Phylogenetic review of the tribal system of Aleocharinae, a mega-lineage of terrestrial arthropods in need of reclassification
Contributing authors: Igor Orlov ([email protected]) and Alfred F. Newton ([email protected])
Abstract
Recently published molecular and total evidence phylogenies of the mega-diverse rove beetle subfamily Aleocharinae defined a backbone for the greatly needed further detailed phylogenetic research and large-scale reclassification of this lineage. Given the enormous species diversity of Aleocharinae, wise taxon sampling is crucial for such research. To facilitate that and place future systematic investigations on a solid phylogenetic basis, here we provide a review of all 62 current tribes of Aleocharinae based on information from 191 references, where we summarize all outstanding phylogenetic questions ever considered about each tribe. For each tribe, we provide current diagnoses as they are understood now and, where possible, we stress emerging monophyletic clades within or across the current legacy tribes and provide their diagnostic features too.
1 INTRODUCTION
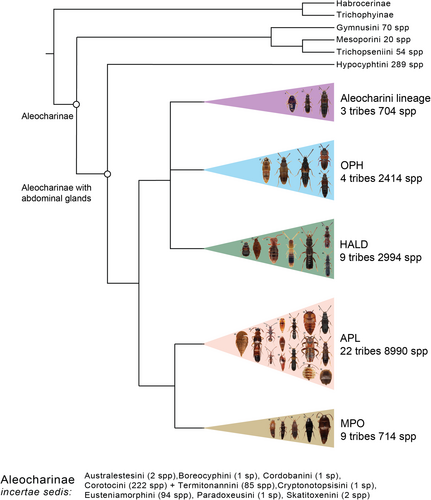
Aleocharinae are one of the biggest groups of the morphologically (Figures 1-11-57-62) and ecologically (Table 1) very diverse terrestrial invertebrates common in any ecological sample, especially from ground-based microhabitats. A significant portion of the aleocharine diversity is formed by highly modified symbionts of ant and termite societies (Figures 1-11, 12-23, 24-34, 35-45, 46-56, 57-62, 20, 25, 32, 37, 39, 44–45, 49–53, 56–62 and Table 1), an unusual feature of this beetle radiation. Naturally, having a well-developed taxonomy and systematics is important for making this notable insect lineage manageable and accessible for biologists of all kinds. Yet, the actual situation is very far from that because Aleocharinae continue to be systematically one of the least known and difficult to study organismal groups, even for specialists within the family Staphylinidae.






| Tribe | Number of described genera/species | Biology | Distribution |
|---|---|---|---|
| 1. Actocharini | 1/3 | Intertidal habitat. Occur on sand beaches | Mediterranean and Atlantic coasts of Europe |
| 2. Aenictoteratini | 10/17 | Myrmecophilous | Oriental region and one species in Australia |
| 3. Akatastopsisini | 1/1 | Biology unknown | Papua New Guinea |
| 4. Aleocharini | 28/677 | Free-living predators in diverse habitats including sea shores, mammal nests and occasionally caves, many coprophilous, some myrmeco- or termitophilous. Larvae of some species are fly parasitoids. | Globally distributed |
| 5. Antillusini | 1/1 | Biology unknown | Dominican Republic |
| 6. Athetini | 244/4104 | Free-living predators in diverse habitats including dung or sea shores, occasionally cavernicolous, some myrmeco- or termitophilous | Globally distributed |
| 7. Australestesini | 2/2 | Biology unknown | Australia |
| 8. Autaliini | 5/54 | Free-living predators associated with fungi and various decaying matter, some myrmecophilous | Globally distributed except Australia and New Zealand |
| 9. Boreocyphini | 1/1 | Riparian habitats in forests | Canada |
| 10. Cordobanini | 1/1 | Biology unknown, possible myrmeco- or termitophilic association | Mexico |
| 11. Corotocini | 67/222 | Termitophilous | Pantropical distribution |
| 12. Crematoxenini | 11/18 | Myrmecophilous | Known only from the New World |
| 13. Cryptonotopsisini | 1/1 | Biology unknown | Malaysia |
| 14. Diestotini | 7/341 | Free living in leaf litter, mosses, apparently predators. Some species are found in bird nests, on flowers, on rotting fruits | Nearly global distribution except for Eastern Palaearctic, New Zealand and South Africa |
| 15. Diglottini | 2/9 | Confined to upper intertidal zone and sea shores | Europe, USA, introduced to Canada, Brazil, Fiji Is., Djibouti. One species in New Zealand |
| 16. Digrammini | 1/1 | Specimens are known from tunnels of wood-boring beetles | New Zealand |
| 17. Dorylogastrini | 2/13 | Myrmecophilous | Afrotropical region. One species in Malaysia |
| 18. Dorylomimini | 4/37 | Myrmecophilous | Afrotropical region and South Africa |
| 19. Drepanoxenini | 1/8 | Termitophilous | Australia |
| 20. Ecitogastrini | 1/6 | Myrmecophilous | Mexico, Panama and Brazil |
| 21. Eusteniamorphini | 3/94 | Apparently free-living predators | Oriental and Afrotropical regions |
| 22. Falagriini | 33/471 | Free-living predators, some coastal, some myrmeco- or termitophilous | Globally distributed |
| 23. Feldini | 10/28 | Termitophilous | Malaysia, Singapore and Indonesia |
| 24. Geostibini | 13/905 | Free-living predators in moss and leaf litter, some cavernicolous or live in mammal nests, some myrmeco- or termitophilous | Nearly worldwide (introduced in Australia, New Zealand, Chile and Argentina) |
| 25. Gymnusini | 8/68 | Free-living predators in wet habitats such as marshes, swampy places beside ponds etc. | Worldwide |
| 26. Himalusini | 3/4 | Adults and larvae of H. thailandensis feed on leaves of sewer vine | China, Japan, Nepal and Thailand |
| 27. Homalotini | 164/2538 | Some species are restricted to coastal habitats, some myrmeco- or termitophilous. Many of Gyrophaenina and Bolitocharina are inhabitants of fresh mushrooms, upon which the larvae and adults feed. Homalotina and Silusina are subcortical and wood-inhabiting | Worldwide |
| 28. Hoplandriini | 24/365 | Many species are predatory on dipteran larvae within decaying organic debris. Also occur in association with flowers of various kinds | Mostly restricted to the Neotropics and the tropical part of the Oriental region |
| 29. Hygronomini | 9/44 | Probably free-living predaceous, in leaf litter | Palaearctic region, South America and Africa (Tanzania and Yemen) |
| 30. Hypocyphtini | 11/228 | Adults often found on flowers and on foliage, in decaying organic matter such as mushrooms, dung, in old hay and grass, and in other decomposing materials, where they feed on mites. Some species are known as associated with ants | Nearly global in distribution |
| 31. Leucocraspedini | 1/106 | Nothing detailed is known about their biology. Probably free-living predaceous | Known from East and Southeast Asia, the Afrotropical and Australian regions, Madagascar and New Guinea |
| 32. Liparocephalini | 8/34 | Exclusively restricted to coastal and intertidal habitats which usually contain decaying seaweed | Pacific coasts of North America, Asia and New Zealand |
| 33. Lomechusini | 224/2518 | Many associated with ants or termites; the majority are probably free-living predators. A few species are strictly cavernicolous. A few found in bird and mammal nests | Known from all regions of the world except oceanic islands, temperate South America and New Zealand |
| 34. Masuriini | 28/1 | Free living, probably predaceous, in leaf litter. Some species are found up to 4000 m above the sea level | Oriental region (India, Nepal, China) |
| 35. Mesoporini | 12/20 | Many species are found under bark or in rotten trees, some species are associated with termites | Nearly worldwide |
| 36. Mimanommatini | 30/171 | Myrmecophilous | Africa and Oriental region |
| 37. Mimecitini | 14/25 | Myrmecophilous | Neotropical region |
| 38. Myllaenini | 14/337 | Free-living predators, some restricted to coastal and intertidal habitats | Worldwide |
| 39. Oxypodini | 173/1864 | Free-living predators, some coastal, cavernicole or found in bird and mammal nests, some species are associated with ants | Worldwide |
| 40. Oxypodinini | 6/39 | Free living, some species apparently pollen-feeding | Madagascar, South Africa and the Neotropical region |
| 41. Paglini | 2/14 | Free living, spore-feeders | Chile, Brazil, Peru |
| 42. Paradoxenusini | 1/1 | Myrmecophilous | Argentina |
| 43. Pediculotini | 1/1 | Free living in leaf litter, probably predaceous | Hungary and Romania |
| 44. Philotermitini | 2/12 | Termitophilous | Nearctic and Neotropical regions |
| 45. Phyllodinardini | 1/3 | Myrmecophilous | Afrotropical region |
| 46. Phytosini | 3/10 | Restricted to coastal habitats | Europe, North Africa, Taiwan, Guadeloupe (West Indies) and New Zealand |
| 47. Placusini | 5/189 | Free living in leaf litter, rotting fruit, in bird nests. A few are myrmecophilous | Nearly global distribution (absent in New Zealand) |
| 48. Pronomaeini | 7/94 | Free living, myrmecophilous, in bird nests | almost global distribution except for Australia and New Zealand |
| 49. Pseudoperinthini | 4/26 | Termitophilous | Australia, Oriental and Pacific regions |
| 50. Pygostenini | 29/268 | Myrmeco- and termitophilous | Africa and the Oriental and West Palearctic regions |
| 51. Sahlbergiini | 4/9 | Myrmecophilous | Oriental and Afrotropical regions |
| 52. Sceptobiini | 2/5 | Myrmecophilous | USA and Mexico |
| 53. Skatitoxenini | 1/2 | Termitophilous | Namibia and Zimbabwe |
| 54. Tachyusini | 27/380 | Free-living predators, a few associated with ants | Global distribution |
| 55. Taxicerini | 3/32 | Free-living predators, some species are restricted to coastal habitats, some are known from bird nests | Europe and the Oriental, Afrotropical and Nearctic regions and Chile (introduced in Australia and New Zealand) |
| 56. Termitodiscini | 2/43 | Termitophilous | Central and South Africa, Oriental region |
| 57. Termitohospitini | 14/40 | Termitophilous | Neotropical, Eastern Palearctic and Oriental regions and Australia |
| 58. Termitonannini | 23/84 | Termitophilous | Neotropical, Oriental, Pacific and Afrotropical regions and Australia |
| 59. Termitopaediini | 19/81 | Termitophilous | Afrotropical and Oriental regions |
| 60. Termitusini | 7/47 | Termitophilous | Afrotropical region including South Africa, a few species occur in Australia and the Neotropical region |
| 61. Trichopseniini | 16/54 | Termitophilous | worldwide except the western Palearctic region and New Zealand |
| 62. Trilobitideini | 1/11 | Myrmecophilous | Afrotropics including South Africa |
There are many reasons for this, one of which is the largely unknown phylogeny of the subfamily resulting in its poorly developed, rather artificial higher classification. Numerous morphological parallelisms in habitus and traditional taxonomic characters such as tarsal formula or structure of the mouthparts in Aleocharinae led to the existence of many polyphyletic, poorly diagnosed tribes, or presence of large tribes without any reasonable internal groupings of numerous genera. Even a tribal identification of an aleocharine specimen may be rather challenging. Seevers (1978) provided an excellent summary of the early development of the classification of Aleocharinae that led to such a situation and made one of the most significant improvements toward a better, less artificial and more phylogeny-based classification. Seevers’ summary, along with Kistner's tremendous input to the study of myrmecophilous and termitophilous aleocharines, were milestone works for the systematics of Aleocharinae in the past century. They were followed by further improvements, first by Ashe and co-authors (e.g., Ahn & Ashe, 2004; Ashe & Newton, 1993) who implemented parsimony-based phylogenetic methods in the morphology-based aleocharine systematics (Ashe, 1992, 2005), and later by researchers employing fast evolving methods of molecular phylogenetics (Elven et al., 2012; Maruyama & Parker, 2017; Osswald et al., 2013). This most recent molecular progress was focused on producing sound phylogenetic hypotheses, while translating these findings into classification lagged far behind, partly because to do so, a critical and time-consuming re-examination of morphological characters in view of molecular clades was required. As a result, early in the 21st century, we have a much better idea about the phylogeny of Aleocharinae, compared to what, for example, Seevers had. Orlov et al. (2020), most recently, further facilitated phylogenetic understanding of Aleocharinae by using a total evidence (morphological and molecular data) approach and, in the form of a detailed richly illustrated morphological character matrix, provided a tool for future research and for translating molecular clades into taxa supported by synapomorphic and diagnostic morphological characters. This emerging phylogeny of Aleocharinae is summed up here in Figures 63, 64, which highlights even more how outdated the current suprageneric classification of this subfamily is.
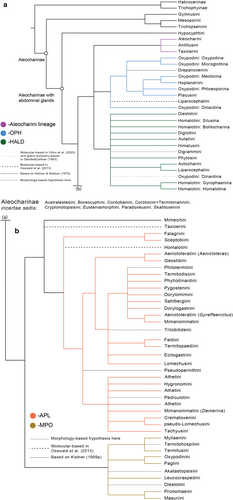
Having now a backbone phylogeny of Aleocharinae, a well-established molecular phylogenetic workflow permitting relatively fast data generation, and a baseline morphological character matrix that can be used either in total evidence phylogenetic inference or in post analysis morphological evaluation of molecular clades, one can design phylogenetic systematic studies of various clades and sub-clades of the subfamily with the aim of improving classification. The purpose of this paper is to facilitate such desired studies even more, by providing a succinct summary of all phylogenetic knowledge and open questions and controversies for all tribes that form the currently accepted conventional system of Aleocharinae (Bouchard et al., 2011, modified in some cases by later works that are cited). We hope that our review will allow anyone who plans a phylogenetic study to optimize their taxon sampling, to make sure to include all critical taxa, to assess their results in view of previous studies that may have used different taxon or character sampling, and to facilitate use of previously published literature.
Our review roughly covers phylogenetic works published between Seevers (1978) and Orlov et al. (2020). We here consider every currently valid tribe, in alphabetical order. Some tribes enjoyed more research attention in the past than others, while a few have never been properly investigated since their erection. The unequal size of the tribes and very different state of their phylogenetic understanding made it difficult to make every tribal account similarly structured here. For example, for many tribes we clearly know their polyphyly already and thus their morphological diagnoses or ecological characteristics have nothing but historical interest. Some lineages that formally belong to one tribe, but in fact are known to be phylogenetic members of another tribe, had to be considered in all respective sections. We tried to optimize all these inconsistencies as much as possible and make our tribal accounts maximally uniform. For each tribe, we tried to provide useful morphological diagnoses, usually at the end of our account, either based on literature (in that case all references provided) or based on our own knowledge. Where necessary or where it was possible, we gave diagnoses of the emerging monophyletic lineages within or across some polyphyletic tribes, rather than poor diagnoses of these tribes alone. For some non-monophyletic tribes, naturally, we were unable to find or provide meaningful diagnoses.
Tribal names are fully referenced to their original descriptions; generic and species names are cited first with author and year, but not referenced. Numbers cited for currently valid genera and species within each tribe are based on Newton (2019), as updated through November 2020 from the active taxon databases maintained by AFN. Currently, Aleocharinae (including 27 extinct taxa) include 16,876 valid species placed in 1328 genera and 62 tribes.
2 REVIEW OF TRIBES
2.1 Actocharini Bernhauer & Schubert (Figure 1)
Actochari Bernhauer & Schubert, 1911: 91, Type genus: Actocharis Fauvel, 1869.
