; Fiera, Habel, Puchałka, et al., 2018; Fiera, Habel, & Ulrich, 2018).
Christiansen and Culver's (1987) biogeographic study of cave springtails of North America showed clear patterns of diminishing geographic range with increased troglomorphy. Two assumptions have resulted: (1) that increasing troglomorphy decreases the ability to disperse, and (2) that increasing troglomorphy indicates increasingly early times of first cave colonization.
Cave springtail species are unevenly distributed in Europe (Figure 2), especially in the most important mountain ranges, like the Alps, the Carpathians, the Pyrenees, the Caucasus, and other European mountains. These mountains present apparently insuperable limits to migration and spread for animals that are only a few millimeters in size, cannot fly, and live in subterranean environments. The isolation of small populations of springtails in the European mountains is best explained by the survival of relict populations during the glacial periods of the Pleistocene. Standing on the edge of the formerly glaciated territories, the most important mountain ranges, like the Alps, the Carpathians, the Pyrenees, the Caucasus, and particularly their subterranean realm, provided a refuge and multiply colonization of both thermophiles during glaciations and cryophiles during interglaciations in the Pleistocene Many endemic species have appeared after these events. Generally, these mountains are considered the richest world hotspots of subterranean biodiversity (Culver & Sket, 2000).
Glacial–interglacial cycles are known as an important motor for species diversification across Europe and North America (Hewitt, 2001) and were shown to be important for cave springtails in Austria (Christian, 2002). Caves acted as important refugia where many species persisted during glacial stages, so that many springtail taxa found in distinct caves are relicts of the glacial period, being endemic to one karst massif or even to a single cave (Kováč et al., 2016). The large number of endemics in caves may be the result of the colonization of a single cave followed by the extinction of the surface population. Most troglobites represent evolutionary products of separate invasions of surface-dwelling species rather than a single colonization followed by subsequent dispersal. Endemism may result, not from the lack of dispersal, but from subterranean dispersal followed by speciation (Christman et al., 2005). Allopatric speciation based on geographic isolation has been shown to occur among cave Collembola species (Katz et al., 2018).
5.1 Fauna of the Alps
The Alps are the highest and most extensive mountain range system that lies entirely in Europe, stretching approximately 1,200 km across Alpine countries (from West to East): France, Switzerland, Italy, Austria, Germany, and Slovenia. Pleistocene glacial periods decimated the fauna of the Northern Calcareous Alps less drastically. Single subterranean species have obviously survived at least the Würmian glaciation (~115,000 years ago) within or near their current ranges in the Northern Calcareous Alps, best indicated by troglomorphic springtails: Deuteraphorura arminiaria (Gisin, 1961), Deuteraphorura trisilvaria (Gisin, 1962), D. quadrisilvaria (Gisin, 1962), and Onychiuroides vornatscheri (Stach, 1946) also occur mainly outside of the Würmian ice cover (Christian & Spötl, 2010).
Christian (2002) compared current distribution patterns of cavernicolous Collembola in Austria and inferred four types of Quaternary range formation: (i) Holocene range expansion into formerly ice-covered regions; (ii) persistence in periglacial refugia (massifs de refuge) or (iii) persistence in underground shelters at the borders of the ice shield, without Holocene range expansion deep into formerly ice-covered regions; and (iv) survival on ice-free peaks and precipices (nunataks) amidst the continuous ice cover. Cribrochiurus cribrosus (Gisin, 1957) exhibits a scattered distribution within the area of Pleistocene glaciation in Switzerland and Austria (Christian & Spötl, 2010). The current distribution of the species Cribrochiurus cribrosus indicates its survival on nunataks (Christian & Spötl, 2010).
