Phylogeographic diversification of the Mesalina olivieri species complex (Squamata: Lacertidae) with the description of a new species and a new subspecies endemic from North West Africa
Contributing authors: Pierre-André Crochet ([email protected]), Philippe Geniez ([email protected]), Fernando Martínez-Freiría ([email protected]), Guillermo Velo-Antón ([email protected]), José Carlos Brito ([email protected])
Zoobank Links: LSID: http://zoobank.org/NomenclaturalActs/dc6c167b-8138-4017-82a4-513d8244072a
Online ISSN: 1439-0469
Abstract
enNumerous molecular studies emphasized how past climatic oscillations in the Sahara-Sahel have left strong imprints on current biodiversity patterns and identified the Atlantic coast and the Northwest African Mountains as refugia and speciation hotspots. Yet, the biodiversity inventory in the region is still far from complete. We use an integrative taxonomy framework to revise the systematics of the Mesalina olivieri species complex; integrating molecular, morphological, and environmental data, we evaluated levels of genetic and phenotypic differentiation among species/lineages and revised the species distribution limits of the M. olivieri complex, refining the distribution of Mesalina simoni, and Mesalina pasteuri. Our study confirmed one previously unidentified speciation event, leading to the description of Mesalina adrarensis sp. nov. Together with this new species, we also describe the south-western Moroccan populations of M. olivieri as Mesalina simoni saharae ssp. nov. Mesalina adrarensis sp. nov. is sympatric with M. pasteuri and parapatric with M. simoni saharae ssp. nov. in Mauritania and southern Morocco. Based on our revised taxonomy, M. simoni now includes most populations of the M. olivieri complex in Morocco, M. olivieri being restricted in Morocco to the east and southeast of the country. We also build on these results to provide further insight on the biogeography of North Africa. Our results point to a diversification of the complex during the late Miocene, that led to the formation of the four species M. simoni, M. olivieri, M. pasteuri, and M. adrarensis sp. nov. After these four speciation events, high intraspecific diversification processes occurred since the beginning of the Plio-Pleistocene transition, in parallel with the beginning of the humid and arid cycles. Through our phylogenetic analysis, we highlight the existence of high levels of undescribed intraspecific diversity in M. olivieri and M. pasteuri that will need to be addressed in future studies. Moreover, we uncover instances of cytonuclear discordances, stressing the need of considering both mitochondrial and nuclear DNA for integrative taxonomic studies to explore biodiversity.
Sommario
itMolteplici studi molecolari enfatizzano come le passate oscillazioni climatiche nel Sahara-Sahel abbiano avuto un forte impatto sulla distribuzione della biodiversità North Africa, ed identificano la costa Atlantica e le montagne del Nordovest Africano come rifugi e hotspot di speciazione. Tuttavia, la catalogazione della biodiversità in quest’area è, ad oggi, ancora lontana dall’essere completa. Attraverso l’approccio multidisciplinare della tassonomia integrativa, è stata revisionata la sistematica del complesso di specie “Mesalina olivieri”. Integrando dati molecolari, morfologici e ambientali, sono stati valutati livelli di differenziazione genetica e fenotipica tra specie/linee evolutive e rivisti i limiti di distribuzione del complesso di specie M. olivieri, rifinendo la distribuzione di Mesalina simoni e M. pasteuri. Questo studio mette in luce un precedente evento di speciazione, ad oggi non ancora confermato, che ha condotto alla descrizione di Mesalina adrarensis sp. nov.. Inoltre, insieme a questa nuova specie, sono descritte le popolazioni di M. olivieri del sud-ovest del Marocco come Mesalina simoni saharae ssp. nov.. Mesalina adrarensis sp. nov è simpatrica con Mesalina pasteuri e parapatrica con M. simoni saharae ssp.nov in Mauritania e Sud Marocco. In base alla nostra tassonomia revisionata, M. simoni ora include la maggior parte delle popolazioni del complesso M. olivieri in Marocco, e la distribuzione di M. olivieri è ora ristretta all’est e sud-est del paese. Ci si è basati su questi risultati per fornire ulteriori conoscenze sulla biogeografia del Nord Africa. I risultati evidenziano una diversificazione del complesso durante il tardo Miocene, il quale ha condotto alla formazione delle quattro specie M. simoni, M. olivieri, M. pasteuri e M. adrarensis sp. nov.. Successivamente a questi quattro eventi di speciazione, processi di alta diversificazione intraspecifica si sono verificati dall’inizio della transizione Plio-Pleistocenica, in parallelo con l’inizio delle passate oscillazioni climatichenel Sahara-Sahel. Attraverso analisi filogenetiche è stata evidenziata l’esistenza di alti livelli di diversità intraspecifica ancora non descritta in M. olivieri e M. pasteuri, la quale necessità ulteriori approfondimenti futuri. Inoltre, questo studio rivela molteplici esempi di discordanza cito-nucleare, sottolineando la necessità di considerare sia il DNA mitocondriale che nucleare per gli studi di tassonomia integrativa.
1 INTRODUCTION
Past climatic oscillations have left strong imprints on current biodiversity patterns worldwide (Bryson et al., 2012) and the Sahara Desert and the neighboring Sahel are no exceptions (Brito et al., 2014). In these ecoregions, dry–humid cycles lead to series of contraction/expansion events in species ranges, with recurrent isolation of populations that promoted diversification and sometimes speciation processes see Brito et al. (2014, for a review). In this context, recent studies unveiled the key role of topographic features (e.g., mountains, valleys) and particular regions (i.e., coastal areas) as major refugia and/or as corridors for many species, facilitating gene flow during favorable climatic conditions (e.g., Gonçalves, Martínez-Freiría, et al., 2018; Gonçalves, Pereira, et al., 2018; Velo-Antón et al., 2018). However, large portions of central Sahara and most Saharan mountains are still widely under-sampled due to their remoteness and long-term regional instability (Brito et al., 2014, 2018). Consequently, knowledge on the biodiversity of the Sahara-Sahel is still relatively scant compared with other biomes (Brito & Pleguezuelos, 2019). The region is heavily affected by the seven types of shortfalls that limit knowledge on biodiversity of the globe (reviewed by Hortal et al., 2015). Indeed, a large fraction of cryptic (and not-cryptic) diversity in North Africa and the Sahara Desert remains undescribed (Brito et al., 2014, 2018), and these regions must be prioritized to reduce biodiversity shortfalls. The genus Mesalina (Lacertidae, Eremiadinae; Gray, 1838) provides an appealing case study to address the influence of geological events and past climatic oscillations on diversification events across the Sahara-Sahel. This genus comprises diurnal, xeric-adapted small lacertids widely distributed from the Atlantic Sahara through North Africa, the Middle East, and the Arabian Peninsula to Pakistan (Sindaco et al., 2008). These fast-moving lizards occur in different habitats (Trape et al., 2012): rocky and mountain areas, sandy habitats or xeric shrublands and mesic regions on the transition between the Sahara and the Mediterranean and Atlantic coasts. Previous studies have addressed the phylogeny, systematics, and biogeography of the genus (Arnold, 1986; Kapli et al., 2015; Simó-Riudalbas et al., 2019; Sindaco et al., 2018; Šmíd et al., 2017) using both molecular and morphological data. The genus currently comprises 19 recognized species (Uetz et al., 2020), subdivided into seven species complexes: (a) Mesalina watsonana (Stoliczka, 1872), (b) Mesalina martini (Boulenger, 1897), (c) the Mesalina olivieri group, (d) Mesalina rubropunctata (Lichtenstein, 1823), (e) the Mesalina adramitana group, (f) the Mesalina brevirostris group, and (g) the Mesalina guttulata group (Simó-Riudalbas et al., 2019).
The ancestor of the M. olivieri complex colonized North Africa and started its diversification around 8 Mya (Kapli et al., 2015) into two well-supported clades: (a) one restricted to Morocco (including the Atlantic Sahara) and Mauritania, and (b) another ranging from Israel to Mauritania. There are currently three recognized species within this species complex: (a) Mesalina simoni (Boettger, 1881), endemic to Morocco (north and west of the Atlas Mountains), (b) Mesalina olivieri (Audouin, 1829), distributed from the Atlantic coast to Iraq and Saudi Arabia, and (c) Mesalina pasteuri (Bons, 1960), scattered distributed across the Sahara in Mauritania, southern Morocco, southern Algeria, Niger, Mali, and western Egypt. This current taxonomy needs to be revised as several mitochondrial DNA (mtDNA) lineages identified by Kapli et al. (2015) render M. olivieri and M. pasteuri paraphyletic. These include (a) one “olivieri” mtDNA lineage from the Atlantic Sahara (AS hereafter) and (b) one “olivieri” lineage distributed from the south of the High Atlas Mountains to the Saharan Atlas and the eastern Anti-Atlas in Morocco (AM) (that both group with the Moroccan endemic species M. simoni); (c) one “olivieri” lineage from Mauritania (ADR) (sister to a clade composed by M. simoni and AS and AM) and (d) a pasteuri-like specimen from the Tagant region in Mauritania embedded in the “olivieri” lineage of the same region (TAG); (e) one “olivieri” lineage from Algeria (ALG1) (clustering with specimens of M. pasteuri from Mauritania). In addition to the mtDNA paraphyly, previous studies (Arnold et al., 2007; Kapli et al., 2008, 2015; Simó-Riudalbas et al., 2019) stressed that the wide distribution and morphological variation (mostly in the scales number and coloration; Hosseinian Yousefkhani et al., 2015; Trape et al., 2012) of the M. olivieri complex could hide several undescribed species. Yet, despite the efforts in gathering information about the morphological diversity in the Mesalina genus, there is no comprehensive revision on the dichotomous characters for the M. olivieri species complex.
In this integrative study, we re-evaluate phylogenetic relationships within the M. olivieri species complex and provide insight on the historical and evolutionary processes that generated its diversity in the Maghreb region. More precisely, we (a) combine mtDNA data with independent nuclear markers to recover the evolution of the M. olivieri species group; (b) identify the role of speciation in generating its current biodiversity, and (c) identify the spatiotemporal drivers of diversification in the context of what is known on the history of the Sahara-Sahel. To do so, we assembled (a) a mtDNA dataset of 378 specimens including all the 19-different species of Mesalina recognized to date; (b) a nuclear concatenated dataset of 84 specimens; and (c) a combined dataset including one mitochondrial and four nuclear genes for 102 specimens of the M. olivieri species complex. We used our datasets to test the monophyly of the lineages AS, AM, ADR, TAG, and ALG1 and addressed their phylogenetic relationships, genetic divergence, and potential reproductive isolation. To assess the species status of the new putative species from Mauritania (ADR and TAG lineages), we examined patterns of allele sharing for the nuclear markers in sympatry or narrow parapatry to evaluate their level of reproductive isolation. We also explored the interspecific morphological difference between this lineage and the other species of the complex and analyzed spatial data to model the distribution of this potential new species and its habitat requirements.