The strictly coastal tribe Actocharini includes only three species in the genus Actocharis (Assing, 1992, 2004; Stenhouse, 2017) from Europe.
Fenyes (1918) placed the genus Actocharis in the Liparocephali group of genera within the tribe Bolitocharini (=Homalotini) based on the number of tarsomeres (4–4–5), antennomeres (11) and segments in the maxillary (four segments) and labial palpi (two or indistinctly three segments). This combination of characters, however, is not unique within Aleocharinae. Later, Actocharis was considered to belong to the subfamily Oxytelinae (Bernhauer & Scheerpeltz, 1926; Winkler, 1925), so Tottenham (1954) had to transfer it back to Aleocharinae (to the tribe Bolitocharini (=Homalotini) again. Moore and Legner (1976) classified Actocharis as a genus of Myllaenini together with Brachypronomaea Sawada, 1956, Myllaena Erichson, 1837, Bryothinusa Casey, 1904, and the genus Halorhadinus Sawada, 1971, which is currently placed in Liparocephalini (Ahn, 2001; Ahn et al., 2010). Moore and Legner (1976) based their decision on the entirely corneous inner and outer lobes of the maxilla, a character that they reported as unique for this group among Staphylinidae. However, they did not consider some other features like the presence of the interdigitating setae of lacinia in Myllaenini and their absence in Actocharis and Halorhadinus. Ahn and Ashe (1996) examined Actocharis readingii Sharp, 1870 (=A. marina Fauvel, 1871) as a genus of the tribe Phytosini together with the genera Arena Fauvel, 1862, Phytosus Curtis, 1838 and Cameronium Koch, 1936 (the latter is currently placed in Homalotini incertae sedis). Since Ahn and Ashe’s (1996) paper was focused on Liparocephalini and they concluded that Actocharis does not share derived characters with that target tribe, they did not include it in their formal cladistic analysis. The recent study of Orlov et al. (2020) revealed a clade which comprises Actocharis together with the genera Diaulota Casey, 1893 and Baeostethus Broun, 1909, of the intertidal tribe Liparocephalini. Based on that and morphological evidence of their affinities (absence of basal lateral pores of epipharynx, two-segmented labial palpi, galea narrower than lacinia, contiguous mesocoxae, a special shape of metendosternite, flared apical lobe of paramere), as well as their shared specialized intertidal habitat, we hypothesize that Actocharis, a single genus of Actocharini, may be a member of Liparocephalini. To address the question in detail, phylogenetic analysis with a broader taxon sampling for this lineage which includes closely related Phytosini is desirable.
The members of Actocharini are small beetles (1–1.5 mm in length, fig. 3 in Stenhouse, 2017) and are characterized by having tarsal formula 4–4–5, galea distinctly shorter than lacinia, labial palpi two-segmented, hind wings rudimental. The beetles are rare, found under seaweed below high-water mark.
2.2 Aenictoteratini Kistner (Figure 2)
Aenictoteratini Kistner, 1993: 242, Type genus: Aenictoteras Wheeler, 1932.
The myrmecophilous tribe Aenictoteratini includes 17 species in 10 genera from the Oriental region (China, Indonesia, Malaysia, Philippines, Thailand, Vietnam) (e.g., Kistner et al., 1997; Maruyama, 2008) and the monotypic genus Weiria Ashe, 2003 from Australia (Ashe, 2003). In the current classification, only three genera (Aenictocupidus Kistner, 1993, Aenictoteras Wheeler, 1932, and Rosciszewskia Kistner, 1993) of several originally included by Kistner (1993) and Kistner et al. (1997) remain in Aenictoteratini. The Aenictoteratini (sensu Kistner, 1993; Kistner et al., 1997) genera Aenictophila Seevers, 1965, Mimaenictus Kistner & Jacobson, 1975, Procantonnetia Kistner & Jacobson, 1975, Steysborgia Kistner & Jacobson, 1975 and Weissflogia Kistner, 1997 were moved to Myrmedoniina, a subtribe of Lomechusini, by Hlaváč et al. (2011) because they share an elongate galea which is a synapomorphy of Lomechusini (Maruyama, 2006). The non-monophyly of Aenictoteratini (sensu Kistner, 1993 and Kistner et al., 1997) was also revealed by preliminary phylogenetic analysis based on morphology and mtDNA by Maruyama (2008), who reported the non-monophyly of Aenictoteratini of which only the genera Aenictoteras, Rosciszewskia and Giraffaenictus Maruyama, 2008 formed a clade. In the molecular phylogenetic study of Maruyama and Parker (2017), five genera of Aenictoteratini were included in the analysis and found nested within the APL (Athetini-Pygostenini-Lomechusini) clade. Of them, four genera formed a clade sister to the Geostibini, and one genus, Giraffaenictus, was sister to the Mimanommatini genus Dorylophila Wasmann, 1904 (as Dorylophilus in the article) within the “Pygostenini” clade. The rounded and shortened lateral apodeme of the labium, one of two autapomorphies mentioned in the paper, is a character shared with “true Lomechusini.”
Kistner (1993) defined the new tribe with only a few character states. He highlighted enlarged first segment of antennae, dense and prominent polygonal microsculpture of the 2nd and 3rd antennal segments, basal segments of abdomen constricted into a petiole and 4th to 7th segments of abdomen enlarged to form a “pseudogaster.” Currently, the tribe includes some of the most highly modified “myrmecoid” (ant-like) body forms found among the Staphylinidae (Maruyama & Parker, 2017). The mentioned antennal characters are common in several other tribes of Aleocharinae, especially in Lomechusini. Since “pseudogaster” is associated with a myrmecoid body shape and also occurs in other tribes, particularly those associated with army ants, all these character states are evidently highly homoplastic and do not support monophyly of Aenictoteratini (Maruyama, 2008).
2.3 Akatastopsisini Pace (fig. 1 in Pace, 2000)
Akatastopsisini Pace, 2000: 112, Type genus: Akatastopsis Pace, 2000.
The tribe includes only one species from Papua New Guinea.
Pace (2000) described a new genus Akatastopsis based on the unique tarsal formula 2–2–5. He reported its habitus similarity with the Myllaenini genera Dimonomera Cameron, 1933 (as Dimonomerus in error in Pace's description), and Myllaena Erichson, 1837. Notably that in the genus Dimonomera (India) the tarsi appear as 1–1–5 (fig. 10 in Ashe, 1999). Pace's illustrations and description of the genus Akatastopsis support its possible close relations with Myllaenini. In particular, it shares with that tribe several characters, which were shown to be phylogenetically important (Ahn & Ashe, 1996; Ashe, 1984, 1992; Hanley, 2002; Maruyama & Parker, 2017; Orlov et al., 2020; Osswald et al., 2013; Sawada, 1972). These are elongate and stylate lacinia with widely dispersed teeth and spines in apical third; elongate, very slender and sclerotized to apex galea with setae only at its apex, asetose on mesal margins; short and entire ligula of prementum; prementum with two discal setae; medial pseudopore field without pseudopores; and mentum with apico-lateral margins produced into spinose processes. One character (a median division of the fourth maxillary palpi) reported by Pace (2000, fig. 3) is unique to Myllaenini (a structure similar to that is present in Oxypodinini) and related tribes (Orlov et al., 2020). Akatastopsisini remains known from a single female specimen, and its phylogenetic relationships within Aleocharinae are unknown.
2.4 Aleocharini Fleming (Figure 3)
Aleocharidae Fleming, 1821: 49, Type genus: Aleochara Gravenhorst, 1802.
The tribe comprises about 677 species in 28 genera. Most of the species (544 described) belong to Aleochara, the largest, globally distributed and best-known genus of the tribe, which has attracted attention due to its peculiar biology as fly parasitoids (Maus et al., 1998; Peschke & Fuldner, 1977). The Aleocharini are divided into three subtribes: Aleocharina (16 genera, e.g., Klimaszewski et al., 2018, 2020), Compactopediina (five genera, Kanao et al., 2011; Kistner, 1970a), and Hodoxenina (one monotypic genus, Hodoxenus Kistner, 1970); six genera of the tribe lack subtribal placement. Members of Compactopediina (Southeast Asia) and Hodoxenina (South Africa) are inquilines in the nests of termites (Kistner, 1970a, 1970b, 1982). One species from Malaysia without a subtribal placement, Myrmecosticta exceptionalis Maruyama, 2011, is myrmecoid and associated with the ant Aenictus sonchaengi Jaitrong & Yamane, 2011 (Maruyama & Parker, 2017). The tribe as a whole has never been the focus of a phylogenetic study. However, there are several morphology-based (Klimaszewski, 1984; Yamamoto & Maruyama, 2015) and molecular phylogenies (Maus et al., 2001; Song & Ahn, 2013, 2014, 2018) devoted to the monophyletic genus Aleochara. In the molecular phylogenetic analyses of Maruyama and Parker (2017), Lü et al. (2019), and Orlov et al. (2020), Taxicerini was recovered as a sister tribe to Aleocharini. The earlier morphology-based (Ashe, 2005; Klimaszewski, 1984; Seevers, 1978) phylogenetic analyses suggested Aleocharini as a lineage closely related to Hoplandriini. Yet, this hypothesis was confidently rejected by the molecular-based work of Osswald et al. (2013) and by the total evidence under parsimony analyses in Orlov et al. (2020) where Aleocharini is shown as a sister group to all remaining Aleocharinae with abdominal glands (often mentioned in the literature as “higher” Aleocharinae) except the Hypocyphtini and Taxicerini.
The tribe is characterized by the presence of apical pseudosegments on both maxillary and labial palpi, which appears as five- and four-segmented, respectively (pseudosegments are absent in the genera Amarochara Thomson, 1858, and Kistnerella Kanao & Maruyama, 2011), by having tarsal formula 5–5–5, and by long flagellum of the median lobe of the aedeagus, longer than median lobe in most species (Klimaszewski et al., 2020). Additionally, Maruyama (2004, fig. 5) pointed out that Aleocharini are characterized by the filiform lateral lobe of the premental apodeme, which he treated as a unique apomorphy of the tribe. At the time of Maruyama (2004) publication, the genera Amarochara and Tinotus Sharp (the latter is currently a subgenus of Aleochara) were not added to this tribe. Yet in fact, Amarochara (pers. observation) and Tinotus (fig. 5 in Yamamoto & Maruyama, 2016) are also characterized by the filiform lateral lobe of the premental apodeme. Osswald et al. (2013) noticed that Amarochara shares a strongly emarginate, sulcate apex of the first antennal segments with the Aleocharini genera Tetrasticta Kraatz, 1857, and Paraleochara Cameron, 1920, and drew attention to the same character state in two oxypodine genera, Porocallus Sharp, 1888, and Paramarochara Cameron, 1952, which may indicate their close relation to the tribe Aleocharini, too. The subgenus Tinotus Sharp, 1883 of the genus Aleochara (Klimaszewski et al., 2018; Yamamoto & Maruyama, 2016) exceptionally has tarsal formula 4–5–5, while the genus Discoxenus Wasmann, 1904 from the subtribe Compactopediina exceptionally has tarsal formula 4–4–5 (Kanao et al., 2011). A pseudosegment on both maxillary and labial palpi is also present in the tribes Antillusini (Pace, 2012) and Himalusini (Klimaszewski et al., 2010). The latter tribe was confidently shown as not closely related to Aleocharini (Maruyama & Parker, 2017; Osswald et al., 2013).
2.5 Antillusini Pace (Figure 4)
Antillusini Pace, 2012: 59, Type genus: Antillusa Pace, 2012.
The tribe includes only one species Antillusa dominicana Pace, 2012 from the Dominican Republic known from a single male specimen. Pace (2012) described this new tribe based on the fusiform body, 4–4–4 tarsal formula, five-segmented maxillary palpi, and four-segmented labial palpi. Although he placed Antillusa near the tribe Paglini from Chile based on the habitus and tarsal segmentation, he also speculated about its close affinity to the tribe Aleocharini because of its five-segmented maxillary palpi, four-segmented labial palpi and ligula in the form of two long lobes. Based on other characters mentioned in Pace's description such as structure of the maxillary and labial palpi, presence of two discal setae of prementum and the large distance between them, an extended compressor plate of the median lobe of aedeagus as well as its general shape, we also hypothesize that Antillusini are closely related to Aleocharini. Antillusa dominicana was never included in any phylogenetic analysis.
2.6 Athetini Casey (Figures 5–7)
Athetae Casey, 1910: 2, Type genus: Atheta C.G. Thomson, 1858.
Athetini is the largest of the aleocharine tribes. It is distributed worldwide and includes more than 244 genera and 4104 species. The genus Atheta alone comprises ca. 1900 species. Recently, the phylogeny of the tribe was a focus of the molecular studies of Elven et al. (2010), Elven et al. (2012), which discovered the ALE-clade (Athetini-Lomechusini-Ecitocharini). Within the ALE-clade, Athetini are found to be paraphyletic with respect to New World Lomechusini (in literature called pseudo-lomechusines or “false Lomechusini”) and Ecitocharini. Currently, the complex of pseudo-lomechusine genera is very difficult to separate from the rest of lomechusines as they all (pseudo-lomechusines and lomechusines) share an elongated galea, a distinct character used to support lomechusines as a tribe. Still, with a few exceptions only, the pseudo-lomechusines and lomechusines can be separated from each other by the former typically having (1) a more elongate (length to width ratio) galea and lacinia (compare figs S3C and S3A in Maruyama & Parker, 2017), and (2) the paramerite constituting a much larger portion of the vellum (while in lomechusines the condylite vellum is proportionally more dominant).
Based on their phylogenetic results, Osswald et al. (2013) synonymized the tribe Ecitocharini with Athetini. Ecitocharini united species with a very distinct habitus formed by their elongated head with a “neck,” prominent eyes, myrmecoid body shape and characteristic sculpturation of the body surface. Apart from molecular evidence of a close relation with Athetini, members of the tribe share with athetines such phylogenetically important morphological structure as an athetine bridge of median lobe of aedeagus. The tribe Ecitocharini was described by Seevers (1965) as a group closely related to the tribe Dorylomimini and later redescribed by Kistner and Jacobson (1990).
As follows from the abovementioned result of reconstructing the Athetini phylogeny, Lomechusini were found polyphyletic by Elven et al. (2012), with some of them (pseudo-lomechusines) nested within the main Athetini clade. On the other hand, since the genera of Geostibina were resolved outside the core Athetini in Elven et al. (2012), they proposed to raise Geostibina, formerly a subtribe of Athetini, to a tribal rank and accordingly thirteen genera were moved from Athetini to Geostibini; three genera of pseudo-lomechusines were moved from Lomechusini to Athetini. The phylogenetic pattern revealed by Elven et al. (2010), Elven et al. (2012) was confirmed by the molecular phylogeny of Maruyama and Parker (2017) where Ecitocharini sensu Kistner and pseudo-lomechusines were found nested within Athetini too. In Orlov et al. (2020), four athetine genera (including the former ecitocharine Ecitomorpha Wasmann, 1889) formed a clade which also included Hygronoma Erichson, 1837 (Hygronomini) and tachyusine genera Tachyusa Erichson, 1837 and Gnypeta Thomson, 1858. Yet, the basal relationships of the whole clade remain unresolved. Finally, it should be mentioned that in all molecular phylogenies, the mega-diverse genus Atheta appeared as non-monophyletic forming three clades (Elven et al., 2012; Maruyama & Parker, 2017).
