Testing the phylogenetic hypotheses of Stevardiinae Gill, 1858 in light of new phenotypic data (Teleostei: Characidae)
Contributing authors: Katiane M. Ferreira ([email protected]), Irani Quagio-Grassiotto ([email protected]), Júlio C. O. Santana ([email protected]), Clarianna Martins Baicere-Silva ([email protected]), Naércio A. Menezes ([email protected])
Abstract
enThe Stevardiinae are a high diverse subfamily of Characidae, the richest family of Neotropical fishes. Many species are inseminating (internal fertilization) and consequently display diverse morphology of reproductive organs and sperm cells. We test the monophyly and internal relationships of the Stevardiinae through a phylogenetic analysis based on a new set of morphological characters, including reproductive traits, combined with publicly available molecular data. We defined 176 characters from general morphology and primary and secondary sexual characters, coded for 54 species. Analyses were made under parsimony using a broad range of extended implied weighting parameters. Given the different morphological characters we use, this analysis provides additional synapomorphies and an independent test for previous hypotheses based on morphological and combined morphological and molecular datasets. Our final hypothesis is a single most parsimonious tree of 6341 steps obtained under three different weighting schemes. This recovers the monophyly of the tribes Creagrutini, Diapomini, Glandulocaudini, Hemibryconini, Landonini (including Eretmobryconini), Stevardiini, and Xenurobryconini. It also supports the recognition of the monotypic tribe Phenacobryconini. Insemination is ambiguously optimized as present in the common ancestor of Stevardiinae and in the common ancestor of all members of the subfamily except for Landonini. That reconstruction constitutes a novel hypothesis about the evolution of insemination within Characidae.
Resumen
ptLos Stevardiinae son una subfamilia altamente diversa de Characidae, la familia más rica de peces neotropicales. Muchas especies son inseminadoras (con fertilización interna) y, en consecuencia, presentan diversas morfologías en los órganos reproductivos y células espermáticas. Testeamos la monofilia y relaciones internas de los Stevardiinae a través de un análisis filogenético basado en un nuevo conjunto de caracteres morfológicos que incluye aspectos reproductivos, combinado con datos moleculares de uso público. Definimos 176 caracteres de morfología general y caracteres sexuales primarios y secundarios, codificados para 54 especies. Los análisis fueron hechos bajo parsimonia en un amplio rango de parámetros de pesos implicados extendidos. Dados los diferentes caracteres morfológicos que usamos, este análisis provee sinapomorfías adicionales y un test independiente para las hipótesis previas basadas en matrices morfológicas y combinadas. Nuestra hipótesis final es un único árbol más parsimonioso de 6341 pasos, obtenido bajo tres diferentes esquemas de pesado. Este árbol recupera la monofilia de las tribus Creagrutini, Diapomini, Glandulocaudini, Hemibryconini, Landonini (incluyendo Eretmobryconini), Stevardiini y Xenurobryconini. También apoya el reconocimiento de la tribu monotípica Phenacobryconini. La presencia de inseminación se optimiza de manera ambigua como presente en el ancestro común de los Stevardiinae y en el ancestro de todos los miembros de la subfamilia excepto los Landonini. Esta reconstrucción constituye una hipótesis novedosa sobre la evolución de la inseminación en Characidae.
1 INTRODUCTION
Characidae is by far the largest characiform family, including more than 1200 valid species (Fricke et al., 2021), widely distributed from southern North America to the Neotropical region. The extant species of Characidae are represented by nine subfamilies and several incertae sedis taxa (Mirande, 2019). Among those subfamilies, the Stevardiinae are the second most speciose, containing 354 valid species in 54 genera (Fricke et al., 2021).
Stevardiinae was proposed by Gill, 1858 (under the name Stevardianae, misspelled) to include the genera Stevardia Gill, 1858, Corynopoma Gill, 1858, and Nematopoma Gill, 1858. Some years later, Günther (1864) indicated that Stevardia and Nematopoma were synonyms of Corynopoma. Eigenmann (1914) proposed the subfamily Glandulocaudinae based mainly on dentition, fin positions, anal-fin length, and sexual dimorphism such as the presence of glandular tissue on the caudal fin and opercle and caudal-fin scales, which are sometimes modified in males. In the Glandulocaudinae, Eigenmann (1914) included Coelurichthys Miranda Ribeiro, 1908, Diapoma Cope, 1894, Gephyrocharax Eigenmann, 1912, Glandulocauda Eigenmann, 1911, Hysteronotus Eigenmann, 1911, Microbrycon Eigenmann & Wilson, 1914, Pseudocorynopoma Perugia, 1891, Pterobrycon Eigenmann, 1913, and Stevardia. In that paper, Eigenmann ignored the subfamily Stevardianae of Gill (1858) and Stevardia had been synonymized by Günther (1864).
Glandulocaudinae has been extensively studied and has undergone nomenclatural changes since its creation (see Castro et al., 2003; Menezes & Weitzman, 2009; Weitzman & Fink, 1985; Weitzman & Menezes, 1998; Weitzman et al., 2005). Major changes were proposed by Weitzman et al. (2005), who restricted the Glandulocaudinae to a tribe composed only of Glandulocauda, Lophiobrycon Castro et al., 2003, and Mimagoniates Regan, 1907. The subfamily Stevardiinae was resurrected to include the tribes Corynopomini, Diapomini, Hysteronotini, Landonini, Phenacobryconini, and Xenurobryconini, earlier allocated in the Glandulocaudinae by Weitzman and Menezes (1998). In Stevardiinae, Weitzman et al. 2005) grouped species characterized by: (1) a hypertrophic extension of the body scales onto the rays of the ventral caudal-fin lobe (vs. onto the rays of the dorsal caudal-fin lobe in Glandulocaudinae) and (2) caudal-gland cells of the caudal organ formed by modified mucous cells (vs. specialized club cells in Glandulocaudinae).
In the last decade, studies based on morphological (Mirande, 2009) and molecular (Thomaz et al., 2015) data proposed nomenclatural modifications to the composition of Stevardiinae. In a phylogenetic study on the Characidae, based mainly on osteological data, Mirande (2009) proposed a broader definition for the subfamily based on three synapomorphies: (1) presence of eight dorsal-fin rays posterior to the leading unbranched two, (2) absence of the epiphyseal branch of the supraorbital laterosensory canal, and (3) presence of nine dorsal-fin pterygiophores. According to Mirande (2009), the Stevardiinae were represented by all the genera belonging to the Glandulocaudinae and Stevardiinae sensu Weitzman et al. (2005), the taxa contained in Clade A of Malabarba and Weitzman (2003), and the genera Aulixidens Böhlke, 1952 Bryconadenos Weitzman et al., 2005 and Nantis Mirande, Aguilera & Azpelicueta, 2006. In a comprehensive molecular study including 153 species and 32 genera, Thomaz et al. (2015) proposed a tribal classification for the Stevardiinae and several generic synonymies and new combinations. Thomaz et al. (2015) recognized seven tribes in Stevardiinae: Creagrutini, Diapomini, Eretmobryconini, Glandulocaudini, Hemibryconini, Stevardiini, and Xenurobryconini. Among them, only the Glandulocaudini maintained the composition and phylogenetic relationships proposed by Weitzman et al. (2005). Vanegas-Ríos (2018) provided some remarks on the phylogeny of the Stevardiinae after conducting a morphology-based phylogenetic analysis focused on Gephyrocharax. After that, an analysis of the Characidae combining morphological characters (mostly osteological, as they were expanded from Mirande, 2010) with publicly available DNA data corroborated the monophyly of Stevardiinae and its tribes (Mirande, 2019). Morphological characters and states from Mirande (2009, 2010, 2019) were not defined with the intention that they would be especially informative within Stevardiinae, given that those analyses focused on the broader context of the family Characidae. Characters from Vanegas-Ríos (2018) were based on Mirande (2010) but modified to be mostly informative in a relatively small clade within the subfamily. Phylogenetic relationships obtained for Stevardiinae by Mirande (2019) were very similar to those previously proposed (Thomaz et al., 2015), except that some species were included in different genera and Hypobrycon Malabarba & Malabarba, 1994, Nantis, and Odontostoechus Gomes, 1947 were resurrected.
Many species in Stevardiinae have conspicuous secondary sexual characters, most notably a pocket-like pheromone organ in the caudal peduncle and fin (Weitzman & Fink, 1985; Weitzman & Menezes, 1998). Such sexual characters exhibit, when present, great morphological variability and are very rare in the Characidae outside of Stevardiinae. Therefore, many of the early classifications (e.g., Eigenmann, 1914) and most of the phylogenetic analyses (Vanegas-Ríos, 2018; Weitzman & Menezes, 1998) of the Stevardiinae relied heavily on sexual characters. However, as noted in the literature, the secondary sexual morphology of many stevardiins is somewhat correlated with different reproductive strategies and also with the development and morphology of reproductive organs and sperm cells (e.g., Burns & Weitzman, 2005; Javonillo et al., 2007; Pecio et al., 2005; Quagio-Grassiotto et al., 2020). This opens a source of variation that is undoubtedly useful for phylogenetic inference at some level. In this contribution, we provide a set of 42 characters from sperm morphology and development, which constitute about one fourth of the morphological block of data. The taxon sampling for this paper prioritized the inclusion of species in which this set of characters could be coded, and analysis of the resulting matrix provides a test for their utility as sources of additional resolution and synapomorphies. Given the lower taxon sampling when compared to previous investigations (Mirande, 2019; Vanegas-Ríos, 2018), our analysis has the power of corroborating previous proposals, inferring the relationships of species not previously analyzed, and reconstructing the evolution of new characters. However, at this stage, it cannot logically refute previous analyses but, rather, to provide an alternative hypothesis.
This article proposes a new hypothesis of the internal relationships of the Stevardiinae based on a new phenotypic dataset (mostly independent from Mirande (2010)), combined with available molecular data. This paper also provides for the first time a block of data from detailed reproductive traits, including ultrastructure and development of sperm cells, and demonstrates that this character system contains variation relevant to phylogenetic reconstruction.
2 MATERIALS AND METHODS
In terms of new data, this contribution is fully phenotypic. The entire morphological partition was based on observations made specifically from inspection of specimens in natural history collections. Morphological characters were coded from 2 to 10 cleared and stained samples of each of the 54 analyzed species, totaling about 250 specimens. Molecular partitions were entirely built from GenBank data, after checking voucher identifications and locality data, when possible. A total of 41 species have sequences of at least one molecular marker, along with the morphological data. No species was analyzed solely from molecular data.
2.1 Morphological characters
A total of 176 phenotypic characters were analyzed. Characters are listed in Appendix 1. Most characters were osteological (115), with the remaining ones derived from sperm (42), external features (14), and histology (5).
The cleared and staining protocol followed Taylor and Van Dyke (1985). Whenever possible, two or more specimens of each species were prepared to check for anomalous features and to allow, whenever possible, the evaluation of sexually dimorphic characters. Osteological terminology follows Weitzman (1962) with the modifications listed by Vari and Harold (2001). The complete list of examined material and museum acronyms is included in Appendix 2.
Testes of specimens, previously fixed in 4% formaldehyde and stored in 70% alcohol, were gradually rehydrated in a decreasing ethanol concentration of distilled water. Once rehydrated, the material was refixed overnight in 2% glutaraldehyde and 4% paraformaldehyde in 0.1 M pH 7.2 Sorensen phosphate buffer. Samples were post-fixed in the dark for 2h in 1% osmium tetroxide in the same buffer, stained in block with an aqueous solution of 0.5% uranyl acetate for 2 h, dehydrated in acetone, embedded in araldite, sectioned, and stained with a saturated solution of uranyl acetate in 50% ethanol and lead citrate. Electron micrographs were obtained using a Carl Zeiss LEO 906 (Carl Zeiss, Germany) transmission electron microscope.
Ovaries were removed, fixed in 10% buffered formalin, and dehydrated in different grades of ethanol and clarified xylene. After processing, the tissues were embedded in paraffin, sectioned to a thickness of 5 µm, and stained with hematoxylin and eosin.
