Integrative taxonomy of insular land snails of the genus Sicradiscus Páll-Gergely, 2013 (Gastropoda, Plectopylidae) with description of a new species
Contributing authors: Chung-Chi Hwang ([email protected]), Josef Harl ([email protected]), Takafumi Nakano ([email protected])
Funding information
The study received funding from Japan Society for the Promotion of Science, Grant/Award Number: JP21J22917 and the Tokyo Metropolitan University Fund for TMU Strategic Research, Grant/Award Number: FY2020–FY2022.
Online ISSN: 1439-0469
Abstract
Many land snail taxa have undergone speciation in the Ryukyu Islands and Taiwan in East Asia. We examined the shell, radular, and genital morphology, and mitochondrial phylogeny of two described Sicradiscus species distributed in Miyako Island and Taiwan, and the newly discovered Sicradiscus pallgergelyi sp. nov. from Iriomote Island. Canonical variate analysis based on adult shell measurements indicated that S. pallgergelyi sp. nov. and the Taiwanese Sicradiscus ishizakii had more similar shell measurements, whereas S. pallgergelyi sp. nov. shared common characteristics of shell sculpture with the Japanese Sicradiscus hirasei. The leave-one-out cross-validation results correctly classified 100%, 71.4%, and 88.0% of S. hirasei, S. ishizakii, and S. pallgergelyi sp. nov., respectively. The radular and genital morphology was similar in these three species. Moreover, molecular phylogenetic analyses showed monophyly of the three species, although the Japanese lineages were more closely related to each other than to the Taiwanese species. Accordingly, the characteristics of shell sculpture are common traits of the two Japanese species, and these findings indicate that shell morphology has significantly diverged in Japan. The different apertural callus lengths among the three species may be an adaptation to predators, and shell flatness may reflect interspecific differences in microhabitats.
1 INTRODUCTION
The land snail family Plectopylidae consists of 113 extant species and seven fossil species which are distributed in Asia from the southern Himalayan region (Nepal) to the Ryukyu Islands and the Malay Peninsula, and their distribution includes northeastern India, Myanmar, Thailand, Laos, Vietnam, China, and Taiwan (MolluscaBase, 2021; Páll-Gergely, 2018b; Páll-Gergely, Budha, et al., 2015; Páll-Gergely & Hunyadi, 2013). Plectopylidae are characterized by having depressed shells with internal plicae and lamellae that are approximately a quarter to a half whorl behind the aperture. Before Páll-Gergely and Hunyadi (2013), the extant members of the family had been classified into four genera, which were originally established as “sections” under the genus Plectopylis Benson, 1860 by Gude (1899). Although the traditional classification was only based on a few shell characters, recent comprehensive examinations of shells, radulae, and reproductive organs resulted in the description of five further extant genera (Páll-Gergely & Hunyadi, 2013; Páll-Gergely, Hunyadi, et al., 2015; Páll-Gergely et al., 2016). Plectopylidae are currently divided into two subfamilies, Plectopylinae and Sinicolinae, mostly based on reproductive anatomical traits and the fine sculpture of the protoconch (Páll-Gergely, 2018b).
The range of the sinicoline genus Sicradiscus Páll-Gergely, 2013 extends from northern Vietnam and the Chinese provinces Sichuan and Yunnan to Miyako Island in the Ryukyu Islands, the easternmost distribution range of the family. Most Sicradiscus species are indigenous to the Asian continent. Only two species, Sicradiscus hirasei (Pilsbry, 1904) and Sicradiscus ishizakii (Kuroda, 1941), were described from Miyako Island in the Ryukyu Islands and Taiwan, respectively, which are the eastern margin of the distribution of the genus (Páll-Gergely & Hunyadi, 2013).
Sicradiscus has two species groups. Four species (Sicradiscus feheri Páll-Gergely & Hunyadi, 2013, Sicradiscus mansuyi (Gude, 1908), Sicradiscus invius (Heude, 1885), and Sicradiscus securus (Heude, 1889)) inhabit Vietnam and the Chinese Sichuan, Yunnan, and Guangxi Provinces; these species are characterized by the presence of an apertural fold and a rounded body whorl (herein, the “western group”). In contrast, the other species group includes five species (Sicradiscus cutisculptus (Möllendorff, 1882), Sicradiscus diptychia (Möllendorff, 1885), S. hirasei, S. ishizakii, and Sicradiscus schistoptychia (Möllendorff, 1886)) that are distributed in the Chinese Hunan, Hubei, Fujian, and Zhejiang Provinces, in Taiwan, and in the Ryukyu Islands; these species are characterized by the absence of an apertural fold and a shouldered body whorl (herein, the “eastern group”) (Páll-Gergely & Hunyadi, 2013). Sicradiscus transitus Páll-Gergely, 2013 from Guangxi and Guizhou Provinces forms a morphological connection between the two groups because the species possesses an apertural fold and a shouldered body whorl (Páll-Gergely & Hunyadi, 2013).
Knowledge regarding the shell, radular, and genital morphology of insular species has been accumulated within the genus. Substantial variation was observed in plical and lamellar morphology of S. hirasei (Páll-Gergely, 2018a). In addition, morphological characters of reproductive organs of the two insular snails were revealed in the 1900s (Azuma & Azuma, 1984; Chang & Ookubo, 1999). Nevertheless, characterization of the reproductive anatomy of S. hirasei was amended in a subsequent publication (Páll-Gergely, 2018a), and that of S. ishizakii in Chang and Ookubo (1999) contains several descriptions which are difficult to interpret. Morphological characters of insular species have not been compared within genera in a series of detailed examinations (e.g., Páll-Gergely & Hunyadi, 2013; Páll-Gergely, Hunyadi, et al., 2015). Accordingly, accumulating information on morphological traits of this genus and conducting comprehensive comparisons can improve our understanding of insular and continental Sicradiscus species.
The first author recently discovered living Sicradiscus snails from Iriomote Island, which is located between Miyako Island and Taiwan. In this study, we performed integrative comparisons using adult shell, radular, and genital morphology, and mitochondrial COI sequences of S. hirasei, S. ishizakii, and the snails from Iriomote Island. As a result of our examination, the information on the two described species has been updated. Furthermore, the detected interspecific morphological variation indicates the existence of different selection pressures on the snails in each island. Finally, the Sicradiscus snails from Iriomote Island were described as a new species, Sicradiscus pallgergelyi sp. nov. by the first and last authors.