2 MATERIALS AND METHODS
2.1 Sampling and study area
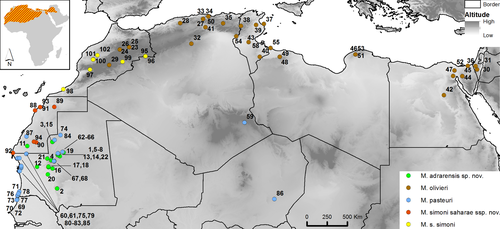
This study focuses on the status of the M. olivieri species complex populations in North West Africa. For comparative purpose, we added representative samples from each major mitochondrial lineage of the M. olivieri complex identified outside our study area by Kapli et al. (2015) and Simó-Riudalbas et al. (2019). A total of 79 samples from M. olivieri (including seven and four samples from the AS and AM lineages, and 21 from the ADR), 39 from M. pasteuri (including one sample from the TAG lineage), and three from M. simoni were amplified and successfully sequenced for this work (samples and species distributions are shown in Figure 1). The complete list of all the new specimens sequenced plus the sequences downloaded from GenBank are provided in Table 1 and Table S1. The distribution and source of the samples are shown in Figure S1.
| No. | Sample code | Country | Latitude | Longitude | Nuc. clades | Mit. clades | Concat. clades |
|---|---|---|---|---|---|---|---|
| Mesalina adrarensis sp. nov. | |||||||
| 1 | BEV.10457 | Mauritania | 21.0150 | −11.7180 | ADR | ADR | ADR |
| 2 | BEV.10823 | Mauritania | 17.3982 | −12.0305 | TAG | TAG | TAG |
| 3 | BEV.14800 | Mauritania | 22.6086 | −12.5569 | – | ADR | ADR |
| 4 | BEV.15060 | Mauritania | 20.5537 | −12.6916 | ADR | ADR | ADR |
| 5 | BEV.15061 | Mauritania | 21.1596 | −11.9362 | ADR | ADR | ADR |
| 6 | BEV.15062 | Mauritania | 21.1596 | −11.9362 | ADR | ADR | ADR |
| 7 | BEV.15063 | Mauritania | 21.1596 | −11.9362 | ADR | ADR | ADR |
| 8 | BEV.15064 | Mauritania | 21.1596 | −11.9362 | ADR | ADR | – |
| 9 | BEV.15163 | Mauritania | 20.5537 | −12.6916 | ADR | ADR | ADR |
| 10 | BEV.T661 | Mauritania | 20.7485 | −13.1276 | ADR | ADR | ADR |
| 11 | CIBIO11440 | Morocco | 22.1557 | −15.3468 | ADR | ADR | ADR |
| 12 | CIBIO11973 | Mauritania | 19.8265 | −14.2555 | ADR | ADR | ADR |
| 13 | CIBIO12011 | Mauritania | 21.1596 | −11.9362 | ADR | ADR | ADR |
| 14 | CIBIO12018 | Mauritania | 21.1502 | −11.9623 | – | ADR | – |
| 15 | CIBIO13640 | Mauritania | 22.6086 | −12.5569 | ADR | ADR | ADR |
| 16 | CIBIO13814 | Mauritania | 19.6289 | −12.5344 | ADR | ADR | ADR |
| 17 | CIBIO1861 | Mauritania | 19.7972 | −12.9980 | ADR | ADR | ADR |
| 18 | CIBIO1862 | Mauritania | 19.8632 | −12.9909 | – | ADR | ADR |
| 19 | CIBIO2902 | Mauritania | 21.4282 | −11.3139 | ADR | ADR | ADR |
| 20 | CIBIO2952 | Mauritania | 18.9849 | −13.0647 | ADR | ADR | ADR |
| 21 | CIBIO5865 | Mauritania | 20.5537 | −12.6916 | – | ADR | – |
| 22 | CIBIO5905 | Mauritania | 21.1521 | −11.9470 | ADR | ADR | ADR |
| Mesalina olivieri | |||||||
| 23 | BEV.10013 | Morocco | 33.1860 | −3.9900 | – | MOR2 | – |
| 24 | BEV.10014 | Morocco | 33.1860 | −3.9900 | – | MOR2 | – |
| 25 | BEV.10015 | Morocco | 33.1860 | −3.9900 | MOR2 | MOR2 | MOR2 |
| 26 | BEV.11948 | Morocco | 32.9297 | −5.0465 | MOR3 | MOR3 | MOR3 |
| 27 | BEV.13322 | Algeria | 36.6245 | 4.8517 | ALG3 | ALG2/ALG3 | ALG2/ALG3 |
| 28 | BEV.13621 | Algeria | 35.8802 | 1.6841 | ALG2 | ALG2/ALG3 | ALG2/ALG3 |
| 29 | BEV.6402 | Morocco | 31.1940 | −6.2100 | – | MOR1 | – |
| 30 | BEV.8796 | Israel | 30.7077 | 34.7845 | – | ISR | – |
| 31 | BEV.8830 | Israel | 31.0858 | 34.6310 | ISR | ISR | ISR |
| 32 | BEV.9225 | Algeria | 33.5914 | 2.9508 | ALG1 | ALG1 | ALG1 |
| 33 | BEV.T3036 | Algeria | 36.3413 | 4.2509 | ALG3 | ALG2/ALG3 | ALG2/ALG3 |
| 34 | BEV.T3037 | Algeria | 36.3413 | 4.2509 | ALG3 | ALG2/ALG3 | ALG2/ALG3 |
| 35 | BEV.T3038 | Algeria | 35.8585 | 6.4908 | ALG1 | ALG1 | ALG1 |
| 36 | BEV.T395 | Egypt | 31.1200 | 33.7600 | – | EGY1 | EGY1 |
| 37 | BEV.T6678 | Tunisia | 35.8006 | 11.0361 | TUN2 | TUN1/TUN2/TUN3 | TUN1/TUN2/TUN3 |
| 38 | CIBIO308 | Tunisia | 35.5822 | 8.4826 | TUN1 | TUN1/TUN2/TUN3 | TUN1/TUN2/TUN3 |
| 39 | NHMC80.3.119.29 | Tunisia | 35.6895 | 10.1501 | – | TUN1/TUN2/TUN3 | – |
| 40 | NHMC80.3.119.10 | Tunisia | 32.1287 | 10.5638 | – | TUN1/TUN2/TUN3 | – |
| 41 | NHMC80.3.119.108 | Algeria | 35.4151 | 4.5190 | – | ALG2/ALG3 | – |
| 42 | NHMC80.3.119.109 | Egypt | 27.8300 | 31.1068 | – | LYB1 | – |
| 43 | NHMC80.3.119.14 | Tunisia | 33.7531 | 9.3350 | – | TUN1/TUN2/TUN3 | – |
| 44 | NHMC80.3.119.16 | Egypt | 29.9651 | 33.1606 | – | EGY2 | – |
| 45 | NHMC80.3.119.19 | Egypt | 29.9651 | 33.1606 | – | EGY2 | – |
| 46 | NHMC80.3.119.2 | Libya | 32.3912 | 21.2404 | – | LYB1 | – |
| 47 | NHMC80.3.119.20 | Egypt | 29.9797 | 32.1187 | – | EGY1 | – |
| 48 | NHMC80.3.119.21 | Libya | 32.1247 | 12.8068 | – | LYB2 | – |
| 49 | NHMC80.3.119.22 | Libya | 32.1247 | 12.8068 | – | LYB2 | – |
| 50 | NHMC80.3.119.23 | Algeria | 35.4151 | 4.5190 | – | ALG2/ALG3 | – |
| 51 | NHMC80.3.119.3 | Libya | 32.3912 | 21.2404 | – | LYB1 | – |
| 52 | NHMC80.3.119.40 | Egypt | 30.5965 | 32.2715 | – | EGY1 | – |
| 53 | NHMC80.3.119.5 | Libya | 32.3912 | 21.2404 | – | LYB1 | – |
| 54 | NHMC80.3.119.9 | Tunisia | 34.4076 | 7.9448 | – | TUN1/TUN2/TUN3 | – |
| 55 | NHMC80.3.164.19 | Libya | 33.0956 | 11.7626 | – | TUN1/TUN2/TUN3 | – |
| 56 | SPM002917 | Egypt | - | - | – | EGY1 | – |
| 57 | SPM002920 | Egypt | - | - | – | EGY1 | – |
| 58 | CIBIO319 | Tunisia | 32.9974 | 10.6080 | TUN3 | TUN1/TUN2/TUN3 | TUN1/TUN2/TUN3 |
| Mesalina pasteuri | |||||||
| 59 | BEV.10179 | Algeria | 24.7839 | 8.8719 | MAU3 + ALG | MAU3 + ALG/MAU4 + NIG | MAU3 + ALG |
| 60 | BEV.10454 | Mauritania | 21.2777 | −15.4703 | MAU2 + MOR | MAU3 + ALG/MAU4 + NIG | MAU4 + NIG |
| 61 | BEV.10455 | Mauritania | 21.2777 | −15.4703 | MAU2 + MOR | MAU3 + ALG/MAU4 + NIG | MAU4 + NIG |
| 62 | BEV.14803 | Mauritania | 21.4866 | −11.4139 | MAU3 + ALG | MAU1/MAU2 + MOR | MAU2 + MOR |
| 63 | BEV.14804 | Mauritania | 21.2970 | −11.9199 | MAU2 + MOR | MAU1/MAU2 + MOR | MAU2 + MOR |
| 64 | BEV.14805 | Mauritania | 21.2970 | −11.9199 | MAU2 + MOR | MAU1/MAU2 + MOR | MAU2 + MOR |
| 65 | BEV.9177 | Mauritania | 21.3321 | −11.9512 | MAU4 + NIG | MAU1/MAU2 + MOR | MAU2 + MOR |
| 66 | BEV.9380 | Mauritania | 20.4600 | −12.3560 | MAU4 + NIG | MAU1/MAU2 + MOR | MAU2 + MOR |
| 67 | BEV.T662 | Mauritania | 20.4563 | −12.3602 | – | MAU4 | – |
| 68 | BEV.T663 | Mauritania | 20.4641 | −12.3790 | MAU1 | MAU1/MAU2 + MOR | MAU1 |
| 69 | CIBIO10706 | Mauritania | 16.2051 | −16.5034 | MAU4 + NIG | MAU3 + ALG/MAU4 + NIG | MAU4 + NIG |
| 70 | CIBIO11653 | Mauritania | 16.6072 | −16.4418 | MAU4 + NIG | MAU3 + ALG/MAU4 + NIG | MAU4 + NIG |
| 71 | CIBIO11656 | Mauritania | 16.6554 | −16.4242 | MAU4 + NIG | MAU3 + ALG/MAU4 + NIG | MAU4 + NIG |
| 72 | CIBIO12821 | Mauritania | 16.1307 | −16.5112 | MAU4 + NIG | MAU3 + ALG/MAU4 + NIG | MAU4 + NIG |
| 73 | CIBIO12822 | Mauritania | 16.1307 | −16.5112 | MAU4 + NIG | MAU3 + ALG/MAU4 + NIG | MAU4 + NIG |
| 74 | CIBIO13770 | Mauritania | 23.3699 | −11.6696 | – | MAU1/MAU2 + MOR | – |
| 75 | CIBIO2765 | Mauritania | 20.8061 | −16.4561 | MAU1 | MAU3 + ALG/MAU4 + NIG | MAU3 + ALG |
| 76 | CIBIO4449 | Mauritania | 17.0674 | −16.2555 | MAU4 + NIG | MAU3 + ALG/MAU4 + NIG | MAU4 + NIG |
| 77 | CIBIO4467 | Mauritania | 16.8484 | −16.3503 | MAU4 + NIG | MAU3 + ALG/MAU4 + NIG | MAU4 + NIG |
| 78 | CIBIO4468 | Mauritania | 16.8484 | −16.3503 | – | MAU3 + ALG/MAU4 + NIG | – |
| 79 | CIBIO5061 | Mauritania | 19.6851 | −16.0641 | MAU3 + ALG | MAU3 + ALG/MAU4 + NIG | MAU3 + ALG |
| 80 | CIBIO5077 | Mauritania | 19.7795 | −16.0390 | MAU2 + MOR | MAU3 + ALG/MAU4 + NIG | MAU4 + NIG |
| 81 | CIBIO5100 | Mauritania | 19.9197 | −16.0280 | MAU2 + MOR | MAU3 + ALG/MAU4 + NIG | MAU4 + NIG |
| 82 | CIBIO526 | Mauritania | 21.2777 | −15.4703 | – | MAU3 + ALG/MAU4 + NIG | – |
| 83 | CIBIO5299 | Mauritania | 20.7972 | −16.2221 | MAU3 + ALG | MAU3 + ALG/MAU4 + NIG | MAU3 + ALG |
| 84 | CIBIO5822 | Mauritania | 22.8350 | −12.3292 | MAU1 | MAU1/MAU2 + MOR | MAU1 |
| 85 | CIBIO6279 | Mauritania | 19.3514 | −16.2004 | MAU3 + ALG | MAU3 + ALG/MAU4 + NIG | MAU3 + ALG |