2.2 Molecular genetic data
DNA sequence data was entirely obtained from public repositories, mostly from GenBank (www.ncbi.nlm.nih.gov/nuccore) except for one sequence provided by Arcila et al. (2017) (Data S1). Four mitochondrial and four nuclear markers were analyzed. Mitochondrial sequences included fragments of the cytochrome c oxidase subunit 1 gene (COX1), cytochrome b gene (CYTB), and the ribosomal 12S and 16S rRNA genes. Nuclear markers were all DNA sequences of protein-coding genes, including portions of myosin heavy chain 6 (MYH6), patched domain containing 1 (PTCHD1), recombination-activating gene 1 (RAG1), and recombination-activating gene 2 (RAG2). Molecular data were aligned with Muscle (Edgar, 2004) using default settings. Gaps were considered as missing data. Potential contamination or mistakes in the identification of GenBank samples were detected through the use of BLAST (Altschul et al., 1990) and parsimony analyses of each molecular partition. When two or more reliable sequences for the same marker and species were available, they were merged using IUPAC codes for polymorphisms with Asado software (Nixon, 2004). Datasets for each individual marker including their GenBank accession numbers are provided as Data S1. When possible, voucher specimens for GenBank sequences were traced back to their ichthyological collections or public databases to account for any changes in the identifications since the publication of the sequences and correct them accordingly. Alignments for each marker are provided as Data S2.
2.3 Dataset and analysis
The complete matrix is available in TNT format as Data S2. The morphological partition is provided as Data S3 and available online at Morphobank P3663 (O’Leary & Kaufman, 2011, 2012). Missing entries in the data matrix are represented by “?” when the character states could not be evaluated, or by “*” for inapplicable character states.
The complete dataset is composed of 6309 characters, with 176 in the morphological partition and the remaining from genetic data. The number of informative characters is 1338, of which 169 are from morphology, 303 from CYTB, 216 from COX1, 93 from MYH6, 60 from PTCHD1, 215 from RAG1, 153 from RAG2, 35 from 12S, and 94 from 16S. The dataset has about 48% of missing entries, mainly occurring for species lacking data for some or all the molecular markers.
Phylogenetic analyses were done under the parsimony criterion with TNT (Goloboff et al., 2008). Searches included tree fusing, sectorial searches (Goloboff, 1999), and parsimony ratchet (Nixon, 1999), stopping each round after reaching three hits to the same optimum. Analyses were performed under extended implied weighting (Goloboff, 2014), which reduces the influence of homoplastic characters with many missing entries relative to equally homoplastic characters with more complete scoring. This analytical choice prevented characters from assuming artificially high weights due to lack of data rather than for congruence with the remaining species. Thus, this method solves a potential problem that arises when the original implied weighting (Goloboff, 1993) is applied to the analysis of combined datasets (Goloboff, 2014). Extended implied weighting also allows weighting each character against the homoplasy of each partition or smaller subdivision, instead of the homoplasy of each individual character. This is useful for molecular data, in which the homology of each phylogenetic character is relative, because it results from an alignment of contiguous and ordered DNA sites. This contrasts with most morphological features, which are recognizable individually, even if dislocated from the specimen. Also, in DNA data homoplasy is commonly concentrated in sets of contiguous sites (see Goloboff, 2014, for details and further justification of the method).
As in Mirande (2017, 2019) and Terán et al. (2020), different weighting schemes were used. Each individual DNA character was weighted according to its own homoplasy (SEP) or to the average homoplasy of a codon (three contiguous columns) (COD), 10 codons (30 contiguous columns) (GRP), or entire markers (BLK). Also, a weighting scheme grouping all first, second, and third codon positions as separate sets in coding (non-ribosomal) sequences (POS) was used. Each morphological character was weighted according to its own homoplasy in all cases. Ribosomal DNA data were weighted according to the homoplasy of the entire partitions in the POS scheme. In addition to the weighting schemes, the regular and extended implied weighting methods allow the user to define weighting strengths (K values), which determine how abruptly a character reduces its influence in the analysis as a function of their number of homoplastic steps (Goloboff, 1993, 2014).
Each of the five abovementioned weighting schemes is herein combined with three different weighting strengths, in which an average character has about 84, 87, and 90% of the weight of a perfectly hierarchic one. This corresponds to concavity constant K values of 7, 9, and 12, respectively. This combination of weighting schemes and strengths results in 15 analytical conditions. The number of analyzed species represents an arguably poor sampling for homoplasy and, hence, precludes the use of stronger (lower) values of K, which in exploratory searches produced obviously artefactual results, as the non-monophyly of the Stevardiinae. Hereafter, each particular search strategy is referred to as its scheme followed by its strength (e.g., POS9 represents POS scheme and K-value = 9).
A search under equal weighting was done for comparative purposes. A metacriterion of stability estimated through SPR distances (Goloboff, 2008) was used to select among the most parsimonious trees (MPTs) obtained under different weighting conditions (see Mirande, 2009, for details). Support was estimated through symmetric resampling (probability of change of 0.33) with values expressed as differences of frequencies (GC values in TNT) (Goloboff et al., 2003).
2.4 Institutional abbreviations
Abbreviations for institutions and collections are as follows: ANSP, Academy of Natural Sciences of Drexel University, Philadelphia; CAS, California Academy of Sciences, San Francisco; CPUFMT, Coleção de Peixes da Universidade Federal de Mato Grosso, Cuiabá; LIRP, Laboratório de Ictiologia de Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto; MCZ, Museum of Comparative Zoology, Washington D.C.; MZUSP, Museu de Zoologia da Universidade de São Paulo, São Paulo; MNRJ, Museu Nacional da Universidade Federal do Rio de Janeiro, Rio de Janeiro; MCP, Museu da Pontifícia Universidade Católica do Rio Grande do Sul, Porto Alegre; MUSM, Museo de Historia Natural de la Universidad Mayor de San Marcos, Lima.
3 RESULTS
One MPT was obtained under each of the 15 analytical conditions of extended implied weighting. Those MPTs ranged from 6341 and 6367 steps, with a single tree of 6341 steps obtained under four of the conditions (BLK12, COD12, POS9, and POS12). This tree was also obtained as the most stable overall and constitutes our final hypothesis (Figure 1). A strict consensus of the 15 MPTs has 39 dichotomous nodes out of the maximum possible of 52, with several branches within the Stevardiinae collapsing into polytomies. Additional searches demonstrated that the final hypothesis stabilized between K = 12–13 in BLK, K = 10–13 in COD, and K = 8–13 in POS. Searches under equal weighting resulted in four MPTs of 6332 steps.
The Stevardiinae were found to be monophyletic under all conditions analyzed. In stronger (lower) values of K than those used for the final searches, Monotocheirodon kontos Menezes et al., 2013 was obtained as the sister group of Deuterodon heterostomus (Eigenmann, 1911), as supported by morphological convergences, rendering the Stevardiinae artefactually non-monophyletic.
In our final hypothesis, the Stevardiinae were obtained as monophyletic and composed of eight units attributable to some of the tribes recognized in the literature: Creagrutini Miles, 1943, Diapomini Eigenmann, 1909, Landonini Weitzman & Menezes, 1998, Glandulocaudini Eigenmann, 1914, Hemibryconini Géry, 1966, Phenacobryconini Weitzman & Menezes, 1998, Stevardiini Gill, 1858, and Xenurobryconini Myers & Böhlke, 1956. Argopleura Eigenmann, 1913 was obtained as incertae sedis in the Stevardiinae.
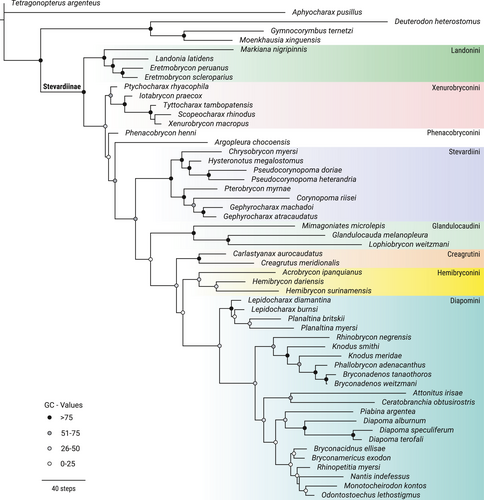
Landonia latidens Eigenmann & Henn, 1914, nominotypical species of the Landonini was reconstructed as belonging to the clade defined as the tribe Eretmobryconini by Thomaz et al. (2015), which would require nomenclatural changes given that Landonini has temporal priority. This clade, composed of Eretmobrycon Fink, 1976, Landonia Eigenmann & Henn, 1914 and Markiana Eigenmann, 1903, has a high support provided by 19 synapomorphies, of which one is morphological: (1) lateral margin of ectopterygoid concave in all its margin (56:0; Figure 2). The Xenurobryconini were recovered as a moderately well-supported clade composed of Iotabrycon Roberts, 1973 Ptychocharax Weitzman et al., 1994, Scopaeocharax Weitzman & Fink, 1985 Tyttobrycon Géry, 1973, and Xenurobrycon Myers & Miranda Ribeiro, 1945. This tribe is supported by four synapomorphies, all of them morphological: (1) posteroventral process of orbitosphenoid absent (28:1; Figure 3a), anterior dentary teeth with the base broader than crown (48:2; Figure 3b), lateral margin of ectopterygoid straight along all its length (57:2; Figure 3c), and elaborations on prezygapophyses and postzygapophyses absent (102:0; Figure 3d,e).
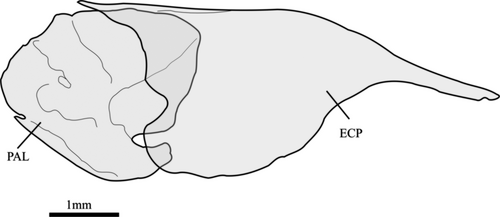
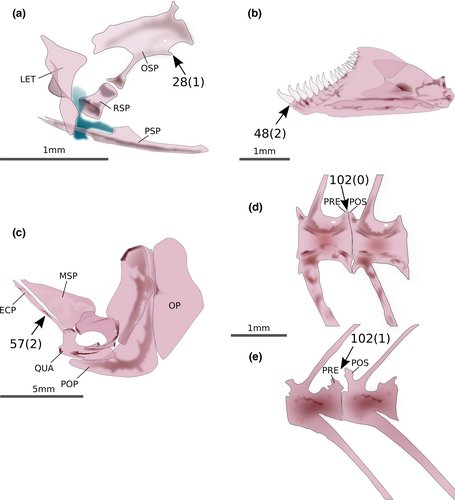
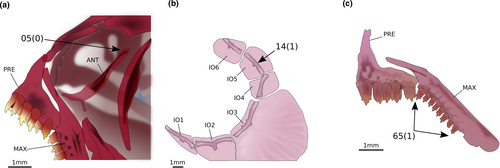
Phenacobrycon henni Eigenmann, 1922 and Argopleura chocoensis (Eigenmann, 1913) are obtained as successive sister clades of the remaining stevardiins. Phenacobrycon Eigenmann, 1922 is a nominotypical genus, and according to our results, the Phenacobryconini should be resurrected. Argopleura, on the other hand, is found as incertae sedis in the Stevardiinae. In our analysis, the monotypic Phenacobryconini, composed only of P. henni, has nine autapomorphies, all of them morphological: (1) anterior margins of ectopterygoid and endopterygoid reaching about the same anterior line (58:1; Figure 4a), (2) foramen in posterior region of metapterygoid forming an incomplete arch, bordered posteriorly by hyomandibular (60:1; Figure 4b), (3) form of metapterygoid-quadrate fenestra round or vertically elongated (63:0; Figure 4b), (4) anterodorsal contact between metapterygoid and quadrate absent, distant from each other (65:1; Figure 4c), (5) eighth pelvic-fin ray unbranched (87:1), (6) anteriormost free anal-fin medial radial on seventh pterygiophore (95:4), (7) presence of one epural (109:0; Figure 4d), (8) pouch scale not hypertrophied (113:0; Figure 4d), and (9) nucleus irregularly shaped in longitudinal section, with re-entrance along its length (164:3; Figure 4e-g).