Taking into consideration the restricted concept of Athetini after Elven et al. (2012), many of its more generalized representatives can be diagnosed based on a combination of several characters: sensillum a of the epipharynx fully developed; galea and lacinia of moderate length; labial palpi three-segmented; tarsal formula 4–5–5; mesocoxae narrowly or moderately separated; mesoventral process narrow; athetine bridge of aedeagus present. The majority of athetines are free living, while a few are associated with ants (e.g., Ecitomorpha) or termites (e.g., subtribes Coptotermoeciina and Termitotelina). Some Atheta and other genera (e.g., Sableta Casey, 1910) are associated with fungi.
2.7 Australestesini Pace (Figure 8)
Australestesini Pace, 2016: 65, Type genus: Australestes Pace, 2016.
This tribe is known from two monotypic genera from Australia (Apimelida Pace, 2016 and Australestes Pace, 2016). In the description of the tribe, Pace (2016) noticed its similarity with Oxypodini (in particular, both tribes share 5–5–5 segmented tarsi), Athetini (in the shape of aedeagus) and Pronomaeini (both tribes share elongated labial palpi of two palpomeres). In fact, it is impossible to speculate about its phylogeny without studying the type specimens.
2.8 Autaliini Thomson (Figure 9)
Autaliides Thomson, 1859: 30, Type genus: Autalia Leach, 1819.
The tribe includes five genera and 54 species distributed everywhere around the globe except Australia and New Zealand. Usually, they are found in humus, dung, leaf litter and fungi (Assing, 1997). Recent molecular studies show close relationships of Autaliini with Homalotini. In Osswald et al. (2013), Autalia longicornis Scheerpeltz, 1947, is a sister taxon to Silusa opaca Fenyes, 1909 (Homalotini: Silusina), within a clade which also includes the subtribe Bolitocharina of Homalotini. In Maruyama and Parker (2017) A. longicornis is a sister taxon to the Himalusini genus Himalusa Pace, 2006, and they together are sister to a clade with Bolitochara Mannerheim, 1830 (Homalotini), Diglotta Champion, 1887 (Diglottini), and liparocephaline genera Amblopusa Casey, 1894, and Liparocephalus Mäklin, 1853. In Lü et al. (2019) A. longicornis is sister to a clade comprising members of Bolitocharini (treated as a tribe, includes Bolitochara + Silusida Casey, 1906), Himalusini (Himalusa Pace, 2006 + Homalota Mannerheim, 1830), and Homalotini (Encephalus Stephens, 1832 + Gyrophaena Mannerheim, 1830). In Orlov et al. (2020), Autalia is a sister taxon to Diglotta and together with Bolitochara, Silusa and liparocephalines form a clade but with unresolved basal relationships. Autalia, by far the largest and most morphologically homogeneous genus of the tribe (37 described species), can be recognized by the following combination of characters: form slender; head broadest at eye level with sides broadly rounded behind to slender neck; ligula very long, narrow, divided into two divergent lobes apically; apical lobes of ligula each bearing a pair of slender processes; pronotum with four basal foveae or furrows and, in some species, a median furrow; elytra each with two conspicuous basal impressions or foveae; abdominal terga III-V transversely impressed at the base, each impression with strong median carina and lateral carinae and tarsi 4–4–5 segmented (Ashe, 2001; Hoebeke, 1988; Hoebeke & Ashe, 1994; Park & Ahn, 2005). These characters do not all apply to other genera assigned to the Autaliini, and the tribe is very difficult to characterize on a worldwide basis (Ashe, 2001). There are no special studies dedicated to the phylogeny of Autaliini or targeting phylogenetic relationships of the tribe within Aleocharinae. Adults of Autaliini are capable of flight, associated with fungi and various decaying matter, and at least some species are known as predators (Klimaszewski, 1992).
2.9 Boreocyphini Klimaszewski & Langor (Figure 10)
Boreocyphini Klimaszewski & Langor, in Klimaszewski et al., 2011: 79, Type genus: Boreocypha Klimaszewski & Langor, 2011.
The tribe was described in Klimaszewski et al. (2011) to accommodate the monotypic genus Boreocypha from Canada. The authors speculated close relations of the Boreocyphini to Oxypodini or Tachyusini (at that time Tachyusina, a subtribe of Oxypodini). Also, they pointed out its similarity in the structure of the aedeagus to the genus “Cypha Fauvel of the tribe Homalotini” (but evidently referring to Cypha Leach, 1819, of the tribe Hypocyphtini, not Cyphea Fauvel, 1863, of Homalotini, based on comments in the generic diagnosis in the same work). The main diagnostic feature of the tribe Boreocyphini is a specific combination of body microsculpture and unique morphology of labial palpi and ligula. So far, the phylogenetic placement of the Boreocyphini remains unknown. The species is found in riparian habitats in forests (Klimaszewski et al., 2011).
2.10 Cordobanini Bernhauer
Cordobanini Bernhauer, 1910: 386, Type genus: Cordobanus Bernhauer, 1910.
This tribe was established based on a single damaged specimen from Mexico (Bernhauer, 1910). In the description, Bernhauer speculated about the possible myrmeco- or termitophilic association of Cordobanus based on its thread-like non-pubescent antennae. Since then, no other species has been assigned to this tribe, and its phylogenetic relationships remain obscure (Santiago-Jiménez et al., 2016). Originally, Cordobanus was placed in the subfamily Tachyporinae because Bernhauer (1910) noticed that it might be closely related to the genus Trichopsenius Horn, 1877, which at that time was assigned to Tachyporinae. Later, Navarrete-Heredia et al. (2002), based on the original description of the type specimen by Bernhauer, suggested moving Cordobanus from Tachyporinae to its own tribe in Aleocharinae.
According to Bernhauer (1910), the tribe has the following diagnostics traits: body broad, with abdomen without distinct paratergites and with abnormally elongated first visible sternite which is nearly as long as all other sternites combined; elytra with poorly distinct epipleura; antennae filiform.
2.11 Corotocini Fenyes (Figure 11)
Corotocini Fenyes, 1918: 61, Type genus: Corotoca Schiødte, 1853.
The notable obligately termitophilous tribe Corotocini includes 222 described species in 67 genera (and possibly over 3500 more undescribed species, see Eloi et al., 2020). The tribe is currently divided into twelve subtribes (Jacobson et al., 1986; Seevers, 1957). Like its hosts (termites from the subfamily Nasutitermitinae), the tribe has a pantropical distribution (Jacobson et al., 1986). Soon after the tribe was proposed by Fenyes (1918), its status was reduced to a subtribe of Hygronomini by Bernhauer and Scheerpeltz (1926) based on the shared tarsal formula 4–4–4 only. Seevers (1957) returned Corotocini the tribal status with a new morphological definition for this tribe and a hypothesis that it is closely related to the tribe Termitonannini. Also, he speculated that free-living Oxypoda Mannerheim, 1830 could be a possible ancestor for the tribe. Jacobson et al. (1986), who revised Corotocini, confirmed its monophyly based on a unique combination of morphological characters (physogastry, free mesocoxae, fused mentum, and submentum). They identified Corotocini and Termitonannini as sister tribes based on shared coeloconic sensillae on the apical segments of the antennae and a unique tergite VII gland (see Pasteels, 1969) and proposed their detailed infra-tribal phylogeny. However, phylogenetic relationships of Corotocini within the rest of Aleocharinae have never been investigated by a formal analysis.
Members of the tribe Corotocini can be recognized by the following combination of characters (Ashe, 2001): tarsal formula 5–5–5 (in some taxa 4–4–4); mentum and submentum fused (suture absent); antennomere 11 with pair of coeloconic sensilla; mesocoxal cavities not margined; hind coxae triangular; abdomen moderately to strongly physogastric in most (figs 1 and 2a in Pisno et al., 2019). The Corotocini are strongly host-specific obligate guests in termite nest (Jacobson et al., 1986). Each species of beetle is found with only one species of termite. In the rare cases where a corotocine is found with more than one host species of termites, the host species are geographically isolated.
2.12 Crematoxenini Mann (Figure 12)
Crematoxenini Mann, 1921: 547, Type genus: Crematoxenus Mann, 1921.
This myrmecoid tribe Crematoxenini consists of 11 genera and 18 species currently known only from the New World (USA, Mexico, Costa Rica, Argentina, Brazil). It can be found with army ants of the genus Neivamyrmex Borgmeier, 1940 (Jacobson & Kistner, 1992). In the description of the new tribe, Mann (1921) already mentioned its close relation with the tribe Myrmedoniini (= Lomechusini). In fact, it is even not easy to separate Crematoxenini from Lomechusini (Ashe, 2001), although its two most peculiar genera (Pulicomorpha Mann, 1924, and Beyeria Fenyes, 1910) once were highlighted as possible members of a different subfamily of Staphylinidae, the Pulicomorphinae (Sanderson, 1943) (now a synonym of Crematoxenini). In the molecular study of Maruyama and Parker (2017), Crematoxenini were represented by four genera and formed a clade which also included unidentified species of Ecitopora Wasmann, 1887 (Athetini incertae sedis), and was sister to New World pseudo-lomechusines. Members of Crematoxenini do not share the elongate galea and lacinia of New World Lomechusini. The mouthpart morphology and body shape of Crematoxenini are highly diverse, which at present make it impossible to satisfactorily define the group morphologically (Maruyama & Parker, 2017).
Jacobson and Kistner (1992) characterized the tribe Crematoxenini with the following combination of features: tarsi 4–5–5 segmented; anterior margin of abdominal sternum IV with well-developed gland reservoirs; the base of abdomen greatly narrowed to form a distinct ant-like "petiole" in most taxa; and pronotum without a distinct median sulcus. Seevers (1978) also noted that mesocoxal cavities are not margined. Most also share the following characteristics with the Lomechusini in the traditional sense: mesosternal process very short, hardly extended between the coxal cavities; metasternal process very long, extended between coxae to the near middle of coxae or more (Ashe, 2001).
2.13 Cryptonotopsisini Pace (fig. 1 in Pace, 2003)
Cryptonotopsisini Pace, 2003: 38, Type genus: Cryptonotopsis Pace, 2003.
This tribe was erected for a single species, Cryptonotopsis rougemonti Pace, 2003, from peninsular Malaysia. In the original description, Pace (2003) placed the new tribe between Leucocraspedini, with which it is similar in body shape, and Deinopsis Matthews, 1838, with which it shares the 3–3–3 tarsal formula. From the shape of the median lobe of the aedeagus and mouthparts in the illustrations of the type specimen provided by Pace, it is not easy to judge about the possible phylogenetic affinity of Cryptonotopsis. The three-segmented tarsi characteristic of C. rougemonti, which are absent in the Aleocharinae with abdominal glands, may suggest its close affinity with Gymnusini sensu Yamamoto and Maruyama (2018).
According to Pace (2003), the tribe Cryptonotopsisini is characterized by the minute size (1.8 millimeters long), densely pubescent fusiform body; the head hidden under the anterior part of the pronotum and not visible from above; 11-segmented antenna; two-segmented labial palpi with very short second segment; four-segmented maxillary palpi; mesocoxae broadly separated from each other; and tarsal formula 3–3–3. Phylogenetic affinities of this tribe within Aleocharinae have never been investigated.
2.14 Diestotini Mulsant & Rey (Figure 13)
Diestotates Mulsant & Rey, 1871: 96, Type genus: Diestota Mulsant & Rey, 1870.
The tribe Diestotini includes about 341 species in seven genera (Diestota, Eudera Fauvel, 1866, Gansia Sharp, 1883, Gansiella Pace, 2008, Parasilusa Bernhauer, 1908, Plesiomalota Pace, 1983, Tachiona Sharp, 1883) with nearly global distribution except for Eastern Palaearctic, New Zealand and South Africa (e.g., Ashe & Lingafelter, 1995). Mulsant and Rey (1870) described Diestota between the genera Gyrophaena Mannerheim, 1830 and Placusa Erichson, 1837. Later, in Casey (1906), Diestota appears as a genus of Gyrophaenina. Ashe (1992) placed Diestota within Homalotini based on several shared apomorphic features: the presence of more or less well-developed denticles in the molar region of the ventral (condylar) side of the mandible; narrowing of the distance between the medial setae of the prementum so that the insertions of the setae are close or contiguous and narrow medial field of pseudopores. In that work he included Diestota in the homalotine subtribe Silusina. In the same year, Diestotini were also presented as a separate tribe in Newton and Thayer (1992). In Ashe (2001) the genus Diestota is the only member of the Homalotini subtribe Diestotina. Finally, in Bouchard et al. (2011) Diestotini is back as a tribe of Aleocharinae.
A recent study of Orlov et al. (2020) is the first attempt to shed light on the phylogenetic placement of Diestotini within Aleocharinae using formal cladistic analysis. In the alternative analyses in this work, Diestota appears either as a member of a clade which also includes Homalotini genera Homalota and Gyrophaena (total evidence BI analysis), or as a sister taxon of a clade comprising the tribes Myllaenini, Pronomaeini, and related tribes (MPO-clade) (morphology only under parsimony). To establish the phylogenetic position of Diestotini, a more detailed examination of the mouthparts of Diestota is needed with particular attention on the presence or absence of interdigitating setae of lacinia (unique character to the MPO-clade).
Recently Asenjo et al. (2019) added six more genera to Diestotini but without providing any discussion for such arrangement. Three genera were moved to Diestotini from Autaliini (Eudera, Gansia and Gansiella; the latter genus was originally described as a diestotine by R. Pace, 2008).
Three other genera (Parasilusa, Plesiomalota, and Tachiona) were moved to Diestotini from Homalotini incertae sedis.
Ashe (2001) defined Diestota by the following complex of characters: tarsi 4–4–5, labial palps of 2 palpomeres, unusually long and filiform or stylate, sutures between palpomeres indistinct in many; ligula divided into two lobes apically; mesocoxal cavities broadly separated by broad meso- and metacoxal processes; metasternal process as long as, or longer than, mesosternal process.
2.15 Diglottini Jakobson (Figure 14)
Diglottina Jakobson, 1909: 529, Type genus: Diglotta Champion, 1899.
The tribe consists of two intertidal genera, Diglotta (coastal, known from Europe, USA, introduced to Canada, Brazil, Fiji Is., Djibouti) with eight species and the monotypic genus Paradiglotta Ashe & Ahn, 2005 from New Zealand. The taxonomic history, biogeography and ecology of the tribe can be found in the review of Diglotta of the world by Haghebaert (1992). For a long time, the phylogenetic position of this tribe within Aleocharinae was a mystery. Seevers (1978) cautiously mentioned its possible relation to Oxypodini. In Lohse (1974) and Klimaszewski (1982) the tribe Diglottini was placed between Myllaenini and Pronomaeini based on the tarsal formula and structure of the mouthparts. Ahn and Ashe (2004) provided convincing evidence that the genus Diglotta is not a member of the Myllaenini or Pronomaeini. Their study suggested that it forms a monophyletic group with the liparocephaline genera Halorhadinus Sawada, 1971, and Amblopusa Casey, 1894. In the molecular phylogenies of Osswald et al. (2013) and Maruyama and Parker (2017) Diglotta is a sister taxon of the Homalotini genus Bolitochara Mannerheim, 1830. In Orlov et al. (2020), Diglotta appears as a sister taxon to Autalia Leach, 1819 within a group of tribes related to Homalotini (HALD clade).