5.2 Fauna of the Carpathians
The Carpathian Mountains form an over 1,500 km long arc stretching in Central and Eastern Europe through NE Austria, E Czech Republic, Slovakia, S Poland, N Hungary, W Ukraine, Romania, and E Serbia. The Western Carpathians with several fragmented karst areas developed in Mesozoic limestones around the Pannonian Basin, involving glacial refugia of cave terrestrial fauna, and constitute the northern boundary for troglomorphic Collembola in Europe. Two types of relictual troglobite Collembola are distinguished here: highly troglomorphic Tertiary relicts (“old” troglobites), derived from ancient thermophilous fauna, and less troglomorphic glacial relicts (“young” troglobites) derived from Pleistocene fauna (Kováč et al., 2016). The majority of troglomorphic taxa are Tertiary relicts. These taxa are predominantly but not exclusively linked to the southern areas around Bükk Mts, Aggtelek, and Slovak Karst (N Hungary and S Slovakia), an area less severely affected by Pleistocene periglacial climate. Tertiary relicts considered to be distinctly troglomorphic are Pseudosinella aggtelekiensis, Pygmarrhopalites aggtelekiensis, P. buekkensis, P. intermedius, and P. hungaricus, distributed predominantly in southern karst areas, as well as troglomorphic such as Deuteraphorura muranensis, Pseudosinella paclti, Megalothorax hipmani, and Neelus koseli occurring north to the Slovak Karst in central karst regions of the Western Carpathians. Pygmarrhopalites aggtelekiensis, a highly troglomorphic Tertiary relict associated with caves in the plateau karst type of southern regions, was also found in caves ~30 km NE (Volovské vrchy Mts.) and ~45 km NW (Slovak Paradise) from the type locality (Baradla Cave), including even the cold parts in Dobšina Ice Cave with a temperature of about 3.5°C (Papáč et al., 2020).
Molecular analyses and the geographical distance between localities of Deuteraphorura kratochvili uncovered high interpopulation divergence, suggesting long isolation of this species, allowing considering it as a descendant of an old phyletic lineage, that is, “old” troglobite. Glacial relicts or “young” troglobites are not distinctly troglomorphic. They are linked with Alpine karst and cold caves (karst or pseudokarst) at lower elevations and represented by Protaphorura janosik, Megalothorax carpaticus, and two undescribed Pseudosinella species (Kováč et al., 2016). Protaphorura janosik, originally described from Mylna Cave in the Polish Tatras (Weiner, 1990), is a widespread Western and Eastern-Carpathian troglobite, and a typical glacial relict. Genetic analysis based on mitochondrial COI genes showed several unique haplotypes meaning high population differentiation, suggesting the existence of geographical isolates (Parimuchová et al., 2017).
The Eastern Carpathians, in their Ukrainian part, contain very limited karst with a relatively small number of caves in fragmented limestone blocks associated with the Marmarosh Klippen Zone. Two troglomorphic species occur here: Pygmarrhopalites carpathicus (with elongated antennae) and P. kristiani (with elongated claws) (Vargovich, 1999, 2005), both in pairs of isolated massifs, thus possibly containing morphologically weakly distinguished cryptic species. In higher elevations of the Ukrainian Carpathians (1,200–1,900 m a.s.l.) lies the eastern limits of Protaphorura janosik distribution (pseudokarstic caves Runa and Petros).
In the Southern and Western Romanian Carpathians with well-developed limestone karst and very numerous caves, the number of troglobitic species is the highest (Gruia, 2003). They are predominantly members of Onychiuridae (12 species) with a rather edaphomorphic appearance without progressive troglomorphic adaptations. Among Arrhopalitidae, the only moderately troglomorphic species, P. subboneti, with an extremely distant disjunct distribution (France and Spain) (Cassagnau & Delamare-Deboutteville, 1953; Selga, 1963) was reported also from Romanian Carpathians (Gruia, 1966). In contrast to the Western Carpathians and Balkan Mountain ranges, the diversity of truly troglomorphic taxa in the Romanian Carpathians is unexpectedly low. Only two species are distinctly troglomorphic “old” troglobites: Oncopodura pegyi and Megalothorax draco, both recorded in the Bihor Mts and possessing slender elongated claws (Gruia, 1994; Papáč & Kováč, 2013).