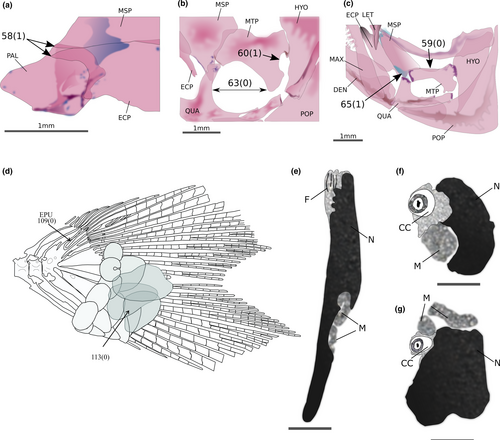
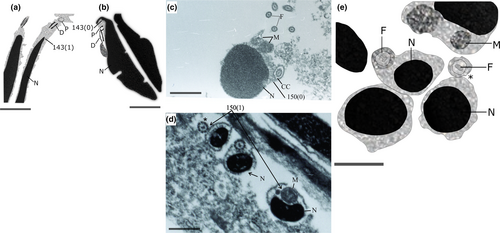
The Stevardiini were obtained as a well-supported monophyletic tribe, composed of two sister clades, one of them including Chrysobrycon Weitzman & Menezes, 1998, Hysteronotus, and Pseudocorynopoma and the remaining one constituted by Corynopoma, Gephyrocharax, and Pterobrycon. This tribe is supported by 30 synapomorphies from DNA and three from morphological data: (1) a well-developed groove above eyes (1:2; Figure 5a), (2) inner premaxillary tooth row with a maximum of five cusps (35:3), and (3) dorsal-fin origin located posterior to vertical line through anal-fin origin (89:2; Figure 5b). The Glandulocaudini are also a monophyletic clade, strongly supported by 40 synapomorphies of which five are morphological: (1) metapterygoid lacking a dorsal concavity (59:0; Figure 4c), (2) absence of contact between metapterygoid and anterodorsal region of quadrate (65:1; Figure 4c), and three reproductive characters: (3) absence of a nuclear fossa (143:1; Figure 6a,b), (4) absence of a midpiece in the sperm cells (150:1; Figure 6c,d), and (5) cytoplasmic canal restricted to the apical region of the sperm (174:0; Figure 6e). As in previous hypotheses, the Glandulocaudini are composed of Glandulocauda, Lophiobrycon, and Mimagoniates.
The Creagrutini are also found as monophyletic and well supported, although only one species of Creagrutus Günther, 1864, plus the monotypic Carlastyanax Géry, 1972 were analyzed herein. This clade is supported by 21 synapomorphies, of which 12 are morphological: (1) ventral end of lateral ethmoid lying posterior to articulation between infraorbitals 1 and 2 (6:2; Figure 7a), (2) infraorbital 3 not reaching the horizontal arm of preopercle (10:1; Figure 7b), (3) a small lamella present in nasal limiting the tubular region (16:0; Figure 7c), (4) presence of three premaxillary tooth rows (31:2; Figure 7d), (5) palatine shorter than ectopterygoid but more than half its length (55:2; Figure 7e), (6) palatine not overlapping the mesopterygoid dorsally (67:1; Figure 7e), (7) posterodorsal angle of hyomandibula lacking any process or notch (69:0; Figure 7f), (8) absence of a dermal bone dorsal to cartilaginous basibranchial 4 (71:0; Figure 7g), (9) base of posteriormost dorsal-fin ray situated anterior or at vertical through anal-fin origin (90:0; Figure 7h), (10) presence of 10 or fewer vertebrae anterior to first dorsal-fin origin (100:0), (11) presence of five supraneurals (107:3), and (12) only one epural (109:0).
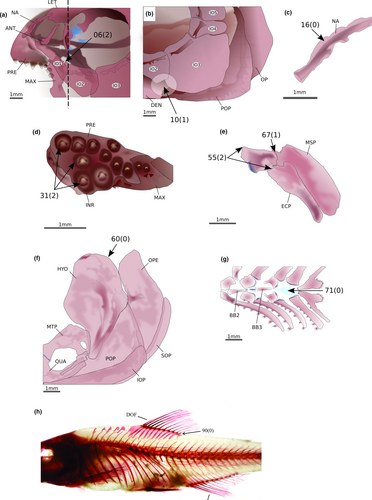
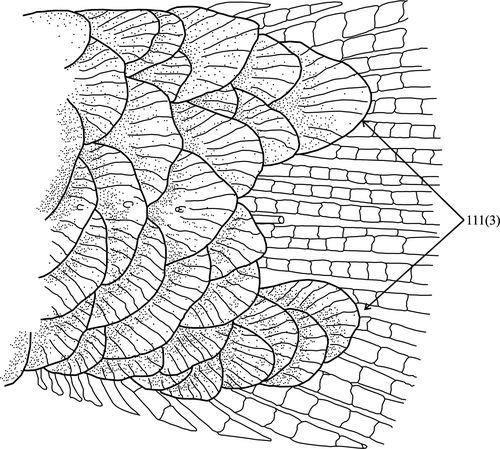
The Hemibryconini and Diapomini are obtained as monophyletic and sister groups, both well supported. The Hemibryconini, represented by Acrobrycon Eigenmann & Pearson, 1924 and Hemibrycon Günther, 1864, are supported by 15 synapomorphies, five of which are morphological: (1) dorsal tip of the antorbital symmetrical (5:0; Figure 8a), (2) infraorbital 5 wider than deep, with the laterosensory canal in a median position (14:1; Figure 8b), (3) toothed portion of maxilla longer than the edentulous region (41:1; Figure 8c), (4) teeth on infrapharyngobranchial 3 present (72:0), and (5) presence of 39 or more vertebrae. The Diapomini are supported by 34 synapomorphies, five of which are morphological: (1) posterior projection of pelvic process relatively reduced (83:1), (2) anterior tip of pelvic bone (basipterygium) located between third and fourth pleural ribs (84:3), (3) scales covering equal portions of dorsal and ventral caudal-fin lobes (111:3; Figure 9), (4) midpiece short (less than 2.0 µm) in the sperm cells (149:0), and (5) midpiece of sperm cell irregularly positioned in relation to the flagellum (151:1).
4 DISCUSSION
The presence and structure of a pocket-like glandular organ in the caudal fin influenced most historical classifications of the species currently included in the Stevardiinae since Eigenmann (1914) erected the subfamily Glandulocaudinae for the species with such a gland. Phylogenetic analyses subsequently corroborated the idea that their sexual dimorphism provided evidence of monophyly (e.g., Weitzman & Fink, 1985; Weitzman & Menezes, 1998). Further available information about insemination (e.g., Burns & Weitzman, 2005) and sperm ultrastructure (Burns et al., 1995, 1998) appeared to support further the monophyly of the stevardiins bearing a caudal gland, given the high correlation between the presence of a caudal-fin gland with the occurrence of insemination and elongate sperm nuclei. Indeed, the caudal-fin bearing stevardiins analyzed by Mirande (2010) from morphological data formed a well-supported clade. Accordingly, the analyses from our morphological partition also show a single origin of the caudal-fin gland, albeit with a later reversion.
Data from DNA sequences were disruptive to previous scenarios about the evolution of the secondary sexual characters in the Stevardiinae and suggested several parallel origins for the caudal-fin gland, the presence of insemination, and some variously correlated sperm features (Mirande, 2019; Oliveira et al., 2011; Quagio-Grassiotto et al., 2020; Thomaz et al., 2015). Our analyses also obtained incongruence between the phylogenetic reconstructions based on morphological and genetic data (see Figure S1). That was expected given the high correlation between several secondary sexual characters, which drive the morphological analyses toward their single origin at the base of a monophyletic group. In any phylogenetic analysis, sets of characters supporting alternative hypotheses compete with that competition resolved in favor of the set that shows greater congruence and especially higher number of characters. Given the conservative morphology of the Characidae, any analysis of the family is prone to be driven by these sets of correlated characters that do not necessarily reflect the true phylogeny of the group. Mirande (2010) explicitly addressed this problem by balancing the amount of characters presumably correlated with sexual dimorphism, predation, or miniaturization with many other morphological characters. The result was a phylogenetic hypothesis that seemed reasonable at the time, but soon found to be incongruent in many details with DNA/rRNA-based analyses (Javonillo et al., 2010; Oliveira et al., 2011).
In combination with molecular sequence data, the same competition between sets of characters occurs, with two important differences. Firstly, DNA sequences are translated to phylogenetic characters with a finer grain and single molecular markers often contain more analyzable data than the entire morphological partition. As a result, sets of congruent molecular DNA data almost always prevail over morphological ones simply due to the greater number of characters. The second difference is that biologists usually assume that DNA is inherited from ancestors to descendants with less influence of extrinsic environmental or ecological constraints than morphology. Systematists therefore often assume that these sets of correlated molecular data truly reflect the phylogeny of the groups that they study. Discussing whether that viewpoint is reasonable or not is beyond the scope of this article. In this study, the inclusion of morphological data in combined phylogenetic analyses of the Characidae was justified by the value inherent in the information itself, namely the possibility of obtaining synapomorphies, deriving diagnoses, and eventually including fossil taxa and time calibration for phylogenies (e.g., Mirande, 2019; Terán et al., 2020).
With subtle differences in the composition and varying nomenclature, the Stevardiinae have been considered monophyletic since Malabarba and Weitzman (2003), with later corroboration from genetic (Javonillo et al., 2010; Oliveira et al., 2011; Thomaz et al., 2015), morphological (Mirande, 2009, 2010), and combined data (Mirande, 2019). The present analysis is based on a different set of species and characters than the previous ones, and therefore, it may be considered as an independent test for the available hypotheses and about the ability of the morphological data to provide synapomorphies for the Stevardiinae and internal clades. The characters from sperm ultrastructure and development are particularly valuable in this respect, because most of them have not been previously analyzed for the Characidae. Some species are herein analyzed for the first time in a phylogenetic context, and therefore, our conclusions are unique in the literature concerning these taxa, which allow us to hypothesize about their relationships independently from previous contributions.
Our results corroborate the monophyly of the Stevardiinae. Their classification in tribes, as obtained herein, is discussed below considering these aspects and comparing our classification with previous proposals.
4.1 Landonini Weitzman & Menezes, 1998, new combination—Type genus Landonia Eigenmann & Henn, 1914
The tribe Landonini, as herein recognized, contains three genera: Eretmobrycon, Landonia, and Markiana. The close relationship between Eretmobrycon and Markiana was first pointed out by Thomaz et al. (2015), who proposed a new tribe Eretmobryconini. According to them, the Eretmobryconini was the sister group of the remaining Stevardiinae. Thomaz et al. (2015), however, did not include Landonia in their analysis and considered it an incertae sedis genus in the Stevardiinae. The monophyly of Eretmobryconini, as well as its position as the sister group of all the other tribes of Stevardiinae, was corroborated by Mirande (2019), who also did not include Landonia and considered this genus as tentatively belonging in the Diapomini, which present study contradicts. Vanegas-Ríos (2018) analyzed Landonia for the first time in a phylogenetic context and found this genus as the sister group of Phenacobrycon, in a different clade than in the present contribution. Our results constitute an alternative hypothesis to the one by Vanegas-Ríos (2018), based on a different set of morphological characters and adding information from DNA/rRNA sequences. Even if our analysis cannot logically refute the hypothesis by Vanegas-Ríos (2018) because both proposals were obtained from different data, he did not propose a tribal classification for Landonia, which was part of his outgroup. Given the high support we found for the inclusion of Landonia in the clade of Eretmobrycon, the absence of the former genus in previous analyses (Mirande, 2019; Thomaz et al., 2015), and the temporal priority of Landonini Weitzman & Menezes, 1998 over Eretmobryconini Thomaz et al. (2015), we propose herein the Eretmobryconini as a junior synonym of Landonini. Mirande (2019) diagnosed the Eretmobryconini based on eight morphological synapomorphies and proposed as additional characters the presence of iii, nine dorsal-fin rays, four teeth in the inner premaxillary row, the infraorbital three reaching the preopercle, the presence of two uroneurals, and the anteroventral angle of the articulation of the second and third infraorbitals. Most (if not all) of these characters are shared also by Landonia, so that the inclusion of this genus would not require major changes in the diagnosis of the tribe.
4.2 Xenurobryconini Myers & Böhlke, 1956 sensu Thomaz et al., 2015—Type genus Xenurobrycon Myers & Miranda Ribeiro, 1945
Based on the similarity of their caudal fins, Myers and Böhlke (1956) proposed the tribe Xenurobryconini for the genera Tyttocharax and Xenurobrycon. Weitzman and Menezes (1998), analyzing mainly the anatomy of the caudal fin in the Glandulocaudinae (sensu Weitzman & Fink, 1985), expanded the composition of Xenurobryconini by including in that tribe the genera Argopleura, Chrysobrycon, Iotabrycon, Ptychocharax, Scopaeocharax, Tyttocharax, and Xenurobrycon. Xenurobryconini was redefined by Thomaz et al. (2015), who excluded Argopleura, considered incertae sedis, and Chrysobrycon, which was transferred to Stevardiini. Mirande (2019) corroborated the monophyly of Xenurobryconini as proposed by Thomaz et al. (2015), with the exception that he also obtained Cyanogaster Mattox et al., 2013 in this clade. However, the actual relationship of Cyanogaster with the Xenurobryconini or even with the Stevardiinae is uncertain and subject of ongoing studies by one of the authors (JMM).