| 86 | CIBIO6692 | Niger | 16.2178 | 12.1985 | MAU4 + NIG | MAU3 + ALG/MAU4 + NIG | MAU4 + NIG |
| 87 | CIBIO7333 | Morocco | 23.2185 | −15.4468 | MAU2 + MOR | MAU1/MAU2 + MOR | MAU2 + MOR |
| Mesalina simoni saharae ssp. nov. | |||||||
| 88 | BEV.10453 | Morocco | 26.1256 | −14.4799 | – | AS | – |
| 89 | BEV.10849 | Morocco | 26.5298 | −12.3364 | AM | AS | AS |
| 90 | BEV.10850 | Morocco | 22.5709 | −14.3544 | AS | AS | AS |
| 91 | BEV.9114 | Morocco | 26.4925 | −13.9198 | AS | AS | AS |
| 92 | BEV.T1242 | Morocco | 21.3963 | −16.9579 | AS | AS | AS |
| 93 | BEV.T1256 | Morocco | 26.4925 | −13.9198 | AS | AS | AS |
| 94 | CIBIO9163 | Morocco | 22.6215 | −14.6044 | AS | AS | AS |
| Mesalina simoni ssp. | |||||||
| 95 | BEV.8508 | Morocco | 32.1900 | −2.2037 | AM | AM | AM |
| 96 | BEV.8509 | Morocco | 32.1750 | −2.1650 | AM | AM | AM |
| 97 | BEV.9429 | Morocco | 30.7076 | −8.3577 | SOUSS | SOUSS | SOUSS |
| 98 | BEV.T12122 | Morocco | 28.4876 | −11.3366 | SOUSS | – | SOUSS |
| 99 | BEV.T353 | Morocco | 31.5740 | −4.7380 | AM | AM | AM |
| Mesalina simoni simoni | |||||||
| 100 | BEV.9430 | Morocco | 31.8301 | −7.9829 | SIM | SIM | SIM |
| 101 | BEV.9431 | Morocco | 31.8129 | −8.0138 | SIM | SIM | SIM |
| 102 | BEV.T6301/E259 | Morocco | 32.3011 | −7.5307 | SIM | SIM | SIM |
Note
- The acronym “BEV” and “BEV.T” indicate vouchers and tissue samples deposited at the Biogéographie et Ecologie des Vertébrés-CEFE (Montpellier, France); the acronym “CIBIO” indicates vouchers and tissue samples deposited at CIBIO/InBIO, Centro de Investigação em Biodiversidade e Recursos Genéticos da Universidade do Porto (Vairão, Portugal); the acronym “NHMC" incates vouchers and tissue samples deposited at the Natural History Museum of Crete. The samples SPM002917 and SPM002920 have been obtained from Simó-Riudalbas et al. (2019).
2.2 Genetic analysis
2.2.1 DNA extraction and amplification
Total genomic DNA was extracted from ethanol-preserved tissue using a proteinase K (10 mg/ml) digestion followed by a standard salt-extraction protocol. Amplifications were performed in 5 μl of 2× MyTaq Mix and 0.4 μM of each primer. The PCR conditions adopted for every primer pair are specified in Table 2. Some samples required minor adjustments to the temperature and time of annealing from the reported conditions. We amplified one fragment from the mitochondrial cytochrome b gene (Cyt-b, 400 bp, Kapli et al., 2015) and four nuclear DNA (nucDNA) gene fragments from the beta fibrinogen intron 7 (β-fib7, 600 bp), melanocortin receptor one gene (MC1R, 630 bp), ornithine decarboxylase gene (OD, 467 bp), and phosphogluconase dehydrogenase intron 7 (PgD7, 414 bp). These markers were selected because they were found to be informative in previous studies (Kapli et al., 2015; Simó-Riudalbas et al., 2019; Sindaco et al., 2018) or during our preliminary analyses. PCR products were cleaned using ExoSAP. Purification, and sequencing were outsourced to GENEWIZ, Leipzig, Germany. Amplified fragments were sequenced for the forward strand only (primers sequences are listed in Table 2). Obtained DNA sequences were checked for errors using Codon-Code Aligner (v. 2.0.6, Codon-Code Corporation). Heterozygote positions of nuclear sequences were coded using IUPAC ambiguity codes. The absence of stop codons was checked with MEGAX (Kumar et al., 2018). DNA sequences were aligned using MAFFT v.7 (Katoh et al., 2019) applying the default parameters (Auto strategy, Gap opening penalty: 1.53, Offset value: 0.0). Homozygous indels were coded with dashes to maintain the overall alignment. The length of each indel was reduced to the minimum to maintain the number of variable positions. All sequences that were newly produced for the present study were deposited in GenBank (Accession numbers from MZ223473 to MZ223857; Table S1).
| Gene | Amplicon length | Alignment size | Primers | Reference | Sequence 5′–3′ | PCR cycling |
|---|---|---|---|---|---|---|
| Cyt-b | 400 bp | 303 bp | Mes_cytb_F | Kapli et al. (2015) | CGWAAACAACACCCVATCCT | 95° (5:00); [95° (0:30); 50 to 53° (0:45)- 72° (1:00)] for 45 cycles; 60° (10:00) |
| Mes_cytb_R | GATATTTGTCCTCADGGHA | |||||
| β-fib7 | 600 bp | 384 bp | BFXF | Sequeira et al. (2006) | CAGGGAGAGCTACTTTTGATTAGAC | 95° (10:00); [95° (0:30); 52° (0:30)- 72° (1:00)] for 38 cycles; 60° (10:00) |
| BF8 | Pinho et al. (2008) | CACCACCGTCTTCTTTGGAACACTG | ||||
| MC1R | 630 bp | 582 bp | MC1RF | Pinho et al. (2009) | GGCNGCCATYGTCAAGAACCGGAACC | 95° (5:00); [94° (0:30); 58° (1:30)- 72° (1:00)] for 40 cycles; 72° (7:00) |
| MC1RR | CTCCGRAAGGCRTAAATGATGGGGTCCAC | |||||
| OD | 467 bp | 425 bp | ODlez F | Friesen et al. (1999) | GCTACACTAAAAACCAGCAG | 95° (5:00); [94° (0:30); 56 to 58° (1:30)- 72° (1:00)] for 40 to 42 cycles; 72° (7:00) |
| ODlez R | CCACCAATATCAAGCAGGTAC | |||||
| PgD7 | 414 bp | 309 bp | PgDP8F | Pinho et al. (2008) | GACATGCAGCTGATCTGTGAGGCC | 95° (5:00); [94° (0:30); 58° (1:30)- 72° (1:00)] for 40 cycles; 72° (7:00) |
| PgDP7R | GAGTCCAGCTCAGTCTTATTCCAC |
A total of 80, 72, 74, 71, and 68 new samples were successfully sequenced for Cyt-b (Alignment S1), β-fib7 (Alignment S2), MC1R (Alignment S3), PgD7 (Alignment S4), and OD (Alignment S5), respectively (Table S1). Three datasets were produced: (a) Dataset S1: a mitochondrial dataset (Cyt-b; 285 bp), including outgroup (listed below) and all sequences available from the most recent publications (Kapli et al., 2015; Simó-Riudalbas et al., 2019; Sindaco et al., 2018; Šmíd et al., 2017) for a total of 378 sequences; (b) Dataset S2: a concatenated multilocus nuclear dataset (1700 bp) with a total of 78 sequences (including only samples with at least three genes) of the M. olivieri species complex, a few representative specimens of the other species of the genus and one specimen of Acanthodactylus erythrurus as outgroup; (c) Dataset S3: a concatenated mt + nucDNA dataset with a total of 86 concatenated sequences (1986 bp) of the M. olivieri species complex (including only samples with at least three genes) plus four species used as outgroup: Gallotia atlantica, Psammodromus algirus, Podarcis pityusensis, and Podarcis lilfordi, this dataset was used to calculate the phylogeny of the species complex and the time of divergence. Specific details of each database, as well as their implementations, are resumed in Table S2.
2.2.2 Phylogenetic analyses
For all datasets, Bayesian inferences (BI) were performed with BEAST 1.10.4 (Suchard et al., 2018). The best-fitting model of nucleotide substitutions for each gene was determined with PartitionFinder v.1.1.1 (Lanfear et al., 2012). The program was set as follows: branch lengths unlinked, only models available in BEAST were evaluated, BIC model selection criterion applied, and all partition schemes analyzed. Each gene was tested independently. The partition scheme and models of sequence evolution selected were β-fib7: HKY+G; MC1R: HKY+I; OD: HKY; PgD7: K80+G; and Cyt-b: HKY+G+I. No partition by codon position was selected for any of the genes.
To test whether the genes studied evolve in a clock-like manner (strict clock) a preliminary BI analyses was run in BEAST 1.10.4 (Suchard et al., 2018) using a relaxed clock for all the markers. The results of this preliminary run were verified using TRACER 1.6. (http://tree.bio.ed.ac.uk/software/tracer). The strict-clock model was rejected when the standard deviation of the uncorrelated lognormal relaxed clock parameter (ucld.stdev) and the coefficient of variation were greater than one. We used a Speciation Yule Process model assuming a constant lineage birth rate for each branch in the tree. Three independent MCMC runs of 100 million generations were implemented for each analysis for each dataset, sampling every 10,000 generations, and 10% of the trees were discarded as burn-in.