Troglobitic springtails of the Serbian Carpathians are poorly known: six Onychiuridae species (Ćurčić et al., 2005; Ćurčić & Lučić, 1997, 1998; Lučić et al., 2008) and non-troglomorphic Pseudosinella problematica (Gisin & da Gama, 1971), all narrow endemics were reported.
5.3 Fauna of the Dinarides
The Dinaric Alps (Dinarides) extend for 650 km in the western part of the Balkan Peninsula along the Adriatic East coast from northwest to southeast through NE Italy, Slovenia, Croatia, Bosnia and Herzegovina, W Serbia, Montenegro, Kosovo, and Albania.
The apparently low number of described troglobitic subterranean Collembola in the Dinarides comparative with other invertebrate groups (Coleoptera) represented by more than 200 troglobitic species (Sket, 2012) is in fact far from the real diversity, which is potentially much richer, often cryptic and significantly underestimated. According to Lukić et al. (2012), the genera of troglobitic Dinaric Collembola are spread in the following distributional patterns: holodinaric (Pseudosinella, Tritomurus, Oncopodura, Troglopedetes, Disparrhopalites, Onychiuroides, and Neelus), northwestern merodinaric (Absolonia), and southeastern merodinaric (Verhoeffiella, Typhlogastrura, Galeriella, and Ongulonychiurus). Recent research on Collembola in the Dinaric Karst uncovered high regional diversification of the cave-dwelling genus Verhoeffiella with well distinguishable adaptive troglomorphic traits in 10 from 14 known species (Lukić et al., 2015, 2018).
Extensive molecular phylogeny unveils its hidden diversity with several lineages of the genus with different levels of troglomorphy. The radiation is estimated as relatively recent, linked to the Messinian salinity crisis and Pleistocene climatic shifts (Lukić et al., 2020). Molecular phylogenetic analysis, showing paraphyletic relationships of the genera Verhoeffiella and Heteromurus, allows explaining enigmatic disjunct distribution and relationships of strictly subterranean species of Verhoeffiella in the Dinarides, Alps, and the Iberian Peninsula. On a continental scale, it is hypothesized that the epigean Heteromurus ancestors, like the widespread H. nitidus, independently colonized caves in different geographically remote regions with consequent subterranean speciation resulting in convergently evolved forms considered as Verhoeffiella. In addition, the extinction of some cave populations, due to Pleistocene climate changes in the Alps, may have occurred. This hypothesis explains the sister group relationship of Catalonian and Cantabrian cave populations as well as the sister group relationship of Iberian to Alpine and Alpine to Dinaric cave populations. Radiation of Verhoeffiella on the local scale within Dinarides might have occurred similarly as on a continental scale pattern (through epigean Heteromurus), as well as through the dispersal of the already adapted troglobitic lineages via continuous subterranean karst cavities and further speciation (Lukić et al., 2020).
Numerous highly specialized relictual endemic forms belong to other genera and families. The large-sized troglomorphic species Absolonia gigantea was recorded from caves of Slovenia and Croatia (Absolon, 1901; Lukić et al., 2012). The distribution of the only highly troglomorphic (blind, with elongated appendages and claws) species of Tomoceridae, microendemic Tritomurus veles, is restricted to deep Amfora jama Cave (down to −430 m) in Biokovo Mt., S Croatia (Lukić et al., 2010). Neelidae collected in 86 Croatian caves show the complex of several troglobitic species, including Neelus cvitanovici and more geographically restricted N. lackovici, both with elongated claws (Papáč et al., 2016). Symphypleona represented in the Dinarides include Galeriella liciniana described from Bosnia and Herzegovina, with extraordinarily elongated appendages (Ćurčić et al., 2007). Low number of troglomorphic Arrhopalitidae as well as Pseudosinella may be the result of lack of taxonomic studies on these taxa in the Dinaric caves. Several highly troglomorphic taxa of the Dinaric Karst, including species of Ongulonychiurus, Disparrhopalites, and even species of Isotomidae (usually rarely troglomorphic) with elongated claws, especially from deep karstic caves, await further taxonomic investigation (Lukić et al., 2012; Sun et al., 2019).
