Does spatial sorting occur in the invasive Asian toad in Madagascar? Insights into the invasion unveiled by morphological analyses
Contributing authors: Franco Andreone ([email protected]), Angelica Crottini ([email protected]), Rodino Fetraharijaona Harison ([email protected]), Gentile Francesco Ficetola ([email protected])
Abstract
enDispersal-relevant traits may be subjected to spatial sorting in rapidly expanding populations, driving the evolution of dispersive phenotypes. This process has been largely documented in invasive species, and ascertaining its presence can have important implications for their management. The Asian common toad was accidentally introduced to Madagascar around 2010 and is a highly problematic invasive species. Exploring the morphology of this species offers the twofold opportunity to test the hypothesis of early onset of spatial sorting and to gather information on the dynamics of this unstudied biological invasion. For these purposes, we analyze morphological data of adult toads collected across the invasive range in Madagascar, and we test the effect of the distance from the introduction point on the relative size of head, radioulna and tibiofibula, and on body size and body condition of toads, respectively. We did not detect evidence of spatial sorting in the morphological traits analyzed. Instead, morphological variation was largely dependent on sex and body size of individuals. Sexual dimorphism was limited in relation to other populations in the native range, and body size did not vary across the invasive gradient, which could indicate that both adults and juveniles are currently contributing to the dispersal in this invasive species. We provide important baseline data for the long-term assessment of morphological variation in the invasive population, and we advocate for further investigations of spatial sorting in life history and behavioral traits.
Riassunto
itNelle popolazioni in rapida espansione i tratti rilevanti per la dispersione possono essere soggetti a spatial sorting, portando all'evoluzione di fenotipi dispersivi. Questo processo è stato ampiamente documentato nelle specie invasive, ed accertarne la presenza può avere importanti implicazioni nella loro gestione. Il rospo asiatico comune è stato accidentalmente introdotto in Madagascar intorno al 2010 ed è una specie invasive altamente problematica. Studiare la morfologia di questa specie offre la duplice opportunità di testare l'ipotesi di insorgenza precoce di spatial sorting e raccogliere informazioni sulle dinamiche di questa invasione poco studiata. A tal fine, analizziamo i dati morfologici di rospi adulti raccolti nella regione del Madagascar interessata dall'invasione, e testiamo l'effetto della distanza dal punto d'introduzione sulla taglia relativa di testa, radioulna e tibiofibula, e sulle dimensioni e la condizione corporea dei rospi. I risultati non mostrano evidenze di spatial sorting nei tratti morfologici analizzati, ma indicano che la variazione morfologica dipende in gran parte dal sesso e la dimensione degli individui. Il dimorfismo sessuale è ridotto rispetto alle popolazioni nell'areale nativo, e la taglia dei rospi non variava lungo il gradiente dell'invasione, il che potrebbe indicare che sia adulti che giovani contribuiscono alla dispersione in questa specie invasiva. In quest'articolo, forniamo dati importanti per una valutazione a lungo termine della variazione morfologica di questa popolazione invasiva e indichiamo l'importanza di ulteriori indagini sullo spatial sorting nei tratti biologici e comportamentali di questa specie.
1 INTRODUCTION
Individuals at the edges of an expanding population may be subjected to differential selective pressures compared to conspecifics from long-colonized areas, which can possibly lead to the emergence of distinctive phenotypic traits (Chuang & Peterson, 2016). This evolutionary mechanism, named spatial sorting, is driven by intrinsic differences of dispersal capabilities among individuals and inevitably brings to the accumulation of dispersal-relevant traits at the leading-edge populations (Phillips & Perkins, 2019; Shine et al., 2011). Spatial sorting can positively select a broad range of traits, including life history, behavioral, physiological, and morphological features (Chuang & Peterson, 2016).