4.3 Phenacobryconini Weitzman & Menezes, 1998—Type genus Phenacobrycon (Eigenmann, 1914)
The tribe Phenacobryconini was proposed by Weitzman and Menezes (1998) to include only the nominotypical genus. The present study corroborates the validity of this tribe, given that Phenacobrycon is not found in a clade with other nominotypical genera having temporal priority. Indeed, according to our results, P. henni is the sister group of a large clade including most stevardiins. Nine morphological autapomorphies are obtained for the monotypic tribe Phenacobryconini, one of them related to caudal-fin pouch-scale morphology (see Results). According to Weitzman and Fink (1985), P. henni has an enlarged caudal scale on the lower caudal-fin lobe. However, the pouch scale present in P. henni is not as hypertrophied as in Acrobrycon, Diapoma, and Planaltina Böhlke (see character 113:0).
Thomaz et al. (2015) and Mirande (2019) did not include Phenacobrycon in their analyses and considered the genus as incertae sedis in Stevardiinae. Vanegas-Ríos (2018) found Phenacobrycon as the sister group of Landonia but did not propose a tribal classification for those genera. According to our results, Phenacobryconini is the sister group of a clade composed of the tribes Creagrutini, Diapomini, Glandulocaudini, Hemibryconini, and Stevardiini. The resurrection of Phenacobryconini does not contradict previous analyses, given that Thomaz et al. (2015) and Mirande (2019) did not analyze P. henni and in the relationships obtained by Vanegas-Ríos (2018), the Phenacobryconini may have been attributed to the sister group of a monotypic Landonini.
4.4 Stevardiini Gill, 1858 sensu Thomaz et al., 2015—Type genus Stevardia Gill, 1858
The Stevardiini was given the level of tribe by Thomaz et al. (2015) to group the Corynopomini and Hysteronotini of Weitzman and Menezes (1998) plus Chrysobrycon, included in the Xenurobryconini (Weitzman & Menezes, 1998). Although Thomaz et al. (2015) did not include Hysteronotus and Pterobrycon in their analysis, they agreed that both genera belong in the Stevardiini and suggested further studies to better understand their relationships with other members of the tribe.
In the present study, Hysteronotus megalostomus Eigenmann, 1911 and Pterobrycon myrnae Bussing, 1974 are analyzed and our results corroborate the hypothesis by Thomaz et al. (2015). The Stevardiini are composed of six genera distributed in two clades. The first clade has Chrysobrycon as the sister group of Hysteronotus and Pseudocorynopoma. The genus Chrysobrycon was proposed by Weitzman and Menezes (1998) to include two species previously described as Hysteronotus hesperus Böhlke, 1958 and Hysteronotus myersi Weitzman & Thomerson, 1970. In the proposal by Weitzman and Menezes (1998), Chrysobrycon was the sister genus of the remaining xenurobryconin genera. The present study strongly contradicts that hypothesis. The similarity between the pouch scale present in males of H. megalostomus and Pseudocorynopoma doriae Perugia, 1891 was first mentioned by Weitzman and Thomerson (1970). The relationship between Hysteronotus and Pseudocorynopoma, primarily described by Weitzman and Menezes (1998), was corroborated by Ferreira et al. (2011), Thomaz et al. (2015), and Mirande (2019).
The second clade obtained in the Stevardiini has Pterobrycon as the sister group of Corynopoma and Gephyrocharax. Relationships among these three genera were first proposed by Weitzman and Menezes (1998), who redefined the tribe Corynopomini (compare with Burns et al., 1995; Weitzman et al., 1988). The hypothesis by Weitzman and Menezes (1998) showed Pterobrycon and Corynopoma as sister taxa. Our results do not corroborate this relationship. Herein, Gephyrocharax and Corynopoma are recovered as sister genera in the final consensus topology, which agrees with the results obtained by Vanegas-Ríos (2018). According to that author, the clade formed by Corynopoma and Gephyrocharax was supported by the presence of spurs formed by hypertrophy of the ventral procurrent rays of the caudal fin. Thomaz et al. (2015) and Mirande (2019) also corroborated a close relationship between Corynopoma and Gephyrocharax, but they did not include Pterobrycon in their analyses.
4.5 Glandulocaudini Eigenmann, 1914 sensu Menezes & Weitzman, 2009—Type genus Glandulocauda Eigenmann, 1911
In the present study, the monophyletic condition of the Glandulocaudini as proposed by Thomaz et al. (2015) is corroborated, including Mimagoniates as the sister group of Glandulocauda and Lophiobrycon. Previous studies (Castro et al., 2003; Menezes & Weitzman, 2009) considered Lophiobrycon as sister group of a clade composed of Glandulocauda and Mimagoniates. Camelier et al. (2018), in a phylogenetic study of the Glandulocaudini based on DNA/rRNA data, found that Glandulocauda was not monophyletic, since that study reconstructed Glandulocauda melanopleura (Ellis, 1911) as sister to Lophiobrycon weitzmani Castro et al., 2003 rather than being included in a clade with Glandulocauda caerulea Menezes & Weitzman, 2009, which turned out to be closely related to Mimagoniates. In our analysis, G. melanopleura and L. weitzmani are sister groups but we did not analyze G. caerulea.
As mentioned earlier, the Glandulocaudini are supported by 40 synapomorphies, of which three are features of spermatozoa. The first sperm synapomorphy is the absence of a depression in the nuclear contour named the nuclear fossa (143:1). The nuclear fossa may be present or absent in species that have type II spermiogenesis. When present, the nuclear fossa can be classified according to the appearance of the contour of the nuclear membrane in longitudinal section: regular (subdivided into single or double concavity) or with irregular projections that penetrate the nucleus. Among the Stevardiinae, the loss of the nuclear fossa is homoplastic in the clade composed of Xenurobrycon and Tyttocharax. The absence of the midpiece (150:1) and the presence of a cytoplasmic canal restricted only to the apical region of the nucleus (174:0) represent the last two sperm synapomorphies. At the end of spermiogenesis, due to nuclear elongation, the midpiece disappears and the cytoplasmic canal remains restricted in the apical region of the nucleus. The mitochondria are anchored in the concave face of the nucleus throughout its length, and there are several mitochondria organized as a row along the nuclear outline. The absence of a midpiece is a homoplastic character in Pseudocorynopoma and the clade of Scopaeocharax, Tyttocharax, and Xenurobrycon. For further explanations about characid sperm and spermiogenesis and how phylogenetic characters were defined for this kind of data, read Quagio-Grassiotto et al. (2020).
4.6 Creagrutini Miles, 1943 sensu Thomaz et al., 2015—Type genus Creagrutus Günther, 1864
Our results corroborate the monophyly of Creagrutini as proposed by Thomaz et al. (2015), where Carlastyanax and Creagrutus were obtained as sister groups. The relationship between Carlastyanax and Creagrutus was first proposed by Mirande et al. (2013). Recently, Mirande (2019) expanded the Creagrutini to include Planaltina britskii Menezes et al., 2003, Lepidocharax burnsi Ferreira et al., 2011, and Microgenys minuta Eigenmann, 1913. In his analysis, Creagrutini was represented by two clades: one including L. burnsi and P. britskii, and the other having M. minuta as the sister group of Carlastyanax and Creagrutus. Although herein we did not include M. minuta, the Creagrutini sensu Mirande (2019) are strongly contradicted by the inclusion of Lepidocharax burnsi and Planaltina britskii in the Diapomini.
4.7 Hemibryconini Géry, 1966 sensu Thomaz et al., 2015—Type genus Hemibrycon Günther, 1864
The tribe Hemibryconini as defined by Thomaz et al. (2015) includes Acrobrycon, Hemibrycon, and tentatively the genus Boehlkea Géry, 1966. We did not include Boehlkea in our analysis, but according to Bertaco and Malabarba (2010), Boehlkea and Hemibrycon share some morphological similarities and are probably closely related.
Acrobrycon was included in the Diapomini, together with Planaltina Böhlke, 1954 and Diapoma Cope, 1894 by Weitzman et al. (1988) based on the presence of two attributes of the pheromone organs overlying the basal portions of the caudal fin: (1) mature males and females possessing organs of equivalent size; (2) presence of three or more series of scales immediately ventral to the lateral line scale series, which form the dorsal border of the pouch opening (see also Arcila et al., 2013). These characters were included in our analysis; however, the tribe Diapomini as proposed by Weitzman et al. (1988) was strongly contradicted.
4.8 Diapomini Eigenmann, 1909 sensu Thomaz et al., 2015—Type genus Diapoma Cope, 1894
The monophyly of the Diapomini sensu Thomaz et al. (2015) is corroborated in the present study. According to our analysis, it includes two clades (Figure 1). The first one shows the non-monophyly of Lepidocharax Ferreira et al., 2011, due to L. burnsi being the sister group of Planaltina. The first hypothesis of relationships of Lepidocharax was erected by Ferreira et al. (2011), who proposed the genus to be sister to all the remaining Stevardiinae (sensu Mirande, 2010), pointing out several similarities of morphological characters and sperm ultrastructure between Lepidocharax and Planaltina. In Thomaz et al. (2015), Lepidocharax appeared as the sister group of the Diapomini and it was considered as incertae sedis in this tribe. Planaltina species were not included in the analysis by Thomaz et al. (2015); however, they tentatively included it in the Diapomini. In Mirande (2019), Lepidocharax and Planaltina were also obtained as closely related to each other but placed within Creagrutini. In the present study, the clade comprising these genera is supported by 11 morphological synapomorphies: (1) presence of a deep groove above eyes containing many neuromasts (1:2), (2) ascending premaxillary process reaching as much as 1/3 of nasal bone length (18:1), (3) lateral wings of urohyal reaching only the middle longitudinal portion of the bone (70:1), (4) origin of first dorsal-fin ray inserted at vertical through anal-fin origin (89:1), (5) anteriormost hemal postzygapophyses present in transitional vertebrae (106:1), (6) nuclear fossa located laterally almost to tip of nucleus (145:3), (7) membranous vesicles in intermediate region of midpiece present (168:0), (8) membranous vesicles in posterior region of midpiece present (169:0), and absence of tubules and vesicles interconnected in (9) anterior (170:1), (10) intermediate (171:1), and (11) posterior (171:2) regions of midpiece.
The second clade of Diapomini is subdivided into two other clades. The first of them is composed of Bryconadenos, Knodus Eigenmann, 1911, Phallobrycon Menezes et al., 2009 and Rhinobrycon Myers, 1944. Rhinobrycon is the sister genus of the remaining ones, Knodus is not monophyletic, and Phallobrycon is the sister taxon of Bryconadenos. We prefer not to follow the classification proposed by Thomaz et al. (2015) concerning the synonymy of Bryconadenos with Knodus, given that neither their analysis nor ours inspected sufficient species of this clade or putatively related genera to support such nomenclatural changes. Also, Thomaz et al. (2015) did not provide an updated diagnosis of Knodus. A close relationship between Knodus and Rhinobrycon was also reported by Thomaz et al. (2015) and Mirande (2019) who did not include Phallobrycon in their studies. Thomaz et al. (2015) considered Phallobrycon as incertae sedis in Stevardiinae.
The sister group of the clade composed of Bryconadenos, Knodus, Phallobrycon, and Rhinobrycon is composed of several genera and supported by eight synapomorphies, of which one is morphological: possession of 18 or fewer branched anal-fin rays (91: 1). This clade is subdivided into two other clades, composed respectively of Attonitus Vari & Ortega, 2000 plus Ceratobranchia Eigenmann, in Eigenmann et al. (1914) and a large group formed by Bryconacidnus, Myers in Eigenmann and Myers (1929), Bryconamericus Eigenmann in Eigenmann et al. (1907), Diapoma, Monotocheirodon Eigenmann & Pearson, 1924, Nantis, Odontostoechus, Piabina Reinhardt, 1867, and Rhinopetitia Géry, 1964.