5.4 Fauna of the Caucasus
The Caucasus Mountains are located on the southern border of Europe and Asia, and there is no generally accepted agreement on which subcontinent they partially or entirely belong to. The mountain chain stretches for about 1,500 km from the NE Black Sea coast to the W Caspian through SW Russia, Georgia, Armenia, and Azerbaijan. The regional distribution of karst in the Great Caucasus is similar to the Alpine, with front ridges composed of Upper Jurassic and Lower Cretaceous limestones encircling the glaciated core zone of igneous rocks (Klimchouk, 2004).
The W Caucasian terrestrial speleofauna is considered related to the Mediterranean, whereas the E Caucasian is closer to the Central Asian one. It was zoogeographically classified as: circummediterranean taxa related to Balkan fauna; autochthonous taxa; species of ancient subterranean groups (Birstein & Ljovuschkin, 1967). The “old” troglobites have been considered relicts of the tropical Tertiary fauna (Birstein & Borutzky, 1950), whereas “young” weakly or non-troglomorphic cave-dwellers could be considered Pleistocene-Holocene glacial relicts.
Cave Collembola of the Caucasus with only 15 described troglobitic species are still poorly known and promise much richer biodiversity. The western part of the Great Caucasus, with abundant karst phenomena including four of the world's deepest caves in the Arabika and Bzyb massifs, harbors the richest relictual speleofauna and particularly hypogean collembolans. The world's deepest subterranean community in Krubera Cave (Arabika Massif) includes Plutomurus ortobalaganensis found at −1980 m depth and Schaefferia profundissima (−1600 m) (Jordana et al., 2012). A high level of troglomorphy shows the large-sized poduromorphs Anurida stereoodorata (Krubera Cave) and Typhlogastrura morozovi (Snezhnaya Cave, Bzyb Massif) (Babenko, 1987; Jordana et al., 2012). Troglaphorura gladiator (Snezhnaya Cave) with extraordinarily elongated claws, found at −1200 m depth, is the world's most troglomorphic species of Onychiuridae (Vargovitsh, 2019). In addition, Arrhopalites macronyx and Troglopalites stygios from the Tsebelda Massif are the most specialized among Arrhopalitidae. The West Caucasus can be considered the center of speciation of Arrhopalitidae with five described (Vargovitsh, 2012, 2013, 2017) and undescribed taxa with different levels of troglomorphy, particularly having a portion of cryptic Pygmarrhopalites species of principalis and pygmaeus groups, presuming multiple colonization during Pliocene, Miocene, and Pleistocene.
Molecular investigation of several Plutomurus species indicated that speciation in the subterranean habitats in Georgia started in Upper Pleistocene (Barjadze, unpublished data). The cave-restricted Plutomurus spp. probably originated here according to the climatic relict hypothesis, when surface populations were extinct during the Upper Pleistocene and Holocene glaciations reported from Georgia (Tielidze, 2017), and their conspecific cave-dwelling populations colonized subterranean habitats successfully and survived. Perhaps speciation in recent historical periods is a reason that troglomorphic characters are poorly developed in the studied Plutomurus species.
The North and East Caucasian fauna are different from W Caucasian, yet poorly studied, and is known only by a few weakly or not troglomorphic Typhlogastrura, Acherontiella, and Plutomurus species (Babenko, 1987; Djanaschvili, 1971; Kniss & Thibaud, 1999). The West Caucasus, with abundant, endemic, relictual, and highly specialized cave fauna, is undoubtedly an important subterranean biodiversity hotspot with high potential of discoveries of new taxa.