Although spatial sorting has been documented in naturally expanding populations, where range shifting can be induced by climate change (e.g., Hassall et al., 2009; Sanford et al., 2006), biological invasions represent the best study system for investigating this phenomenon, as invasion speeds can be manifold faster than natural expansions of species ranges. Invasive populations of the cane toad Rhinella marina (Linnaeus, 1758) in Australia are an impressive example of such a phenomenon. In this invasive species, spatial sorting has occurred on a whole set of dispersal-related traits. Leading-edge populations expanding in monsoonal floodplain and savannah areas of northern Australia show enhanced dispersal abilities due to several behavioral (Alford et al., 2009; Brown et al., 2007), locomotor, and morphological changes (Hudson et al., 2016, 2020; Phillips et al., 2006). As a result of spatial sorting, cane toads from these invasion forefronts have proportionally narrower heads, longer tibiofibulas and radioulnas, and better dispersal ability (Hudson et al., 2018; Hudson, McCurry, et al., 2016; Phillips et al., 2006). Furthermore, larger and heavier adult cane toads are leading the invasion forefront, as they have longer dispersal than smaller individuals and juveniles, which arrive months later, revealing clear spatiotemporal patterns of invasion (Brown et al., 2013). Limb proportions show a complex pattern across the whole invasive range, initially decreasing from the central to the intermediate populations, where natural selection promotes short-legged individuals. The trend sharply reverses near the invasion front, where individuals have significantly longer limbs and better dispersal ability (Hudson et al., 2016; Stuart et al., 2019). This reveals how trade-offs between contrasting evolutionary forces (i.e., natural selection vs. spatial sorting) determine complex patterns of phenotypic variation across populations (Clarke et al., 2019; Hudson, McCurry, et al., 2016). In addition, environmental features of the recipient ecosystems (Urban et al., 2008) can promote or hinder species dispersal, determining the spatial sorting onset.
Spatial sorting can strongly influence multiple features and the dispersal of invasive populations. Therefore, ascertaining the occurrence of this pattern helps to gather important information on the invasion dynamics and to identify better management responses.
The Asian common toad Duttaphrynus melanostictus (Schneider, 1799) is a smaller relative of the cane toad. Native to Southeast Asia, it was accidentally introduced to Madagascar around 2010 (Moore et al., 2015). The recent invasion of the Asian common toad in Madagascar is posing unprecedented threats to Malagasy biodiversity. First, these toads are toxic and can cause the poisoning of native predators (Marshall et al., 2018). Furthermore, due to its high fecundity, the toad could attain high abundances with multiple potential impacts on native species, especially amphibians, including competition and the transmission of pathogens (Licata et al., 2020).
The invaded area is highly suitable for this species (Vences et al., 2017), characterized by the widespread presence of human settlements associated with rice paddies, which are favorable foraging and breeding habitats for the invasive toad (Jørgensen et al., 1986; Licata et al., 2019), that have granted a successful establishment and are likely to ensure optimal conditions for its dispersal, and the consequent onset of spatial sorting. This invasion provides a unique opportunity of analyzing spatial sorting since the earliest phases of the invasion, thus greatly improving our understanding of the drivers and consequences of this process.
Here, we performed the first broad-scale analysis of morphological variation of the Asian toad across the whole invasive range in Madagascar, to characterize dispersal-relevant morphological traits and test the spatial sorting hypothesis. In so doing, we assessed whether there is a relationship between these traits and the distance from the introduction point. Furthermore, we explored invasion dynamics by testing whether size and body condition of adult toads changed with the distance from the introduction point.
2 MATERIAL AND METHODS
2.1 Study system
The invasive population of D. melanostictus in Madagascar belongs to a species complex of Asian medium-sized toads (Wogan et al., 2016), which have been accidentally introduced by humans to several regions worldwide, and, in particular, in the Wallacea (Othman et al., 2020; Reilly et al., 2017). This terrestrial, lentic-breeding amphibian is an ecological generalist and thrives in human-dominated landscapes (van Dijk et al., 2004). Females reproduce seasonally, producing over 9500 eggs per clutch (Ngo & Ngo, 2013), and, as in most anurans, are larger than males, but size and sexual dimorphism can vary considerably across populations (range of average body size across populations: females = 58.0–107.1 mm; males = 50.8–90.6 mm; Guo et al., 2019). The invasive population established in Madagascar originates from an area between Vietnam and Cambodia (Vences et al., 2017) and was likely introduced via accidental stowaway in commercial containers around 2010 (Moore et al., 2015). The putative introduction point of the toad was identified close to the SW suburbs of Toamasina (Moore et al., 2015). From here, the species expanded in all landward directions with a spreading rate of 1.6–2.5 km/year and, in 2017, it already invaded an area of 549 km2, with average abundances of 184 toads/ha (Figure 1; Licata et al., 2019). In 2014, when the invasive range was estimated at only 108 km2 (Moore et al., 2015), the total population abundance was estimated to be already >7 million post-metamorphic individuals (Reardon et al., 2018).