To root and calibrate the mt + nucDNA tree, four species were used as outgroup: G. atlantica, P. algirus, P. pityusensis, and P. lilfordi. The calibration points (in millions of years ago) and priors applied to the divergence time estimation correspond to those used Simó-Riudalbas et al. (2019): (a) the split between G. atlantica and P. algirus (age of the of the Canary Islands Fuerteventura and Lanzarote; normal distribution, mean 18, SD 2); (b) the split between P. pityusensis and P. lilfordi (end of the Messinian Salinity Crisis; normal distribution, mean 5.32, SD 0.05).
2.2.3 Haplotype networks reconstruction and genetic distances
Haplotype networks were reconstructed for each nuclear marker. Nuclear sequences were initially phased per lineage using the PHASE algorithm, implemented in DNASP 5.10.01 (Librado & Rozas, 2009). The algorithm was run five times, for 10,000 iterations, with a burn-in of 1000. The most probable reconstructed haplotypes were used to create the haplotype networks. Haplotype networks were then reconstructed in TCS (Clement et al., 2000) with a 95% parsimony threshold for the nuclear genes: β-fib7 (186 sequences), MC1R (152 sequences), OD (120 sequences), and PgD7 (124 sequences), and then displayed in tcsBU (Múrias dos Santos et al., 2016).
Computation of sequence divergence (uncorrected p-distances) for the Cyt-b fragment was performed in MEGAX (Kumar et al., 2018) to provide an overview of the genetic divergence among taxa. The grouping referred to the topology of the Cyt-b tree built using a database containing the sequences from this study and those published by Kapli et al. (2015).
2.3 Additional analyses for the species description
2.3.1 Morphological analyses
For the morphological data, we examined voucher specimens housed in the collection of the Biogeography and Ecology of Vertebrates (BEV) in the CEFE lab in Montpellier. A total of 32 morphological variables were measured in 252 specimens: 138 of M. olivieri (including eleven specimens from the AS/AM linages and 18 from the ADR lineage), 37 of M. pasteuri (including the TAG lineage) and 21 of M. simoni (samples localities are given in Table 1 and Table S1). Variables were selected according to their relevance as diagnostic characters of the genus Mesalina in previous sources (e.g., Hosseinian Yousefkhani et al., 2015; Trape et al., 2012) or based on our own examination of specimens of the various lineages. We scored each individual for: (a) five quantitative biometric variables; (b) 11 quantitative pholidotic variables; (c) 10 semi-quantitative (ordinal) chromatic variables; (d) four semi-quantitative (ordinal) variables describing morphological states (Table 3). Scale nomenclature, scale counts, and measurements follow Bons and Geniez (1995). All morphological data were obtained by the same observer (CP).
| Type | Acronym | Extended name |
|---|---|---|
| Biometry (Quantitative) | SVL | Snout-vent length (mm) |
| TL | Tail length (mm) | |
| HL | Head length (mm) | |
| HW | Head width (mm) | |
| HH | Head height (mm) | |
| Pholidotic (Quantitative) | D | No. of longitudinal rows of dorsal scales counted around midbody |
| V | No. of transvers rows of ventral plates | |
| G | No. of gular scales in one straight line from the collar to the infralabials (collar included) | |
| Pf (Dx and Sx) | No. of femoral pores on the right and left sides | |
| Lam | No. of lamellae beneath the fourth toe | |
| NTS | No. of rows of temporal granulae (average of left and right side) | |
| TR | No. of scales around the tail at the 10th scale ring | |
| SL | No. of supralabials in contact with the subocular on the right and left sides | |
| IL (Dx and Sx) | No. of infralabials on the right and left sides | |
| Col | No. of enlarged scales forming a collar | |
| EL | No. of enlarged scales on the lower eyelid (forming the palpebral disks, average of left and right side) | |
| Chromatic (Ordinal) | EBL | Black line surrounding the eyelids scales (0 = absent; 1 = present) |
| DBF | Dark bands along the flanks (0 = absent; 1 = fragmented; 2 = continuous) | |
| PSDBF | Pale spots inside the dark bands along the flanks (0 = absent; 1 = small pale spots 2 = ocelli) | |
| PDLL | Pale dorsolateral line (0 = absent; 1 = fragmented; 2 = continuous) | |
| SPDLL | No. of scales included into the width of the pale dorsolateral line | |
| DDSLL | Dark supra-dorsolateral line (0 = absent; 1 = fragmented; 2 = continuous) | |
| SDDLB | No. of scales included into the width of the dark supra-dorsolateral line | |
| PSDDSLL | Pale spots within the dark supra-dorsolateral line (0 = absent; 1 = small pale spots 2 = ocellus) | |
| DSO | Small ocelli arranged in rows along the mid dorsum 0 = absent; 1 = small pale spots 2 = ocelli) | |
| TC | Under tail coloration (0 = white; 1 = yellowish; 2 = yellow) | |
| Morphological (Ordinal) | TE | Tail enlargement (from 0 to 2) |
| RN | Raised nostrils (from 0 to 2) | |
| PS | Pointed snout (from 0 to 2) | |
| DS | Shape of the dorsal scales (0 = flat; 1 = pointed; 2 = weakly carinated (tectiform); 3 = well carinated) |
Note
- Variables were selected according to their relevance as diagnostic characters in the genus Mesalina (Hosseinian Yousefkhani et al., 2015; Trape et al., 2012) or based on our own examination of specimens of the various lineages.
To identify diagnostic characters and to quantify the amount of morphological differentiation between the ADR and TAG lineages and the other species of the olivieri complex, we conducted multivariate analyses over three morphological datasets: biometry (Table S3), pholidosis (Table S4), and coloration (Table S5). Multivariate analyses were restricted to adult specimens to reduce variation due to the strong ontogenetic modifications in color patterns in the genus Mesalina. Both sexes were treated separately, as preliminary analyses revealed significant sexual dimorphism for many variables in every clade (results not shown). Due to the small sample size, statistical tests were not conducted. An exploratory investigation was first performed with Excel using a heating map to facilitate an immediate visualization of the most relevant interspecific differences. Then, Principal Component Analyses (PCAs) were run on each database for a pairwise comparison of the potential new species with the other species of the complex. The data were normalized to zero mean and unit variance prior to PCAs. All analyses were run on PAST 3.24 Software (Hammer et al., 2001).
2.3.2 Distribution modeling and habitat comparison
An ecological niche model was performed to assess the potential distribution and characterize the realized ecological niche of the ADR and TAG lineages. For this analysis, we aimed to include all the known localities of these two lineages. To do so, we considered an area of approximately 1,979,127 km2 (varying between 28°N, 17°W to 14°N, 4°W) that includes southern Morocco, South-western Algeria, the full extent of Mauritania, South-western Mali, and North-eastern Senegal, comprising the whole distribution of the potential new species (known to date). Models were based on the 28 presence records confirmed by genetic or morphological assignment. Morphological identifications of unsequenced specimens were based on pictures of live unvouchered animals or from the direct examination of specimens deposited in museum collections. Only records based on adult specimens exhibiting distinctive characteristics of the ADR lineage were treated as valid. To remove duplicated observations from the same geographic locations, data were thinned reducing to 20 the number of observations to build models. Models were built using a spatial resolution of 1 km.
Variables used for the modeling were: (a) Terrain Roughness Index (TRI) calculated by upscaling a digital elevation model (USGS, 2006) from 90 meters to 1 km; (b) 18 land-cover categories (Table S6) adapted from Campos and Brito (2018) and up-scaled from 30 m to 1 km; (c) four bioclimatic variables, maximum temperature of warmest month (BIO5), minimum temperature of coldest month (BIO6), temperature annual range (BIO7), and annual total precipitation (BIO12) from Hijmans et al. (2005). All variables were uncorrelated (r < 0.75).
The models were developed using the Maximum Entropy approach implemented in Maxent v.3.3 (Phillips et al., 2006) with the following settings: 5000 maximum number of iterations; regularization multiplier equal to 1; 10,000 maximum number of background points; 20 replicates selected by bootstrap. The area under the receiver-operating curve (AUC) of each replicate run was taken as a measure of model accuracy and ensemble models were generated by averaging the 20 model replicates. Response curves and jackknife analyses were performed to assess the importance of each variable in each model replicate (e.g., Vale et al., 2014). Finally, the minimum training presence threshold was applied to the ensemble model given that less restrictive thresholds should be applied for conservation purposes (Liu et al., 2005). The resulting binary map (depicting presence/absence areas) was used to calculate the extent of occurrence and area of occupancy following IUCN guidelines for assessing Red List categories (IUCN, 2017).
The percentage of presences of each lineage of the olivieri complex in each land-cover unit was taken as a measure of the biogeographic affinities of each group (Brito et al., 2009). Selection among land-cover units was quantified from the percentages of training observations using the Standardized Levin's B measure of niche breadth: Bs ¼ B_1/n_1, where B is the Levin's index and n the total number of land-cover units. “B” is given by 1/P(p2), where p is the proportion of observations in each land-cover unit. The standardized index was used because of unbalanced sample size among groups. Eight land-cover units selected were sandy areas (51.1% of the study area), compact soil (6.1%), rocky plateaus (16.8%), bare rocks (6.3%), gravel and sand floodplains (1.9%), grasslands (9.1%), savannah (2.9%), and croplands (5.7%).
2.3.3 Taxonomic ranking
We (the authors) do not necessarily adhere to the same species concept. While some follow the Unified Species Concept (USC) of De Queiroz (2007), some prefer the general framework of the Biological Species Concept (BSC; Mayr, 1970). However, we all recognize reproductive isolation as the primary operational criterion for the delimitation of species and we all agree that, while every species is a lineage, not every lineage is a species. The approach used in this paper can be defined either as following the BSC framework, or as applying a Biological Species Criterion under the USC. We thus treated sympatric or parapatric lineages that do not exchange genes when they are not isolated by extrinsic geographical barriers as species. Moreover, this study adopts the framework of integrative taxonomy based on the assumption that divergences in any of the attributes can provide evidence for the species' existence (Dayrat, 2005; Padial et al., 2010). We have divided our dataset into six categories that can each be regarded as distinct lines of evidence. They can be combined and compared in order to assess the congruence of the putative species limits among the olivieri complex: (a) the mtDNA data can be used to test the criteria of reciprocal monophyly and the presence/absence of barcoding gaps, (b) the multilocus nucDNA data can be used to test, independently from the mtDNA set, the criteria of reciprocal monophyly, notably in coalescence theory framework, (c) the morphological data allows to test the criteria of morphological divergence, and (d) habitat and v) distribution range and vi) land-cover datasets can be used as a complementary approach to test the criteria of isolation and distinct habitat requirements.