5.5 Fauna of the Pyrenees and Cantabrian range
The calcareous massifs in the northern Iberian Peninsula emerged during the Eocene period, around 45 million years ago, forming the Pyrenaic range and the Basque mountains, which are part of the Pyrenees and continues along the Cantabrian range. The progressive rising of the range is a consequence of the collision and knitting together of the continental Iberian and European plates. The region is included in a midlatitude ridge of high biodiversity in troglobitic fauna (Culver et al., 2006), extending in Europe from Slovenia (in the Balkans) and N of Italy, through the SE of France, Pyrenees, Basque Country, and Cantabrian range. The northern Iberian range is an important factor which controls and explains the colonization and later evolution of the troglobitic fauna, common to Europe, and different from that inhabiting other Iberian karst.
Following Bellés (1987), from West to East, four biospeleological districts can be recognized in the Pyrenees: Cantabrian (provinces of Asturias, Cantabria, west of Bizkaia, and Araba in Spain), Basque (east of the provinces of Bizkaia and Araba, Gipuzkoa, and Navarra in Spain; department de Pyrénées Atlantiques in France), Central Pyrenees (province of Huesca in Spain; departments of Hautes Pyrénées et Ariége in France), and Oriental Pyrenees (provinces of Lleida and Girona in Spain; department of Pyrénées Orientales in France; Andorra) with 15, 48, 17, and 6 troglobitic species, respectively. The Basque biospeleological district is the richest in troglobitic species, an extremely high proportion of which are endemic (48 species with 43 district endemics). In the other biospeleological districts, a high proportion of endemism is also recorded: Central Pyrenees (17 troglobitic species with 13 district endemics), Cantabrian (15 species with 12 district endemics) and Oriental Pyrenees (6 species with 4 district endemics).
At least three relictual monospecific genera of Collembola are cave restricted with spectacular troglomorphic characters, including Ongulonychiurus and Ongulogastrura, and Bessoniella. Ongulonychiurus colpus is endemic to the Cantabric district, while Ongulogastrura longisensilla and Bessoniella procera are endemic to the Basque district. The richest genus in troglobitic species is Pseudosinella with 17 endemic species in the Basque district, 8 endemics in the Cantabrian district, 6 endemics in the Central Pyrenees, and 2 endemics in the Oriental Pyrenees. The widespread genus Lepidocyrtus has one relictual troglobitic species known only from a single cave in the Central Pyrenees (L. pseudosinelloides). Species of Deuteraphorura and Micronychiurus have the center of radiation in the Basque district. The genus Deuteraphorura has only been found in the caves of the Basque district with 10 species belonging to the group of D. boneti (Beruete et al., 2001).
To conclude, this paper presents the synthesis of the existing knowledge on troglobitic species of European mountains and may serve as a basis for establishing multiscale relationships between diversity of Collembola and environmental, biotic, and spatial factors.
6 FUTURE LINES OF RESEARCH
Poulson and White (1969) considered caves as “natural laboratories,” as they can be used to understand the principles governing the dynamics of more complex environments. Subterranean habitats have been regarded as ideal systems for investigating many of the unresolved questions in ecology, biogeography, and evolutionary and subterranean biology (Juan et al., 2010; Mammola et al., 2020). In this perspective, the checklist of European troglobitic springtails herein presented aims at setting an in-depth review of all reports in order to provide a sound basis for future studies. Here, we suggest some future themes to stimulate further research on subterranean Collembola.