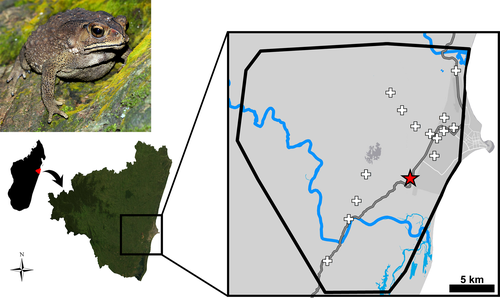
The invasive range is located at low-mid elevations (0–500 m a.s.l.) and is intersected by the Ivondro and Ivoloina rivers, on the southern and northern edges of Toamasina, respectively (Figure 1). Apparently, the Ivoloina river could have acted as a partial barrier, temporarily slowing down the northern front of the invasion (Licata et al., 2019). The invaded area is characterized by a human-modified landscape, with more than 1500 villages or small groups of huts scattered throughout the area, and is largely occupied by croplands (Zhang et al., 2020). Uncultivated land is mainly covered by grasslands and shrubland, with only a few remnant patches of forest (Zhang et al., 2020). Climatic conditions in the invasive range are highly suitable for this species (Pearson, 2015; Vences et al., 2017), being characterized by a tropical rainforest climate (Peel et al., 2007), alternating dry (August–November) and wet season (December–July), and monthly temperatures ranging from 21°C (July) to 27°C (February) (Merkel, 2021).
2.2 Sampling
Sampling took place during two different periods (October 2016–January 2017 and October 2018–May 2019), in parallel with other ecological field studies. Localities were chosen opportunistically, mainly along the principal route connecting Toamasina to the capital Antananarivo (Route Nationale 2), which crosses the invasive range from north to south, and also in the urban and suburban districts of Toamasina and in the inland areas of the countryside (Figure 1). We hand-collected adult toads (> 50.2 mm; Ngo & Ngo, 2013) encountered during night time, conducting spotlight surveys (visual encounter surveys; Crump & Scott Jr, 1994). We collected the furthermost toads in a locality on the southern invasion frontline along the Route Nationale 2 (18.274°S, 49.254°E). Additional visual encounter surveys in this area, visiting other localities farther south, did not detect any further toad. One of us (F.L.) measured the collected toads using a vernier caliper (±0.1 mm), recording four morphometric parameters: snout–vent length (SVL), head width (HW), tibiofibular length (TBL), and radioulnar length (RUL). All animals were weighed using a digital balance (precision: 0.01 g). Sex determination was based on the external characters, with females larger and heavier than males, which in turn have thicker forearms, nuptial pads and develop an orange gular region (Gordon, 1933; Licata et al., 2020).
2.3 Statistical analysis
We calculated the body condition of each toad using the Residual Index (RI), which is considered a reliable body condition index (Labocha et al., 2014), and is obtained by extracting the residuals of the regression of mass against SVL (both ln-transformed). We ln-transformed all continuous variables prior to analyses, to improve normality and reduce skewness.
We used simple linear, second-, third-, and fourth-order polynomial regressions to check for allomorphic relations between morphological traits (HW, TBL, and RUL) and SVL, including the effect of sex. We used the Akaike's Information Criterion to identify the curve with the best fit (i.e., AIC; Burnham & Anderson, 2002).
We performed linear mixed-effect models (LMMs) to test the spatial sorting hypothesis, by assessing relationships between morphometric features and distance from the introduction point. First, we tested the relationship between SVL and distance from the introduction point, while taking into account differences between sexes. Second, we used TBL, RUL, and HW as dependent variables and distance from the introduction point as an independent variable, while taking into account the effect of sex and including SVL as a covariate, to correct for the size effect. For graphical purposes, we plotted the relative TBL, RUL, and HW as percentages, by dividing these measures by SVL and multiplying by 100. Finally, we assessed the variation of RI. In this case, the independent variables included distance from the introduction point, SVL, sex, day of the year and its quadratic term, to take into account potential seasonal variation in body condition. In all the mixed models, we considered the year of sampling as a random effect to take into account possible variation through time.