3 RESULTS
3.1 Phylogenetic relationships within the Mesalina olivieri species complex
Since not all samples were successfully amplified for all genes (see Table S1), some samples were absent from the concatenated mt + nucDNA dataset and/or not included in the comparison between nuclear and mtDNA results. Consequently, some of the mtDNA M. olivieri lineages (see Figure 2a, Figure S2 and Table 1) are absent from the mt + nucDNA results (Figure 3 and Figure S4). The trees based on concatenated mtDNA and nuDNA recover several lineages that occupy distinct regions of the distribution of the M. olivieri complex (including M. simoni and M. pasteuri). These lineages are depicted in Figures 2-4 (see also Table 1) and identified by acronyms corresponding with their distribution: ADR from Adrar and adjacent areas in Mauritania and southern Morocco; TAG from the Tagant mountains in Mauritania; SOUS from the Sous-Massa region (Morocco); SIM the terra typica lineage of M. simoni from north and west of the Atlas Mountains in Morocco; AM from south and southeast Morocco; AS from Atlantic Sahara, ALG1 from Algeria; ISR from Israel; the terra typica lineage of M. olivieri from Egypt (EGY1); TUN1/TUN2/TUN3 from Tunisia; ALG2/ALG3 from western Algeria; MOR2 and MOR3 from eastern Morocco; a pasteuri clade from the center of Mauritania (MAU1); a pasteuri clade from the Atlantic Sahara region (MAU2 + MOR); a pasteuri clade from the Atlantic coast of Mauritania and inland Algeria (MAU3 + ALG); a pasteuri clade from the Atlantic coast of Mauritania and inland Niger (MAU4 + NIG).
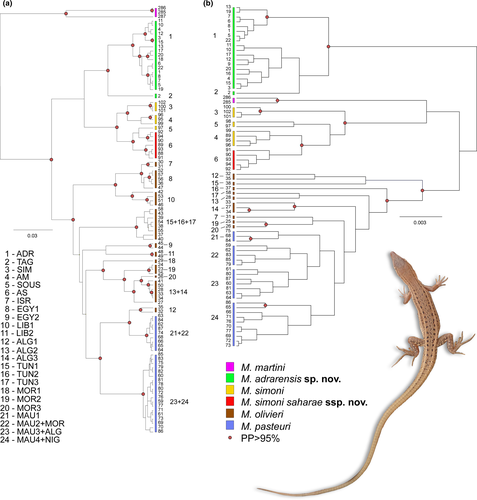
All the species used as outgroup always clustered outside the olivieri complex, except for M. martini. The latter was recovered as: (a) basal to the olivieri complex when mtDNA was analyzed alone (Dataset S1, Figure 2a and Figure S2), and for the mt + nucDNA results (Dataset S3; Figure 3 and Figure S4); (b) sister to the M. simoni clade (M. simoni and olivieri lineages AS/AM) for the nucDNA Dataset S2 (Figure 2b and Figure S3).
In all the trees that include mtDNA (Figures 2a and 3, Figures S2 and S4), the olivieri species complex is divided into two deep and well-supported clades: (a) one clade including the ADR and TAG lineages, M. simoni and the two olivieri lineages AS and AM (sister taxa of M. simoni), and (b) one clade including the remaining M. olivieri and M. pasteuri. In all trees the AS and AM lineages are well-defined, except for the sample BEV.10849 (sample number 89). This sample (collected 76 km past Laayoune toward Smara) clusters in the AS lineage in all trees (Figures 2a and 3 and Figures S2 and S4) with the exception of the concatenated nucDNA tree (Figure 2b and Figure S3). The position of the clade made of the ADR and TAG lineages also differs between nuclear and mtDNA: basal to the M. olivieri species complex for the nuclear dataset (Dataset S2, Figure 2b and Figure S3) or sister to the M. simoni clade for the mitochondrial and for the mt + nucDNA datasets (Datasets S1 and S3, Figures 2a and 3, Figures S2 and S4).
The results also revealed a case of discordance between markers: the olivieri lineage ALG1 was recovered (with strong support, PP > 95%) as sister to the continental lineage of M. pasteuri in mtDNA (Figure 2a and Figure S2), but basal to M. pasteuri and M. olivieri (together with a sample from Tunisia, 308 = TUN1) when nucDNA was analyzed alone (Figure 2b and Figure S3). As a result, its position (basal to M. pasteuri and M. olivieri) in the mt + nucDNA concatenated tree is not meaningful (Figure 3 and Figure S4).
3.2 Nuclear haplotype networks and mitochondrial genetic divergence
Haplotype network reconstructions for nuclear loci (Figure 5) indicate that none of the alleles found in the ADR and TAG lineages (marked in green in Figure 5) is shared with the other lineages. However, haplotype sharing was detected between M. simoni and the two olivieri lineages AS and AM for all markers. All the haplotypes found in M. simoni + AS and AM lineages are private and completely separated from the other species in β-fib7 and PgD7 networks. A connection between M. simoni, AS and AM lineages and the other species is recovered in MC1R and OD where they still diverge from the other M. olivieri by one and two mutational steps, respectively. In the network analysis for MC1R, the terra typica lineage of M. simoni and the SOUS lineage (yellow dot detached from the other haplotypes in the MC1R network, Figure 5) shows a divergence from the two olivieri lineages AS and AM of five mutational steps.

The level of mitochondrial genetic divergence (p-distance) between ADR and TAG lineages and the other species of the complex was recorded to be always above 8.9%. The genetic divergence between the olivieri lineages AS and AM and M. simoni was always below 5% (Table 4).
| Mesalina adrarensis sp. nov. | Mesalina olivieri | Mesalina pasteuri | Mesalina simoni ssp. | Mesalina guttulata | |
|---|---|---|---|---|---|
| Mesalina olivieri | 0.14 | ||||
| Mesalina pasteuri | 0.13 | 0.11 | |||
| Mesalina simoni ssp. | 0.08 | 0.11 | 0.11 | ||
| Mesalina guttulata | 0.20 | 0.19 | 0.22 | 0.18 | |
| Mesalina rubropunctata | 0.21 | 0.19 | 0.18 | 0.21 | 0.16 |
| Mesalina simoni | Mesalina simoni ssp. AM | |
|---|---|---|
| Mesalina simoni ssp. AM | 0.03 | |
| Mesalina simoni saharae ssp.nov. (AS lineage) | 0.04 | 0.05 |
Note
- Analyses were conducted using the Maximum Composite Likelihood model. This analysis involved 118 nucleotide sequences of the Cyt-b fragment. Codon positions included were 1st + 2nd + 3rd + Noncoding. All ambiguous positions were removed for each sequence pair (pairwise deletion option). There was a total of 286 positions in the final dataset. Distances in Mesalina simoni ssp. include the two subspecies.
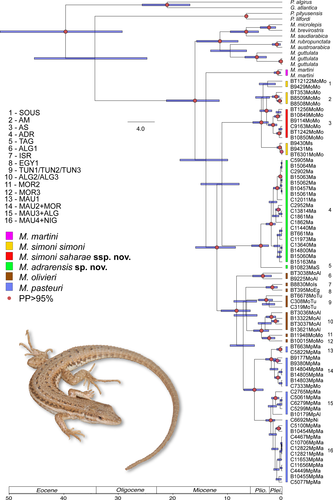
3.3 Time of divergence
Based on the mtDNA + nuDNA concatenated tree (Figure 3 and Figure S4), estimation of divergence times indicates that the olivieri complex started to diversify in the Late Miocene, ca. 9.6 Mya (6.92–12.87 Mya, 95% highest posterior densities [HPD]; Figure 3) in two main clades: (a) one including the ancestor of M. simoni and the lineages ADR, TAG, AS, and AM and (b) the other one including the ancestor of M. olivieri and M. pasteuri. Our estimation shows that the latter has undergone a first split (c.a. 6.77 Mya) between the olivieri lineage ALG1 and the ancestor of M. olivieri and M. pasteuri, and that these two species diversify afterward from each other around 5 Mya. The clade made of M. simoni and the lineages AS, AM, ADR, and TAG started its diversification around 8.94 Mya (6.30–12.8 Mya, 95% HPD). Moreover, most of the intraspecific diversification of the complex took place during the Plio-Pleistocene transition (Figure 3). For instance: (a) the split between M. simoni and the lineages AS and AM is dated to 3.09 Mya (2.05–4.37 Mya 95% HPD); (b) the north–south diversification between the lineages ADR and TAG occurred ca. 3.17 Mya (2.01–4.54 Mya 95%); (c) a second, more recent and well-supported split delineated the eastern (samples no.: 1, 5–8, 13, 16–20) and the north-western (samples no: 3, 4, 9–12, 15) lineages, ca. 1.63 Mya (1.01–2.4 Mya 95% HDP).
3.4 Additional results for the species description
3.4.1 Morphological analyses
Measurements of all individuals examined for this work are given in Tables S3–S5. Descriptive statistics of the morphological data are shown in Tables 5–7 and Table S7. Morphological differences were found between the two lineages (ADR and TAG) representing the putative new species from Mauritania, and the remaining species of the complex (Figures S5–S8; Tables 5–7 and Table S7).
| Males | Mesalina adrarensis sp. nov. vs. Mesalina olivieri | Mesalina adrarensis sp. nov. vs. Mesalina pasteuri | Mesalina adrarensis sp. nov. vs. Mesalina simoni ssp. | |||
|---|---|---|---|---|---|---|
| PC 1 | PC 2 | PC 1 | PC 2 | PC 1 | PC 2 | |
| SVL | −0.039 | −0.295 | −0.133 | −0.125 | −0.060 | −0.134 |
| HL | 0.118 | 0.711 | 0.150 | 0.013 | 0.139 | 0.349 |
| HW | −0.107 | 0.390 | 0.176 | 0.569 | −0.112 | 0.421 |
| HH | −0.301 | 0.322 | −0.096 | 0.206 | −0.182 | −0.012 |
| TE | −0.059 | 0.364 | 0.199 | 0.725 | −0.030 | −0.436 |
| RN | 0.448 | 0.138 | 0.599 | −0.290 | 0.333 | 0.649 |
| PS | 0.824 | 0.004 | 0.722 | −0.096 | 0.905 | −0.266 |
| % variance | 66.773 | 19.973 | 66.209 | 17.556 | 74.348 | 13.783 |
| Females | PC 1 | PC 2 | PC 1 | PC 2 | PC 1 | PC 2 |
|---|---|---|---|---|---|---|
| SVL | −0.002 | −0.232 | −0.134 | −0.216 | −0.166 | −0.216 |
| HL | 0.074 | 0.480 | 0.260 | 0.400 | 0.345 | 0.626 |
| HW | −0.220 | 0.475 | 0.299 | 0.403 | 0.168 | 0.475 |
| HH | −0.283 | 0.208 | 0.546 | 0.405 | 0.026 | 0.362 |
| TE | −0.107 | 0.622 | 0.441 | −0.242 | 0.398 | 0.062 |
| RN | 0.469 | −0.002 | −0.413 | 0.421 | 0.225 | −0.136 |
| PS | 0.797 | 0.245 | −0.404 | 0.481 | 0.785 | −0.427 |
| % variance | 52.963 | 39.385 | 70.650 | 21.405 | 45.815 | 31.321 |
Notes
- Loading scores and percentage of variance explained in the first three principal components extracted. Comparison between male and female individuals of Mesalina adrarensis sp. nov. with the other species of the Mesalina olivieri species complex.