6.1 Phylogeographic patterns
Caves are ideal to understand the evolutionary processes that shape patterns of biological diversity (Culver & Pipan, 2009). Phylogeography, the study of processes that influence the contemporary geographic distributions of species' populations by utilizing genetic data, can provide insights into the relative influences of evolutionary factors driving patterns of genetic isolation and divergence in cave springtails (Katz et al., 2018). Using two ecologically distinct groups of terrestrial cave-dwelling springtails (Collembola) in the genera Pygmarrhopalites (Arrhopalitidae) and Pogonognathellus (Tomoceridae), Katz et al. (2018) assessed the evolutionary history across a geologically complex karst landscape in N America (Illinois and Missouri). Patterns of genetic differentiation in troglobites are likely driven primarily by isolation due to physical barriers and reflect vicariance.
Other cases can be illustrated in Europe. The tested populations of Deuteraphorura kratochvili using molecular techniques were well isolated both geographically and genetically (Parimuchová et al., 2020). Deuteraphorura kratochvili was considered to be a complex of cryptic species in the Western Carpathians in which long-term isolation in individual karst systems likely led to gene-flow cutoff (Parimuchová et al., 2020). Another study regarding the phylogenetic analysis revealed a complex relationship between the European subterranean genus Verhoeffiella and the surface species Heteromurus nitidus (Lukić et al., 2020). Phylogenetic patterns of subterranean springtail species using broad taxonomic samples, both cave and epigean species, could be a promising line of inquiry.
6.2 Ecological drivers of subterranean springtails
Subterranean fauna species usually have restricted distributions and do not move outside their specific habitats due to poor dispersal ability and the discontinuous nature of their habitats. Studies to show whether species richness and diversity of subterranean Collembola in European subterranean habitats are driven by macro-scale (e.g., latitude, past glacial dynamics), are still missing. Cave arthropod diversity can be correlated with habitat availability, that is, the number of caves, at both local and regional scales (Christman & Culver, 2001).
At a micro-scale, the presence of subterranean fauna is generally strongly linked to underlying geology and hydrology, and the availability of suitable microhabitats. Microclimate plays a major role in the distribution and variety of cave arthropods (Poulson & Culver, 1969; Christiansen, 1970). Most troglobites are both stenothermic and stenohygrobic (e.g., Barr, 1968). Troglobitic springtail species, restricted to climatic-stable cave conditions, retain a functional thermal resistance, that is, the genetically determined ability to tolerate relatively broader temperature ranges (Raschmanová et al., 2018). The number of cave springtail species appears to increase significantly with cave air temperature and decrease with latitude. In addition, a latitudinal effect on the diversity of cave Collembola in the Western Carpathians, with a decline in the species number from the southern to the northern regions, was observed (Kováč et al., 2016). In the Biokovo Mts in Southern Croatia, Lukić and Deharveng (2008) observed an increase in the number of troglobitic taxa with cave elevation. Cave length is often linked with habitat diversity (Culver et al., 2006). Local richness of cave Collembola (country level: Romania) increased allometrically with cave length and cave depth, while altitude showed no significant effect on springtail richness (Fiera, Habel, & Ulrich, 2018).
The greatest complexity of interactions appears to occur in troglobitic species. The deepest part of the cave is normally the most stable region and has the narrowest range of physical variables. This stability (i.e., predictability) may lower the extinction rate of small populations, thus bringing about greater biological complexity (Christiansen & Bullion, 1978).
6.3 Functional assemblages of subterranean springtails
Studies on functional assemblages of subterranean springtail communities are still lacking. In a classical way, focusing on the functional feeding groups, four fundamental trophic levels may be recognized in terrestrial habitats: primary producers, primary consumers (herbivores), decomposers, and predators. Of these levels, only two (decomposers and predators) are significantly represented in subterranean food webs (Mohr & Poulson, 1966). Trophic linkages within subterranean food webs indicate extensive omnivory. In that way, subterranean food webs are often truncated at the top with few or no strict predators (Gibert & Deharveng, 2002). Analyses of matter and energy fluxes from soil to cave, including Collembola as troglobite detritivorous group, were investigated by Gers (1998).