We assessed the spatial autocorrelation of the residuals of our models using a Moran's I test (Legendre, 1993). Lastly, we calculated the sexual dimorphism index (SDI), following the formula proposed by Lovich and Gibbons (1992) (i.e., SDI = [(Larger sex/Smaller sex) – 1].
We conducted all the analyses in R environment (version 4.0.3; R Core Team, 2020) and made publicly available the code used (http://doi.org/10.5281/zenodo.4779685). Linear-mixed models were performed using the package lme4 (Bates et al., 2015) and test statistics were obtained using the lmerTest package (Kuznetsova et al., 2017), while spatial autocorrelation analysis was conducted using the ecoGenetics package (Roser et al., 2017). All graphics were obtained using the package ggplot2 (Wickham, 2011).
3 RESULTS
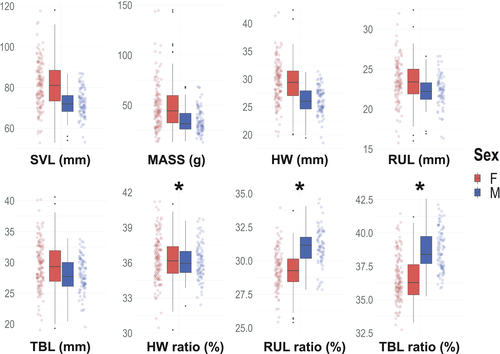
We measured 228 adult toads (89 males and 139 females), captured at distances of 3.7–13 km from the putative introduction point (Moore et al., 2015; Figure 1). Morphology of adult toads varied significantly between sexes (Figure 2), with females larger than males for both total length and all the other morphometric parameters (Figure 2, Table S1). Males showed proportionally longer radioulnas and tibiofibulas than females, which, however, showed slightly bigger head proportions (Figure 2; Table S1). Overall, the sexual dimorphic index SDI was 0.120.
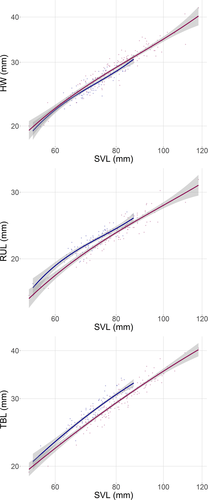
All the relationships between SVL and the other morphometric parameters showed a significant non-linear pattern (Figure 3; Table S2). The relationship between SVL and TBL was best described by a second-order polynomial curve (adjusted R2 = 0.893), indicating a deceleration of the relationship between SVL and TBL in larger individuals (Figure 3). The relationships between SVL and the other morphological traits were best fitted by a third-order polynomial curve (adjusted R2: HW =0.891; RUL =0.863), which indicate complex changes in trait proportions during toad ontogenesis (Figure 3).
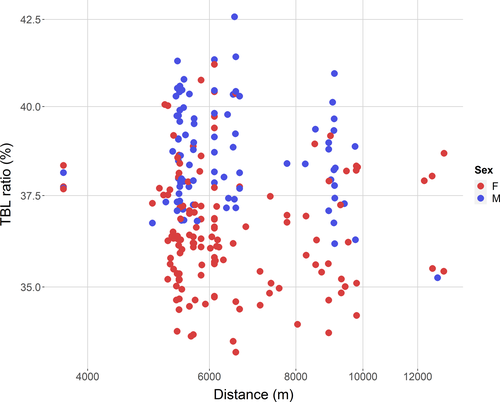
The distance from the introduction point was unrelated to any of the morphological traits taken into consideration, with variation mostly attributable to the sex and/or the size of individuals (see Figure 4; Table 1). RI showed a significant linear relationship with the day of the year, indicating an increase of body conditions toward the end of the dry season.