2 MATERIALS AND METHODS
2.1 Sample collection
In total, 64 Sicradiscus snails were newly collected. Twenty-five specimens of S. pallgergelyi sp. nov., 17 of S. hirasei, and 21 of S. ishizakii were examined morphologically; among these specimens, six individuals of S. pallgergelyi sp. nov., six of S. hirasei, and three of S. ishizakii were used for the molecular phylogenetic analysis (Table 1). In addition to the newly collected specimens, the morphological examination was performed for a lectotype of S. hirasei and a paratype of S. ishizakii. The field survey was conducted by the first and second authors from four localities in Japan and Taiwan, in 1997–2020 (Table 1; Figure 1). Sicradiscus hirasei was collected from crevices in the limestone on the ground in the inland natural forest of Miyako Island. On Iriomote Island, the new species was found in the inland natural forest at an elevation of around 200 m; however, S. pallgergelyi sp. nov. was not found from three limestone areas at low elevations whose environments are similar to the habitat of S. hirasei in Miyako Island. All of the snails collected by the first and second authors were found on the forest ground by eye. Because S. hirasei is protected by a Miyakojima City ordinance on Miyako Island, the snails were collected with permission by the city. In addition to the three insular species, a specimen of the continental S. schistoptychia (the type species of Sicradiscus) was included in the phylogenetic analysis.
| Sample | Voucher # | Collection locality | INSD accession # |
|---|---|---|---|
| In-group | |||
| Sicradiscus hirasei | |||
| ANSP 87632 (lectotype) | Miyakojima, Ryukyu (Pilsbry, 1904) | ||
| HM04, 08–17 | KUZ Z3942 | Miyakojima City in Miyako Island, Japan | |
| HM01 | KUZ Z3990 | Miyakojima City in Miyako Island, Japan | LC638865 |
| HM02 | KUZ Z3991 | Miyakojima City in Miyako Island, Japan | LC638866 |
| HM03 | KUZ Z3992 | Miyakojima City in Miyako Island, Japan | LC638867 |
| HM05 | KUZ Z3993 | Miyakojima City in Miyako Island, Japan | LC638868 |
| HM06 | KUZ Z3994 | Miyakojima City in Miyako Island, Japan | LC638869 |
| HM07 | KUZ Z3995 | Miyakojima City in Miyako Island, Japan | LC638870 |
| Sicradiscus ishizakii | |||
| NCP−244 (paratype) | Tentana, Tikuto, Sintiku-syu (Kuroda, 1941) | ||
| IT04–05 | KUZ Z3943 | JianShih Town, Xinzhu County, Taiwan | |
| IT06–19 | KUZ Z3944 | JianShih Town, Xinzhu County, Taiwan | |
| IT20 | KUZ Z3945 | JianShih Town, Xinzhu County, Taiwan | |
| IT21 | KUZ Z3946 | JianShih Town, Xinzhu County, Taiwan | |
| IT01 | KUZ Z3996 | JianShih Town, Xinzhu County, Taiwan | LC638856 |
| IT02 | KUZ Z3997 | JianShih Town, Xinzhu County, Taiwan | LC638857 |
| IT03 | KUZ Z3998 | Guanxi Town, Xinzhu County, Taiwan | LC638858 |
| Sicradiscus pallgergelyi sp. nov. | |||
| SI25 | KUZ Z3947 (holotype) | Taketomi Town in Iriomote Island, Japan | |
| SI06 | KUZ Z3948 (paratype) | Taketomi Town in Iriomote Island, Japan | LC638862 |
| SI19 | KUZ Z3949 (paratype) | Taketomi Town in Iriomote Island, Japan | |
| SI21 | KUZ Z3950 (paratype) | Taketomi Town in Iriomote Island, Japan | |
| SI23 | KUZ Z3951 (paratype) | Taketomi Town in Iriomote Island, Japan | |
| SI01, 05, 09–18, 20, 22, 24 | KUZ Z3952 | Taketomi Town in Iriomote Island, Japan | |
| SI02 | KUZ Z3999 | Taketomi Town in Iriomote Island, Japan | LC638859 |
| SI03 | KUZ Z4000 | Taketomi Town in Iriomote Island, Japan | LC638860 |
| SI04 | KUZ Z4001 | Taketomi Town in Iriomote Island, Japan | LC638861 |
| SI07 | KUZ Z4002 | Taketomi Town in Iriomote Island, Japan | LC638863 |
| SI08 | KUZ Z4003 | Taketomi Town in Iriomote Island, Japan | LC638864 |
| Outgroup | |||
| Sicradiscus schistoptychia | |||
| SC01 | HNHM 104887 | Jiuyi National Park, Ningyuan County, Yongzhou, Hunan, China | LC638871 |
Note
- All sequences were newly obtained in the present study. Acronyms: ANSP, Academy of Natural Sciences, Philadelphia, USA; HNHM, Hungarian Natural History Museum, Budapest, Hungary; KUZ, Zoological Collection of Kyoto University, Kyoto, Japan; NCP, paratype collection of the Nishinomiya Shell Museum, Hyogo, Japan.
Specimens from Iriomote and Miyako Islands were separated into shells and soft parts after being boiled in hot water at 80°C for 12 s. The extracted soft bodies were frozen until dissection after cutting off the foot tip. Separated tissues were preserved in 99% ethanol for molecular phylogenetic analysis. Snails from Taiwan were preserved in 95% ethanol after boiling. The newly collected specimens in this study were deposited in the Zoological Collection of Kyoto University, Kyoto, Japan (KUZ) and Hungarian Natural History Museum, Budapest, Hungary (HNHM).
2.2 Morphological examination
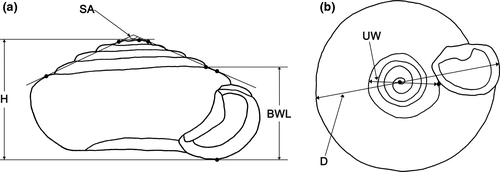
Morphometric characters of the newly collected specimens and the type specimens of S. hirasei and S. ishizakii were examined. Fully matured adults were observed and measured following the methods in previous studies (Páll-Gergely, Hunyadi, et al., 2015). The nomenclature of plicae and radulae follows Páll-Gergely and Hunyadi (2013) and Páll-Gergely (2018b), respectively. Newly collected specimens were first photographed using a Nikon D7100 camera with a Tamron SP 90 mm f/2.8 1:1 macro lens for Nikon. All specimen measurements were obtained from digital images of dorsal, ventral, and lateral sides of shells using ImageJ 1.51k (Schneider et al., 2012). The apertural callus length was obtained from photos taken of the callus in the frontal position and was measured at the longest part of the callus. The spire angle was measured as the angle between the lines connecting shoulders of the apical and body whorls (Figure 2a). The umbilicus width was measured by extending the line connecting the base of the thickened aperture with the center of the spiral (Figure 2b). The whorl number of adult shells was counted in intervals of 0.25 following the method described by Sawada and Nakano (2021). The numbers of specimens used for each character are shown in Table 2.
| Number/characters | Sicradiscus hirasei | Sicradiscus ishizakii | Sicradiscus pallgergelyi sp. nov. |
|---|---|---|---|
| Number of specimen examined (CL/EN/HL/other characters) | 10/6/1/17 | 12/3/3/21 | 12/8/3/25 |
| Body whorl length (BWL, mm) | 2.2–2.5 (2.4 ± 0.1) | 2.4–2.9 (2.6 ± 0.2) | 2.4–2.7 (2.6 ± 0.1) |
| Apertural callus length (CL, mm) | 0.30–0.51 (0.36 ± 0.06) | 0.17–0.43 (0.32 ± 0.08) | 0.35–0.54 (0.44 ± 0.04) |
| Shell diameter (D, mm) | 5.4–6.2 (5.8 ± 0.2) | 5.7–7.2 (6.5 ± 0.4) | 6.2–6.9 (6.5 ± 0.2) |
| Embryo number (EN) | 1–5 (3.0 ± 1.5) | 2–7 (4.7 ± 2.5) | 3–6 (4.3 ± 1.3) |
| Shell height (H, mm) | 2.8–3.3 (3.0 ± 0.1) | 2.8–3.6 (3.1 ± 0.2) | 3.1–3.4 (3.2 ± 0.1) |
| Hair length (HL, mm) | 0.40 | 0.27–0.41 (0.35 ± 0.07) | 0.29–0.42 (0.38 ± 0.08) |
| The proportion of shell height to shell diameter (HD) | 0.50–0.55 (0.52 ± 0.01) | 0.45–0.50 (0.48 ± 0.01) | 0.45–0.52 (0.49 ± 0.01) |
| Spire angle (SA, degrees) | 132.9–143.2 (138.9 ± 3.0) | 142.9–154.2 (149.1 ± 3.1) | 139.1–150.4 (144.9 ± 2.5) |
| The proportion of umbilicus width to shell diameter (UD) | 0.35–0.39 (0.37 ± 0.01) | 0.36–0.40 (0.38 ± 0.01) | 0.36–0.43 (0.39 ± 0.02) |
| Umbilicus width (UW, mm) | 2.0–2.4 (2.2 ± 0.1) | 2.1–2.8 (2.5 ± 0.2) | 2.2–2.9 (2.5 ± 0.2) |
| Whorl number (WN) | 5.25–5.50 (5.3 ± 0.1) | 5.00–5.50 (5.3 ± 0.2) | 5.00–5.50 (5.3 ± 0.2) |
Note
- Measurements and counts: minimum–maximum value (mean ± SD).