- Significant values are indicated in bold.
| Males | Mesalina adrarensis sp. nov. vs. Mesalina olivieri | Mesalina adrarensis sp. nov. vs. Mesalina simoni ssp. | Mesalina adrarensis sp. nov. vs. Mesalina pasteuri | |||
|---|---|---|---|---|---|---|
| PC 1 | PC 2 | PC 1 | PC 2 | PC 1 | PC 2 | |
| V | 0.311 | 0.012 | 0.078 | 0.008 | 0.067 | 0.234 |
| D | −0.713 | −0.050 | 0.892 | −0.158 | 0.897 | −0.182 |
| DS | 0.049 | −0.158 | 0.039 | 0.254 | 0.174 | −0.231 |
| TR | 0.213 | 0.179 | 0.092 | −0.072 | 0.039 | 0.085 |
| SL(Sx) | 0.046 | 0.074 | −0.015 | 0.000 | −0.099 | 0.059 |
| SL(Dx) | 0.067 | 0.100 | −0.024 | 0.011 | −0.077 | 0.082 |
| IL(Sx) | 0.051 | 0.049 | −0.003 | −0.006 | 0.020 | −0.090 |
| IL(Dx) | 0.064 | 0.059 | −0.038 | −0.022 | −0.014 | −0.052 |
| G | 0.425 | −0.693 | 0.191 | 0.667 | 0.103 | 0.477 |
| Col | 0.190 | 0.048 | 0.235 | −0.159 | 0.111 | −0.055 |
| EL | 0.161 | 0.385 | 0.010 | −0.316 | 0.112 | −0.080 |
| NTS | 0.100 | −0.031 | 0.154 | −0.130 | 0.228 | 0.023 |
| Pf(Sx) | −0.024 | 0.112 | 0.201 | 0.097 | 0.137 | 0.416 |
| Pf(Dx) | −0.059 | −0.011 | 0.162 | 0.450 | 0.165 | 0.303 |
| Lam | 0.271 | 0.522 | −0.068 | 0.325 | −0.090 | −0.572 |
| %variance | 43.31 | 17.31 | 52.948 | 19.840 | 62.349 | 12.411 |
| Females | PC 1 | PC 2 | PC 1 | PC 2 | PC 1 | PC 2 |
|---|---|---|---|---|---|---|
| V | 0.231 | 0.015 | 0.627 | 0.403 | 0.231 | 0.015 |
| D | 0.699 | −0.495 | 0.482 | −0.582 | 0.699 | −0.495 |
| DS | 0.033 | 0.013 | −0.079 | 0.043 | 0.033 | 0.013 |
| TR | 0.061 | −0.210 | 0.185 | 0.199 | 0.061 | −0.210 |
| SL(Sx) | −0.033 | 0.011 | −0.159 | 0.032 | −0.033 | 0.011 |
| SL(Dx) | −0.058 | 0.027 | −0.168 | 0.029 | −0.058 | 0.027 |
| IL(Sx) | 0.048 | −0.080 | 0.108 | 0.033 | 0.048 | −0.080 |
| IL(Dx) | 0.031 | −0.032 | 0.056 | 0.137 | 0.031 | −0.032 |
| G | 0.210 | 0.374 | −0.373 | −0.164 | 0.210 | 0.374 |
| Col | 0.166 | 0.104 | −0.108 | −0.132 | 0.166 | 0.104 |
| EL | 0.047 | −0.559 | −0.003 | 0.489 | 0.047 | −0.559 |
| NTS | 0.106 | 0.073 | 0.193 | 0.106 | 0.106 | 0.073 |
| Pf(Sx) | 0.299 | 0.311 | −0.157 | 0.252 | 0.299 | 0.311 |
| Pf(Dx) | 0.324 | 0.203 | −0.222 | 0.273 | 0.324 | 0.203 |
| Lam | 0.406 | 0.309 | −0.047 | 0.029 | 0.406 | 0.309 |
| %variance | 35.895 | 19.620 | 94.81 | 2.50 | 35.895 | 19.620 |
Notes
- Loading scores and percentage of variance explained in the first three principal components extracted. Comparison between male and female individuals of Mesalina adrarensis sp. nov. with the other species of the Mesalina olivieri species complex.
- Significant values are indicated in bold.
| Males | Mesalina adrarensis sp. nov. vs. Mesalina olivieri | Mesalina adrarensis sp. nov. vs. Mesalina pasteuri | Mesalina adrarensis sp. nov. vs. Mesalina simoni ssp. | |||
|---|---|---|---|---|---|---|
| PC 1 | PC 2 | PC 1 | PC 2 | PC 1 | PC 2 | |
| EBL | 0.255 | −0.201 | 0.326 | −0.182 | −0.035 | 0.262 |
| DBF | 0.555 | −0.031 | 0.098 | −0.116 | −0.046 | 0.465 |
| PSDBF | −0.262 | −0.003 | 0.000 | 0.556 | 0.165 | −0.329 |
| PDLL | −0.210 | 0.232 | −0.332 | 0.146 | 0.030 | −0.213 |
| SPDLL | −0.122 | 0.746 | −0.110 | −0.062 | 0.114 | −0.087 |
| DDSLL | 0.298 | −0.110 | −0.176 | −0.395 | 0.052 | 0.318 |
| SDDLB | 0.340 | −0.165 | 0.852 | −0.012 | 0.956 | 0.206 |
| PSDDSLL | −0.452 | −0.511 | 0.000 | 0.000 | 0.127 | −0.592 |
| DSO | 0.300 | 0.217 | 0.078 | 0.680 | −0.152 | 0.243 |
| %variance | 38.513 | 23.754 | 61.52 | 18.176 | 54.423 | 23.086 |
| Females | PC 1 | PC 2 | PC 1 | PC 2 | PC 1 | PC 2 |
|---|---|---|---|---|---|---|
| EBL | −0.006 | 0.159 | 0.249 | −0.174 | −0.033 | 0.057 |
| DBF | 0.422 | 0.114 | 0.000 | 0.000 | −0.082 | 0.032 |
| PSDBF | −0.153 | 0.227 | 0.000 | 0.000 | 0.122 | 0.051 |
| PDLL | −0.320 | 0.268 | −0.433 | 0.429 | 0.504 | 0.033 |
| SPDLL | −0.213 | 0.486 | 0.000 | 0.000 | 0.619 | −0.017 |
| DDSLL | 0.285 | −0.143 | −0.036 | 0.485 | −0.183 | −0.037 |
| SDDLB | 0.663 | 0.588 | 0.858 | 0.194 | −0.147 | 0.965 |
| PSDDSLL | −0.306 | 0.454 | 0.000 | 0.000 | 0.525 | 0.226 |
| DSO | 0.192 | −0.175 | −0.111 | −0.716 | −0.095 | −0.096 |
| %variance | 62.727 | 24.348 | 82.388 | 15.487 | 37.915 | 24.499 |
Notes
- Loading scores and percentage of variance explained in the first three principal components extracted. Comparison between male and female individuals of Mesalina adrarensis sp. nov. with the other species of the Mesalina olivieri species complex.
- Significant values are indicated in bold.
Compared with the other species of the M. olivieri complex, individuals of the ADR and TAG lineages have: (a) a narrower and more pointed snout (Figures S5 and S6; Table 6 and Table S7), (b) more protuberant nostrils (Figures S5 and S6; Table 5 and Table S7), (c) more dorsal and gular scales (Figure 7 and Figure S7; Table 6 and Table S7), (d) more femoral pores (Figure S7; Table 6 and Table S7), and (e) a higher number of lamellae beneath the fourth toe (Figure S7; Table 6 and Table S7). Moreover, (f) the shape and disposition of the scales composing the eyelid in the new species resemble those of Mesalina guttulata (Lichtenstein, 1823) (1–2 clearly enlarged, transparent scales in the lower eyelids versus several sub-equal scales in the M. olivieri complex) although most of the specimens of the ADR and TAG lineages lack the well-defined dark lines edging the large eyelid scales found in M. guttulata.
Major differences were also found in the dorsal coloration. The PCAs on these characters including specimens of M. olivieri and the putative new species highlights the presence of pale dorsal spots on the dorsum (PSDDSLL, more obvious in both male and females of the potential new species) and the pattern of dark bands on the dorsum (SDDLB) and on the flanks (DBF) both thicker and better defined in M. olivieri than in the putative new species. Similarly, the dorsal pattern differs between the new species and M. pasteuri: in M. pasteuri the dorsal patterns is mostly made of obvious pale and dark continuous longitudinal stripes, whereas in the putative new species the two dark supra-dorsolateral lines are highly fragmented (forming irregular small patches) and the two pale dorsolateral lines are thin and fragmented (Figures S5 and S8; Table 7).
3.4.2 Distribution modeling and habitat comparison
The 20 replicate ecological models exhibited good predictive accuracy (average AUC = 0.906, Table S8). The most important environmental predictors were terrain ruggedness index (42.6% contribution) and land-cover category (35.4%) (Figure S9; Table S8). The highest probability of occurrence is in the intermediate levels of terrain ruggedness and in bare rock habitats (Figure 6 and Figure S9). These findings fit well with our field observations, as samples of the ADR and TAG lineages were always collected in rocky habitats on slopes or plateaux, while M. pasteuri was only found in the adjacent plains (pers. obs.).
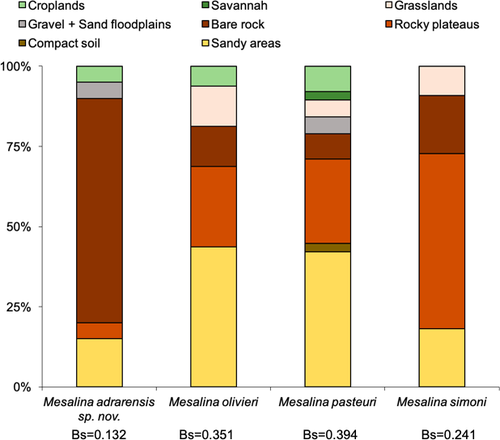
We found significant differences in number of observations in each land-cover category (χ2; p = 0.006; df = 21; Figure 6; Table S9) for the area considered in this study: (a) the lineage representing the putative new species from Mauritania (ADR-TAG) was mostly found (70% of observations) in bare rocks, a restricted land-cover category in the study area (6.3% of the area) but showing similarities with M. guttulata habitat; (b) M. simoni and the olivieri lineages AS and AM were most frequently found in rocky plateaus; (c) M. olivieri occurred in almost all units; and (d) M. pasteuri appeared to be most related to sandy areas. The niche breadth estimation was under 0.5 in all cases indicating that all taxa are specialized in habitat selection, and that the olivieri lineages ADR and TAG are the most specialized ones.