The close relationship of Attonitus and Ceratobranchia obtained in the present study corroborates the hypotheses by Thomaz et al. (2015) and Mirande (2019) in which Bryconamericus pachacuti Eigenmann, 1927 and Bryconamericus pectinatus Vari & Siebert, 1990 (transferred to Bryconacidnus by Thomaz et al., 2015) appeared as related to Attonitus and Ceratobranchia. Bryconamericus pachacuti was not included in the present study, and the status of Bryconacidnus will be discussed below. In the analysis by Thomaz et al. (2015), Ceratobranchia was obtained as the sister group of Attonitus, B. pachacuti, and B. pectinatus, while in Mirande (2019), Ceratobranchia was the sister group of B. pectinatus and B. pachacuti nested with Attonitus. The clade of Attonitus and Ceratobranchia is herein supported by 25 synapomorphies, of which seven are morphological: (1) two premaxillary tooth rows (31:1), (2) inner premaxillary teeth with maximum of three cusps (35:1), (3) inner premaxillary teeth with base narrower than crown (36:0), (4) dentary teeth with three or more cusps (53:1), (5) contact ectopterygoid-quadrate absent (54:1), (6) palatine bone one half as long as ectopterygoid or shorter (55:2), and (7) presence of eight branched dorsal-fin rays (88:1).
The remaining Diapomini comprises a clade including Diapoma plus Piabina and a larger monophyletic group formed by Bryconacidnus, Bryconamericus, Monotocheirodon, Nantis, Odontostoechus, and Rhinopetitia. The close relationship of Diapoma and Piabina was already proposed by Thomaz et al. (2015) but also includes Piabarchus Myers, 1928, which was not analyzed herein. In Mirande (2019), Piabina was the sister group of a more comprehensive clade that also included Diapoma. This clade is supported by nine synapomorphies, one of them morphological: (1) All anal-fin proximal radials posterior to first hemal spine (93:0).
The final clade within Diapomini clade includes Bryconacidnus plus Bryconamericus as the sister group of Monotocheirodon, Nantis, Odontostoechus, and Rhinopetitia. In Thomaz et al. (2015), a group of terminal taxa attributed to the genus Bryconacidnus formed the sister group of Attonitus and B. pachacuti. Mirande (2019) included B. pectinatus (as Bryconacidnus pectinatus) in his analysis, and this species appeared as related to Ceratobranchia, “Bryconamericus” diaphanus (Cope, 1878), “Bryconamericus” pachacuti, and the species of Attonitus. Bryconamericus was related to Diapoma, Piabarchus, and Piabina in Thomaz et al. (2015). In Mirande (2019), Bryconamericus, together with Piabarchus Myers (not included here), was the sister group of Nantis and Odontostoechus, as in the present study. According to Menezes and Netto-Ferreira (2019), the species of Rhinopetitia were more closely related to Bryconacidnus, Monotocheirodon, and Odontostoechus than to the remaining species of the Stevardiinae. That study reconstructed Bryconacidnus as the sister group of the other three genera, which the present study corroborates. This clade is supported by 14 synapomorphies, one of them morphological: scales extended posteriorly only to base of caudal fin (111:0).
Hypobrycon, Nantis, and Odontostoechus had been synonymized with Bryconamericus by Thomaz et al. (2015) due to the relationships they obtained for Bryconamericus exodon Eigenmann, 1907, the type species of the genus. Mirande (2019: 16) resurrected these three genera, after a comprehensive phylogenetic analysis of the Characidae. Bryconamericus exodon is very similar to B. stramineus Eigenmann, 1908 and, among the available information, only some DNA data exclude it from a sister group relationship with that species. Available sequences attributed to B. exodon in the literature and deposited in GenBank were produced by Oliveira et al. (2011) from specimens in lot LBP 7123, Pereira et al. (2013) from lots LBP 2893, 6392, 7111, and 19824, Thomaz et al. (2015) from a single specimen in the lot UFRGS 13571, and García-Melo et al. (2019) from lots LBP 5118, 12926, 13408, 13463, 13565, and 13598. Among these specimens, the lots from Oliveira et al. (2011) and Pereira et al. (2013) are currently assigned to either Bryconamericus iheringii Boulenger, 1887 or B. aff. iheringii in the collection databases, while the voucher from Thomaz et al. (2015) and most lots from García-Melo et al. (2019) are still catalogued as B. exodon. One of the authors (JMM) corroborated the current identification of some of the LBP lots. All the information attributed to B. exodon as analyzed by Thomaz et al. (2015) is sequences obtained from a single specimen still identified as B. exodon (UFRGS 13571). Analysis of sequences from this individual resulted in several taxonomic changes in the genus, but the identification of the specimen itself is suspect. Most of these sequences are very similar to DNA/rRNA samples of B. stramineus and Piabarchus analis (Eigenmann, 1914) (to B. stramineus RAG1: 99.03%, RAG2: 98.73%, 12S: 98.57%; to P. analis 16S: 99.28%), and indeed, B. exodon nests with those two species in gene-by-gene parsimony analyses. However, the COX1 sequence of B. exodon analyzed by Thomaz et al. (2015) has a similarity of 99.23% with a GenBank sample of Bryconamericus ikaa Casciotta et al., 2004. Indeed, the 100 most similar samples in GenBank database are from species in the clade of B. iheringii, rather distantly related to B. exodon among the Stevardiinae. The closest presumably true B. exodon COX1 sequence in GenBank has a similarity of 90.98% with this one, analyzed by Thomaz et al. (2015). Parsimony analyses of the complete dataset of Thomaz et al. (2015), under equal and implied weighting using medium-to-mild concavity values (K), resulted in the sister group relationship of B. exodon with the clade of B. iheringii, which included also Hypobrycon, Nantis, and Odontostoechus, in congruence with the results of Thomaz et al. (2015) under model-based analyses. Strong weighting against homoplasy (K < 10), instead, put B. exodon in the clade of B. stramineus and P. analis. Either removing the COX1 sequence of B. exodon from the dataset of Thomaz et al. (2015) or replacing it with one from García-Melo et al. (2019) resulted in a close relationship of B. exodon with B. stramineus and P. analis under any analytical condition. This makes evident a contamination of the COX1 sequence of B. exodon in Thomaz et al. (2015), which consequently invalidates the synonymy of Hypobrycon, Nantis, and Odontostoechus and implies the exclusion from Bryconamericus of the species found in the “Bryconamericus iheringii clade” of Mirande (2019). Among these three genera, Odontostoechus Gomes, 1947 has priority over Hypobrycon Malabarba & Malabarba, 1994 and Nantis. However, the generic reassignment of the species of the “Bryconamericus iheringii clade” (Mirande, 2019) to Odontostoechus still depends on the corroboration by broader analyses of the relationships herein obtained for Monotocheirodon Eigenmann & Pearson and, eventually, the position of Othonocheirodus Myers, 1927, which has yet to be included in any phylogenetic analysis. Both genera have temporal precedence over Odontostoechus.
This contribution constitutes a rather independent test for the phylogenetic relationships in the Stevardiinae. It also includes a set of characters related to sperm morphology and development for the first time in a phylogenetic analysis of the subfamily. This set of characters is particularly interesting for the Stevardiinae given that this clade includes species with insemination and presumably correlated variation in the morphology of sperm cells (e.gBurns & Weitzman, 2005; Javonillo et al., 2007; Pecio et al., 2005; Quagio-Grassiotto et al., 2020). Among the stevardiins previously included in phylogenetic analyses, insemination was reported for the species of Glandulocaudini, Stevardiini, Xenurobryconini, and Acrobrycon (Hemibryconini), Argopleura (incertae sedis), Attonitus, Bryconacidnus, some species of Bryconamericus, Diapoma, and Knodus (Diapomini), and some species of Creagrutus (Creagrutini) (Thomaz et al., 2015). The evolution of insemination, as optimized by parsimony on the tree topology obtained by Thomaz et al. (2015) would imply at least seven instances of parallel evolution with reversals at the common ancestor of Hemibrycon and the tribes Creagrutini and Hemibryconini. In this analysis, we found the presence of insemination ambiguous at the common ancestor of the Stevardiinae and most parsimoniously reconstructed as present in the clade comprising all components of the subfamily except for the Landonini. The ancestral state of the Landonini is also ambiguous given that insemination is present in Landonia, absent in Markiana, and unknown in Eretmobrycon. Thus, in one of the most parsimonious reconstructions of the character, the insemination had a single origin in the Stevardiinae, with multiple reversals to external fertilization. Spermatozeugmata are unencapsulated sperm packets present in many inseminating characid species (Pecio et al., 2005), but not in all (e.g., inseminating species of Diapoma, and species of Attonitus and Gephyrocharax). Therefore, not only their distribution in the Stevardiinae is more restricted than insemination, but also there are inseminating species in which this feature is unknown (e.g., Landonia), which renders the reconstruction of this character somewhat tentative at this point. According to the available data, the spermatozeugmata had also an origin in the clade including all the Stevardiinae excepting the Landonini, followed by four reversals to absence and an additional gain in the clade of Bryconadenos and Phallobrycon. However, as this feature is unknown for Landonia, the presence of spermatozeugmata in the common ancestor of all the Stevardiinae is still possible. We have not yet completely understood the evolutionary mechanisms and conditions that allowed the great diversification of the Characidae and particularly of the Stevardiinae, but is highly probable that insemination was one of them. Its comprehension may provide a clue to understand the evolution of this subfamily.
As mentioned, this dataset is somewhat exploratory in its search for additional characters informative about internal relationships of the Stevardiini. Therefore, we intentionally made no formal taxonomic changes in the cases wherein our results contradicted previous analyses. In the future, it will be valuable to expand the coding of characters from sperm and spermiogenesis to a broader extent in the family and also to combine the set of morphological characters herein proposed with those from the literature (Mirande, 2019; Vanegas-Ríos, 2018). However, that would require a minute comparison character by character and state by state to prevent redundancies and, after that, a huge work coding the missing entries derived from the different taxon samplings of the involved approaches. This contribution provides an intermediate step toward that broader goal.
The inspected characters behaved as expected, recovering most of the relationships in the literature and providing additional synapomorphies for most clades. The monophyly of the Stevardiinae and most of its tribes, as proposed in the literature, were corroborated. The different set of characters and taxa analyzed herein provided new morphological synapomorphies for the subfamily and their tribes and also imply some forthcoming taxonomic changes in the Stevardiinae. The most important taxonomic finding with respect to previous classifications is the recognition of the tribes Landonini, as a senior synonym of Eretmobryconini, and the monotypic Phenacobryconini. However, the main focus of this analysis was on the phenotypic characters and especially on those related to insemination, sperm, and spermiogenesis. This analysis results in a reinterpretation of the evolution of the presence of insemination and partially correlated characters, as the spermatozeugmata. Until now, several parallel acquisition of those reproductive traits in the Stevardiinae were suggested or, at least, implicitly considered. Our results are compatible with a single acquisition of insemination and related features, with several reversals in the subfamily. This hypothesis, however, should be further tested, given that information about reproductive strategies and morphology of the associated organs and reproductive cells is missing for many species in the Stevardiinae and Characidae in general. Also, potentially informative details about sperm and spermiogenesis remain to be studied and coded for many species. This contribution serve as step forward in that sense.
ACKNOWLEDGMENTS
The following people helped us with loan of specimens: Mário C.C. de Pinna, Alessio Datovo, and Osvaldo T. Oyakawa (MZUSP); Francisco Langeani (DZSJRP); David Catania (CAS), Ricardo Macedo Corrêa e Castro, and Flávio Alicino Bockmann (LIRP); Roberto Reis and Zilda Margarete Lucena (MCP); Karsten E. Hartel (MCZ); Luiz R. Malabarba (UFRGS); Marcelo Britto (MNRJ); Francisco Provenzano (MBUCV); Hernán Ortega (MUSM); and Mark Sabaj and John Lundberg (ANSP). Alexandre Cunha Ribeiro helped us with the preparation of all the drawings of fish structures. The authors want to thank Brian Sidlauskas (OSU) for careful reading of the manuscript. The authors receive financial support from the Fundação de Amparo a Pesquisa do Estado de São Paulo-FAPESP: KMF (fellowship 07/52756-0), Conselho Nacional de Desenvolvimento Científico e Tecnológico: NAM (fellowship 302585/2019), and Agencia Nacional de Ciencia y Tecnología: JMM (PICT-2016-0275). This paper is part of the postdoctoral of the first author (KMF), in which Dr. Naércio A. Menezes was the supervisor.
CONFLICT OF INTEREST
The authors declare no competing interests.
AUTHOR CONTRIBUTIONS
KMF conceptualized the study and wrote the first draft. JMM performed analyses, and wrote part of the results and discussion. NAM supervised the project and the writing–review process. KMF, JMM, IQG, JCOS, and CMBS contributed data. All authors critically contributed to the writing by adding to the text and suggesting edits.