After obtaining measurements, microstructures of the protoconch and ventral side were observed in 10 adult shells of each species with a Hitachi TM1000 scanning electron microscope (SEM; Hitachi). The plical and lamellar morphology was studied in eight specimens of each species by carefully cracking open the shells. These inner structures of holotype of S. pallgergelyi sp. nov. were observed from the outside of the shell using transmitted light. Additionally, soft parts of three S. ishizakii specimens were extracted by breaking shells. Six S. hirasei, three S. ishizakii, and eight Iriomote Island specimens were dissected under a Leica M125C stereoscopic microscope. The reproductive organ structure of the Japanese snails was photographed after fixation with 70% ethanol. Following the dissection of reproductive organs, the radulae were extracted by soaking oral tissues in 1 M sodium hydroxide solution for a day. Extracted radulae were coated with gold using a JFC-1200 Fine Coater (JEOL) and photographed with an SEM.
A canonical variate analysis (CVA) was conducted to clarify the relationships of shell measurements among the three Sicradiscus species. The CVA was performed on the datasets using five measurements, apertural callus length, shell diameter, shell height, and whorl number, and spire angle. Missing values of apertural callus length were substituted with the measurement average. The CVA data were also used to assign specimens with a leave-one-out cross-validation to estimate the expected actual error rates in classifying the insular Sicradiscus species. The CVA was conducted with PAST 4.04 (Hammer et al., 2001).
Abbreviations. Museum collections: ANSP, Academy of Natural Sciences, Philadelphia, USA; NCP, paratype collection of the Nishinomiya Shell Museum, Hyogo, Japan. Morphometrics: BWL, body whorl length; CL, apertural callus length; D, shell diameter; EN, the number of embryos; H, shell height; HD, the proportion of H to D; SA, spire angle; UD; the proportion of umbilicus width to D; UW, umbilicus width; WN, whorl number. Radulae: EcL, ectocone of a lateral tooth; EcM, ectocone of a marginal tooth; EnL, endocone of a lateral tooth; EnM, endocone of a marginal tooth.
2.3 PCR and DNA sequencing
The phylogenetic relationships of the insular Sicradiscus species and the continental S. schistoptychia were estimated using a mitochondrial marker, a fragment of the cytochrome c oxidase subunit I (COI) gene. Genomic DNA was extracted from muscle tissue of feet following the method described in Okamoto et al. (2006). A 706 bp section of the mitochondrial COI gene which contains the DNA barcoding region was amplified in 16 Sicradiscus snails by polymerase chain reaction (PCR) using a Takara Ex Taq Kit (Takara Bio) and the primers shown in Table 3 with a GeneAmp PCR System 9700 (Thermo Fisher Scientific). The thermocycling regime was an initial denaturation step at 96°C for 1 min, followed by 35 cycles of 1 min at 96°C, 30 s at 42°C, and 1 min at 72°C, and a final extension at 72°C for 7 min. The PCR products were purified as described by Okamoto and Hikida (2009).
Nucleotide sequencing was conducted for PCR products using a BigDye Terminator v3.1 Cycle Sequencing Kit (Thermo Fisher Scientific). The sequencing mixtures were heated to 96°C for 1 min, followed by 25 cycles at 96°C (10 s each), 45°C (30 s each), and 60°C (4 min each). Sequencing was then performed using an Applied Biosystems 3130xl Genetic Analyzer (Thermo Fisher Scientific). The final alignment for the phylogenetic tree reconstructions included 16 sequences which had a length of 655 sites (see Alignment S1). The sequences were deposited in the International Nucleotide Sequence Database Collaboration through the DNA Databank of Japan (LC638856–LC638871).
2.4 Molecular phylogenetic analyses
Phylogenetic trees were reconstructed using maximum likelihood (ML) with IQ-TREE v. 1.6.12 (Nguyen et al., 2015). The following best-fit models for each partition of the COI sequence were identified based on the corrected Akaike information criterion and the greedy algorithm: HKY+F for the 1st position, K2P for the 2nd position, and F81+F for the 3rd position. The robustness of the ML phylogenetic tree was inferred by 1000 non-parametric bootstrap replicates. Mean genetic distances among the three insular Sicradiscus species were estimated with the Kimura two-parameter (K2P) in MEGA v. 10.2.4 (Stecher et al., 2020).
3 RESULTS
3.1 Morphological analyses
The morphometric character measurements are described in Table 2. The EN, H, UD, and WN measurements largely overlapped among the three Sicradiscus species. Although S. ishizakii shared similar BWL, D, HD, and UW measurements with S. pallgergelyi sp. nov., these characters distinguished S. hirasei from the two former species. The characters of CL and SA separated the three species with a slight overlap.
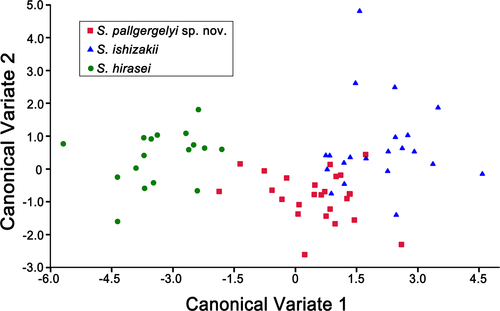
The first and second canonical components explained 90.5% and 100% of the total variance, respectively (Table 4). The variance of CV1 was mainly explained by SA and D. The two canonical components clearly distinguished S. hirasei from the other two congeners. The CVA results also showed that the shell morphology of S. pallgergelyi sp. nov. partly overlapped with that of S. ishizakii (Figure 3). The results of the leave-one-out cross-validation correctly classified 100%, 71.4%, and 88.0% of S. hirasei, S. ishizakii, and S. pallgergelyi sp. nov., respectively.
| CV1 | CV2 | |
|---|---|---|
| Eigenvalue | 4.72 | 0.49 |
| Percent of explained variance | 90.5 | 9.5 |
| Cumulated percent of explained variance | 90.5 | 100.0 |
| Contributions of the variables to the factors: | ||
| Apertural callus length | −0.18 | −1.36 |
| Shell diameter | 1.15 | −1.85 |
| Shell height | 0.26 | 1.40 |
| Spire angle | 1.41 | 0.99 |
| Whorl number | −0.37 | 0.20 |
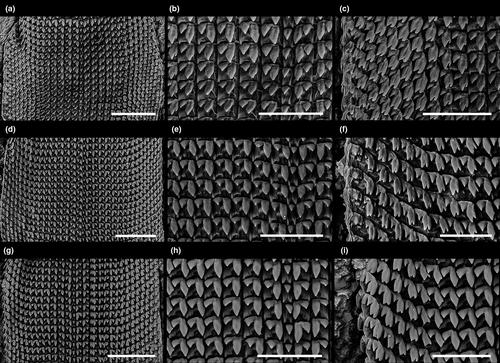
The upper whorl surface of the protoconch was smooth or very finely ribbed in S. hirasei and the new species, whereas S. ishizakii usually possessed remarkable ribs across its entire protoconch surface (Figure 4a,e,i). Sicradiscus ishizakii was also clearly distinguishable from its two congeners by the microstructure of the shell's ventral side (Figure 4b,f,j). Sicradiscus ishizakii had a glossed ventral side with rudimental radial striations, whereas the two other species had strong spiral striations reticulated with radial sculptures.