3.4.3 Taxonomic implications
The ADR and TAG lineages form a well-supported, monophyletic clade. In all datasets and all analyses, they share the same morphological features and habitat that distinguish them from the other species of Mesalina from northwest Africa. We thus treat them as one evolutionary unit for the time being, pending further studies and larger sampling of the TAG lineage. Given the observed divergences in genetics, morphology, and ecology from the other species of the genus Mesalina, and the lack of alleles sharing with the sympatric M. pasteuri and parapatric M. simoni (Figure 5 and Figure S10) demonstrating reproductive isolation, we treat the evolutionary unit made of the ADR and TAG lineages as a valid species that we describe here.
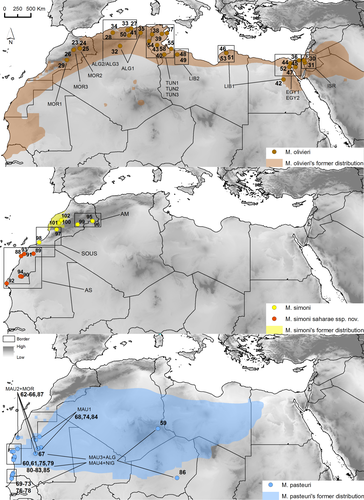
Although the current taxonomy of the genus Mesalina assigns populations of the complex from southern Morocco and the Atlantic Sahara to M. olivieri, both mtDNA and nucDNA data unambiguously assign the lineages AS and AM to M. simoni rather than to M. olivieri sensu stricto (s.s.). We, thus, formally assign, here, all the populations of the M. olivieri complex from southern and south-western Morocco to the Atlantic Sahara to M. simoni. We thus restrict M. olivieri to the populations distributed from central and eastern Morocco to Israel (in brown in Figure 4; see also Figures 2, 3 and 5). We suspect that M. olivieri, as restricted here, is itself a species complex and that several species-level units are still merged under this name.
In the concatenated mtDNA + nucDNA trees, there are four reciprocal monophyletic lineages within M. simoni: (a) SOUS, (b) AS, (c) AM, and (d) M. simoni s.s. (Figures 3 and 4). We have sequenced or examined very few specimens of the SOUS and AM lineages and thus refrain from proposing a distinct taxonomic status for the time being. From now on, we will refer to these lineages as M. simoni ssp. “SOUS” and M. simoni ssp. “AM”. On the contrary, the AS lineage exhibits a distinct morphology, with most sub-adult or adult specimens being diagnosable from M. simoni s.s. (Figure S5). However, the four lineages are not distinct on the nuclear networks and specimens from the northern Atlantic Sahara or extreme south-western Morocco appear intermediate morphologically between M.simoni s.s. and AS (pers. obs.). We thus refrain from treating the AS lineage as a distinct species and formally recognize it as a new subspecies of M. simoni.
The nomen simoni was based on seven specimens collected “inter urbes Mogador et Marocco” (= between the cities of Mogador and Marocco) and one specimen from “prope urbem Casablanca” (= not far from the city of Casablanca, see Böttger, 1880). Mogador is the city currently known as Essaouira and Marocco was used at the time for the city of Marrakech. As far as we know, there has been no type locality restriction for this nomen so the type locality of M. simoni remains as “between Essaouira and Marrakech and near Casablanca.” This area is inhabited by the lineage currently designed as M. simoni s.s. All the nomina currently allocated to the synonymy of M. olivieri were based on specimens from the eastern clade of M. olivieri, and we were unable to identify any nomen available for the lineages of the M. olivieri complex inhabiting Mauritania or south-western Morocco. We, thus, need to create new nomina to name the new species from Mauritania (ADR and TAG linages) and the new subspecies from south-western Morocco (AS lineage).
Consequently, we suggest the following nomenclatural and taxonomic actions: (a) designate all the populations of the lineages ADR and TAG as a new species here described as Mesalina adrarensis sp. nov.; (b) designate as subspecies of M. simoni all the M. olivieri present in the Atlantic Sahara region, here described as Mesalina simoni saharae ssp. nov.; (c) allocate all populations of M. simoni SOUS and AM (from north of the High Atlas and west of the Middle Atlas) to M. simoni. The two subspecies Mesalina simoni simoni and M. simoni saharae ssp. nov. are bridged by populations of intermediate morphology and genetic background between the High Atlas and the north of the Atlantic Sahara; specimens of M. simoni ‘SOUS’ (inhabiting the area around Tan-Tan and El Ouatia) are morphologically close to typical saharae but seem already admixed genetically with M. s. simoni (see specimen B9429 Figure 2a and Figure S2). The populations currently treated as M. simoni ‘AM’ could conceivably be included in M. simoni saharae ssp. nov., as they are genetically grouped with this taxon, but we prefer to analyses more specimens before we can conclude.
Mesalina adrarensis sp. nov.

Zoobank registration
http://zoobank.org/NomenclaturalActs/dc6c167b-8138-4017-82a4-513d8244072a
Holotype
Adult female (Figure 7) with code MNHN-RA-2020.0017 preserved in the Muséum national d'Histoire naturelle, in Paris, France. Collected in the Tiris Zemmour (Mauritania) by Philippe Geniez, Olivier Peyre, Pierre-André Crochet and José Carlos Brito on 7th April 2017.
Type locality
Mauritania, 440 m south-west of the Guelta Oumm el Habâl, 17.4 km east-southeast of F'derick. 22.60636°N/−12.55743°W/372 m a.s.l.
Paratypes
BEV.15163, adult female collected in the Adrar Atar, 44 km before Chinguetti coming from Atar (Mauritania, 20.5537°N/12.6916°W) by F. Martínez-Freiría, J.C. Brito, D.V. Gonçalves, J.C. Campos, Z. Boratyński, C.G. Vale, T.L. Silva, X. Santos, J.M. Pleguezuelos, M. Feriche and A.S. Sow on the 29th October 2011; BEV.15060, adult male, same locality, collected by J.C. Brito, Z. Boratyński, S. Lopes, J. Marques and F. Martínez-Freiría on the 12th September 2015; BEV.15061, adult female, BEV.15062, adult male, BEV.15063, adult male, BEV.16064, adult male, all collected in the Adrar Atar, Oumm Lekhterat (Mauritania, 21.1596°N/11.9362°W) by J.C. Brito, Z. Boratyński, S. Lopes, J. Marques and F. Martínez-Freiría on the 13th September 2015, all preserved in the BEV collection in Montpellier.
Etymology
The species epithet “adrarensis” refers to the Adrar Atar region because the new species was first suspected when seeing specimens from this region.
Diagnosis
A species of the M. olivieri complex characterized by the following combination of characters: (a) low number of eyelid scales (5–6); (b) with two clearly larger scales, like M. guttulata (several sub-equal scales or rarely 1–2 larger scales in the other species of the M. olivieri complex); (c) small black dots on the edge of the eyelids (mostly visible in dead animals preserved in ethanol); (d) more elongated snout with more prominent nostrils than the other species of the olivieri complex; (e) adult coloration in life (Figure 7) different from all other species of the complex (see Figure S5 and comparison below).
Coloration in life (adults)
Dorsum coloration mostly brown, from sandy-brown to dark-brown or rufous-brown, with a dorsal pattern made of two central longitudinal lines of small whitish spots (usually partially edged with dark-brown or black) then two supra-dorsolateral longitudinal rows of black blotches edged externally by narrow dorsolateral fragmented whitish lines, sometimes nearly continuous, sometimes reduced to series of small elongated dots; the flanks show small pale spots partially edged by dark coloration, sometimes fusing in a near complete dark band; on the lower part of the flanks a more continuous longitudinal white line separates the dorsal and ventral parts of the body. The pattern from the center of the back fades on the anterior part of the dorsum, especially on the nape, then disappears on the head. Dorsal pattern more contrasted in females than in some males; in some males, this pattern is reduced to a series of whitish and dark-brown spots aligned along the body. The color of the head is similar to the body coloration with faintly spotted pileus and a dark line running though the sides of the head through the eye, bordered below by a pale line reaching to the eye. Forelimbs coloration uniformly brown, hind legs brownish with whitish ocelli. Uniformly brown tail with a median dark stripe, more or less visible, disappearing at the first third of its length, this median stripe is externally edged on each side by a light stripe resulting from the prolongation of each light dorsolateral stripe. Underparts of the head and the legs pinkish-beige, belly overall similar but paler on central belly, underparts of the tail whitish, sometimes yellowish especially in males. Juveniles present a pale background coloration, striped with wide and continuous pale and dark (sometimes white and pure black) bands along the body, then very much like M. pasteuri (this pattern is probably common to all juveniles of the M. olivieri species complex).
Comparison
Mesalina adrarensis sp. nov. resembles (sometimes strongly) M. guttulata, possibly due to adaptation to similar environmental conditions. In comparison with M. guttulata, M. adrarensis sp. nov. has a browner background coloration and a less densely spotted dorsal pattern that often fades on the neck without exhibiting the remarkable pattern of irregularly arranged ocelli that characterizes M. guttulata. Contrary to M. guttulata, M. adrarensis sp. nov. has small light spots and dark marks organized in longitudinal lines. In both species, the eyelids are composed of 2 (rarely 1) large translucent scales, situated above 0 to 8 smaller ones; while in M. guttulata these scales are always edged with a continuous black stripe, in M. adrarensis sp. nov. this black coloration is mostly absent or made of spots along the edges of the scales. Compared with M. olivieri and M. simoni, M. adrarensis sp. nov. has more prominent nostrils, a longer snout, and more flattened head. Dorsal coloration in juveniles of all species of the M. olivieri species complex is characterized by continuous stripes. Meanwhile M. olivieri and M. pasteuri (but less frequent in M. simoni) maintain this striped coloration in their adult forms, these dorsal stripes in adults of M. adrarensis sp. nov. are fragmented. The eyelids of M. simoni and M. olivieri are made of 6–12 medium and small scales but no or (rarely) 1–2 clearly larger scales (cf. Figure 7i).
Adults of M. adrarensis sp. nov. can be easily distinguished from the sympatric M. pasteuri by the lack of obvious dorsal and dorsolateral stripes that characterize the adults of the latter species, and by the brownish coloration typical of M. adrarensis sp. nov. (M. pasteuri has a typical sand color in accordance with its sandy habitat; Figure S5).
Genetic and phylogenetic remarks
The phylogenetic analyses by Kapli et al. (2015), Simó-Riudalbas et al. (2019) and the phylogenetic and nuclear network analyses performed in this study (Figures 2, 3 and 5 and Figures S2–S4; Table 4) validate the specific status of M. adrarensis sp. nov.. The amount of genetic divergence (p-distance) for the Cyt-b gene between the new species and the other members of the M. olivieri complex ranges from 9% (from its sister species M. simoni) to 14% M. olivieri (Table 4). The network analysis of the nuclear genes indicates that, despite the large number of samples of the M. olivieri species complex included in the analysis (70, 85, 71,and 69 for β-fib7, MC1R, PgD7, and OD, respectively), all haplotypes of M. adrarensis sp. nov. are private (Figure 5).