The tribe Diglottini can be recognized by the combination of: tarsi 4–4–4 (4–4–5 in North American species); tarsal claws scythe-like; labial palps very long, thin and stylate; maxillary lobes very long and slender with widely scattered teeth on lacinia; head large, broadly rounded, as broad as or broader than pronotum, with small eyes; pronotum strongly narrowed posteriorly; abdomen broadly oval in dorsal outline, broader at the widest point than elytra (Ashe, 2001).
2.16 Digrammini Fauvel (Figure 15)
Digrammini Fauvel, 1900: 123, Type genus: Digrammus Fauvel, 1900.
The tribe includes only the monotypic genus Digrammus, endemic of New Zealand. Since the original description, the genus Digrammus was never revised or included in any phylogenetic analysis of the subfamily. Digrammus resembles members of Homalotini or Phytosini, to which it is probably closely related, in the presence of a dense patch of spinules at the molar ventral area of mandibles (our personal observation), in two-segmented labial palpi, the shape of the ligula and the tarsal formula 4–4–5. According to Fauvel (1900), the tribe has the following diagnosis: antennae inserted on sides of the frons under its margin; eighth abdominal segment retracted; tarsal formula 4–4–5.
2.17 Dorylogastrini Wasmann (Figure 16)
Dorylogastrini Wasmann, 1916a: 103, Type genus, Dorylogaster Wasmann, 1904.
Currently, the tribe consists of two myrmecophilous genera, the monotypic genus Berghoffia Kistner, 2003, from Malaysia and Dorylogaster Wasmann, 1904 with 12 species from the Afrotropical region. Seevers (1965) considered this tribe as part of the Dorylomimus-group of the tribe Dorylomimini. Newton and Thayer (1992) considered it a synonym of the tribe Mimanommatini. Kistner (1993) resurrected it from synonymy and provided a new description.
At present, it is only the molecular study of Maruyama and Parker (2017) that sheds light on the phylogeny of the tribe. There, the Dorylogastrini genus Dorylogaster was found in the Pygostenini clade together with Dorylomimini (e.g., Dorylomimus Wasmann, 1902, Dorylocratus Wasmann, 1916), Sahlbergiini (Malaybergius Kistner, 1993), Mimanommatini (e.g., Siafumimus Kistner, 1993), and Pygostenini (e.g., Anommatoxenus Wasmann, 1904 and Sympolemon Wasmann, 1900) with maximal statistical support. However, interrelationships between many of the descendent lineages are unclear and weakly supported. Earlier, Seevers (1965) and Kistner (1993) already noted the similarities between Dorylogastrini and Dorylomimini but without providing any phylogenetic analysis to support their relationships.
The tribe is characterized by having unique single-segmented tarsi without pretarsal claws. These single-segmented tarsi bear numerous spatulate setae, which resemble the same in some Pygostenini (our personal observation). Members of the tribe have asymmetrical mandibles, nearly oval labrum, two coeloconic sensilla on the apical segment of the antennae, and petiolate abdomen in which the petiole is formed by segment II and the anterior portion of segment Ill. The males have a median sternal gland and reservoir under abdominal sternite VII, which is lacking in females (Kistner, 1993).
2.18 Dorylomimini Wasmann (Figure 17)
Dorylomimini Wasmann, 1916a: 99, Type genus: Dorylomimus Wasmann, 1902.
Currently, the myrmecophilous tribe Dorylomimini consists of four genera with 37 species known from the Afrotropical region and South Africa. So far, only the molecular work of Maruyama and Parker (2017) included Dorylomimini in a phylogenetic analysis (two genera, Dorylocratus and Dorylomimus) and resolved them in a “Pygostenini” clade with maximal statistical support.
The following characters are shared by all members of this tribe: labrum with a deeply notched anterior edge; galea foliose in that the setae are strongly modified in a unique way to act as a sponge for liquid food; mentum with rounded edges; tarsal formula 4–4–4 with at least some tarsomeres bearing specially modified setae which are reminiscent of those of Pygostenini (our personal observation); female abdominal segment IX with a uniquely modified sclerotized foot-like structure which may function in disengaging the male genitalia; tergal defense gland egress points displaced posteriorly on tergite VII (Kistner, 1993).
2.19 Drepanoxenini Kistner & Watson (fig. 1A in Kistner & Watson, 1972)
Drepanoxenini Kistner & Watson, 1972: 2, Type genus: Drepanoxenus Kistner & Watson, 1972.
The termitophilous tribe Drepanoxenini includes only the genus Drepanoxenus with eight species (five described from larvae only) from Australia. Since the description, the tribe was never revised or included in any phylogenetic analysis. In the description of the tribe, the authors hypothesized its possible close relation to the Oxypodina genus Calodera Mannerheim, 1830 (by that time a genus of Caloderae, a subtribe of Aleocharini) based on the generalized tarsal formula 5–5–5 and small but distinct paraglossae.
The tribe can be characterized by the tarsal formula 5–5–5; maxillae reduced and partially membranous; maxillary palpi four-segmented, the segments small but swollen; labial palpi three-segmented; labium reduced, thickened, highly modified, the apical segment of the palp reduced to a small membranous ring with a sclerotized rim; labrum greatly swollen, the edges membranous; mandibles reduced, without median teeth, and with many pores; clypeus membranous; gula, submentum, and mentum fused, without evidence of former articulation; abdomen slightly physogastric, one pair of paratergites on each of segments III-VII, segment IX trilobed with a sternite in male; median lobe of aedeagus unspecialized (Kistner & Watson, 1972).
It is noteworthy that the larva of Drepanoxenus (figs 1B, 8 and 9 in Kistner & Watson, 1972) has two-segmented antennae, while all other known larvae of Aleocharinae have three- or four-segmented antennae.
2.20 Ecitogastrini Fenyes (Figure 18)
Ecitogastrini Fenyes, 1918: 74, Type genus: Ecitogaster Wasmann, 1899.
The tribe consists only of the genus Ecitogaster (Mexico, Panama, and Brazil) and includes six myrmecophilous species associated with the ant genus Labidus Jurine, 1807. Seevers (1965) noticed a close resemblance of Ecitogaster to certain genera of the Old World Pygostenini, but he mentioned these similarities as a probable result of convergence. The only attempt to infer the phylogenetic relationships of the tribe was the study by Orlov et al. (2020) where Ecitogaster was confidently placed in the Athetini-Pygostenini-Lomechusini clade (APL clade). It is an interesting fact that the unusually large median lobe of the aedeagus and the shape of parameres of Ecitogaster resembles those of the genus Neodioxeuta Seevers, 1957, of the tribe Termitopaediini (compare fig. 32E in Seevers, 1965, and fig. S29F in Orlov et al., 2020), the latter tribe was also confidentially placed in the APL clade in Orlov et al. (2020).
The genus Ecitogaster is characterized by having tarsi 4–4–4 segmented; galea and lacinia moderately long; ninth tergite deeply incised to form two strongly sclerotized processes (fig. 32D in Seevers, 1965).
2.21 Eusteniamorphini Bernhauer & Scheerpeltz (fig. 45 in Pace, 1984)
Eusteniamorphini Bernhauer & Scheerpeltz, 1926: 517, Type genus: Eusteniamorpha Cameron, 1920.
The tribe consists of the genera Eusteniamorpha (67 species from the Oriental and Afrotropical regions (Pace, 1984, 2002), Eustenidia Pace, 1984 (26 species from the Afrotropical region including Madagascar (Pace, 1999)) and the monotypic genus Pseudeustenia Levasseur, 1971, from the Afrotropical region. Members of the tribe have the tarsal formula 3–4–4 (Eusteniamorpha) or 4–5–5 (Eustenidia and Pseudeustenia), four-segmented maxillary and three-segmented labial palpi, as well as short, slender, and bilobed ligula. The Eusteniamorphini were never the focus of any phylogenetic study. Cameron (1939), Levasseur (1971), and Pace (1984) noticed the similarity of the habitus between Eusteniamorphini and Falagriini, but so far, we do not have strong evidence of their close affinity.
2.22 Falagriini Mulsant & Rey (Figure 19)
Falagriates Mulsant & Rey, 1873: 8, Type genus: Falagria Leach, 1819.
The tribe is cosmopolitan in distribution and includes 33 genera and about 471 species. Some members of Falagriini are restricted to seashores (e.g., Bryobiota Casey, 1893, Myrmecopora Saulcy, 1864). Seevers (1978) delimited Falagriini based on eight characters, among them “condylite velum clearly separated from the paramerite velum,” and further he proposed the affinity of this tribe with the Sceptobiini. Hoebeke (1985) revised Falagriini of America north of Mexico and proposed phylogenetic relationships among the North American genera. He discussed the tribe Falagriini as a monophyletic group based on five synapomorphies: slender neck of the head capsule, presence of a median sulcus of the pronotum, appreciably narrowed pronotum behind the middle, unusually enlarged mesospiracular peritremes (fig. 268.22 in Ashe, 2001), and separation of the velum of the paramerite from condylite of the paramere. In the morphology-based phylogeny of Sceptobiini (Danoff-Burg, 1994), two genera of Falagriini (Cordalia Jacobs, 1925 and Falagriota Casey, 1906) were sister to the Sceptobiini. Ahn and Ashe (1995) discussed the systematic position of Bryobiota with a revised phylogeny of Falagriini of America North of Mexico. In the molecular phylogeny of Maruyama and Parker (2017), the Falagriini genera Myrmecopora Saulcy, 1865, and Cordalia formed a clade with Sceptobiini genera Sceptobius Sharp, 1883, and Dinardilla Wasmann, 1901, with high support. But in the same study, two species of the Falagriini genus Pheigetoxenus Kistner, 1983, were found nested within the “true Lomechusini” clade. In Orlov et al. (2020), Cordalia obscura (Gravenhorst, 1802) is resolved as a sister to the APL clade.
According to Ashe (2001), Falagriini can be recognized based on the following character combination: tarsi 4–5–5 (4–4–5 in Bryobiota); head with well-defined neck, neck narrow, less than 1/2 head width; pronotum narrowed basally, with a slight to very strong medial longitudinal sulcus; prosternum usually elongate behind procoxae, often extending laterally to pronotal hypomera; peritremes present around mesosternal spiracles, peritreme size ranging from very small and only narrowly surrounding spiracles, to very large and conspicuous but not contiguous along the midline, to very large and contiguous along midline; male copulatory organ with velum of paramerite and condylite clearly separated into two lobes; and anterior margin of abdominal sternum IV with distinctive gland opening (see Ahn & Ashe, 1995).
2.23 Feldini Kistner (Figure 20)
Feldini Kistner, 1972: 2, Type genus: Felda Blackwelder, 1952 (=Termitobiella Wasmann, 1916).
The termitophilous tribe Feldini includes 10 genera and 28 species known from Malaysia, Singapore and Indonesia. Feldini were revised by Kistner (1975b) who later (Kistner, 2007) reconstructed phylogenetic relationships among the genera of the tribe. Since Zyras haworthi (Stephens, 1832) was the only outgroup taxon in Kistner (2007), that analysis did not bring any information about a sister group for Feldini. Orlov et al. (2020) included one feldine species, Termitobaena bryanti Bernhauer, 1915, in their phylogenetic analyses and found it sister to Termitopaediini (represented by genera Dioxeuta Sharp, 1899 and Neodioxeuta Seevers, 1957), altogether belonging to the APL clade. Such a result confirms the previous hypothesis of Seevers (1957) and Kistner (2001, 2007) about the possible close relationships of Feldini and Termitopaediini.
Members of Feldini can be recognized by the following combination of characters (Kistner, 1972): head capsule lacking a neck; submentum, gula and mentum fused together; antennae 11-segmented, apical segment without coeloconic sensillae; maxillary palpi four-segmented, labial palpi three-segmented; mesocoxal cavities widely separated; tarsal formula 4–5–5.
2.24 Geostibini Seevers (Figure 21)
Geostibae Seevers, 1978: 126, Type genus: Geostiba C. G. Thomson, 1858.
The tribe includes 13 genera and 905 free-living species nearly worldwide (introduced in Australia, New Zealand, Chile and Argentina). Members of the tribe were included in several recent phylogenetic studies (Elven et al., 2012; Maruyama & Parker, 2017; Orlov et al., 2020). One of the major discoveries of the molecular phylogeny of Elven et al. (2012) was that the Athetini subtribe Geostibina actually is sister to the so-called “true Lomechusini,” that respective clade being sister to Athetini. Thus, in the same article Geostibina was raised to the tribal rank based on the reduced sensillum a of the epipharynx. Notably, Geostiba, the type genus of the tribe with about 455 described species, does not appear monophyletic in this work. In the molecular phylogeny of Maruyama and Parker (2017), the Geostibini genera Aloconota Thomson, 1858, Callicerus Gravenhorst, 1802, Earota Mulsant & Rey, 1873, and Geostiba formed a clade outside the clade of Athetini but not sister to the “true Lomechusini.” Instead, there Geostibini is sister to Aenictoteratini and both together are sister to Athetini. In Orlov et al. (2020), Geostiba is sister to a clade which includes “true Lomechusini,” Pygostenini and related tribes. Gusarov (2018) reported non-monophyly of Geostibini where Pelioptera sp. prope micans was sister to the clade Geostibini + (Pygostenini + “true” Lomechusini).
The main diagnostic character to separate Geostibini from any other members of the APL clade is reduced sensillum a of the epipharynx, which is a putative synapomorphy for this tribe (Elven et al., 2012).
2.25 Gymnusini Heer (Figure 22)
Gymnusida Heer, 1839: 302, Type genus: Gymnusa Gravenhorst, 1806.
The tribe Gymnusini includes 68 species worldwide organized into eight genera; two of which (Cretodeinopsis Cai & Huang, 2015 and Electrogymnusa Wolf-Schwenninger, 2004) are extinct and known from fossils (Yamamoto & Maruyama, 2018). All species of Gymnusini are associated with riparian habitats along the margins of marshes, bogs, ponds, and streams.
Gymnusini belong to a group of tribes which are united by lacking the defensive abdominal tergal glands and related structures on abdominal tergum VII in adults and tergum VIII in larvae (Ashe, 2005; Steidle & Dettner, 1993; usually mentioned in the literature as “basal” or “lower” Aleocharinae). Before being united under the name Gymnusini, two small aleocharine tribes, Gymnusini and Deinopsini, were believed to be a clade sister to the rest of the Aleocharinae (Ashe, 2005; Ashe & Newton, 1993; Hammond, 1975; Klimaszewski, 1979). Due to their importance for elucidating aleocharine phylogeny, these tribes have been well investigated phylogenetically (Ashe, 2000, 2005; Hammond, 1975; Klimaszewski, 1979). The recent work of Yamamoto and Maruyama (2018) is the most comprehensive morphology-based phylogeny of Gymnusini that confidently shows monophyly of the tribe. At the same time, none of the molecular-based phylogenies (e.gLü et al., 2019; McKenna et al., 2015; Osswald et al., 2013; Yamamoto et al., 2017) could show the monophyly of the group. Furthermore, in Osswald et al. (2013), the genera Gymnusa and Deinopsis neither form a clade nor are even placed inside Aleocharinae. We tend to explain this by the insufficient taxon sampling of the Gymnusini in these molecular studies which were targeting other groups. In Orlov et al. (2020), three gymnusine genera (Deinopsis, Gymnusa, and Stylogymnusa Hammond, 1975) formed a well-supported clade sister to the Aleocharinae with abdominal glands. For a diagnosis of the tribe and genera, see Yamamoto and Maruyama (2018).
2.26 Himalusini Klimaszewski, Pace & Center (Figure 23)
Himalusini Klimaszewski, Pace & Center, In Klimaszewski et al., 2010: 3, Type genus: Himalusa Pace, 2006.