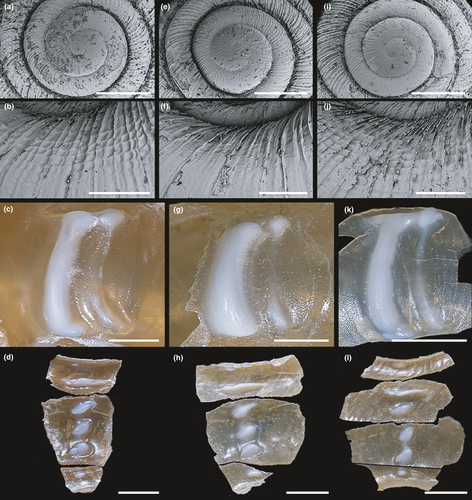
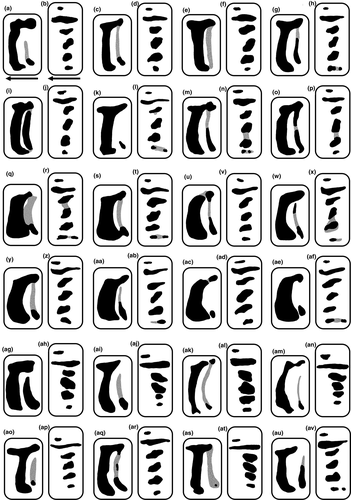
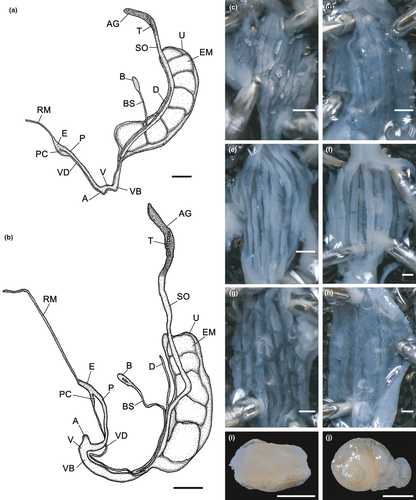
The lamellae shapes were approximately similar in the two Japanese species, although their morphology showed large variation (Figures 4c,g,k and 5a,c,e,g,i,k,m,o,q,s,u,w,y,aa,ac,ae,ag,ai,ak,am,ao,aq,as,au). The anterior lamellae of S. hirasei and S. pallgergelyi sp. nov. were narrow and elongated, and T-shaped (Figure 4c,k). Alternatively, S. ishizakii had thick C-shaped anterior lamellae (Figure 4g). Among the three species, no significant differences were detected in the plicae (Figures 4d,h,l and 5b,d,f,h,j,l,n,p,r,t,v,x,z,ab,ad,af,ah,aj,al,an,ap,ar,at,av), radulae (Figure 6), general reproductive organ morphology, and inner penial wall (Figure 7a–c,e,g). Prominent folds were observed in the inner vaginal wall of S. ishizakii, whereas fine ventral structures were observed in the two Japanese species (Figure 7d,f,h).
3.2 Molecular phylogenetic analyses
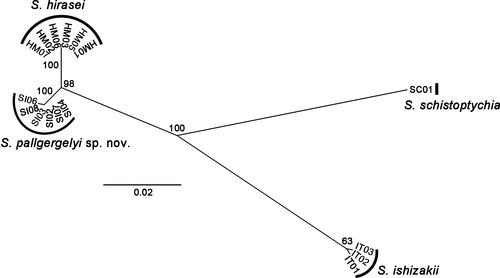
The 655-bp COI section was successfully sequenced for six S. hirasei (LC638865–LC638870), six S. pallgergelyi sp. nov. (LC638859–LC638864), three S. ishizakii (LC638856–LC638858), and one S. schistoptychia (LC638871). All S. hirasei snails had the same haplotype, whereas S. ishizakii and the new species had two different haplotypes each. In the obtained ML tree (Figure 8), each of the Japanese species appears as a strongly supported clade (BS = 100%). A well-supported clade was also formed by the Japanese species (BS = 98%). The two Japanese species had the closest relationship (1.6% of mean K2P distance), and the mean K2P distances among S. ishizakii and the two other species were 6.7% (vs. S. pallgergelyi sp. nov.) and 7.0% (vs. S. hirasei).
3.3 Systematics
It was suggested that a genetic distance of more than 3% is an appropriate boundary between intra- and interspecific differentiation of the COI gene (Hebert et al., 2003). This COI distance is generally accepted in Stylommatophora (e.g., Criscione et al., 2012; Kameda et al., 2007). However, it is also known that the interspecific distances can be as low as 1% in stylommatophoric snails (Davison et al., 2009). In contrast, 18.4% of the genetic distances have been revealed within Orcula dolium (Harl et al., 2014). Although the genetic divergence between S. hirasei and the new species was 1.6% and is thus lower than 3%, they were clearly discriminated by their shell morphology. Their unique shell morphology and the substantial difference in their habitats indicate that they adapted to their habitats upon allopatric divergence and are on independent evolutionary trajectories (see section 4). Therefore, we consider S. pallgergelyi sp. nov. an independent species, although further genetic study is needed.
Plectopylidae Möllendorff, 1898
Sinicolinae Páll-Gergely, 2018
Sicradiscus Páll-Gergely, 2013
Type species: Plectopylis schistoptychia Möllendorff, 1886 by original designation
Sicradiscus hirasei (Pilsbry, 1904)
- Plectopylis (Sinicola) hirasei Pilsbry, 1904: 58–59; Minato, 1980: 88–89, figures 7–10; Minato, 1988: 135; Azuma, 1982: 210, figure 624; Azuma & Azuma, 1984: 89–90, unnumbered figure (genital anatomy); Higo & Goto, 1993: 481; Nature Conservation Division, Department of Environmental Affairs, Okinawa Prefectural Government, 2017: 457.
- Plectopylis hirasei—Pilsbry & Hirase, 1904: 616; Iwakawa, 1919: 207; Brooks & Brooks, 1931: 212; Hirase & Taki, 1951: pl. 124, figure 6; Baker, 1963: 215; Hsieh, 2003: 143, unnumbered figure (adult shell).