APPENDIX 1
List of morphological characters
Orbital region
(1) Head groove above eyes (Ferreira et al., 2011, character 36): (0) absent; (1) reduced, shallow, not well delineated and containing only few neuromasts; (2) developed, deep, well delineated and containing numerous neuromasts.
(2) Antorbital (Ferreira et al., 2011, character 81): (0) in contact with infraorbital 1; (1) not contacting infraorbital 1.
(3) Antorbital canal (Ferreira et al., 2011; character 82): (0) absent; (1) present.
(4) Ventral expansion of antorbital: (0) absent; (1) at ventral margin of antorbital; (2) at middle half of antorbital.
(5) Shape of antorbital dorsal region (Ferreira et al., 2011, character 84): (0) dorsal tip symmetrical; (1) dorsal end asymmetrical, with tip oriented posterodorsally.
(6) Lateral view of lateral ethmoid ventral end (Ferreira et al., 2011, character 85): (0) anterior to dorsal articulation between infraorbitals 1 and 2; (1) just dorsal to articulation between infraorbitals 1 and 2; (2) posterior to articulation between infraorbitals 1 and 2.
(7) Length of infraorbital 1 (Ferreira et al., 2011, character 86): (0) shorter than infraorbital 2; (1) about same length of infraorbital 2; (2) longer than infraorbital 2.
(8) Infraorbital 1 laterosensory canal (Ferreira et al., 2011, character 87-modified): (0) not reaching anterodorsal margin of bone; (1) reaching anterodorsal margin of bone.
(9) Posterior margin depth of infraorbital 1 (Ferreira et al., 2011, character 88-modified): (0) as deep as anterior margin of infraorbital 2; (1) shallower than anterior margin of infraorbital 2.
(10) Infraorbital 3 ventral extension (0) reaching preopercle in its anteroventral margin; (1) not reaching preopercle in its anteroventral margin.
(11) Infraorbital 4 (Ferreira et al., 2011, character 89): (0) rectangular, deeper than long; (1) approximately as long as deep; (2) rectangular, longer than deep; (3) triangular, with dorsal and ventral margins converging.
(12) Posterior margin depth of infraorbital 4 (Ferreira et al., 2011, character 90): (0) shallower than anterior margin; (1) as large as anterior margin or slightly deeper.
(13) Posterior branch of laterosensory canal of infraorbital 4 (Ferreira et al., 2011, character 91): (0) present; (1) absent.
(14) Infraorbital 5 laterosensory canal (Ferreira et al., 2011, character 93): (0) near anterior margin of bone; (1) in middle of a wider than deep infraorbital 5; (2) in middle of a deeper than wide infraorbital 5.
(15) Size of infraorbital 6 (Ferreira et al., 2011, character 94-modified): (0) smaller than infraorbital 5; (1) larger in surface than infraorbital 5.
Neurocranium
(16) Nasal bone lateral lamellae (Ferreira et al., 2011, character 95): (0) present, lateral to tubular region; (1) absent.
(17) Nasal bone anterior extension (Ferreira et al., 2011, character 96): (0) not reaching lateral wings of mesethmoid; (1) reaching lateral wings of mesethmoid; (2) extending slightly anterior to lateral wings of mesethmoid; (3) extending anterior to mesethmoid lateral wings in ¼ of its length.
(18) Extension of ascending premaxillary process (Ferreira et al., 2011, character 97): (0) not reaching nasal bone; (1) reaching 1/3 or slightly less of nasal bone length; (2) reaching ½ or more of nasal bone length.
(19) Pores size of frontal laterosensory canal (Ferreira et al., 2011; character 98): (0) smaller than laterosensory canal width; (1) as wide as laterosensory canal.
(20) Anterior frontal fontanel (Ferreira et al., 2011; character 99): (0) delimited by posterior margin of mesethmoid; (1) delimited by confluence of contralateral frontals.
(21) Anterior cranial fontanel (Ferreira et al., 2011; character 100): (0) absent, completely occluded by frontal; (1) extending for about 1/3 of distance between epiphyseal bar and mesethmoid; (2) extending for about half way distance from bar to mesethmoid; (3) reaching mesethmoid anteriorly.
(22) Epioccipital bridge over posttemporal fossa (Ferreira et al., 2011, character 101): (0) present; (1) absent.
(23) Posttemporal fossa lateral view (Ferreira et al., 2011, character 102): (0) dorsal and ventral openings about same size from lateral view; (1) dorsal opening larger than ventral one from lateral view; (2) dorsal opening smaller than ventral one from lateral view.
(24) Supraoccipital posterior extension (Ferreira et al., 2011, character 103-modified): (0) short, not reaching posterior end of Weberian apparatus; (1) extending posterior to line through Weberian apparatus margin.
(25) Vomer anterior region (Ferreira et al., 2011, character 104-modified): (0) almost twice as wider as posterior laminar region; (1) only slightly wider than posterior region.
(26) Rhinosphenoid: (0) absent; (1) present.
(27) Rhinosphenoid dorsal extension (Ferreira et al., 2011, character 105): (0) absent; (1) present.
(28) Orbitosphenoid posteroventral process (Ferreira et al., 2011, character 106): (0) absent; (1) present.
Jaws and dentition
(29) Premaxilla ascending process (Ferreira et al., 2011, character 124-modified): (0) straight or slightly curved; (1) conspicuously convex.
(30) Premaxilla margin at articulation point with mesethmoid and contralateral premaxilla (Ferreira et al., 2011, character 125-modified): (0) following a continuous line; (1) with a conspicuous angle between both articulations.
(31) Number of premaxillary tooth rows (Ferreira et al., 2011, character 126): (0) one; (1) two; (2) three.
(32) Number of inner row premaxillary teeth (Ferreira et al., 2011, character 127-modified): (0) three; (1) four; (2) five; (3) six or more.
(33) Size of outer row premaxillary teeth (Ferreira et al., 2011; character 128-modified): (0) smaller than those of inner row; (1) same size or larger than those of inner row.
(34) Diastemas (spaces between teeth) of premaxillary outer row (Ferreira et al., 2011, character 129): (0) as broad as a teeth of that row; (1) all spaces more slender than teeth.
(35) Maximum number of cusps of inner row premaxillary teeth (Ferreira et al., 2011, character 130-modified): (0) one cusp, conical teeth; (1) three; (2) four; (3) five; (4) seven.
(36) Shape of inner row premaxillary teeth (Ferreira et al., 2011, character 131-modified): (0) base narrower than crown; (1) base and crown about same size; (2) base broader than crown.
(37) Form of inner row premaxillary teeth (Ferreira et al., 2011, character 132-modified): (0) broad; (1) slender.
(38) Extension of posterior margin of maxilla (Ferreira et al., 2011, character 133-modified): (0) not reaching ventral tip of lateral ethmoid; (1) posterior to ventral tip of lateral ethmoid.
(39) Maxillary teeth (Ferreira et al., 2011, character 134): (0) absent; (1) present.
(40) Length of alveolar ramus in relation to ascending process of maxilla (Ferreira et al., 2011, character 135-modified): (0) about same size or alveolar ramus slightly longer; (1) alveolar ramus twice long than ascending process; (2) about same size than edentulous portion of alveolar ramus.
(41) Extension of toothed portion of maxilla (Ferreira et al., 2011, character 136-modified): (0) shorter than edentulous portion of alveolar ramus; (1) longer than edentulous portion of alveolar ramus; (2) edentulous posterior process of maxillary about same size as toothed portion.
(42) Maxillary teeth: (0) with a variable number of cusps; (1) cusps absent, plate-shaped.
(43) Number of maxillary teeth (Ferreira et al., 2011, character 137): (0) 1–4; (1) 5–10 (2) 11 or more.
(44) Number of maxillary teeth cusps (Ferreira et al., 2011; character 138 - modified): (0) maxillary teeth conical; (1) with two cusps; (2) with three cusps; (3) with five cusps; (4) with seven cusps.
(45) Size of maxillary teeth cusps (Ferreira et al., 2011; character 139-modified): (0) cusps absent, teeth conical; (1) all cusps with similar sizes; (2) median cusps larger than lateral ones.
(46) Number of fossae at interdigitation anteriorly connecting both halves of dentary: (0) three; (1) four; (2) five; (3) six; (4) seven.
(47) Relative size of anterior toothed and posterior edentulous portions of lower jaw (Ferreira et al., 2011, character 141): (0) anterior portion longer than posterior; (1) anterior portion shorter than posterior.
(48) Form of four larger anterior dentary teeth (Ferreira et al., 2011, character 142-modified): (0) base and crown of about same size; (1) base narrower than crown (2) base broader than crown.
(49) Shape of cusps of dentary teeth (Ferreira et al., 2011; character 143 - modified): (0) straight and perpendicular to axis of dentary; (1) posteriorly curved.
(50) Size of cusps of four larger anteriormost dentary teeth (Ferreira et al., 2011, character 144): (0) cusps with about same size; (1) median cusps longer than others.
(51) Number of cusps of anteriormost four dentary teeth (Ferreira et al., 2011, character 145-modified): (0) teeth conical; (1) three; (2) five; (3) six; (4) seven.
(52) Anteriormost dentary teeth (Ferreira et al., 2011; character 146): (0) deeply implanted, with markedly convex base; (1) shallow insertion, with slightly convex base; (2) inserted dorsally, with base straight in lateral view.
(53) Number of cusps of posterior dentary teeth (Ferreira et al., 2011, character 147): (0) conical; (1) with three or more cusps.
Suspensorium
(54) Ectopterygoid-quadrate contact (Ferreira et al., 2011, character 107): (0) present; (1) absent.
(55) Ectopterygoid and palatine relative sizes (Ferreira et al., 2011, character 108-modified): (0) palatine one half or less ectopterygoid length; (1) palatine and ectopterygoid about same size; (2) palatine shorter than ectopterygoid but longer than one half.
(56) Shape of ectopterygoid (Ferreira et al., 2011, character 109): (0) broad anteriorly, tapering abruptly on posteriorly end; (1) narrow anteriorly, tapering gradually on posterior end; (2) narrow and about same width in all its length.
(57) Lateral margin of ectopterygoid (Ferreira et al., 2011, character 110): (0) concave in all its length; (1) concave only on posterior portion; (2) straight, without concavity.
(58) Ectopterygoid and endopterygoid anterior margins: (0) ectopterygoid margin anterior to endopterygoid; (1) ectopterygoid margin reaching about vertical line crossing endopterygoid anterior margin (2 endopterygoid margin anterior to ectopterygoid margin.
(59) Metapterygoid dorsal margin (Ferreira et al., 2011, character 112 - modified): (0) absent, straight or convex; (1) present.
(60) Posterior metapterygoid foramen (Ferreira et al., 2011; character 113, Mirande, 2010; character 168): (0) piercing metapterygoid or partially limited by cartilage; (1) forming an incomplete arch, bordered posteriorly by hyomandibula.
(61) Position of metapterygoid fenestra for pseudobranch artery (Ferreira et al., 2011, character 114): (0) on middle depth of metapterygoid; (1) on ventral region of metapterygoid.
(62) Posteroventral contact between metapterygoid and quadrate (Ferreira et al., 2011, character 115-modified): (0) absent; (1) present.
(63) Metapterygoid-quadrate fenestra (Ferreira et al., 2011; character 116): (0) round or vertically elongate; (1) horizontally elongate; (2) three times or more as long as its width.
(64) Metapterygoid dorsal groove (Ferreira et al., 2011, character 117-modified): (0) restricted to middle length of metapterygoid; (1) along entire dorsal surface of metapterygoid.
(65) Metapterygoid and quadrate anterodorsal contact (Ferreira et al., 2011; character 118): (0) present or bones close to each other; (1) absent, bones distant from each other.
(66) Palatine fenestra (Ferreira et al., 2011, character 120): (0) present; (1) absent.
(67) Palatine position (Ferreira et al., 2011, character 121-modified): (0) overlapping endopterygoid dorsally; (1) contacting endopterygoid but not overlapping it dorsally.
(68) Palatine shape (Ferreira et al., 2011, character 122-modified): (0) middle portion narrow, forming a constriction in bone; (1) square or rectangular, without a constriction in its middle length.
(69) Hyomandibular posterodorsal margin (Ferreira et al., 2011, character 123-modified): (0) without notch and process; (1) with a notch and a short process oriented posterodorsally; (2) with notch and a process almost reaching dorsal hyomandibula.
Branchial apparatus
(70) Urohyal lateral wings extension (Ferreira et al., 2011, character 150): (0) reaching posterior half of bone; (1) reaching only middle portion of bone; (2) restricted to anterior third of bone.