The Himalusini includes only three genera and four species known from China, Japan, Nepal and Thailand. One of these species, Himalusa thailandensis Klimaszewski, Pace & Center, 2010, is obligately phytophagous, having been found feeding on sewer vine, Paederia pilifera Hook.f., 1881, in Thailand (Klimaszewski et al., 2010). Phytophagy in Himalusa is the only definitive example of this feeding type within the mega-diverse family Staphylinidae. Himalusa thailandensis, one of two species from the type genus of the tribe, was included in the molecular phylogeny of Osswald et al. (2013) and Maruyama and Parker (2017). In the former case, H. thailandensis is a sister taxon to Gyrophaena congrua Erichson, 1837 (Homalotini) and in the latter to Autalia longicornis Scheerpeltz, 1947 (Autaliini). In Lü et al. (2019) H. thailandensis is sister to Homalota plana (Homalotini). In all these phylogenies, Himalusini is confidently placed in the HALD clade.
The tribe Himalusini was erected based on the following combination of characters: tarsal formula 4–4–5; maxillary palpi with four true segments and a pseudosegment; ligula in the form of a small lobe; labial palpi with two true segments and a very short pseudosegment; median lobe of aedeagus slightly asymmetrical, with uniquely shaped structures of the internal sac and rigid flagellum; and apical lobe of paramere attached medially to the paramerite, very long, with serrate internal edge (Klimaszewski et al., 2010; Maruyama et al., 2014).
2.27 Homalotini Heer (Figures 24–26)
Homalotida Heer, 1839: 305, Type genus: Homalota Mannerheim, 1830.
The tribe Homalotini includes about 2538 free-living and fungi-associated species grouped in 164 genera currently classified into five subtribes: Bolitocharina, Dinardopsina, Gyrophaenina, Homalotina, and Silusina (Kim & Ahn, 2016). Genera Caloderina, Euryusa, Gastrophaena, and Dinardopsis are myrmecophilous. The genus Coenonica is termotophylous. Adults and larvae of Silusa rubiginosa Erichson, 1837, feed on Diptera larvae (Furst von Lieven 1999). Some species of the genera Cameronium, Paractocharis, Thinobiosus, Heterota, Linoglossa, and Pseudopasilia are restricted to sea shores. Many genera are not assigned to any subtribe (Kim & Ahn, 2016). Discussion of the phylogenetic relationships of the genera of Bolitocharina can be found in Ashe (1992), Gyrophaenina in Ashe (1984), Homalotina in Kim and Ahn (2016). The molecular phylogeny of Osswald et al. (2013) showed that Homalotini is not a monophyletic tribe. In their analysis Homalota plana (Gyllenhal, 1810), the type species of Homalotini is a member of the APL clade while the subtribes Gyrophaenina, Bolitocharina and Silusina are grouped in a separate clade not even sister to the APL. The molecular phylogeny of Maruyama and Parker (2017) also showed that Homalotini is a polyphyletic taxon where the subtribe Homalotina (including H. plana) was found far away from Bolitochara pulchra (Gravenhorst, 1806) (type species of Bolitocharina). Finally, in Orlov et al. (2020), Homalotina is sister to Gyrophaenina, and both are phylogenetically distant from Bolitocharina and Silusina. Still, they are part of the HALD clade, rather than APL. A more detailed study with broader taxon sampling is obviously needed to infer the phylogenetic relationships of the taxa forming the current tribe Homalotini.
Currently (Ashe, 2001), most of the members of the Homalotini can be recognized by the following combination of characters which, naturally, works rather poorly for a non-monophyletic group: tarsi 4–4–5; mandible with a patch or rows of denticles in the ventral molar region; bases of medial setae of prementum very close together, setae displaced one behind the other in some; medial pseudopore field of prementum very narrow.
2.28 Hoplandriini Casey (Figure 27)
Hoplandriae Casey, 1910: 170, Type genus: Hoplandria Kraatz, 1857.
This tribe includes three subtribes (Hoplandriina, Platandriina and Pseudoplandriina) and 24 genera (many not placed to subtribe), with 365 species (Hanley, 2002, 2003a, 2003b).
Historically, Hoplandriini have been included in Myrmedoniini (=Lomechusini) (Bernhauer & Scheerpeltz, 1926; Casey, 1910; Fenyes, 1918, 1921), an artificial group first erected to house all aleocharine genera with 4–5–5 tarsal segmentation (Hanley, 2002). Seevers (1978), based on the mouthparts and structures of male genitalia, proposed Hoplandriini to be most closely related to the tribe Aleocharini and this concept was also followed by Klimaszewski (1984). By the end of the 20th century, only Muona (1997) questioned a close relationship between Aleocharini and Hoplandriini. All formal phylogenetic analyses of the next century, however, rejected this sister group relationship. First, the molecular phylogeny of Maus et al. (2001) did not confirm the sister relationships between these tribes. Next, morphology-based analyses did not provide unequivocal evidence for their sister group relationship (Ahn & Ashe, 2004; Ashe, 2005; Paśnik, 2010). Hanley (2002) discussed possible sister groups of Hoplandriini and, based on the preliminary phylogenetic analysis, hypothesized Oxypodini as a sister group to Hoplandriini. The molecular phylogeny of the tribe Oxypodini by Osswald et al. (2013) did not support a close relationship between Oxypodini and Hoplandriini, but the authors pointed out that inclusion of the additional genera of Hoplandriini in the analysis would be needed in order to clarify possible sister relationships of this tribe. In the latest molecular phylogeny of Aleocharinae by Maruyama and Parker (2017), a single included species of that tribe, Hoplandria lateralis, is a sister taxon to the Oxypodini subtribe Meoticina. A similar topology was obtained by Orlov et al. (2020) where Hoplandria and Meotica Mulsant & Rey, 1873 (Oxypodini: Meoticina) are sister taxa placed in the OPH-clade in a series of total evidence analyses.
Members of the tribe can be recognized by the presence of a distinct pseudosegment on the last palpomere of the maxillary and (in most) labial palps (as a poorly sclerotized band near apex) so that they appear to have 5 and 4 palpomeres, respectively; ligula generally with a well-defined fork at apex; the tarsal formula 4–5–5 in most genera (5–5–5 in Pseudoplandria Fenyes, 1921, 4–4–4 in some Hoplandriina, and 4–4–5 in some Microlia Casey, 1910) (after Hanley, 2002).
2.29 Hygronomini Thomson (Figure 28)
Hygronomides Thomson, 1859: 31, Type genus: Hygronoma Erichson, 1837.
The tribe Hygronomini includes 9 genera and about 44 species (including one fossil) assigned into two subtribes (Hygronomina and Saphoglossina) from the Palaearctic region, South America and Africa (Tanzania and Yemen). One genus, Eymekesina Pace, 2015, remains without subtribal assignment. Hygronoma dimidiata (Gravenhorst, 1806), the type species of the tribe, was included in several recent phylogenetic studies (Maruyama & Parker, 2017; Orlov et al., 2020; Osswald et al., 2013), where it was firmly placed in a clade together with Athetini and Tachyusini.
Members of the tribe can be recognized by having the tarsal formula 4–4–4, and the athetine bridge in the median lobe of the aedeagus. All the recent phylogenies suggest that Hygronomini should be included in Athetini, which is also supported by shared morphological features between those two tribes (first of all by presence of a well-marked athetine bridge of the median lobe of aedeagus, as well as the segmentation of the mouthparts.
2.30 Hypocyphtini Laporte (Figure 29)
Hypocyphtidae Laporte, 1835: 135, Type genus: Hypocyphtus Gyllenhal, 1827 (=Cypha Leach, 1819).
Hypocyphtini include 11 genera and 288 species (including one extinct genus and species, Baltioligota electrica Paśnik, 2005 from Baltic amber, 35–55 million years old). This tribe of minute species is nearly global in distribution. Some Hypocyphtini occur on vegetation because they are predators of phytophagous mites (Ashe, 2001).
They usually can be recognized by having 10-segmented antennae and tarsal formula 4–4–4 (Ashe, 2001). As was shown by Ahn and Ashe (2004), Ashe (2005), Osswald et al. (2013), Lü et al. (2019) and Orlov et al. (2020), the Hypocyphtini is a monophyletic lineage sister to the rest of Aleocharinae with abdominal glands. It is notable that in the Maruyama and Parker (2017) phylogeny, Hypocyphtini were resolved within the clade ((Adinopsis + Deinopsis) + Trichopseniini) sister to the Aleocharinae with abdominal glands.
2.31 Leucocraspedini Fenyes (Figure 30)
Leucocraspedini Fenyes, 1921: 34, Type genus: Leucocraspedum Kraatz, 1859.
The tribe includes only the genus Leucocraspedum with 106 very similar to each other species which are known from East and Southeast Asia, the Afrotropical and Australian regions, Madagascar and New Guinea. There are no known species from North or South America. Maruyama et al. (2014) provided a recent diagnosis of Leucocraspedum which they considered arboreal based on the Japanese fauna: body limuloid, hairy; head strongly deflexed and concealed under pronotum; large, long, erect black macrosetae scattered on abdomen; tarsal formula 5–5–5. The total evidence analysis of Orlov et al. (2020) is the only phylogenetic work on Aleocharinae which includes Leucocraspedini. The genus Leucocraspedum was resolved there within the MPO-clade sister to the Myllaena (Myllaenini) + Termitusa Wasmann, 1905 (Termitusini) clade.
2.32 Liparocephalini Fenyes (Figure 31)
Liparocephali Fenyes, 1918: 18, Type genus: Liparocephalus Mäklin, 1853.
The flightless intertidal tribe Liparocephalini includes eight genera and 34 species from the Pacific coasts of North America, Asia and New Zealand. Since Ahn and Ashe (1996) there has been a widely accepted hypothesis (even though not discussed in details and not supported by synapomorphies) that Liparocephalini is a tribe closely related to Homalotini, while the most suitable outgroup to infer character polarity and phylogeny of this tribe were the Homalotini genera Leptusa Kraatz, 1856 and Heterota Mulsant & Rey, 1874 and the Phytosini genus Phytosus Curtis, 1838. The authors even hypothesized that “it may seem more logical to treat the liparocephalines as a subtribe within the more inclusive tribe Homalotini.” Later, Liparocephalini were the focus of several morphology-based phylogenetic studies using the mentioned outgroup (Ahn, 2001, 2004; Ahn & Ashe, 1996; Leschen et al., 2002). Using cladistic methods, these studies confidently demonstrated the monophyly of Liparocephalini and showed that several taxa with doubtful systematic position (e.g., Halorhadinus Sawada, 1971, Ianmoorea Ahn, 2006, Baeostethus Broun, 1909) were liparocephalines as well. In the total evidence phylogenetic study, Ahn et al. (2010) redefined the tribe Liparocephalini and tested the phylogenetic position of the genus Salinamexus Moore & Legner, 1977. Although this genus demonstrates some liparocephaline characters, Ahn et al. (2010) did not resolve it in the Liparocephaline clade, pending more detailed study on this topic. Three species (Amblopusa magna Zerche, 1999, Diaulota densissima Casey, 1894 and Liparocephalus cordicollis LeConte, J.L., 1880) were included in the molecular phylogeny of Osswald et al. (2013) which resolved them nested in a well-supported clade with Oxypodini, Hoplandriini and Placusini. Since the basal relationships of this entire clade remained unresolved, Liparocephalini were not downgraded to a subtribe of the Oxypodini then. In the molecular phylogeny of Maruyama and Parker (2017) two liparocephaline species (Liparocephalus cordicollis and Amblopusa magna) were included and resolved nested in a clade together with the tribes Himalusini, Homalotini, Diglottini and Autaliini. Nearly the same topology was revealed in the total evidence analysis by Orlov et al. (2020) where Liparocephalini were resolved as a member of the HALD clade together with the tribes Actocharini, Autaliini, Diglottini, and Homalotini.
Liparocephalines can be recognized by having tarsi 4–4–5 (4–4–4 in some Diaulota); body densely covered with very fine, short setae; elytra very short, flight wings absent; labial palps with two palpomeres (first and second palpomeres fused); ligula of prementum long, slender and entire at apex; galea shorter than lacinia; mandibles without denticles in the ventral molar area; metasternum greatly shortened; middle coxae contiguous (after Leschen et al., 2002 and Ahn et al., 2010).
2.33 Lomechusini Fleming (Figures 32–33)
Lomechusidae Fleming, 1821: 49, Type genus: Lomechusa Gravenhorst, 1806.
The tribe comprises 2518 extant species in 224 genera arranged into three subtribes. Lomechusines are found in all regions of the world except oceanic islands and temperate South America (Hlaváč et al., 2011). This tribe is also sometimes referred to in the literature as Myrmedoniini or Zyrasini. Newton and Thayer (1992) pointed out that the name Lomechusini has priority as the correct name for this tribe because Lomechusidae Fleming, 1821 has priority over Myrmedoniina Thomson, 1867 and Zyrini Bradley, 1930. The first phylogeny where Lomechusini were one of the target groups was the molecular phylogeny of Elven et al. (2010) that targeted Athetini and discovered the Athetini-Lomechusini-Ecitocharini clade (ALE-clade). The following work of Elven et al. (2012) investigating the basal phylogenetic relationships among the ALE- clade revealed the polyphyly of Lomechusini. They found out that the so-called “true Lomechusini” from the Old World was sister to Geostibini, and together, they were sister to Athetini. The so-called “false Lomechusini” clade from the New World were confirmed as members of the Athetini clade a few nodes away from the “true Lomechusini.” Osswald et al. (2013) recovered the tribe Pygostenini as a sister to “true Lomechusini.” Maruyama and Parker (2017) confirmed the division of Lomechusini into two non-related “true” and pseudo-lomechusines. In the total evidence analysis of Orlov et al. (2020), “true Lomechusini” is the sister group to a clade comprising Pygostenini, Phyllodinardini and Termitodiscini and all together are sister to Geostiba (Geostibini). Such topology is entirely congruent with the previous molecular phylogenies of Elven et al. (2012) and Osswald et al. (2013). Moreover, Orlov et al. (2020) discovered the tribes Ecitogastrini, Feldini, and Termitopaediini as members of the Lomechusini-Pygostenini clade.
Morphologically, members of Lomechusini are well characterized, even though this is not a monophyletic group (Elven et al., 2012). They are recognized by a combination of the following characters: sensillum a of the epipharynx fully developed, galea significantly elongate, metaventral process long and apically truncate, mesocoxae broadly separated, tarsal formula 4–5–5, median lobe of aedeagus with athetine bridge, and compressor plate covered by extension from base of bulbus. At present, the morphological basis for separation of the “true” and pseudo-lomechusines is not explored.
2.34 Masuriini Cameron (Figure 35)
Masuriini Cameron, 1939: 24. Type genus: Masuria Cameron, 1928.
The tribe includes only the genus Masuria with 28 species from the Oriental region (India, Nepal, China) (e.g., Assing, 2012). In the morphology-based phylogenetic study of Ahn and Ashe (2004), the Masuriini forms a clade sister to Myllaenini. In Orlov et al. (2020), Masuria plumbea Cameron, 1928, the type species of the type genus of the tribe, was found sister to Pronomaea Erichson, 1837 (Pronomaeini).
Members of Masuriini can be recognized by having labrum rounded in front, labial palpi three and maxillary palpi four segmented, ligula short and slender with entire apex, thorax rather strongly sinuate before the posterior angles, tarsal formula 4–5–5, and elytra sinuate within postero-external angles (Cameron, 1928).