- Sicradiscus hirasei—Páll-Gergely & Hunyadi, 2013: 2, 50, 57; Páll-Gergely & Asami, 2014: 558–559; Páll-Gergely, Hunyadi, et al., 2015: 4, 107; Páll-Gergely, 2018a: 86–89, figure 1b,c,f–h; Páll-Gergely, 2018b: 105.
| Characters | Sicradiscus hirasei | Sicradiscus ishizakii | Sicradiscus pallgergelyi sp. nov. |
|---|---|---|---|
| Body whorl length (BWL, mm) | Small (2.4 ± 0.1) | Large (2.6 ± 0.2) | Large (2.6 ± 0.1) |
| Apertural callus length (CL, mm) | Middle (0.36 ± 0.06) | Short (0.32 ± 0.08) | Long (0.44 ± 0.04) |
| Shell diameter (D, mm) | Small (5.8 ± 0.2) | Large (6.5 ± 0.4) | Large (6.5 ± 0.2) |
| The proportion of shell height to shell diameter (HD) | Large (0.52 ± 0.01) | Small (0.48 ± 0.01) | Small (0.49 ± 0.01) |
| Spire angle (SA, degrees) | Small (138.9 ± 3.0) | Large (149.1 ± 3.1) | Middle (144.9 ± 2.5) |
| Umbilicus width (UW, mm) | Small (2.2 ± 0.1) | Large (2.5 ± 0.2) | Large (2.5 ± 0.2) |
| Surface of the protoconch | Smooth or very finely ribbed | Remarkably ribbed | Smooth or very finely ribbed |
| Gloss of the shell ventral side | Absent | Present | Absent |
| Microstructure of the shell ventral side | Both spiral and radial striations | Only radial striations | Both spiral and radial striations |
| Anterior lamellae | Narrow, elongated, T-shaped | Thick, C-shaped | Narrow, elongated, T-shaped |
Note
- Measurements show mean ± SD.

Material examined. Lectotype, ANSP 87632 (photos examined). Newly collected materials. 17 adult shells collected from crevices in the limestones on the ground in the inland natural forest in Miyako Island, Miyakojima City, Okinawa Prefecture, Japan on October 18, 2020, KUZ Z3942.
Amended diagnosis. Shell very small, lenticular, thin, dextral (D 5.8 ± 0.2 mm [mean ± SD]; H 3.0 ± 0.1 mm) with slightly keeled body whorl; apex moderately protruding (HD: 0.52 ± 0.01; SA: 138.9 ± 3.0 degrees); periostracal folds short, on dorsal side of shell (HL 0.40 mm); teleoconch surface matte, not glossy, with strong ribs and spiral striations on ventral side; parietal callus mildly elevated (CL: 0.36 ± 0.06 mm); apertural fold very weak or absent; parietal wall with slightly curved and elongated anterior lamella and weaker vestigial posterior one; palatal plicae in one row, slender, rounded, and oblique; reproductive system with penial caecum; inner penial wall with longitudinal folds that form pocket-like pouches.
Measurements of lectotype. Adult shell: Measurements: BWL 2.3 mm; D 5.7 mm; H 3.0 mm; HD 0.53; SA 129.3 degrees; UD 0.39; UW 2.2 mm; WN 5.25.
Description of newly collected materials. Adult shell: Measurements are shown in Table 2. Shell lenticular, with bluntly shouldered body whorl and with conical and moderately protruding apex; light brown, translucent; 5.25–5.50 whorls separated by shallow suture; first 0.25 whorl smooth or very finely ribbed; remaining 1.75–2.00 whorl finely ribbed (Figure 4a); radial and spiral lines of comparable strength on dorsal side; same structure on the upper half of the body whorl; ventral side with stronger radial sculpture and spiral striations (Figure 4b); periostracal fold visible in fresh specimen on keel (HL 0.40 mm); umbilicus deep and wide; apertural margin thickened and strongly reflected; parietal callus mildly to strongly elevated; aperture without or with very weak fold connected to the parietal callus.
Lamellae and plicae (Figures 4c,d and 5a–p): Parietal wall with two vertical lamellae; moderately curved and elongated anterior lamella variable from near straight line to C- to T-shaped line; dorsal end of anterior one elongated anteriorly and posteriorly; weaker vestigial posterior one usually connected to anterior one; palatal plicae in one row with six horizontal or oblique plicae; first and sixth plicae on dorsal side small and horizontal; 6th plicae usually split in 2 horizontal parts; second longest and most slender; thick third one slender to rounded; thick, rounded, and oblique 4th and 5th rarely connected with weak prominence.
Radulae (Figure 6a–c): pointed central teeth same length as or slightly longer than EcL; lateral tooth in six rows with pointed EcL, EnL, EnL 2–2.5 times longer than EcL; 8–10 row marginal tooth bear pointed EcM, EnM. EnMs with incisions 1/4 to 1/6 the length of the tooth 2.5–3 times longer than EcM.
Genitalia: Atrium short; penis consisting of thicker proximal and slimmer distal portions, of comparable length; internally with elevated longitudinal folds that form pocket-like pouches (Figure 7c); calcareous crystals observed in penis lumen in three specimens of six examined ones; penial caecum well-developed, approximately half as long as proximal part of penis; slender elongated retractor muscle inserts on apical part of penial caecum; epiphallus joining penis laterally at base of caecum; vas deferens slender, long, distal end gradually thickened to the base of spermoviduct; vagina with slightly thickened vaginal bulb, slightly slender near atrium; several weak fibers attaching vaginal bulb to body wall; inner vaginal wall with fine folds (Figure 7d); calcareous crystals found in vagina lumen in two specimens; narrow stalk of the bursa copulatrix branch off from the base of spermoviduct connect to elongated oval bursa copulatrix; narrow diverticulum start from slight distal portion of stalk of the bursa copulatrix extends almost the same length as the end of bursa copulatrix; spermoviduct long, approximately the same thickness as the proximal portion of penis; uterus contained 1–5, well-developed embryo consisting of 2.5 whorls; talon relatively small; albumen gland short.
Remarks. The reproductive anatomy of S. hirasei was first shown in Azuma and Azuma (1984). Although the genital characteristics examined in that study excluded the penial caecum, vaginal bulb, and diverticulum, those characters were recently described (Páll-Gergely, 2018a). Reproductive organs of the newly collected snails in this study almost corresponded to those in Páll-Gergely (2018a) because a penial caecum, vaginal bulb, and diverticulum were present. However, the vaginal bulb was not well developed and was almost as thick as the vagina.
Sicradiscus hirasei can be discriminated from S. pallgergelyi sp. nov. by its smaller shell with more protruding apex (larger HD, smaller SA) and less elevated parietal callus (middle CL; Table 5). The keeled body whorl and absence of strong folds in the aperture distinguishes S. hirasei from the western group species of the genus and S. transitus. Sicradiscus hirasei is also distinct from S. ishizakii and the other continental congeners by the matte ventral side, a slightly curved and elongated anterior lamella, and palatal plicae in one row consists of rounded and oblique plicae.
Sicradiscus ishizakii (Kuroda, 1941)
- Plectopylis ishizakii Kuroda, 1941: 188–189, pl. 7 figures 42, 43; Hwang et al., 2008: 58, figure 5a,b; Nature Conservation Division, Department of Environmental Affairs, Okinawa Prefectural Government, 2017: 457.
- Plectopylis (Sinicola) ishizakii—Minato, 1980: 89; Chang & Ookubo, 1999: 21–28, plates 1–2, figures 1–3.
- Plectopylis (Chersaecia) ishizakii—Higo & Goto, 1993: 481.