| Trait | Variable | β | df | t-value | p-value |
|---|---|---|---|---|---|
| SVL | Sex M | −0.107 | 1,224 | −6.491 | <0.001 |
| Distance | 0.034 | 1,225 | 0.99 | 0.323 | |
| RI | SVL | −0.015 | 1,217 | −0.179 | 0.858 |
| Sex M | 0.025 | 1,217 | 1.146 | 0.253 | |
| Distance | 0.026 | 1,217 | 0.603 | 0.547 | |
| Day | 0.413 | 1,217 | 2.766 | 0.006 | |
| Day | −0.120 | 1,149 | −0.672 | 0.503 | |
| TBL | SVL | 0.868 | 1,223 | 41.567 | <0.001 |
| Sex M | 0.044 | 1,223 | 7.942 | <0.001 | |
| Distance | −0.007 | 1,223 | −0.695 | 0.488 | |
| RUL | SVL | 0.787 | 1,181 | 32.612 | <0.001 |
| Sex M | 0.035 | 1,181 | 5.683 | <0.001 | |
| Distance | 0.011 | 1,181 | 0.921 | 0.358 | |
| HW | SVL | 0.818 | 1,222 | 37.423 | <0.001 |
| Sex M | −0.021 | 1,224 | −3.523 | <0.001 | |
| Distance | −0.012 | 1,222 | −1.085 | 0.279 |
- Significant p-values are highlighted in bold.
- Abbreviations: HW, head width; RI, residual index; RUL, radioulnar length; SVL, snout-to-vent length; TBL, tibiofibular length.
We did not detect spatial autocorrelation in the residuals of the models with SVL or relative size of head, radioulna and tibiofibula (for all the models, Moran's I < 0.1; p > 0.1). Conversely, the residuals of the model with RI as a dependent variable showed significant autocorrelation at lags up to 1.3 km (Moran's I = 0.13; p = 0.01). This indicates that, after taking into account the effect of sex and SVL, toads living in nearby areas have similar body condition values.
4 DISCUSSION
Dispersal-related morphological traits of the Asian common toad in Madagascar apparently did not change with distance from the introduction point, but were mostly related to the sex and/or ontogenetic phase of individuals, suggesting that spatial sorting is currently not affecting the features of this invasive population. Average body size of adult toads and body conditions did not vary significantly nearby the invasion forefront (see Table 1), unlike what has been documented at the cane toad northern invasion forefront in Australia, where larger adults can be found during the first months following toad arrival (Brown et al., 2013; Phillips & Shine, 2005). In fact, juvenile toads were also found to be extremely abundant at the invasion front in Madagascar, often outnumbering adults (F.L., pers. obs.), suggesting that they are currently contributing to the range expansion. Further data collection at invasion front localities is required, as well as studies on toad abundances and dispersal abilities. In particular, dispersal ability at different ontogenetic stages needs to be ascertained, to identify the life history stages that contribute most to the range expansion.
After taking into account the distance from the introduction point, the body condition index showed a significant spatial autocorrelation at lags up to 1.3 km, suggesting that it is affected by spatially structured environmental features (e.g., food resources) not included in our analyses. Future studies should also include the effect of habitat type on the body condition of the invasive toad, as native populations exhibit lower body condition in human-modified habitats compared with forested habitats (Karraker et al., 2018).
Furthermore, toads were significantly heavier toward the end of the year (see Table 1), namely at the end of the dry season. In its native range in central Vietnam, D. melanostictus shows reproductive seasonality, breeding mainly between April and July (i.e., during the auxiliary rainy season; Ngo & Ngo, 2013), when its body condition decreases (Church, 1960). The variation of body condition index could indicate a better foraging activity before the breeding season and/or gonadal maturation, but more analyses are required to clarify the breeding phenology of this species in Madagascar.