2.35 Mesoporini Cameron (Figure 36)
Mesoporinae Cameron, 1959: 119. Type genus: Mesoporus Cameron, 1959.
Mesoporini is a small tribe of the glandless Aleocharinae consisting of 20 species in 12 genera distributed nearly worldwide (including monotypic genera Mesosymbion Yamamoto et al., 2016, Burmese amber, about 99 million years old; Palaeomesoporus Yamamoto & Maruyama, 2017 from 35–55 million years old Baltic amber; Ambracyptus Seevers, 1971, from Oligocene/Miocene amber and one extinct species from Baltic amber from the extant genus Dictyon Fauvel, 1900). Some Mesoporini are known as termitophiles (e.g. Cai et al., 2017; Kim et al., 2011; Naomi & Iwata, 1996). Members of this tribe were included in several morphology-based phylogenetic studies (Ahn & Ashe, 2004; Ashe, 2005; Yamamoto & Maruyama, 2017, 2018; Yamamoto, Maruyama, & Parker, 2016, 2017), where monophyletic Mesoporini were found sister to Trichopseniini. The odd New Zealand genus Paraconosoma Bernhauer, 1941, was originally placed in Tachyporini but recognized as Aleocharinae by Steel (1960). Paraconosoma polita Steel, 1960, included in the phylogenetic analysis of Orlov et al. (2020), was firmly resolved within the lineage of glandless Aleocharinae. According to Ashe (2001), members of Mesoporini are mostly small to minute beetles (about 1 millimeter in length), tear-drop shaped to sublimuloid aleocharines with 5–5–5 tarsi; without a tergal gland on the anterior margin of abdominal tergum VII; with antennomeres 3–7 minute, 8–10 much larger, forming a distinct "club"; and with a large lamella on the posterior coxa that covers the base of the femur.
2.36 Mimanommatini Wasmann (fig. 47 in Kistner, 1993)
Mimanommatinae Wasmann, 1912: 478, Type genus: Mimanomma Wasmann, 1912.
The myrmecophilous tribe Mimanommatini currently consists of 30 genera and 171 species organized into two subtribes, Dorylophilina (28 genera, from Africa and Oriental region) and Mimanommatina (two genera from Africa). The evolution of the genera of the tribe was discussed by Kistner and Jacobson (1979). Later, Kistner (1993) mentioned Athetini as the tribe most closely related to Mimanommatini. In Maruyama and Parker (2017), the Mimanommatini genera Siafumimus Kistner, 1993, Rodylopora Kistner, 1966, and Dorylophila Wasmann, 1904 (as Dorylophylus in the paper) were resolved in a large well-supported clade together with the members of the tribes Dorylomimini, Dorylogastrini, Sahlbergiini, and Pygostenini. Inside this clade, the Aenictoteratini genus Giraffaenictus Maruyama, 2008, was found within Mimanommatini, rendering the latter tribe paraphyletic. In Orlov et al. (2020), a single included species of Mimanommatini, Demerina bickmanni (Reichensperger, 1922) the sister taxon to Ecitomorpha arachnoides Wasmann, 1889 (Athetini). Seevers (1965) and Kistner (1993) mentioned the absence of athetine bridge of median lobe of aedeagus as an important character to distinguish Mimanommatini from Athetini.
2.37 Mimecitini Wasmann (Figure 37)
Mimecitonini Wasmann, 1909: 55, Type genus: Mimeciton Wasmann, 1893.
The myrmecophilous Mimecitini includes 14 genera and about 25 species in four subtribes known from the Neotropical region and associated with army ants of the genera Labidus Jurine, 1807, Neivamyrmex Borgmeier, 1940 or Nomamyrmex Borgmeier, 1936, of Ecitonini. Due to the extreme morphological modifications and reductions of various characters, including eyes, wings, elytra and genitalia, it was impossible to establish sister group relationships of this tribe based on morphology only (Jacobson & Kistner, 1991). Jacobson and Kistner (1991) considered Crematoxenini as the group most closely related to Mimecitini. In the molecular phylogeny of Maruyama and Parker (2017), Mimecitini were resolved as a sister group of the APL clade.
Members of this tribe are characterized by the absence of paratergites, tarsal formula 4–4–4, and the presence of an abdominal petiole in all genera (Jacobson & Kistner, 1991).
2.38 Myllaenini Ganglbauer (Figure 38)
Myllaenini Ganglbauer, 1895: 317, Type genus: Myllaena Erichson, 1837.
The tribe Myllaenini includes 14 genera and about 337 free-living species worldwide.
Based on the habitus, structure of the mouthparts and the habitat, Seevers (1978) and Klimaszewski (1982) proposed Myllaenini as not a member of Aleocharinae with abdominal glands but as a group with close affinity to Gymnusini. Ashe and Newton (1993), on the contrary, presented evidence that the tribe Myllaenini is a member of the Aleocharinae with abdominal glands based on the very small opening of the tergal gland of Myllaena. Based on this, Dettner (1993) suggested that Myllaenini are the least derived group of Aleocharinae with abdominal glands. The question of the monophyly and sister group relationships of Myllaenini was addressed in the morphology-based phylogeny of Ahn and Ashe (2004) where they were recovered as monophyletic with the tribe Masuriini as a possible sister group. One species, Myllaena audax Casey, 1911 was included in the molecular phylogeny of Osswald et al. (2013), where it was found in a clade together with Thendelecrotona Paśnik, 2007 (Oxypodinini), and Pagla Blackwelder, 1952 (Paglini). Maruyama and Parker (2017) did not support such a relationship and placed Myllaenini far away from the Paglini +Oxypodinini clade. In Orlov et al. (2020), the tribe Myllaenini is sister to Termitusini and with four other aleocharine tribes form a MPO-clade sister to the APL clade.
Members of Myllaenini share 4–4–5 segmented tarsi; labial palps and maxilla very long and stylate; galea very slender with setae only at the apex; lacinia long and stylate with widely scattered teeth internally; mentum with anterolateral margins produced into moderate to strong spinose processes; and tarsal claws not scythe-like (Ashe, 2001).
2.39 Oxypodini Thomson (Figures 39–41)
Oxypodides Thomson, 1859: 36, Type genus: Oxypoda Mannerheim, 1830.
This large tribe comprises 1864 species from all over the world in 173 genera grouped in six subtribes (but with half of the genera not assigned to subtribe). Diagnoses of each of the subtribes of Oxypodini can be found in the supplementary materials in Osswald et al. (2013). The Oxypodini has never been the focus of any morphology-based phylogenetic studies. Usually, only the type genus Oxypoda and at most one-two additional genera represented this tribe in such analyses (Ahn & Ashe, 2004; Paśnik, 2010). In any other, non-morphology-based phylogenetic studies that included more than one oxypodine species, the tribe was never resolved as monophyletic (Maus et al., 2001; Steidle & Dettner, 1993; Thomas, 2009). In the most comprehensive molecular phylogeny of Oxypodini by Osswald et al. (2013), this tribe was represented by 56 species assigned to 39 genera, covering all previously proposed suprageneric groups. The oxypodines were found there in a clade together with the tribes Hoplandriini, Liparocephalini and Placusini with unresolved basal relationships where the oxypodine subtribe Meoticina were resolved as a clade separate from the rest of the Oxypodini. In Maruyama and Parker (2017), the Oxypodini were found divided into two non-sister clades. The first clade included the subtribe Meoticina and the tribe Hoplandriini and the second clade was comprised of the rest of the oxypodines included in that analysis. Orlov et al. (2020) obtained similar topology where Hoplandria (Hoplandriini) was resolved with Meotica (Oxypodini: Meoticina) in a separate clade from the type species of the tribe. Affiliation of the subtribe Dinardina to oxypodines is questionable. As was shown by Lü et al. (2019) and Orlov et al. (2020), the members of that subtribe may not belong to Oxypodini. In fact this was proposed long ago by Steidle and Dettner (1993), based on the chemistry of the abdominal glands. Furthermore, Francke and Dettner (2004) reported that the genus Dinarda Leach, 1819 and Bolitocharini share specific combination of chemicals of defensive secretions. In particular they share predominantly long-chain fatty acid esters (ethyl octadecanoate to ethyl octadecadienoate, and ethyl hexadecanoate). This observation corroborates the unorthodox placement of Dinarda in Orlov et al. (2020). In the series of analyses (molecular-based BI, total evidence-based MP and total evidence-based BI), Dinarda was consistently found as a member of the HALD clade. Overall, polyphyly of Oxypodini is well established now, while the current diagnosis of the tribe available in Seevers (1978) and Ashe (2001) is poor and based on plesiomorphic and homoplastic characters: tarsi 5–5–5, 4–5–5, or 4–4–4; head without a distinct neck in most, neck present in some; frontal suture present or absent; terminal antennomere with or without coeloconic sensilla; mouthparts generalized; mesocoxae narrowly separated in most, moderately separated in a few; intercoxal processes slender in most; abdominal tergum IX narrowly subdivided at base (see Seevers, 1978 for description and discussion); parameres of aedeagus with striated velums; and median lobe of aedeagus with an elongate compressor plate and without an athetine bridge.
2.40 Oxypodinini Fenyes (Figure 42)
Oxypodinini Fenyes, 1918: 18, Type genus: Oxypodinus Bernhauer, 1901.
The tribe Oxypodinini includes 39 free-living species in six genera known from Madagascar, South Africa and the Neotropical region. At least some oxypodinines were found apparently pollen-feeding (Assing, 2006). The tribe has never been revised or studied phylogenetically. Some of the species of the Oxypodinini were described as members of Thamiaraeini (Assing, 2006) or treated as members of the Athetini (Peliusa Erichson, 1839 in Newton et al., 2005). The genus Thendelecrotona, described by Paśnik (2007) in Thamiaraeini, was treated as Aleocharinae incertae sedis in Elven et al. (2010) before it was finally recognized as a member of Oxypodinini in Elven et al. (2012) and placed sister to the Myllaenini genus Myllaena. In Osswald et al. (2013) this clade also comprised the genus Pagla Blackwelder, 1952 (Paglini) which was not included in the previous analyses. In the resulting tree of Maruyama and Parker (2017), Thendelecrotona is sister to Pagla but in a distant position from Myllaena. Orlov et al. (2020) recovered Oxypodinus anxius Bernhauer, 1902 within the MPO clade. O. anxius shares the presence of interdigitating setae of lacinia with some other members of the MPO clade.
Members of the tribe Oxypodinini can be recognized by the presence of a median division of the apical segment of the maxillary palpi (not the same as in Aleocharini or Hoplandriini), so they appear as five-segmented, and two-segmented labial palpi. The tarsal formula is quite variable among genera. It can be 4–4–4 (Heterotaxus Bernhauer, 1915), 4–4–5 (Taraxetaxus Pace, 2006), 4–5–5 (Oxypodinus Bernhauer, 1902, Peliusa Erichson, 1839 and Thendelecrotona) or 5–5–5 in Pseudoxypodinus Pace, 2006.
2.41 Paglini Newton & Thayer (Figure 43)
Paglini Newton & Thayer, 1992: 54, Type genus: Pagla Blackwelder, 1952.
The tribe Paglini consists of the genera Pagla (13 species from Chile and Brazil) and Paglhoplasymma Pace, 2015 (monotypic, from Peru). Lipkow and Betz (2005) mentioned the members of the genus Pagla as obligate spore-feeders. A single undetermined species of Pagla was included in the molecular phylogenies by Osswald et al. (2013) and Maruyama and Parker (2017). In Osswald et al. (2013), Pagla sp. formed a clade with the Oxypodinini genus Thendelecrotona and the Myllaenini genus Myllaena. In Maruyama and Parker (2017), Pagla sp. was sister to the Oxypodinini genus Thendelecrotona alone. No morphology-based phylogenies are known for the Paglini. According to Pace (2015), members of the tribe Paglini are characterized by having tarsal formula 4–4–4, two-segmented labial palpi (three-segmented in Paglhoplasymma Pace, 2015), and short, broad, and whole ligula (narrow, long and divided to the apex in Paglhoplasymma).
2.42 Paradoxenusini Bruch (fig. 1 in Bruch, 1937)
Paradoxenusini Bruch, 1937: 354, Type genus: Paradoxenusa Bruch, 1937.
The monotypic myrmecophilous tribe Paradoxenusini is known only from Argentina. The tribe has never been revised or included in any phylogenetic study of the Aleocharinae. Its single species, P. silvestrii Bruch, 1937 (figs 1–4 in Bruch, 1937), is characterized by having a trilobite-like body shape, antennae 11-segmented, the tarsal formula 4–4–5, labial palpi two-segmented, maxillary palpi four-segmented.
2.43 Pediculotini Ádám (fig. 1 in Vogel, 1999)
Pediculotini Ádám, 1987: 156, Type genus: Pediculota Ádám, 1987.
The monotypic tribe Pediculotini is based on a single species P. montandoni (Roubal, 1909) described from Hungary and Romania. The reason why Ádám (1987) decided to erect a new tribe is a bit confusing. Ádám pointed out that by having tarsal formula 4–4–4 and unlobed tarsomeres, the new species can be assigned to Hygronomini, but further he argued that Hygronoma in fact has the tarsal formula 5–5–5 and bilobed tarsomere IV. Apart from this, the new genus differs from Hygronoma dimidiata in habitus and way of life. In the description, Ádám (1987) also mentioned its habitus similarity with Alpinia Brundin, 1948 (Athetini), and, to some extent, Leptusa (Homalotini) as well as Poromniusa Ganglbauer, 1895, Zoosetha Mulsant & Rey, 1873 or Leptusina Bernhauer, 1900 (=Tectusa Bernhauer, 1899), all from the Oxypodini. Vogel (1999) redescribed the genus and species and illustrated genitalia, but did not resolve the tribal status. The tribe was never revised or included in any phylogenetic study of Aleocharinae.
2.44 Philotermitini Seevers (fig. 1 in Kistner, 1971)
Philotermitini Seevers, 1957: 250, Type genus: Philotermes Kraatz, 1857.
The termitophilous tribe Philotermitini includes two genera, Philotermes with seven species from the Nearctic and Pseudophilotermes Bernhauer, 1934 with five species from the Neotropical regions. These genera are easily distinguished from each other by their tarsal formulas which are 4–4–5 in Philotermes and 4–5–5 in Pseudophilotermes. Although the tribe was revised by Kistner (1971), it was never included in any phylogenetic analysis. Members of the Philotermitini are characterized by pronotum very broadly transverse with a distinctive pubescence pattern, microsetae sparse or absent medially, macrosetae increasing in number and distinctly longer laterally, microsetae directed laterally and anterolaterally; mandibles without a distinct patch of denticles in ventral molar region; mesocoxae contiguous; mesocoxal cavities not margined behind (Ashe, 2001). Up to date there are no hypotheses on the phylogenetic relationships of Philotermitini. But we would like to note the amazing similarity of the median lobe and spermatheca of Pseudophilotermes (=Neophilotermes Seevers) (fig. 4 in Kistner, 1971) and Termitodiscus Wasmann (Termitodiscini) (e.g., figs 22–26 in Kistner, 1967). In his monograph on the termitophilous Staphylinidae, Seevers (1957) placed Philotermitini before Termitodiscini but without discussion on its possible affinity. Based on exceptional similarity of the median lobe of aedeagus and the way of life of both tribes, we agree with Seevers and hypothesize here their possible close affinity.