- Sicradiscus ishizakii—Páll-Gergely & Hunyadi, 2013: 2, 50; Páll-Gergely & Asami, 2014: 558–559; Páll-Gergely, Hunyadi, et al., 2015: 4, 107, 111; Páll-Gergely, 2018a: 86–89, figure 1a, d–e; Páll-Gergely, 2018b: 105.
Material examined. Paratype, NCP-244 (photos examined). Newly collected materials. 5 adult shells collected from JianShih Town, Xinzhu County, Taiwan, on July 19, 2017, KUZ Z3943. Fourteen adult shells collected from the same region on January 29, 1997, KUZ Z3944. 1 adult shell collected from the same region on March 21, 2017, KUZ Z3945. 1 adult shell collected from Guanxi Town, Xinzhu County, on August 31, 2017, KUZ Z3946.
Amended diagnosis. Shell small, lenticular, rather thick, dextral (D 6.5 ± 0.4 mm; H 3.1 ± 0.2 mm) with slightly keeled body whorl; apex bluntly protruding (HD 0.48 ± 0.01; SA 149.1 ± 3.1 degrees); periostracal folds short on dorsal side of shell (HL 0.35 ± 0.07); entire protoconch remarkably ribbed; teleoconch surface glossy, with strong ribs and without or with very fine spiral striations on ventral side; callus mildly elevated (CL 0.32 ± 0.08 mm); apertural fold absent or very weak; parietal wall with moderately curved, thick, C-shaped anterior lamella and weaker vestigial posterior one; palatal plicae in one row with slender, rounded, and oblique plicae; reproductive system with penial caecum; inner penial wall with longitudinal folds that form pocket-like pouches.
Measurements of paratype. Adult shell: Measurements: BWL 2.7 mm; D 6.7 mm; H 3.3 mm; HD 0.49; SA 145.7 degrees; UD 0.39; UW 2.6 mm; WN 5.50.
Description of newly collected materials. Adult shell: Measurements are shown in Table 2. Shell lenticular; with bluntly keeled body whorl and with conical and slightly protruding apex; pale yellow or light brown, translucent; whorls separated by shallow to medium depth suture; entire protoconch remarkably ribbed (Figure 4e); dorsal side with prominent radial sculpture, weak spiral lines; same structure on the upper half of the body whorl; ventral side with strong radial ribs, without or with rudimental spiral striations (Figure 4f); short periostracal folds visible in fresh specimens on keel (HL 0.27–0.41 mm); umbilicus deep and rather narrow; apertural margin thickened and strongly reflected; parietal callus mildly elevated; aperture without or with very weak fold connected to the parietal callus.
Lamellae and plicae (Figures 4g,h and 5q–af): Parietal wall with two vertical lamellae; moderately curved, thick anterior lamella C-shaped line; dorsal end of anterior one elongated anteriorly and posteriorly; weaker vestigial posterior one usually connected to anterior one; palatal plicae in one row with 6 horizontal or rounded plicae; first and sixth plicae on dorsal side small and horizontal; 6th plicae rarely split in 2 horizontal parts; second longest and most slender; rounded, and oblique third, 4th, and 5th rarely connected with weak prominence.
Radulae (Figure 6d–f): pointed central teeth same length as or slightly longer than EcL; lateral tooth in seven rows with pointed EcL, EnL, EnL 2–3 times longer than EcL; 9–10 row marginal tooth bear pointed EcM, EnM. EnMs with incisions 1/4 to 1/5 the length of the tooth 2.5–3 times longer than EcM.
Genitalia (Figure 7a,e,f,i): Atrium short; penis consisting of thicker proximal and slimmer distal portions, of comparable length; internally with elevated longitudinal folds that form pocket-like pouches; calcareous crystals observed in penis lumen in all three examined specimens; penial caecum well-developed, approximately half as long as proximal part of penis; slender elongated retractor muscle inserts on apical part of penial caecum; epiphallus joining penis laterally at base of caecum; vas deferens slender, long, distal end gradually thickened to the base of spermoviduct; vagina with slightly thickened vaginal bulb, slightly slender near atrium; several weak fibers attaching vaginal bulb to body wall; inner vaginal wall with prominent folds; calcareous crystals found in vagina lumen in one snail; narrow stalk of the bursa copulatrix branch off from the base of spermoviduct connect to elongated oval bursa copulatrix; narrow diverticulum start from slight distal portion of stalk of the bursa copulatrix extends almost the same length as the end of bursa copulatrix; spermoviduct long, approximately the same thickness as the proximal portion of penis; uterus contained 2–7, well-developed embryo consisting of 2.5 whorls; talon relatively small; albumen gland short.
Remarks. The radular morphology and reproductive anatomy of S. ishizakii were described by Chang and Ookubo (1999). Although there was variation in number of tooth rows was observed in this study, the radular morphology of this species was concordant with the description of the previous study.
Genital morphologies of the newly collected specimens possessed several discordances with the preceding study; that is, there was a penial caecum and diverticulum, which were not previously found. In this study, there was a penis branched off from a vagina near the atrium; the preceding study observed a penis at a distal portion of a vagina. Prominent folds observed in the inner vaginal wall distinguished the examined S. ishizakii from the two other species. However, the seasonal change in the characteristics should be clarified because reproductive organ morphology has been proposed to vary relative to mating season (Páll-Gergely, Hunyadi, et al., 2015).
Sicradiscus ishizakii can be distinguished from the other insular species by a flatter shell (smaller HD, larger SA), a less developed parietal callus (smaller CL), and a glossy ventral side (Table 5). The shell with keeled body whorl and the lack of apertural fold discriminates this species from the western group species of Sicradiscus and S. transitus. Sicradiscus ishizakii is also distinguishable from the other continental congeners by a moderately curved, thick, C-shaped anterior lamella and palatal plicae in one row with slender, rounded, and oblique plicae.
Sicradiscus pallgergelyi Sawada & Nakano, sp. nov.
Tables 2 and 5; Figures 4i–l, 5ag–av, 6g–i, 7b,g,h,j, 9g–l and 10.

(New Japanese name: Yaeyama-itokakemaimai)
http://zoobank.org/urn:lsid:zoobank.org:act:E3250AC4-6494-438C-B591-1F5C99F9AA0E
Material examined. Holotype. KUZ Z3947, adult shell collected from gaps in fallen leaves on the ground in the inland natural forest at an elevation of around 200 m in Iriomote Island, Taketomi Town, Yaeyama County, Okinawa Prefecture, Japan, on October 15, 2020. Paratypes. Four specimens collected with the holotype, KUZ Z3948–Z3951. Additional materials. Twenty adult specimens collected with the holotype, KUZ Z3952.
Diagnosis. Shell small, lenticular, rather thick, dextral (D 6.5 ± 0.2 mm; H 3.2 ± 0.1 mm) with bluntly keeled body whorl; apex moderately protruding (HD 0.49 ± 0.01; SA 144.9 ± 2.5 degrees); periostracal folds on dorsal side of shell (HL 0.38 ± 0.08 mm); teleoconch surface matte, not glossy, with strong ribs and spiral striations on ventral side; callus very strongly elevated (CL 0.44 ± 0.04 mm); apertural fold absent; parietal wall with slightly curved and elongated anterior lamella and weaker vestigial posterior one; palatal plicae in one row with rounded and oblique plicae; reproductive system with penial caecum; inner penial wall with longitudinal folds that form pocket-like pouches.