A synthetic classification based on Collembola species traits, which indicate dispersal ability and the degree of adaptation to cave life (body color, body size, reproduction and furca type, number of ocelli per head side, and the presence of pseudocelli (Fiera, Habel, Puchałka, et al., 2018)) and habitat affinities (trogloxenes, troglophiles, and troglobites), may demonstrate its efficiency to test response of springtails communities to ecological changes, natural, and human disturbances, as well as for generating testable hypotheses about the process of adaptation to extreme environmental conditions found in caves. How these traits are organized in a community has antagonistic implications: the higher the functional diversity, the greater the efficiency in resource use, but the lower the resilience of ecological processes (Tilman et al., 1997).
6.4 Competition and niche partitioning
The distribution in caves is unique for each species. Culver and Pipan (2015) demonstrated that interspecific competition, a divergent selective force, is important in shaping morphology when competing species are present in the caves. The facts that in caves, more highly specialized species replace less specialized ones over large geographic areas, and that closely related species are rarely found in the same environment, indicating that competition may play a major role in the Collembola group (Cassagnau, 1961; Christiansen, 1960). Cases of apparent competitive exclusion can be explained based on differing ecological requirements, as is demonstrated by the detailed analysis of troglobitic species: Pseudosinella theodoridesi Gisin & da Gama, 1969, or by P. virei Absolon, 1901. This high degree of niche individuality also permits a good deal of co-occurrence, most strikingly seen in the case of P. theodoridesi and Tomocerus problematicus Cassagnau, 1964 (Christiansen & Bullion, 1978).
Knowledge about feeding habits of troglobitic species of Collembola is very limited. Troglobitic collembolans apparently rarely feed on decaying wood but commonly on the hyphae of polypores and other wood-rot fungi (Christiansen, 1964). Some species are strict guanobionts Pseudacherontides spelaeus (Ionesco, 1922), while others (Protaphorura janosik Weiner, 1900) occurs in a wider spectrum of cave microhabitats, including cave sediment and rotten wood (Parimuchová et al., 2018). Therefore, further studies have to be conducted for a better understanding of how competition between cave-dwelling Collembola influences the large-scale distribution.
6.5 Reproduction
Caves generally allow the maintenance of small populations as the result of food scarcity and a lower biodiversity than can reduce interspecific predation (Parimuchová et al., 2021). It thus has a strong impact on life history traits such as the reproductive lifespan, aging, and number and size of offspring (Culver & Pipan, 2009). Cave Collembola have lower fecundity than surface forms. For example, in the troglobite Bonetogastrura balazuci (Delamare-Deboutteville, 1951) a female has a potential reproduction of 50,000 individuals in three generations, in 3 years. In contrast, Ceratophysella denticulata (Bagnall, 1941), a hemiedaphic-troglophile, one female has a potential reproduction of 5000,000 individuals in three generations, in only 2 years (Thibaud, 1970).
6.6 Interspecific relationships
Interactions between different species of troglobitic Collembola and other animal groups are poorly known. Delamare-Deboutteville (1952) reported a case of phoresy whereby aggregations of nematodes Rhabditophanes schneideri (Bütschli, 1873) were found spiraling around leg setae and articulations of guano-dwelling Tritomurus catalanus. Other case of phoresy was observed with Bonetogastrura balazuci (Delamare-Deboutteville & Théodoridès, 1951). Some years ago, the first report of a cave springtail (Collembola, Paronellidae) parasitized by a mite (Parasitengona, Microtrombidiidae) in the Brazilian caves was published by Oliveira et al. (2016). A recent study using molecular methods (NGS) to decipher the food web in the caves (Parimuchová et al., 2021) revealed that springtails were very frequent in the diet of spiders (Centromerus cavernarum (Koch, 1872) mainly had the DNA of springtails). The effect of interspecific relationships on distributional patterns in caves has not been studied yet.