2.45 Phyllodinardini Wasmann (Figures 44–45)
Phyllodinardini Wasmann, 1916a: 105, Type genus: Phyllodinarda Wasmann, 1916.
The myrmecophilous tribe Phyllodinardini includes only the genus Phyllodinarda with three species from the Afrotropical region. The tribe was revised by Kistner (1965) where he assumed that Phyllodinarda is actually a member of Lomechusini. One species of Phyllodinardini was included in the analyses of Orlov et al. (2020) where, together with Pygostenini and Termitodiscini, it formed a clade sister to “true Lomechusini.”
According to Kistner (1965) members of Phyllodinardini have many unusual features and cannot be confused with any other staphylinids. Their distinguishing characteristics include very small, geniculate and compressed antenna; dorsoventrally flattened body; unique setae on the head, pronotum, elytra, and abdomen; and the way in which the eye has a ventral window; tarsal formula is 4–5–5.
2.46 Phytosini Thomson (Figure 46)
Phytosides Thomson, 1867: 206, Type genus: Phytosus Curtis, 1838.
The coastal tribe Phytosini includes 10 species in three genera known from Europe, North Africa, Taiwan, Guadeloupe (West Indies) and New Zealand. The tribe was revised by Moore (1956). Before his revision Phytosus has been a catch-all group for numerous sea-shore species, some of which were later removed to other genera. Moore stabilized the tribe by providing a description of the tribe entirely based on Phytosus spinifer Curtis, 1838, type species of Phytosini. Based on a few phylogenies where P. spinifer (Ahn & Ashe, 2004; Kim & Ahn, 2016; Orlov et al., 2020), or P. balticus Kraatz, 1859 (Ahn et al., 2010), were included in their analyses, they appear as related to Homalotini and the HALD clade units.
Diagnosis (after Moore, 1956): body elongate, parallel, somewhat depressed; labrum variable, truncate or slightly emarginate; mandibles each with a median tooth, with the tip curved ventrally; mentum truncate, outer lobe of maxillae with a membranous finger-like process at tip, inner lobe setose internally at apex, labial palpi distinctly three-segmented, ligula simple and slender; tarsal formula 4–4–5; anterior and middle tibiae with several series of long thick setae externally, which are interspersed with the pubescence.
2.47 Placusini Mulsant & Rey (Figure 47)
Placusates Mulsant & Rey, 1871: 102, Type genus: Placusa Erichson, 1837.
The tribe Placusini includes five genera and 189 species with nearly global distribution (absent in New Zealand) (e.g., Santiago-Jiménez & Santiago-Navarro, 2016). Historically, Placusa was recognized as a taxon closely related to Homalotini based on 4–4–5 tarsal formula and subcortical biology (Fenyes, 1918; Lohse, 1974; Mulsant & Rey, 1871; Seevers, 1978). Another Placusini genus Euvira Sharp, 1883 was treated as closely related to the autaliine genera Autalia and Eudera Fauvel, 1866 (Fenyes, 1918), or as its own species group within Homalotini (Seevers, 1978). Ashe (1991) in his study of the systematic position of Placusa and Euvira confidently showed these two genera as a monophyletic lineage and rejected their close relationship with the tribe Homalotini. In the molecular phylogenies of Elven et al. (2010, 2012), Placusa was resolved as a sister taxon to the oxypodine Oxypoda praecox Erichson, 1839. A close relationship between the Placusini and the core Oxypodini was confirmed by the analyses of Osswald et al. (2013), Lü et al. (2019) and Orlov et al. (2020).
Placusini are recognized by the following character combination: tarsi 4–4–5; labrum rounded medially with small a-sensillum; mandible with dorso-basal "velvety patch" modified to transverse rows of large teeth; mandibles without rows of denticles in ventral molar region; ligula short, broad, and apically rounded; two medial setae of prementum with bases distant from each other; labial palps short, of two palpomeres; mesocoxae narrowly separated by slender intercoxal processes, or mesocoxae virtually contiguous (Ashe, 2001).
2.48 Pronomaeini Mulsant & Rey (Figure 48)
Pronoméates Mulsant & Rey, 1873: 8, Type genus: Pronomaea Erichson, 1837.
The tribe Pronomaeini includes 7 genera and 94 free-living species with almost global distribution except for Australia and New Zealand. Based on the tarsal formula and structure of the mouthparts, the Pronomaeini were considered closely related to Myllaenini and Diglottini (Lohse, 1974), or to Masuriini and Myllaenini based on the mouthparts alone (Seevers, 1978).
In a study of the phylogeny of the Myllaenini and related taxa, Ahn and Ashe (2004) included six Pronomaeini genera and recovered them as a monophyletic lineage not sister to either Myllaenini or Diglottini. However, they did not resolve phylogenetic relationships among the genera of Pronomaeini. Osswald et al. (2013) included four species from the tribe Pronomaeini in their phylogenetic analysis and resolved them as a well-supported clade inside a bigger clade with poorly resolved internal relationships that also included Myllaenini, Oxypodinini and Paglini. In Orlov et al. (2020) Pronomaeini, represented by Pronomaea picea Heer, 1841, was resolved sister to the type species of Masuriini, Masuria plumbea Cameron, 1928.
2.49 Pseudoperinthini Cameron (fig. 4 in Bourguignon & Roisin, 2006)
Pseudoperinthinae Cameron, 1939: 1, Type genus: Pseudoperinthus Wasmann, 1916.
Four genera and 26 species of the termitophilous tribe Pseudoperinthini are known from Australia and the Oriental and Pacific regions. Wasmann (1916b) placed the genus Pseudoperinthus in Aleocharinae, but Cameron (1939) transferred it to a new subfamily in the belief that its head structure does not conform to the aleocharine pattern. He noted that the antennae are inserted under an arcade and that there is a fine, cariniform line across the front. Further, Seevers (1957) studied Pseudoperinthus and noticed that its head is by no means as unusual as Cameron implied and that the genus certainly belongs to the Aleocharinae. He first used this group as a tribe of Aleocharinae. The tribe was revised by Kistner and Pasteels (1970) and Kistner (1999a). The New Guinean species were revised by Bourguignon and Roisin (2006). The members of the tribe were never included in any phylogenetic analysis, but Kistner (1999a) proposed close relations of Pseudoperinthini with the tribes Athetini and Lomechusini based on several shared characters between these tribes: the margined mesocoxal acetabulae, the distinct mentum, the distinctly margined maxillary acetabulae, the shape of the maxillae, and the 4–5–5 tarsal formula (Kistner & Pasteels, 1970). Members of Pseudoperinthini can be easily distinguished from athetines and lomechusines by the presence of a vertexal arcade (figs. 1–3 in Bourguignon & Roisin, 2006), which is a shelf-like extension of the vertex that covers the clypeus and the antennal fossae.
2.50 Pygostenini Fauvel (Figures 49–51)
Pygostenini Fauvel, In Raffray & Fauvel, 1899: 5, Type genus: Pygostenus Kraatz, 1858.
The myrmecophilous or termitophilous (e.g., Odontoxenus Kistner, 1958) tribe Pygostenini includes 29 genera and 268 species which are mostly known from Africa and the Oriental and West Palearctic regions. The tribe was a focus of several revisionary studies by Kistner with co-authors (Jacobson & Kistner, 1975b; Kistner, 1958; Kistner & Taylor, 2009). Only a few phylogenetic works included representatives of Pygostenini. In the molecular phylogenies by Osswald et al. (2013) and Maruyama and Parker (2017) the tribe Pygostenini was recovered as a sister clade to the “true Lomechusini.” The “Pygostenini” clade of the latter phylogeny also included the tribes Dorylomimini, Dorylogastrini, Sahlbergiini and Mimanommatini. In Orlov et al. (2020) Pygostenini formed a clade with Phyllodinardini and Termitodiscini, altogether sister to “true Lomechusini.” Most members of the tribe have a characteristic limuloid habitus (e.g., Anommatoxenus Wasmann, 1904, Doryloxenus Wasmann, 1898, Mimocete Fauvel, 1899). Some genera are of the typical staphylinid shape and resemble some lomechusines (e.g., Anommatophilus Wasmann, 1904, Neopygostenus Kistner, 1958, Sympolemon Wasmann, 1900).
Members of Pygostenini can be recognized by: antennae with the pedicels covered by projections of the lateral edges of the segments anterior and posterior to the pedicels; antennal fossae sunk within the head between the eyes (the degree to which they are sunk varies from one genus to another, but they are always sunk at least to the degree exhibited by Typhloponemys Rey, 1886); the tarsal formula variable, 4–5–5, 4–4–4 (may appear to be 3–3–3 if the long fourth segment is broken off as it often is); postclypeus greatly reduced and thinly sclerotized, inserted between the large antennal fossae; median lobes of the male genitalia divided into anterior and posterior parts which articulate on each other; and ninth abdominal segment highly modified with a median dorsal lobe and two lateral plates, which in turn can be divided into two median dorso-lateral and two ventro-lateral lobes (Kistner, 1958).
2.51 Sahlbergiini Kistner (Figure 52)
Sahlbergiini Kistner, 1993: 315, Type genus: Sahlbergius Bernhauer, 1927.
The myrmecophilous tribe Sahlbergiini consists of 4 genera and 9 species known from the Oriental and Afrotropical regions (Hlaváč & Maruyama, 2004). Kistner (1993) hypothesized Lomechusini as the most closely related tribe to Sahlbergiini based on the similarity of their maxillae, labium, the shape of the mandibles, and the 4–5–5 tarsal formula. He also provided a cladistic study of the genera of Sahlbergiini with Zyras haworthi (Stephens, 1832) used as an outgroup. Maruyama and Parker (2017) included one species of Malayloeblius Hlaváč & Maruyama, 2004 as a representative of Sahlbergiini in their phylogenetic analysis and found it nested within “Pygostenini” clade with high support. The characters which unite members of this tribe are the following (Kistner, 1993): mandibles are largely symmetrical without median teeth; 4–5–5 tarsal formula and the absence of spatulate setae on the tarsal segments; mesothoracic peritremes large and loose, not fused to the prosternum; hind coxae elongated with special flanges which proceed dorsally; and long legs. This tribe is easily distinguished from Lomechusini by the ant-like form and the structure of the mesothoracic peritremes.
2.52 Sceptobiini Seevers (Figure 53)
Sceptobiini Seevers, 1978: 148, Type genus: Sceptobius Sharp, 1883.
The myrmecophilous tribe Sceptobiini includes two genera and five species known from the New World (Danoff-Burg, 1996). Already in the description of the tribe, Seevers (1978) mentioned its affinity with the Falagriini based on the distinctive shape of the parameres. Later, Danoff-Burg (1994) revised the Sceptobiini using cladistic analysis, where he used two genera of Falagriini and Atheta (Athetini) as the outgroups and five sceptobiine species as an ingroup. The Sceptobiini were resolved as monophyletic group sister to Falagriini. Later, Ahn and Ashe (1995) used genera of Sceptobiini as the closest outgroup in their analyses of the position of the falagriine genus Bryobiota Casey, 1893, but without showing the final resolution of the outgroup in the paper. In the molecular-based aleocharine phylogeny of Maruyama and Parker (2017), the Sceptobiini genera Sceptobius and Dinardilla Wasmann, 1901, were found sister to each other with high support, and together their clade was sister to the genera Cordalia Jacobs, 1925, and Myrmecopora Saulcy, 1865, of Falagriini.
Sceptobiini are characterized by antennae 11-segmented; maxillary palpi four-segmented; labial palpi three-segmented; lacinia slightly longer than galea; tarsal formula 4–5–5; and condylite velum of parameres clearly separated from the paramerite (Danoff-Burg, 1994).
2.53 Skatitoxenini Kistner & Pasteels (fig. 1 in Kistner, 1975a)
Skatitoxenini Kistner & Pasteels, 1969: 1190, Type genus: Skatitoxenus Kistner & Pasteels, 1969.
The tribe consists of only two termitophilous species in the genus Skatitoxenus known from Namibia and Zimbabwe. Members of the tribe were never included in any phylogenetic analysis. Kistner and Pasteels (1969) speculated that Feldini (Feldina of Myrmedoniini =Lomechusini in their paper) could be the closest relatives of Skatitoxenini. Nevertheless, Feldini can be easily distinguished from Skatitoxenini by the remarkable mouthparts of members of the latter tribe with highly modified palpi and short lacinia and galea. The authors also pointed out the similarity between Skatitoxenus and Corotocini. However, absence of the coeloconic sensillae on the apical antennomere and separated (not fused) mentum and submentum in Skatitoxenus distinguish members of these two tribes as well. In the description of the second species of Skatitoxenus, Kistner (1975a) mentioned that it superficially resembles the tribe Corotocini and some physogastric members of Myrmedoniini (=Lomechusini) and Termitopaediini.
2.54 Tachyusini Thomson (Figure 54)
Tachyusides C. G. Thomson, 1859: 34, Type genus: Tachyusa Erichson, 1837.
About 27 genera and 380 species from all over the world are assigned to the tribe Tachyusini. The position of this group has been controversial up to the last decade. Based on the habitus, Tachyusa and closely related genera have been originally treated as members of the tribe Falagriini (Lohse, 1974). Seevers (1978), however, demonstrated that they lack important diagnostic characters of that tribe. Since then, the Tachyusina have been treated as either a subtribe of the Athetini (Pace, 2006; Sawada, 1987), or Oxypodini (Ashe, 2001; Newton & Thayer, 1992; Seevers, 1978), or as a separate tribe Tachyusini (Lohse, 1989; Paśnik, 2010). Paśnik (2010) studied the phylogeny of Tachyusini based on morphological characters. In his analysis, Tachyusini, represented by 28 genera, were recovered as a sister clade to the six Oxypodini genera, but with low support (Paśnik, 2010). One of the goals of the molecular phylogeny of Osswald et al. (2013) was to check the phylogenetic position of Tachyusini (at that time a subtribe of Oxypodini again). The monophyletic Tachyusini were found outside the Oxypodini lineage and nested within a clade that also included the core members of Athetini and Hygronomini. The subsequent phylogenies by Maruyama and Parker (2017), Lü et al. (2019) and Orlov et al. (2020) confirm a close affinity of Tachyusini and Athetini. In the Tachyusina, the athetine bridge is considered absent (Seevers, 1978), at least in its typical form. However, in some species of Gnypeta the sclerotized parameral side of the median lobe of the aedeagus has two short processes that could be interpreted as remains of the athetine bridge (V. Gusarov, unpublished observation in Osswald et al., 2013).
Members of Tachyusini can be recognized by tarsal formula 4–5–5; frontal suture absent; terminal antennomere with or without coeloconic sensilla; medial pore field of epipharynx with less than 25 pseudopores; apical third on lacinia with 6–8 contiguous spines and 2–5 clearly isolated spines below; maxilla with medial sclerite of stipes with three long setae; aedeagus very distinctive: basal bulb deeply incised by V-shaped unsclerotized region and median lobe of aedeagus with distinct, prominent bladelike process (Paśnik, 2010).
2.55 Taxicerini Lohse, 1989 (Figure 55)
Taxicerina Lohse, 1989: 210, Type genus: Taxicera Mulsant & Rey, 1873.