(71) Dermal bone lamella dorsal to basibranchial 4: (0) absent; (1) present.
(72) Teeth on pharyngobranchial 3 (Ferreira et al., 2011, character 152): (0) present; (1) absent.
(73) Basihyal anterior expansion (Ferreira et al., 2011, character 153-modified): (0) anterior margin slightly wider than posterior margin; (1) anterior margin about twice or more wider than posterior margin.
(74) Gill gland in mature males formed by fusion of contiguous gill filaments (Ferreira et al., 2011, character 12-modified): (0) present; (1) absent.
(75) Gill gland extension in mature males (Ferreira et al., 2011; character 13-modified): (0) restricted to anterior portion of gill filaments on ceratobranchial of first gill arch; (1) covering almost entirely gill filaments on first gill arch.
Pectoral girdle
(76) Postcleithrum 1 (Ferreira et al., 2011, character 55): (0) present; (1) absent.
(77) Postcleithrum 1 size (Ferreira et al., 2011; character 56-modified): (0) equivalent in size or larger than scales from opercular region; (1) smaller than scales from from opercular region.
(78) Postcleithrum 2 (Ferreira et al., 2011, character 57): (0) present; (1) absent.
(79) Postcleithrum 3 posterior lamella (Ferreira et al., 2011, character 58-modified): (0) absent; (1) present.
(80) Coracoid ventral expansion (Ferreira et al., 2011, character 59): (0) absent; (1) present.
Pelvic fin
(81) Total number of pelvic-fin rays (Ferreira et al., 2011, character 37): (0) eight (i7 or i6i); (1) seven (i6).
(82) Position of dorsal longitudinal ridge of pelvic bone (Ferreira et al., 2011, character 39-modified): (0) Close to external margin (1) lateral but separated from margin of bone; (2) medial along bone axis.
(83) Bony posterior pelvic process extension (Ferreira et al., 2011, character 40-modified; Weitzman & Menezes, 1998, character 19-modified): (0) elongate; (1) relatively reduced.
(84) Pelvic bone anterior tip position (Ferreira et al., 2011, character 41; Weitzman & Menezes, 1998-modified from character 20): (0) anterior to first pleural rib; (1) between first and second pleural ribs; (2) between second and third pleural ribs; (3) between third and fourth pleural ribs; (4) between fourth and fifth pleural ribs; (5) between fifth and sixth pleural ribs.
(85) Cartilaginous portion of pelvic-fin posterior process (Ferreira et al., 2011, character 42; Weitzman & Menezes, 1998, character 36): (0) present; (1) absent.
(86) Seventh pelvic-fin ray: (0) branched; (1) unbranched.
(87) Eighth pelvic-fin ray: (0) branched; (1) unbranched.
Dorsal fin
(88) Number of dorsal-fin rays posterior to unbranched leading two rays (Ferreira et al., 2011, character 43): (0) seven; (1) eight; (2) nine.
(89) Dorsal-fin origin (Ferreira et al., 2011, character 44-modified): (0) anterior to vertical through anal-fin origin; (1) at vertical through anal-fin origin; (2) posterior to vertical through anal-fin origin.
(90) Origin of last dorsal-fin ray (Ferreira et al., 2011, character 45): (0) anterior to or at vertical through anal-fin origin; (1) posterior to vertical through anal-fin origin.
Anal fin
(91) Number of branched anal-fin rays (Ferreira et al., 2011, character 48): (0) more than 18; (1) 18 or fewer.
(92) Anal-fin anterior lobe (Ferreira et al., 2011; character 46-modified): (0) not expanded, without lobe or expansions; (1) with prominent lobe.
(93) Number of proximal anal-fin radials anterior to first hemal spine (Ferreira et al., 2011, character 50-modified): (0) none; (1) up to two; (2) three or four; (3) five or more.
(94) Large anterior keel on anal-fin proximal radial (Ferreira et al., 2011, character 51): (0) restricted to first radial; (1) present on first radial and also on posteriormost sixth or seventh.
(95) Anteriormost free anal-fin median radial (Ferreira et al., 2011, character 52): (0) on third pterygiophore; (1) on fourth pterygiophore; (2) on fifth pterygiophore; (3) on sixth pterygiophore; (4) on seventh pterygiophore; (5) on eleventh pterygiophore.
(96) Glandular tissue on anal fin (Ferreira et al., 2011, character 53): (0) absent; (1) present.
(97) Position of anal-fin hooks on mature males (Ferreira et al., 2011, character 54): (0) along posterior margin of anal-fin rays; (1) lateral to anal-fin rays; (2) both lateral and along posterior margin of anal-fin rays.
Vertebrae and ribs
(98) Total number of vertebrae (Ferreira et al., 2011, character 73): (0) 33 or less; (1) 34 to 38; (2) 39 or more.
(99) Relative number of precaudal vertebrae (Ferreira et al., 2011, character 74-modified): (0) equal to or greater than caudal vertebrae; (1) fewer than caudal vertebrae.
(100) Number of vertebrae anterior to first dorsal-fin pterygiophore (Ferreira et al., 2011, character 75): (0) 10 or less; (1) 11–13; (2) 14–17; (3) 18 or more.
(101) Number of transitional vertebrae (Ferreira et al., 2011, character 76, modified): (0) one; (1) two.
(102) Elaborations on prezygapophyses and postzygapophyses (Ferreira et al., 2011, character 77-modified): (0) absent; (1) present.
(103) Articulation between neural zygapophysis of posteriormost vertebrae (Ferreira et al., 2011, character 78-modified): (0) absent; (1) postzygapophyses articulated with prezygapophyses.
(104) Number of ribs articulating with transitional vertebrae: (0) absent; (1) one pair; (2) two pairs.
(105) Size of ribs articulating with transitional vertebrae (Ferreira et al., 2011, character 79-modified): (0) reduced, half size of remaining ribs; (1) same size of remaining ribs.
(106) Anteriormost hemal postzygapophysis (Ferreira et al., 2011, character 80): (0) on penultimate or last precaudal rib; (1) on transitional vertebrae.
Supraneurals
(107) Number of supraneurals (Ferreira et al., 2011, character 46): (0) eight or more; (1) seven; (2) six; (3) five; (4) four; (5) three.
(108) Shape of supraneurals (Ferreira et al., 2011, character 47): (0) cylindrical; (1) expanded dorsally through anterior and posterior expansions.
Caudal fin
(109) Number of epurals (Ferreira et al., 2011, character 60-modified): (0) one; (1) two; (2) three.
(110) Caudal-fin glandular organ (Weitzman & Menezes, 1998, character 1-modified): (0) absent; (1) present.
(111) Caudal-fin scales (Ferreira et al., 2011, character 1-modified): (0) present only at base of caudal fin; (1) extended only through ventral lobe; (2) extended only through dorsal lobe; (3) covering equal portions of dorsal and ventral lobes.
(112) Pouch scales (Ferreira et al., 2011, character 3-modified; Weitzman & Menezes, 1998, character-modified): (0) absent; (1) present.
(113) Development of pouch scales (Ferreira et al., 2011, character 3-modified; Weitzman & Menezes, 1998, character 3-modified): (0) not hypertrophied; (1) hypertrophied relative to remaining scales.
(114) Anteroventral process of pouch scales (Ferreira et al., 2011, character 10-modified; Weitzman & Menezes, 1998; character 32-modified): (0) absent; (1) present.
(115) Scales dorsal to pouch opening when present (Weitzman & Menezes, 1998, character 18-modified): (0) few modified scales; (1) multiple series of scales forming dorsal border of pouch opening.
(116) Scales on dorsal caudal-fin lobe (Ferreira et al., 2011, character 4): (0) not involved in caudal-fin organ; (1) involved in caudal-fin organ.
(117) Scales on ventral caudal-fin lobe (Ferreira et al., 2011, character 5): (0) not involved with a caudal-fin organ; (1) involved in a caudal-fin organ.
(118) Posterior lobes of pouch scale (Ferreira et al., 2011; character 6-modified): (0) only a central lobe; (1) two lobes, one central and one ventral.
(119) Caudal organ in females, in species in which it is present (Ferreira et al., 2011, character 7): (0) absent or much less developed than in males; (1) as developed as in males.
(120) Number of radii on pouch scale (Ferreira et al., 2011, character 9-modified): (0) fewer than 35 on posterior field of scale; (1) more than 35 on posterior field of scale.
(121) Distribution of pouch-scale radii (Ferreira et al., 2011, character 11): (0) over entire posterior field of pouch scale; (1) restricted to posteroventral margin of scale.
(122) Vertical extent of pouch scale (Weitzman & Menezes, 1998, character 51): (0) from lateral-line tube to middle of ventral lobe caudal-fin rays; (1) from caudal-fin ray 12 to ventral caudal-fin rays; (2) from ray 12 to ventral procurrent caudal-fin rays.
(123) Scales bordering posterolateral margin of caudal organ opening (Ferreira et al., 2011, character 62; Weitzman & Menezes, 1998; character 8): (0) none; (1) several regularly-sized scales with no hypertrophied radii.
(124) Form of caudal-fin rays 11 and 12 (Ferreira et al., 2011, character 63; Weitzman & Menezes, 1998; character 9): (0) not involved in a caudal pump, parallel to remaining rays; (1) decurved; (2) together with rays 13 (and sometimes 14) forming a caudal pump.
(125) Parhypural and hypural one (Ferreira et al., 2011, character 64; Weitzman & Menezes, 1998, character 12): (0) separated from each other; (1) contacting or fused to each other.
(126) Caudal-fin rays 11 to about 14 (Ferreira et al., 2011, character 66; Weitzman & Menezes, 1998, character 30): (0) similar to remaining rays; (1) narrow distally and sometimes shortened.
(127) Principal caudal-fin rays 1–8 and 16–19 plus largest ventral procurrent ray (Ferreira et al., 2011, character 67; Weitzman & Menezes, 1998, character 31): (0) not expanded in sagittal plane; (1) expanded in sagittal plane.
(128) Distance between bases of caudal-fin rays 10 and 11 (Ferreira et al., 2011, character 68-modified; Weitzman & Menezes, 1998, character 44-modified): (0) greater than posterior margin of hypural 3; (1) equal to or less than posterior margin of hypural 3.
(129) Distal halves of caudal-fin rays 7–10 (Ferreira et al., 2011, character 69; Weitzman & Menezes, 1998, character 29): (0) not arched or curved ventrally; (1) strongly curved ventrally.
(130) Caudal-fin hooks on mature males (Ferreira et al., 2011, character 70): (0) absent; (1) present.
(131) Spur formed by last ventral procurrent rays: (0) absent; (1) present.
(132) Laminar expansions of caudal-fin lateral line canal (Ferreira et al., 2011, character 71-modified): (0) absent; (1) present.
Sperm
(133) Spermatozeugmata (Ferreira et al., 2011, character 35): (0) absent; (1) present.
(134) Insemination (Weitzman & Menezes, 1998, character 2): (0) absent; (1) present.
(135) Tripartite condition on testis (Baicere-Silva, 2013, character 1): (0) absent; (1) present.
(136) Spermatozoa disposition in luminal compartment (Baicere-Silva, 2013, character 2): (0) random; (1) in bundles; (2) forming spermatozeugmata.
(137) Site of formation of spermatozoa organization (Baicere-Silva, 2013, character 3): (0) into luminal compartment; (1) inside spermatocysts.
(138) Secretion of Sertoli cells, as detected by histochemical techniques (Baicere-Silva, 2013; character 4): (0) undetected; (1) protein (2) polysaccharide.
(139) Site of spermiogenesis completion (Baicere-Silva, 2013, character 5): (0) cyst (inside cyst); (1) in luminal compartment (partially cystic).
(140) Initial position of flagellum formation in relation to nucleus in initial spermatid (Baicere-Silva, 2013, character 8): (0) eccentric to medial; (1) lateral.
(141) Nucleus with movement toward flagellum, subsequent movement over centriolar complex (Baicere-Silva, 2013, character 12): (0) absent; (1) present.
(142) Movement of nucleus over centriolar complex (Baicere-Silva, 2013, character 14): (0) absent; (1) present.
(143) Nuclear fossa (Ferreira et al., 2011, character 18): (0) present; (1) absent.
(144) Shape of nuclear fossa in longitudinal section (Baicere-Silva, 2013, character 18-modified): (0) double concavity (1) single concavity.
(145) Position of nuclear fossa (Ferreira et al., 2011, character 19-modified): (0) slightly eccentric; (1) strongly eccentric; (2) lateral; (3) lateral and located almost at tip of nucleus; (4) anterior.