Description of holotype. Adult shell (Figure 9g–i): Measurements: BWL 2.6 mm; D 6.3 mm; EN 6; CL 0.47 mm; H 3.1 mm; HD 0.49; SA 147.7 degrees; UD 0.39; UW 2.5 mm; WN 5.50. Shell lenticular; with slightly keeled body whorl and with conical and moderately protruding apex; pale yellow, translucent; whorls separated by rather deep suture; first 0.25 whorl smooth; remaining 1.75 whorl very finely ribbed; radial and spiral lines of comparable strength on dorsal side; same structure on the upper half of the body whorl; ventral side with stronger radial sculpture and spiral striations; periostracal folds visible in fresh specimens on keel (HL 0.42 mm); umbilicus deep and wide; apertural margin thickened and strongly reflected; parietal callus very strongly elevated; aperture without fold connected to the parietal callus.
Parietal lamellae: Not examined.
Palatal plicae: one row with 6 horizontal or rounded plicae; first and sixth plicae on dorsal side small and horizontal; second longest, most slender, and horizontal; remaining ones thick, rounded, and oblique.
Radula: pointed central teeth same length as or slightly longer than EcL; lateral tooth in seven rows with pointed EcL, EnL, EnL three times longer than EcL; nine row marginal tooth bear pointed EcM, EnM. EnMs with incisions 1/5 the length of the tooth three times longer than EcM.
Genitalia: Atrium short; penis consisting of thicker proximal and slimmer distal portions, of comparable length; internally with elevated longitudinal folds that form pocket-like pouches; penis lumen without calcareous crystals; penial caecum well-developed, approximately half as long as proximal part of penis; slender elongated retractor muscle inserts on apical part of penial caecum; epiphallus joining penis laterally at base of caecum; vas deferens slender, long, distal end gradually thickened to the base of spermoviduct; vagina with slightly thickened vaginal bulb, slightly slender near atrium; several weak fibers attaching vaginal bulb to body wall; inner vaginal wall with fine folds; vagina lumen without calcareous crystals; narrow stalk of the bursa copulatrix branch off from the base of spermoviduct connect to elongated oval bursa copulatrix; narrow diverticulum start from slight distal portion of stalk of the bursa copulatrix extends almost the same length as the end of bursa copulatrix; spermoviduct long, approximately the same thickness as the proximal portion of penis; uterus contained 6, well-developed embryo consisting of 2.5 whorls; talon relatively small; albumen gland short.
Variation. Adult shell (Figure 9j–l): Measurements are shown in Table 2. Shell pale yellow or light to dark brown; first 0.25–0.50 whorl smooth; remaining 1.50–1.75 whorl very finely ribbed; parietal callus moderately to very strongly elevated; aperture without or with very weak fold connected to the parietal callus.
Lamellae and plicae (Figures 4k,l and 5ag–av): Parietal wall with two vertical lamellae; slightly to moderately curved and elongated anterior lamella variable from near straight line to C- to T-shaped line; dorsal end of weaker vestigial posterior one elongated anteriorly and posteriorly and usually connected to anterior one; palatal plicae in one row with 6 horizontal or rounded plicae; first and sixth plicae on dorsal side small and horizontal; second longest and most slender; remaining ones thick, rounded, and oblique.
Radulae (Figure 6g–i): lateral tooth in 6–7 rows with pointed EcL, EnL, EnL 2–2.5 times longer than EcL; nine rows marginal tooth bear pointed EcM, EnM. EnMs with incisions 1/4 to 1/6 the length of the tooth 2.5–3 times longer than EcM.
Genitalia (Figure 7b,g,h,j): calcareous crystals found in penis lumen in 3 specimens of 8 examined ones; calcareous crystals found in vagina lumen in 1 specimen; uterus contained 3–6, well-developed embryo consisting of 2.5 whorls.
Distribution and ecology. The new species was found from a narrow area in the interior of Iriomote Island, Japan. The fossil recorded from Ishigaki Island and identified as S. hirasei may belong to this species (Nature Conservation Division, Department of Environmental Affairs, Okinawa Prefectural Government, 2017).
Etymology. The specific name is dedicated to Dr. Barna Páll-Gergely, who greatly contributed to the systematics of the family Plectopylidae.
Remarks. The new species can be discriminated from S. hirasei by the larger shell size (larger BWL, D, H, UW) of the former species (Table 5). The new species also possesses a flatter shell (smaller HD, larger SA) and a more developed parietal callus (larger CL). Although the shell morphology of S. pallgergelyi sp. nov. is similar to that of S. ishizakii because it has a larger and flatter shell (similar values in BWL, D, H, HD, UW), the new species exhibits a more protruding apex (larger SA) and a more developed parietal callus (larger CL) than the Taiwanese species. The new species has a shell whose ventral side is not glossy, and has strong ribs and spiral striations. In contrast, the ventral side of S. ishizakii has a glossy appearance because it lacks spiral striae.
The matte ventral side of the new species distinguishes this species from its congeners. The new species is distinguishable from S. feheri, S. mansuyi, S. invius, S. secures, which belong to the western group of Sicradiscus and S. transitus by the presence of keeled body whorls and absence of strong folds from the aperture. The new species is also distinct from seven Chinese and Vietnamese congeners, S. feheri, S. invius, S. cutisculptus, S. schistoptychia, S. transitus, S. diptychia, S. securus, and S. mansuyi by the following internal structures: elongated and slender anterior lamella; vestigial and slender posterior lamella; and palatal plicae in one row consists of rounded and oblique plicae that are not usually connected.
4 DISCUSSION
4.1 Genital morphology of insular Sicradiscus species
The genus Sicradiscus has been subdivided into two species groups based on shell characters (Páll-Gergely & Hunyadi, 2013). The new species is clearly a member of the eastern group of the genus based on its slightly keeled body whorl and no fold in the aperture. Observations of reproductive organs in previous studies (Azuma & Azuma, 1984; Páll-Gergely, 2018a; Páll-Gergely & Asami, 2014; Páll-Gergely & Hunyadi, 2013; Páll-Gergely, Hunyadi, et al., 2015) revealed that each group has different genital characteristics (Páll-Gergely, 2018a). The western group species have a very weakly developed penial caecum and pockets in their inner penial wall, whereas the eastern group species have a well-developed penial caecum and parallel folds in their inner penial wall.
In the present study, the general morphology of S. hirasei genitalia was mostly concordant with that of Páll-Gergely (2018a) (see Remarks of S. hirasei). However, the previously overlooked penial caecum and diverticulum were discovered in the reproductive organs of S. ishizakii. The new species also possessed a clearly distinguishable penial caecum. Accordingly, it is even more likely that the members of the eastern group share the characteristics of a well-developed penial caecum, although the reproductive anatomy of S. cutisculptus and S. diptychia is still not known.
This study revealed the presence of slit-like pockets in the inner penial wall of the three examined species. In contrast to the penial caecum results, these pockets were not previously detected in the eastern group. As indicated in Páll-Gergely, Hunyadi, et al. (2015), the inconsistencies between the present and previous observations may be due to seasonal changes because the samples were collected in different seasons [Páll-Gergely (2018a), December; this study, October]. Therefore, the inner penial wall structure is unlikely to significantly differ between the groups, and further investigation is necessary to clarify the seasonal change in these reproductive structures.