The tribe Taxicerini includes three genera (Discerota Mulsant & Rey, 1873, Halobrecta Thomson, 1858 and Taxicera Mulsant & Rey, 1873) and 32 species from Europe and the Oriental, Afrotropical and Nearctic regions and Chile (introduced in Australia and New Zealand). Originally, this tribe was erected as a subtribe of Athetini for the genera Taxicera and Discerota (Lohse, 1989). The genus Halobrecta was traditionally placed in the tribe Athetini (e.g., Lohse, 1974), based mainly on the 4–5–5 tarsal formula. However, Gusarov (2004) demonstrated that the genus lacks the athetine bridge of the aedeagus while sharing some characters with the tribe Oxypodini. In the molecular phylogeny of Athetini by Elven et al. (2010) Halobrecta was found outside the Athetini–Lomechusini–Ecitocharini (ALE) clade and was tentatively moved to Oxypodini. In Elven et al. (2012), another Taxicerini genus Discerota (which also lacks the athetine bridge of the aedeagus and has both male and female genitalia similar to some Oxypodini) was added into the analysis. The genus Discerota formed a well-supported clade with Halobrecta outside the ALE-clade. For these reasons, Discerota was moved from Athetini and placed tentatively in Oxypodini as well. Finally, in the following molecular study of Oxypodini by Osswald et al. (2013) Halobrecta was recovered as a sister to Taxicera and the study rejected a close relationship of Halobrecta + Taxicera to either Oxypodini or Athetini. Therefore, the Athetini subtribe Taxicerina was raised in the rank of a tribe and expanded to include Halobrecta. In contrast, all three of the latest phylogenetic works (Lü et al., 2019; Maruyama & Parker, 2017; Orlov et al., 2020) resolved Taxicerini and Aleocharini as sister clades, but no strong morphology-based evidence in support of that is known so far.
The tribe Taxicerini can be recognized by the tarsal formula 4–5–5, the distinct shape of ligula (Gusarov, 2004), spermatheca (Gusarov, 2004; Kapp, 2005), and aedeagus (Gusarov, 2004; Kapp, 2005), the latter lacking the athetine bridge (Osswald et al., 2013).
2.56 Termitodiscini Wasmann (Figure 56)
Termitodiscinae Wasmann, 1904: 656, Type genus: Termitodiscus Wasmann, 1899.
The tribe consists of 43 termitophilous species in the genera Termitodiscus and Termitogerrus Bernhauer, 1932 known from Central and South Africa and from the Oriental region. The phylogenetic position of Termitodiscini within Aleocharinae has never been explored. Termitodiscini were proposed as a tribe of Aleocharinae by Seevers (1957). Originally, Wasmann (1899) placed Termitodiscus in the Aleocharinae without tribal assignment, and then, Wasmann (1904) erected a new subfamily for this genus. In his revision of this group of small limuloid beetles, Seevers (1957) confidently recognized this genus as a member of Aleocharinae and placed it in its own tribe Termitodiscini. Kistner (1967) revised Termitodiscini and supported Seevers’ judgment that Termitodiscini should not be a subfamily, suggesting their affinity to Myrmedoniini (=Lomechusini) based on five shared characters. The type species of the type genus of the tribe, Termitodiscus heimi Wasmann, 1899, was included in the recent total evidence phylogeny by Orlov et al. (2020) where it was resolved in a clade together with Phyllodinarda xenocephala Wasmann, 1916 (Phyllodinardini) and Typhloponemys sp. (Pygostenini). That clade, in turn, is sister to “true Lomechusini.”
According to Seevers (1957), the tribe Termitodiscini can be diagnosed as follows: body limuloid; head concealed beneath the very large pronotum, hypognathous; antennae inserted in deep fossae medial to the eyes, their scapes resting in these fossae; antennae 9-, 10-, or 11-segmented, their second and third segments small, the fourth to terminal segment forming a compact "club"; pronotum approximately twice as broad as long, its sides and apex continuously arcuate; pronotal hypomera very large, but not visible from the side; elytra broad, their epipleura very large; prosternum keeled; mesocoxae set in margined acetabula, narrowly or broadly separated; hind coxae broad, produced laterally to meet the elongated metepimera; tarsi 4–5–5-segmented; abdomen acuminate; and aedeagus of a typically aleocharine structure.
2.57 Termitohospitini (Figures 57–58)
Termitohospini Seevers, 1941: 331. Type genus: Termitohospes Seevers, 1941.
The termitophilous tribe Termitohospitini includes 40 species in 14 genera from the Neotropical, Eastern Palearctic and Oriental regions and Australia (Kanao et al., 2012). Seevers (1941) erected this tribe to accumulate Neotropical termitophilous bolitocharines (currently Homalotini) with 4–4–5 segmented tarsi. Later, Seevers (1957) expanded Termitohospitini to include termitophiles from other biogeographical regions: Termitohospitina Seevers, 1941 for Neotropical members; Hetairotermitina Seevers, 1957, for Australian and Oriental members; Termitospectrina Seevers, 1957 for Pantropical members; and Termitusina Seevers, 1957 for Ethiopian members. Kistner and Jacobson (1976) subsequently raised Termitusina to a tribe with Termitospectrina as a subtribe within it, based on 4-segmented maxillary and 3-segmented labial palpi. Therefore, Termitohospitini is now divided into two subtribes, Termitohospitina and Hetairotermitina. Species of Termitohospitini differ from all other Aleocharinae, including Myllaenini, in the following putative synapomorphies: anterior tentorial arms of the head migrated anteriorly and disassociated from antennal fossae (Seevers, 1941); 2) antennal fossae dorsally obscured by enlarged vertex (Seevers, 1941); 3) lacinia with basal paired cuticular processes reduced in size (Kanao et al., 2012); processes of anterolateral angles of mentum reduced in size (Kanao et al., 2012); and ligula broad and reduced in length (Kanao et al., 2012). At the same time in Termitohospitini and Myllaenini, there are a median division of the fourth maxillary palpomere and a sensory patch on lateral surface of the third maxillary palpomere. These are unique structures that are found only within the MPO clade (Orlov et al., 2020), suggesting close affinity of Termitohospitini to Myllaenini.
2.58 Termitonannini Fenyes (fig. 40 in Kistner, 2004)
Termitonannini Fenyes, 1918: 75, Type genus: Termitonannus Wasmann, 1902.
The termitophilous tribe Termitonannini includes 23 genera and 84 species arranged into the subtribes Perinthina and Termitonannina and known mostly from the Neotropical region, but also from the Oriental, Pacific, and Afrotropical regions and Australia. Seevers (1957) cautiously mentioned that the presence of the almost identical coeloconic sensillae on the apical antennomere in Termitonannini and Corotocini might suggest an affinity of both tribes. Pasteels and Kistner (1970) were certain about a close relation between Termitonannini and Corotocini. So far, the probably monophyletic Termitonannini (Kistner, 2004) were never included in any phylogenetic analysis, and the relationships of the tribe within Aleocharinae are largely unknown. Kistner (1999b, 2004) analyzed phylogenetic relationships among genera of Perinthina and discussed its monophyly, as well as the monophyly of the whole tribe.
Termitonannini can be recognized by: tarsal formula 4–4–4 (Perinthina) or 4–4–5 (Termitonannina); coeloconic sensillae on terminal segments of the antennae (absent in Termitopelta Borgmeier, 1950); antennae 10 (Termitonannina) or 11 (Perinthina) segmented; mentum fused; enlarged hind coxae; and the presence of the median gland reservoir on the anterior border of abdominal segment VII (absent in Termitopelta) (Kistner, 2004).
2.59 Termitopaediini Seevers (Figures 61–62)
Termitopaediini Seevers, 1957: 214, Type genus: Termitopaedia Wasmann, 1911.
The termitophilous tribe Termitopaediini includes 19 genera and 81 species from the Afrotropical and Oriental regions. Seevers (1957) speculated that members of the Termitopaediini might be closely allied to the genus Termitobiella Wasmann, 1916, of the Feldini. Kistner (1968) suggested that the sister group of Termitopaediini could be established after the complete revision of the Lomechusini, Mimanommatini and Homalotini. The most comprehensive work on the Termitopaediini was done by Kistner (2001) and included a cladistic analysis of the entire tribe with Zyras haworthi added as an outgroup. Two species of Termitopaediini, Dioxeuta sp. and Neodioxeuta sp., were included in Orlov et al. (2020) where they formed a clade sister to the Feldini genus Termitobaena.
The characters which link the genera of the tribe Termitopaediini are as follows: tarsi 4–5–5 segmented; maxillary acetabula not as extremely developed as in the Lomechusini; galea and lacinia moderate in length (in contrast to the Lomechusini), the galea usually foliate and membranous; terminal antennal segments without coeloconic sensilla; mesocoxal acetabula large, shallow, faintly margined externally or not at all; the broad mesosternal process rounded or angulate; middle coxae narrowly or moderately widely separated; hind coxae subtriangular, not produced beyond lateral articulation; and metepimera obliquely truncated posteriorly and not prolonged beyond coxal articulation (Kistner, 2001). The mentum is free in Termitopaedia Wasmann, 1911, and related genera, but fused with the submentum in other genera (Seevers, 1957).
2.60 Termitusini Fenyes (fig. 1 in Kistner, 1974)
Termitusae Fenyes, 1918: 18, Type genus: Termitusa Wasmann, 1905: 199.
Seven genera and 47 species in the subtribes Termitospectrina and Termitusina are currently assigned to the termitophilous tribe Termitusini. They are mostly known from the Afrotropical region including South Africa, while a few species occur in Australia and the Neotropical region. The Termitusini was defined as a subtribe of Termitohospitini by Seevers (1957) based on a complex of morphological characters. Kistner (1974) revised this subtribe, while Jacobson and Kistner (1975a) provided a phylogenetic analysis of its genera. In the next year, Kistner and Jacobson (1976) raised Termitusina to a tribal rank and separated them from Termitohospitini by the absence of median division of fourth maxillary palpomere. Termitusa sjoestedti Wasmann, 1905, the type species of the type genus of Termitusini, was included in the phylogenetic study of Orlov et al. (2020). There, it was found sister to Myllaena (Myllaenini) with maximal statistical support. This affinity is also supported by a shared putative synapomorphy among Termitusini and Myllaenini: presence of a unique sensory patch on the lateral surface of the third maxillary palpomere (fig. 7H3 in Orlov et al., 2020). The same structure was reported by Kanao et al. (2012) for Termitohospitini of which Termitusini is a former subtribe.
Termitusini share with Termitohospitini a 4–4–5 tarsal formula; the labial palps with the first two segments long and slender and the third segment reduced in length; the distinct mentum; the slender ligula; and the large and margined mesocoxal acetabulae. Termitusini are distinguished from Termitohospitini by pronotum not covering entire head; metasternum much longer than the mesosternum; and antennae with distinct petioles not covered by extensions of the sides of the segments (Kistner, 1974).
2.61 Trichopseniini LeConte & Horn (Figure 59)
Trichopsenii J. L. LeConte & G. H. Horn, 1883: 100, Type genus: Trichopsenius G. H. Horn, 1877.
The termitophilous tribe Trichopseniini includes 54 species in 16 genera distributed worldwide except the western Palearctic region and New Zealand. Of these, one monotypic genus (Cretotrichopsenius Cai et al., 2017) and four species of Prorhinopsenius Pasteels & Kistner, 1971, are known from amber fossils; the former is the oldest record (98.79 ± 0.62 million years old) for the tribe Trichopseniini (Cai et al., 2017). The group Trichopsenii was first proposed by LeConte and Horn (1883) as a subgroup of Tachyporinae (back then the tribe Tachyporini of Staphylininae). Much later, Seevers (1941) raised the rank of Trichopseniini to a subfamily of Staphylinidae. Subsequent authors either accepted its subfamily status (Kistner, 1998; Pasteels & Kistner, 1971) or considered it as a tribe of Aleocharinae (Newton & Thayer, 1992). In the phylogenetic analyses by Ahn and Ashe (2004) and Ashe (2005), the Trichopseniini were clearly recovered as a lineage of glandless Aleocharinae. The latest molecular phylogeny of Aleocharinae (Maruyama & Parker, 2017) also recovered the Trichopseniini as a glandless lineage of the subfamily.
According to Ashe (2001), the diagnosis of Trichopseniini is as follows: tarsi 5–5–5; paratergites absent; posterior coxae absent (possibly fused to the metasternum) so that the trochanters appear to articulate directly with the metasternum; and presence of a large lamella on the metasternum covering at least the base of the hind femur.
2.62 Trilobitideini Fauvel (Figure 60)
Trilobitideidae Fauvel, In Raffray & Fauvel, 1899: 3, Type genus: Trilobitideus Raffray, 1898.
The myrmecophilous tribe Trilobitideini comprises a single genus Trilobitideus with 11 species known from the Afrotropics including South Africa. Earlier, the genus Trilobitideus was placed in a monotypic subfamily Trilobitideidae related to Aleocharinae (Wasmann, 1916a). Later Seevers (1965) downgraded Trilobitideini to a tribe of the Aleocharinae. Seevers cautiously admitted that the Phyllodinardini genus Phyllodinarda might be closely related to Trilobitideus, but the evidence of the relationships was too weak. The genus Trilobitideus was revised by Kistner (2006) who did not find support for a close relationship between Trilobitideus and Phyllodinarda and provided a cladistic analysis for the known species of Trilobitideus. The Trilobitideini were never included in any broader phylogenetic analyses. We could get a specimen dissected by Dr. Kistner for study. Based on our observations and illustrations provided in Kistner (2006), we assume Trilobitideini to be a member of the APL clade. In particular, Trilobitideus shares with some Athetini or Lomechusini a sclerotized flap over base of compressor plate and the overall shape of aedeagus, scale-like structures at the molar area of mandibles and large distance between two discal setae of prementum. By its habitus (fig. 1 in Kistner, 2006), Trilobitideus is similar to Phyllodinarda, from which it can be distinguished by the ten segmented antennae (Seevers, 1965; but reported as 11-segmented in Kistner, 2006) and tarsal formula 4–4–4 (11-segmented antennae and 4–5–5 tarsal formula in Phyllodinarda). Members of the tribe are also characterized by the most unusual in Aleocharinae body surface sculpturing. The dorsal surface of their body is covered with rows of furrow protuberances, which makes Trilobitideus difficult to confuse with any other aleocharines.
3 CONCLUSIONS
Recent progress in phylogenetic research confirmed the monophyly of Aleocharinae and revealed major clades within this large subfamily that clearly showed the outdated nature of its current classification (Figures 63 and 64). This research laid the foundation for further in-depth phylogenetic studies to be accompanied by a classification upgrade. Our review here provides a platform for informed taxon selection in such studies which inevitably have to be restricted to key specimens representing the enormous diversity of Aleocharinae. We highlight a number of tribes, such as for example Akatastopsisini, Antillusini, Australestesini, Cryptonotopsisini, or Cordobanini, for which the available material is so limited that a collecting effort must be made prior to their efficient phylogenetic study.
ACKNOWLEDGEMENTS
We sincerely thank our friend and colleague Richard Leschen who encouraged us to focus on Aleocharinae and undertake research which also led to this paper. We thank the Danish Beetle Bank (danbiller.dk) for the opportunity to use some of the Aleocharinae images here. Josh Jenkins Shaw and Janina Lisa Kypke, former PhD office mates of IO, are greatly acknowledged for all support, advice, and inspirational discussions, which were useful on the way to this paper too. Vladimir Gusarov and Kee-Jeong Ahn, who served as opponents at IO PhD defense, are sincerely acknowledged for their constructive criticism and useful suggestions that facilitated transformation of this work into its final form. The PhD scholarship for IO that initiated this paper was funded by the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement no. 642241. This research was continued and finished under support from the Russian Science Foundation grant 20-14-00097 to IO and AS.