(146) Insertion of centriolar complex in double nuclear fossa (Baicere-Silva, 2013, character 21): (0) proximal centriole inserted and distal partially inserted; (1) only proximal centriole partially inserted; (2) no centrioles inserted.
(147) Striated rootlets in centriolar complex (Baicere-Silva, 2013, character 22): (0) absent; (1) present.
(148) Chromatin final aspect (Ferreira et al., 2011; character 17-modified): (0) compact granular material (1) not compact aggregates with a flocculent appearance.
(149) Length of midpiece (Ferreira et al., 2011; character 26): (0) short (longitudinal axis <2.0 µm); (1) intermediate (longitudinal = 2.1–3.0 µm); (2) long (longitudinal axis >3.0 µm).
(150) Midpiece (Baicere-Silva, 2013; character 24): (0) present; (1) absent.
(151) Position of midpiece in relation to flagellum (Ferreira et al., 2011; character 27, modified): (0) regular; (1) irregular.
(152) Organelles distribution in relation to flagellum (transversal section), in proximal region of midpiece (Baicere-Silva, 2013, character 26): (0) asymmetrical, with mitochondria and vesicles slightly more concentrated in one side of midpiece; (1) strongly asymmetrical, with mitochondria and vesicles concentrated in only one side of midpiece.
(153) Organelles distribution in relation to flagellum (transversal section), in intermediary region of midpiece (Baicere-Silva, 2013, character 27): (0) asymmetrical, with mitochondria and vesicles slightly more concentrated in one side of midpiece; (1) strongly asymmetrical, with mitochondria and vesicles concentrated in only one side of midpiece.
(154) Organelles distribution in relation to flagellum (transversal section), in terminal region of midpiece (Baicere-Silva, 2013, character 27): (0) asymmetrical, with mitochondria and vesicles slightly more concentrated in one side of midpiece; (1) strongly asymmetrical, with mitochondria and vesicles concentrated in only one side of midpiece.
(155) Cytoplasmic sleeve in midpiece (Baicere-Silva, 2013, character 30): (0) absent; (1) present.
(156) Number of mitochondria (Ferreira et al., 2011, character 30): (0) few (1–5); (1) moderate (5–10); (2) many (more than 10).
(157) Shape of mitochondria (Baicere-Silva, 2013, character 31-modified): (0) oblong; (1) elongate; (2) spherical.
(158) Elongate mitochondria (Baicere-Silva, 2013, character 36): (0) cylindrical; (1) helical; (2) irregular unbranched.
(160) Mitochondria in nuclear region (Baicere-Silva, 2013, character 37): (0) absent; (1) present.
(161) Mitochondrial position in relation to nucleus (Baicere-Silva, 2013, character 38): (0) peripheral; (1) interposed.
(160) Mitochondrial distribution along length of nucleus (Baicere-Silva, 2013, character 39): (0) only in basal region; (1) from medial region; (2) from apical region.
(162) Mitochondrial position in relation to flagellum in anterior region of midpiece (Baicere-Silva, 2013, character 43): (0) only in one side of flagellum; (1) mostly in one side of flagellum.
(163) Mitochondrial position in relation to flagellum in intermediate region of midpiece (Baicere-Silva, 2013, character 44): (0) only in one side of flagellum; (1) mostly in one side of flagellum.
(164) Nucleus shape in longitudinal section (Baicere-Silva, 2013, character 80): (0) circular; (1) elliptical; (2) drop shaped; (3) irregular with re-entrance along length; (4) arched; (5) wavy.
(165) Shape of membranous vesicles (Baicere-Silva, 2013, character 54): (0) spherical; (1) elongate.
(166) Membranous vesicles in perinuclear region (Baicere-Silva, 2013, character 58): (0) absent; (1) present.
(167) Membranous vesicles in anterior region of midpiece (excluding cytoplasmic sleeve) (Baicere-Silva, 2013, character 59): (0) present; (1) absent.
(168) Membranous vesicles in intermediate region of midpiece (excluded cytoplasmic sleeve) (Baicere-Silva, 2013, character 60): (0) present; (1) absent.
(169) Membranous vesicles in posterior region of midpiece (excluded cytoplasmic sleeve) (Baicere-Silva, 2013, character 61): (0) present; (1) absent.
(170) Tubules and vesicles interconnected in anterior region of midpiece (Baicere-Silva, 2013, character 62): (0) present; (1) absent.
(171) Tubules and vesicles interconnected in intermediate region of midpiece (Baicere-Silva, 2013, character 63): (0) present; (1) absent.
(172) Tubules and vesicles interconnected in posterior region of midpiece (Baicere-Silva, 2013, character 64): (0) present; (1) absent.
(173) Tubules and vesicles interconnected, feature in cross section (Baicere-Silva, 2013, character 65): (0) alveolar; (1) tracery.
(174) Extension of cytoplasmic canal lateral to nucleus (Baicere-Silva, 2013, character 91): (0) only in apical region; (1) until medial region; (2) throughout nucleus length.
(175) Relative position of centriolar complex in initial spermatid (Baicere-Silva, 2013, character 92): (0) perpendicular to proximal centriole in medial position in relation to distal; (1) oblique at an acute angle; (2) oblique at obtuse angle; (3) parallel to proximal centriole anterior to distal.
Musculature
(176) Development of interradialis between caudal-fin rays 12 and 13 (Vanegas-Ríos, 2018, character 489): (0) interradialis little developed posteroventrally, resulting in moderate separation between rays; (1) interradialis greatly developed posteroventrally, resulting in strong separation between rays.
APPENDIX 2
List of examined specimens
Material examined
Acrobrycon ipanquianus (MUSM 13232) Peru, CU, La convención, Quillabamba; (CPUFMT 685) Argentina, Tucumán, Trancas, Rio Vipos, 5 km from Ruta Nacional 9. Aphyocharax pusillus (MZUSP 80044) Brasil, Amazonas, Tefé, Boca do Lago Caiambé, abaixo de Tefé. Argopleura chocoensis (CAS 68360) Colombia, Manigru, Atantic Slope. Attonitus irisae (MUSM 30270) Peru, PA, Oxapampa, Palcazu, cc. nn. La Raya, R. Raya, cca Palcazu. Bryconacidnus ellisae (MUSM 11628) Peru, PU, Sandia, ZRTC, Cuenca Ebehuabaeji. Bryconadenos tanaothoros (MZUSP 98979) Brasil, Mato Grosso, Nova Ubiratã, Xingu, Rio Von den Steinen. Bryconadenos weitzmani (MZUSP 96559) Brasil, Pará, Altamira, Xingu, Rio Curuá, bacia do Iriri, na Vila de Castelo dos Sonhos. Bryconamericus exodon (MZUSP 90715) Brasil, Mato Grosso, Barra do Bugres, Paraguai, Sepotuba. Ceratobranchia obtusirostris (MUSM 22306) Peru, CU, La Convencion, S.N. Megantoni, Cuenca Bajo Urubamba, Rio Ticumpinía. Chrysobrycon myersi (MUSM 18908) Peru, PA, Oxapampa, Pto Bermudez, Qda Atas. Corynopoma riisei (MZUSP 108441) Venezuela, Carabobo, Lago de Velencia, Hacienda Monte Sacro. Creagrutus cf. meridionalis (MZUSP 78789) Brasil, Mato Grosso, Reserva do Cabaçal, Rio Cabaçal, abaixo da cachoeira. Deuterodon heterostomus (MZUSP 7904) Brasil, São Paulo, Santa Branca Paraíba, Represa de Santa Branca, rio Paraíba do Sul. Diapoma alburnum (MCP 19501) Brasil, Rio Grande do Sul, Jacui, Rio Taquari em Cruzeiro do Sul. Diapoma speculiferum (MCP 16617) Brasil, Rio Grande do Sul, Jacui, Arroio Santa Bárbara, 12 km a oeste da Vila do Segredo. Diapoma terofali (MCP 11491) Brasil, Rio Grande do Sul, Uruguai, Arroio Guarupa, divisa Quarai/Alegrete. Gephyrocharax atracaudata (MZUSP 19702) Panamá, córrego que deságua no lago de Miraflores, zona do canal. Glandulocauda melanopleura (CPUFMT 684) Brasil, São Paulo, Boracéia, Afuente do Rio Guaratuba. Gymnocorymbus ternetzi (LIRP 2018) Brasil, São Paulo, Luis Antônio, Lagoa do Diogo, Bacia do Rio Mogi-Guaçú. Hemibrycon surinamensis (MZUSP 30530) Brasil, Pará, Tocantins, Rio Itacaiunas, estrada de Ferro, 10 km leste do N-4 (05°52′00″S 05°32′00″W). Hysteronotus megalostomus (MZUSP 85978) Brasil, Minas Gerais, Montes Claros, São Francisco, Rio Verde Grande. Iatobrycon praecox (MCZ 50602) Equador, Rio Vinces. Knodus meridae (MZUSP 99146) Venezuela, Mérida, Río Chama, puente n° 4, aprox. 20 km, NW de Mérida. Knodus smithi (LIRP 5886) Brasil, Rondônia, Porto Velho, Igarapé Belmonte, alfluente do Rio Madeira. Landonia latidens (MZUSP 49195) Equador, Guayas, Rio Daule, 200 metros acima de Coliares. Lepidocharax diamantina (MNRJ 21997) Brasil, Bahia, Palmeiras, Santo Antônio. Lepidocharax burnsi (MCP 34828) Brasil, Minas Gerais, São Francisco, Rio Paraopeba (Toca). Lophiobrycon weitzmani (MZUSP 83353) Brasil, Minas Gerais, Delfinópolis, Rio Grande Basin, Estância Carmem Silva, córrego Bom Jesus. Mimagoniates microleps (MZUSP 79914) Brasil, São Paulo, Ilha Comprida Leste, Rio Candapuí, Vila de Juruvauva. Moenkhausia cf. xinguensis (MZUSP 36806) Brasil, Pará, Altamira, Cachoeira do Espelho, rio Xingu. Monotocheirodon kontos (MUSM 11416) Peru, MO, Tambopata, ZRTC, Rio Malinowski, Quebrada Venado. Odontostoechus lethostigmus (MCP 13657) Brasil, Rio Grande do Sul, Tramandai, Arroio Água Parada, tributário do rio Maquiné em Maquiné. Phallobrycon adenacanthus (MZUSP 98902) Brasil, Mato Grosso, Paranatinga, Xingu, Rio Culuene. Phenacobrycon henni (MCZ 48660) Equador, Rio Vinces. Piabina argentea (MZUSP 88433) Brasil, Ipeúna, Ribeirão Passa Cinco, afluente do Rio Corumbataí prox. da SP-310. Planaltina britskii (MZUSP 100643) Brasil, São Paulo, Alto Paraná, Rio Cubatão, ponte entre Elisiário e Mirapoema. Planaltina myersi (MZUSP 100642) Brasil, Alto Paraná, Rio dos Patos. Pseudocorynopoma doriae (MZUSP 4479) Brasil, Rio Grande do Sul, São Leopoldo, Rio dos Sinos, Porto do Vicente. Pseudocorynopoma heterandria (MZUSP 37273) Brasil, São Paulo, Ribeira, Riacho Arataca, afluente rio Jacupiranga, estrada Pariquera-açu-Iguape, na divisa dos municípios (24°45′00″S 47°48′00″W). Pterobrycon myrnae (ANSP 164243) Costa Rica, Puntarenas. Ptychocharax rhyacophila (MZUSP 108442) Venezuela, Amazonas, Rio Siapa. Rhinobrycon negrensis (MUSM 4925) Peru, MD, Manu, PNM, Quebrada Culli. Rhinopetitia myersi (DZSJRP 8499), America do Sul, Brasil, Mato Grosso: Ponte Branca, Rio Araguaia (16°43′15″S 52°45′59″W). Scopaeocharax rhinodus (MUSM 20370) Peru, SM, Tarapoto, Morales, San Antonio, Rio Cumbaza. Tetragonopterus argenteus (MZUSP 18798) Brasil, Mato Grosso, Barão de Melgaço, Boca do Croará, Rio Cuiabá (16°11′S 55°57′W). Tyttocharax tambopatensis (MZUSP 26351), America do Sul, Peru, Cachoeira Copal, Jenaro Herrera, Prov. Requena, Departamento Loreto. Xenurobrycon macrops (MZUSP 96715) Brasil, Mato Grosso, Barão de Melgaço, Paraguai, Rio Mutum, entre Vila de Mimoso e Joselândia.