In the study population, females were significantly larger than males which, instead, had proportionally longer limbs (Figure 2), a common pattern in toads and other amphibians (Wells, 2010). The marked female-biased sexual dimorphism in body size is well known in this and several other toad species (Guo et al., 2019; Ngo & Ngo, 2013; Wells, 2010). The observed sexual dimorphism index of the invasive toad of Madagascar (SDI = 0.12) stands among the lowest recorded for this species, where the median is 0.201 (range = −0.033–0.353; Guo et al., 2019). This is due to the larger average size of males in Madagascar (SVL = 72.5 mm; Table S1) with respect to native populations (SVL = 66.4 mm; Guo et al., 2019), whereas females are only slightly bigger than the native ones (SVL = 81.1 vs. 79.9 mm; Table S1; Guo et al., 2019). Variation of sexual size dimorphism could be determined by age differences between sexes (Liao et al., 2013). Further studies will be needed to verify whether the low SDI could be linked to altered selective pressures, which could determine an increased survival rate of adult males, reducing the size gap between sexes and, consequently, sexual size dimorphism.
The lack of spatial sorting in the dispersal-relevant traits considered is likely attributable to the short invasion history of the Asian common toad in Madagascar. Duttaphrynus melanostictus was introduced approx. 10 years before the beginning of our field activities. In its native range, the Asian common toad apparently attains sexual maturity during the second year of life (Nayak et al., 2007). In Madagascar, this process could be faster, as preliminary skeletochronological analysis suggested a continuous physiological growth of individuals (F.M. Guarino, pers. Comm.), contrary to native populations where lines of arrested growth are observed (Nayak et al., 2007). Preliminary estimates reported a rate of invasion spread ranging between 1.6 and 2.5 km/year (Licata et al., 2019), which is coherent with the annual dispersal rate obtained from radiotracking data on adult toads (approx. 2 km/year; F. Licata, unpublished data), therefore, invasion front toads could be at most from the fifth or sixth generation. Simulation studies predict that conditions at the leading edges of an expanding population can maximize the allelic expression of genes which enhance the spatiotemporal fitness of individuals in approx. 10–15 generations (Phillips & Perkins, 2019). In fact, rapid morphological changes resulting from spatial sorting have still not been documented in other invasive species with similar short invasion histories (see Brownscombe & Fox, 2012; Merwin, 2019), while studies of different species indicate that rapid changes are more likely to emerge in dispersal behavior rather than external morphology (see Lombaert et al., 2014; Merwin, 2019; Ochocki & Miller, 2017). Now, it will be important to test the occurrence of spatial sorting for behavioral or life history traits, for instance through telemetric studies, laboratory experiments, or common-garden breeding experiments, which allow investigating evolutionary changes by reducing the potential environmental influence of maternal effect. Furthermore, morphological analysis following a systematic random sampling design (e.g., SECR; Royle et al., 2013), targeting discrete and independent localities, could complement such studies and allow ascertaining the lack of spatial patterns in the morphometric traits of the invasive population.
The study of biological invasions is a key source of information on contemporary evolution (Melotto et al., 2020; Reznick et al., 2019). The implementation of long-term studies of the Asian common toad in Madagascar will allow monitoring of variation of its populations during the invasion, providing a great opportunity to understand the evolutionary dynamics of invasive species. In turn, understanding multiple processes occurring during invasion dynamics is essential for informed management strategies.
ACKNOWLEDGMENT
We thank the Malagasy authorities (in particular to the former Ministère de l’Environnement, Ecologie et des Forêts, now Ministère de l’Environnement et du Développement Durable) for granting the research permits (n. 263/18/MEEF/SG/DGF/DSAP/SCB. Re; 100/19/MEDD/SG/DGF/DSAP/SCB. Re), and the Madagascar Institut pour la Conservation des Ecosystèmes Tropicaux (MICET), Serge H. Ndriantsoa, Andolalao Rakotoarison, Tsanta F. Rakotonanahary and all the staff of Madagascar Fauna and Flora Group for the logistic help. A first draft of the manuscript was significantly improved by the constructive criticisms of two anonymous reviewers. Our fieldwork was made possible with the support of the Saint Louis Zoo’s Field Research for Conservation program (FRC# 2018.01) of the Wildcare Institute and of Portuguese National Funds through FCT—Fundação para a Ciência e a Tecnologia—that supports the PhD fellowship to FL (SFRH/BD/131722/2017) and the research contract to AC (2020.00823.CEECIND). Open Access Funding provided by Universita degli Studi di Milano within the CRUI-CARE Agreement. [Correction added on 28 May 2022, after first online publication: CRUI funding statement has been added.]