Calcareous granules were observed in the inner penial and/or vaginal wall of the three Sicradiscus species. It was suggested that the penial granules function as a mating apparatus for disposable males and are lost when snails bear offspring (Páll-Gergely, Hunyadi, et al., 2015). In this study, however, all nine individuals with granules on the inner penial wall possessed embryos, and no significant difference was detected in the number or size of embryos between the snails with or without the granules. The vaginal granules observed in the three Sicradiscus species have only been previously described from two other species in the family, Halongella schlumbergeri (Morlet, 1886) and Halongella fruhstorferi (Möllendorff, 1901). These granules have also been proposed to be seasonal and associated with mating season (Páll-Gergely, Hunyadi, et al., 2015). In this analysis, some snails had penial and vaginal granules and some did not, even within the same population collected on the same day; this indicates that the reproductive cycle of each snail may also affect granule appearance.
4.2 Morphological evolution of insular Sicradiscus species
The present morphological examination discriminated the three Sicradiscus species with the measurements of the apertural callus length and the spire angle. The four other quantitative characters separated S. hirasei from the other species. CVA based on the measurements of five characters also differentiated S. hirasei from S. pallgergelyi sp. nov. and S. ishizakii, which indicates that S. hirasei has a unique shell morphology. Moreover, S. ishizakii was distinguished from S. hirasei and the new species by the ventral sculpture of its shell and characteristics of its protoconch, lamellae, and plicae. The molecular phylogenetic analyses revealed that shell measurements of the insular Sicradiscus species seem to have higher plasticity than shell structure. In addition, the ventral sculpture characteristics can be estimated to be a common trait of Japanese lineages, and the Taiwanese species share characteristics with the continental congeners.
The CL measurements were largest in the new species and smallest in S. ishizakii. Although the function of the apertural callus is still unclear, it may help prevent natural enemies from entering the aperture if the apertural folds serve a similar function in land snails as they do in Vertiginidae and Diapheridae (Gittenberger, 1995; Solem, 1972). In the genus Satsuma A. Adams, 1868, it was suggested that strong apertural folds evolved by predation pressure from snail-eating snakes of family Pareatidae in the Ryukyu Islands and Taiwan (Hoso & Hori, 2008). Even though snakes are not predators of Sicradiscus species due to the snails' small size, morphological evolution in the apertural callus may be promoted by predation pressures from snail-eating fireflies, which occur on each of the Ryukyu Islands (Ohba, 2004). The relationship between aperture size and water loss revealed that the apertural callus may also prevent water loss from the aperture by reducing the amount of air that passes through the aperture (Machin, 1967).
The proportion of shell height to shell diameter of S. hirasei found from gaps in limestone was larger than that of the new species, which was found in gaps in fallen leaves on the ground. Land snails with a low-spired shell tend to prefer low-angle or horizontal surfaces, whereas the species with intermediate shell shapes are active on a wide variety of angles (Cain & Cowie, 1978; Cameron, 1978). It was also theorized that a low-spired shell is well-balanced in both horizontal and vertical substrates (Okajima & Chiba, 2009). In addition, it is known that a flat shell morphology is more advantageous in narrow spaces such as rock crevices (Goodfriend, 1986). Because Sicradiscus species are small, it is unclear whether shell balance significantly contributes to shell morphology. However, the present results of the larger HD of the rock dweller may be explained by their habitat use: S. hirasei is active on rocks with various angular conditions, whereas the other species are restricted to the vicinity of the horizontal ground. It was also shown that land snails in limestone areas tend to thicken their shells in response to calcium availability (Owen, 1965), although the S. hirasei shells were the thinnest among the three species. Because shell thickness is also related to predation pressure (Moreno-Rueda, 2009), different predation pressures among islands may potentially affect the shell thickness of Sicradiscus species.
The two Japanese species are phylogenetically closely related, which indicates that major morphological shifts occurred in the two species, even though Sicradiscus species are estimated to experience only slight conchological changes (Páll-Gergely & Hunyadi, 2013). The aforementioned morphological differences between S. hirasei and the new species strongly indicate that the common ancestor of the Japanese lineage expanded to different islands and underwent different selection pressures, which resulted in speciation.
4.3 Radular morphology
The radular morphology of Sicradiscus was examined in S. ishizakii and four other species by Chang and Ookubo (1999) and Páll-Gergely, Hunyadi, et al. (2015), and the latter study also reported that the shape of the radulae was highly preserved among related genera. In this examination, significant differences were not observed in radular morphology among the three Sicradiscus species, and the S. ishizakii radulae corresponded to those described by Chang and Ookubo (1999). The radulae of the three species consisted of a relatively large central tooth, a lateral tooth with ectocones as large as cusps of a central tooth, and tricuspid marginal teeth with pointed cusps and deep incisions between the cusps; these characteristics are similar to those of the radulae of other congeners and members of Sinicola and Gudeodiscus (Páll-Gergely, Hunyadi, et al., 2015).
4.4 Taxonomic position of other insular populations of Sicradiscus
The new species was discovered in a well-preserved natural forest at an elevation of approximately 200 m on Iriomote Island. In addition to the record on the island, fossil specimens previously identified as S. hirasei were found on Ishigaki Island between Miyako Island and Taiwan (Nature Conservation Division, Department of Environmental Affairs, Okinawa Prefectural Government, 2017). Although only fossil specimens have been collected from Ishigaki Island, extant Sicradiscus snails may be distributed on the island because the natural forests are preserved on the island and are similar to the habitat of the new species. Taking into account the similarity of the land snail faunas between the two islands (Habe & Chinen, 1974), the fossil records on Ishigaki Island likely belong to S. pallgergelyi sp. nov.
The length of the apertural callus distinguishes the three insular species. Although the apertural structure may facilitate self-protection, a population without callus was also found in central Taiwan (Lee & Chen, 2003). This population also has a smaller number of ribs on the ventral side of the shell compared with S. ishizakii of northern Taiwan and the Japanese species. Because there are other species in northern and central Taiwan (Hsieh, 2003) and the length of the callus may be related to different selection pressures, this central population may be an independent species with a unique evolutionary history. The systematic position of the populations in Ishigaki Island and central Taiwan should be examined in future studies.
ACKNOWLEDGEMENTS
We are very grateful to Dr. Barna Páll-Gergely (Plant Protection Institute, Centre for Agricultural Research) for providing materials and helpful comments on our manuscript. We also thank two anonymous reviewers for their constructive comments on the manuscript. We are also grateful to Miyakojima City for providing permission for Sicradiscus sample collection. We also thank Jamen Uiriamu Otani, Taiji Kurozumi, Takashi Hosoda, and Kanji Ookubo for providing information on Sicradiscus. The first author is grateful to Jamen Uiriamu Otani, Yuji Nakahara, Wei Lin, Dr. Yuta Morii (Kyoto University; KU), Tatsuki Nishioka (KU), and Iriomote Station of Ryukyu University, Japan, for assisting with sample collection. The first author also thanks Dr. Taku Okamoto, Tomohisa Makino, Yusuke Sugawara, Tomoki Kadokawa, Professor Minoru Tamura (KU), and Dr. Takahiro Hirano (Tohoku University) for supporting SEM observations, preparation of the figure of the map, and morphological and phylogenetic analyses. This study was financially supported in part by JSPS KAKENHI Grant Number JP21J22917 and the Tokyo Metropolitan University Fund for TMU Strategic Research (Leader: Professor Noriaki Murakami at TMU; FY2020–FY2022). We thank Dr. Eckstut Mallory (Edanz) for editing a draft of this manuscript.