6.7 Conservation and management policies
Troglobitic species are endemics with small ranges, are unique, rare, and highly vulnerable (Juberthie, 2000) and must be considered as important units for conservation. It was indicated recently that a cave must be equally protected if it has one or more rare and strictly endemic cave species (Moldovan et al., 2021). Besides, the detection of short-range endemics, genetic isolation, and apparent cryptic diversity of Collembola has major conservation implications (Katz et al., 2018). High level of endemism in caves requires specific protection measures for maintaining their ecological integrity and biological diversity. Endemic species serve as reliable indicators of the undisturbed cave environment. Subterranean habitat loss and degradation is primarily due to surface activities, such as agricultural expansion and intensification, urbanization, and mining activities (Reboleira et al., 2013). Human activities inside caves may also constitute localized threats, with recreational use and tourism activities being of particular concern (Faille et al., 2015). Reduced dispersal capacity observed for cave springtail Pygmarrhopalites can increase their susceptibility to human disturbances such as land use practices, climate change, pollution, and invasive species—all of which pose major threats to fragile cave ecosystems (Culver & Pipan, 2009).
The current incomplete state of knowledge on troglobitic springtail species and the lack of conservation strategies constrains the implementation of protection policies. Because conservation resources are limited, it is impossible to protect all caves inhabited by troglobitic springtails in Europe. Endangered cave arthropods, including Collembola, are often not considered in national or international conservation policies. The status of conservation of most subterranean springtails is unknown, given that a few species have been assessed according to the IUCN criteria (Moldovan et al., 2021). There is an urgent need for IUCN assessments of European cave springtails and the development of specific conservation programs (Borges et al., 2012; Cardoso et al., 2011). Therefore, there is a need to publish a list of European cave springtails species that are under threat.
7 CONCLUSIONS
With respect to Collembola, there is a significant lack of knowledge concerning eco-evolutionary processes within caves. This is largely driven by insufficiency of functional ecology studies on Collembola species, the weakness of trait-based approaches (Fiera, Habel, Puchałka, et al., 2018; Zhang et al., 2018), the lack of sampling in many places in Europe (see Figure 2), and also the paucity of systematic sampling techniques for most taxonomic groups living in caves (Culver et al., 2004; Wynne et al., 2019; Zagmajster et al., 2008). Although many research questions from macroecology and biogeography about subterranean Collembola and again for other cave groups are still unanswered (Mammola et al., 2020), this paper represents a strong potential for fast advancements in our understanding to clarify the indices of endemism, as well as to generate data for future biogeographic studies.
The detection of narrowly distributed endemics, genetic isolation, and cryptic diversity of Collembola from caves also has major conservation implications. In addition, this work can help in understanding the impact and risk of future environmental scenarios in light of recent environmental changes (climate change and pollution) on known springtail species. Hence, there is an increasingly urgent need to accurately map the geographic distributions (species occurrences as well as derived parameters: densities and species richness) of various springtail species and their subterranean habitats of Europe.
ACKNOWLEDGEMENTS
Fiera C. acknowledge support by a grant of the Ministry of Research, Innovation and Digitization, CNCS/CCCDI—UEFISCDI, project number PN-III-P1-1.1-TE-2019-0358, within PNCDI III”. Barjadze S. was supported by the Institutional Grant of Ilia State University, Tbilisi, Georgia: “Taxonomy, fauna and ecology of the invertebrates in the long and biospeleologically poorly investigated caves of Imereti and Samegrelo regions.” We are thankful to Marko Lukić (Croatian Biospeleological Society, Zagreb, Croatia), who provided us important information about the status of some cave species. We are grateful to Sahlean Constantin-Tiberiu ("Grigore Antipa" National Museum of Natural History, Bucharest, Romania) for creating the maps; special thanks go to Charlene Janion-Scheepers (Department of Biological Sciences, University of Cape Town, South Africa) for proofreading our English. We would like also to thank the reviewers and editor for their thoughtful comments and efforts toward improving our manuscript.