Description of the holotype
Adult female (Figure 7). Slender body slightly depressed in the caudal part. Long and pointed snout with slightly prominent nostrils; SVL = 44 mm, Tail length (entire, not regenerated) = 86 mm, total length = 130 mm, Head length = 10 mm, Head width = 6.4 mm, Head height = 4.3 mm, midbody dorsal scales = 44, small smooth, and regular temporal and dorsal scales, transverse rows of ventral plates = 32 arranged in eight longitudinal rows, enlarged plates in collar = 7, supralabials = 4, infralabials = 7, gular scales (counted along a line from the infrasupralabials to the collar) = 24, femoral pores 15 + 14, lamellae beneath the 4th toe = 19 (slightly keeled). Eyelid disks with five barely translucent scales (two large + three small) not edged with black.
Distribution and habitat
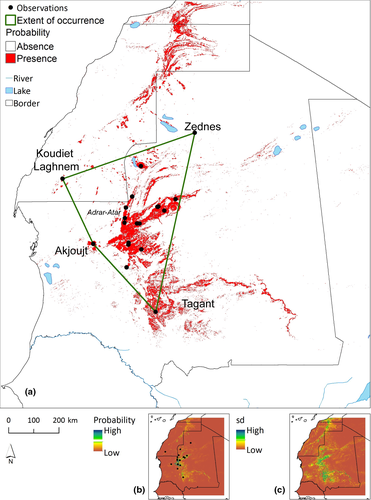
The known range of M. adrarensis sp. nov. encompasses the mountain rocky areas of the Adrar Atar in Mauritania, further extending to the north up to the plateau of Zednes (Observation 13758; Table S10), to the Northwest up to Koudiet Laghnem (Morocco), and to the south down to the central Tagant Mountain (Figure 8). The observation 13758 is a sight record that is unsupported by any photo or tissue sample but has been identified in the field by its typical habitus and habitat (J. C. Brito pers. obs.)
The Area of Occupancy (AOO) calculated from the ecological models was of 34,766 km2, while the Extent of Occurrence (EOO) was of 175,445 km2 (Table S8).
The highest probability of occurrence is at an intermediate levels of terrain ruggedness and in bare rock habitats (Figure 6 and Figure S9). The type locality is a flat and rocky area located on the top of a plateau in the Adrar Atar (Figure 8 and Figure S10b,c). The area is very scarcely vegetated with many stones, mostly low and sparse shrubs and, rarely, Acacia sp. trees (Figure 7j).
Ecology
Specimens were observed active among stones mostly from 11:00 am to 17:00 pm. If threatened it usually seeks shelter under a rock or in spiny bushes. Diet and reproductive features are still unknown.
Conservation status
The AOO calculated from the ecological models was 34,766 km2, while the EOO was 175,445 km2. Most of the habitats are for the moment devoid of major human impact and we are not aware of any direct threat to the species such as direct destruction of human exploitation. Although we do not have any information on the species' population trends, we do not suspect any marked decline based on the lack of direct or indirect menace. Following IUCN guidelines (2017), we thus propose here that the conservation status of M. adrarensis sp. nov. should be Least Concern (LC), based on the high values for the area of occupancy and extend of occurrence and the lack of observed or forecasted population decline. However, the area of occupancy is highly fragmented due to the ecology of the species (Figure 8) and the amount of knowledge currently available for this species remain limited.
Notes
The specimens BEV.10823 (GenBank code KM411138) and CIBIO2952 (GeneBank code KM411139) correspond to a juvenile (BEV.10823) and to a sub-adult (CIBIO2952) M. adrarensissp. nov., previously identified as M. pasteuri in Kapli et al. (2015) and Simó-Riudalbas et al. (2019). The assignment of these two specimens to M. pasteuri was due to their striped color pattern, typical of M. pasteuri. However, both our, Kapli et al. (2015) and Simó-Riudalbas et al. (2019) genetic results assign these two specimens to M. adrarensis sp. nov.. The difference in coloration between these two specimens and the other specimens of M. adrarensis sp. nov. results from strong ontogenetic variation of coloration in M. adrarensis sp. nov. (contrastingly striped coloration in juveniles).
Mesalina simoni saharae ssp. nov.
(Figures 1-5 and 9; Tables 1, 3–6)

Zoobank registration
http://zoobank.org/NomenclaturalActs/30f33118-ec56-48a6-87b9-1d811dac016e
Holotype
Adult male (Figure 9), with code MNHN-RA-2020.0018 (ex BEV.9114) preserved in the Muséum national d'Histoire naturelle, in Paris, France. Collected by Pierre-André Crochet and Julien Renoult on 10th September 2006.
Type locality
Morocco, Atlantic Sahara, road N1, 69 km past Boujdour toward Laayoune, 26.4925°N/−13.9198°W/60 m a.s.l.
Paratypes
Adult male BEV.10849, from 6 km E of Sidi Kathari, 26.5298°N/−12.3364°W, collected by Pierre-André Crochet on 21st March 2010; adult female BEV.10850, from 4 km past Awserd toward Dakhla, 22.5709°N/−14.3544°W, collected by Pierre-André Crochet on the 18th March 2010, all preserved in the BEV collection in Montpellier.
Etymology
The epithet “saharae” refers to the Atlantic Sahara region where this new subspecies is distributed.
Diagnose
A member of the M. olivieri species complex closely related to the nominotypical M. simoni, but with the following combination of characters: (a) eyelids with 5–6 large transparent scales (b) not edged in black (rarely indistinct spots on their edges), (c) dorsal coloration in life generally sandy, sandy-brown, or sandy gray with (d) a poorly marked dorsal pattern mostly composed of longitudinal rows of whitish spots; spots narrowly edged internally by dark coloration (dark inner edge typically 1–2 or 2–3 scales wide), sometimes bordered by a pale grayish dorsolateral line, (e) a dark continuous or near continuous band from the nostril along the flanks to the hind legs constituting the darkest element in the pattern and often including pale spots and their dark edges, (f) a distinctive delicate habitus with elongated body and neck and flattened head (Figure 9 and Figure S5).
Genetic and phylogenetic remarks
The phylogenetic analyses by Kapli et al. (2015), Simó-Riudalbas et al. (2019) and the phylogenetic and nuclear network analyses performed in this study (Figures 2, 3 and 5 and Figures S2–S4; Table 4) support the hypothesis that the populations of M. simoni saharae ssp. nov. belong to the species M. simoni and not to the species M. olivieri. A network analysis of the nuclear genes indicates that, despite the large number of samples of the M. olivieri species complex included in the analysis (70, 85, 71, and 69 for β-fib7, MC1R, PgD7, and OD, respectively), all haplotypes of M. simoni saharae ssp. nov. are shared with M. s. simoni and not with M. olivieri (Figure 5). The amount of genetic divergence (p-distance) in the Cyt-b gene between the new subspecies and the other members of the M. olivieri complex ranges between 4% from M. simoni and 11% from M. olivieri (Table 4). One specimen from the Souss valley, geographically located between the ranges of both subspecies, is morphologically similar to M. s. simoni but is somewhat intermediate in the nuclear and concatenated trees, suggesting a lack of reproductive isolation between simoni and saharae. Based on this and on their level of genetic divergence, we treat saharae and simoni as conspecific pending more detailed analyses of their interactions in contact zones.
Description of the holotype
An adult male (Figure 9) with well-developed femoral pores. Slender and elongated body and slender head; SVL = 41 mm, regenerated tail, Head length = 9.6 mm, Head width = 5.9 mm, Head height = 4.2 mm, midbody dorsal scales = 34, medium sized, slightly pointed, not shining; transverse rows of ventral = 28, longitudinal rows of ventral = 8, enlarged plates in collar = 6, supralabials = 4 + 5, infralabials = 7 + 9, gular scales (counted along a line from the infrasupralabials to the collar) = 25, femoral pores = 10 + 13, lamellae beneath the 4th toe = 20. Eyelid disks with five translucent scales not edged with black.
Coloration in life
See diagnose above. In addition, pileus almost uniform, with faded spots, sometimes distinctly spotted with small dark spots. Fore limbs uniformly sandy-brown, hind legs brownish with faded whitish ocelli. Almost uniform sandy/brown tail with indistinct spots disappearing in the second half of its length. Ventral coloration white, sometimes turning to yellow on the undertail, at least in the specimens from the north of the range.
Distribution and habitat
As understood here, this subspecies' distribution encompasses approximately 173,970 Km2 across the west of the Sahara, from the Mauritanian border to the Tan–Tan area, and as far inland as Awserd (see Figure 4). The current knowledge on this distribution is clearly constrained by the reduced accessibility of the region and is doubtless more extensive than currently known. In its distribution range, this subspecies mostly dwells semi-deserts and coastal steppes with scattered low bushes on loess, coarse sand, or gravel substratum (Figure 9).
Notes
Individuals from Tan–Tan to the Sous-Massa region (Morocco; 1-SOUS in Figures 2-4 and Table 1), seem to be genetically admixed between M. simoni saharae ssp. nov. and M. s. simoni; morphologically, specimens from the Tan–Tan area are rather typical of M. simoni saharae ssp. nov. though while our specimen from the Souss is rather typical of M. s. simoni. More detailed studies are needed to clarify the extant of gene flow between these two subspecies and the taxonomy of these populations that inhabit the area between the cores of the range of the two subspecies.
Taxonomic notes and updated distribution for Mesalina simoni
Our results provide the first direct evidence that M. olivieri and M. simoni are valid, reproductively isolated species (based on lack of allele sharing for nuclear markers in the parapatry area in Morocco, see below). The taxon simoni was recognized as a valid subspecies of M. olivieri by Pasteur and Bons (1960) but was restricted to the population of the M. olivieri complex inhabiting Morocco north and west of the Atlas Mountains (corresponding with our M. s. simoni here). Mesalina simoni was first elevated to species rank by Arnold (1986) based on considerable differences in hemipenial structure between specimens assigned to either M. s. simoni or Mesalina simony olivieri. Further genetic data (e.g., Kapli et al., 2015) failed to recover M. olivieri and M. simoni as reciprocally monophyletic in mtDNA, suggesting an inadequate taxonomy for the complex. Our new taxonomy (which includes in M. simoni most populations of the M. olivieri complex from Morocco) solves these issues as M. simoni and M. olivieri become reciprocally monophyletic. Our nuclear data suggest that there is reproductive isolation in their contact zone in south-eastern Morocco: localities 29 (M. olivieri) and 99 (M. simoni) do not share any alleles in OD or β-fib7 despite their distribution overlapping. The distribution of M. olivieri now stops in the west on the eastern and southern foothills of the High Atlas of Morocco, reaching as far west as the surroundings of Boumalne Dades (see Figure 4). Our understanding of the distribution limits of M. simoni and M. olivieri in north-western Africa is still hampered by the lack of diagnostic external morphological features to separate them. We have examined numerous specimens of the two species and have failed to find any stable morphological difference. Clearly, the morphological characters reported in the literature (see for ex. Hosseinian Yousefkhani et al., 2015) were based on an inadequate appraisal of the considerable morphological variation in M. simoni (see for ex. Figure S5). While M. simoni saharae ssp. nov. is usually reasonably easy to identify, M. s. simoni (north and west of the Atlas Mountains in Morocco) and the various populations of M. simoni ssp. (SOUS and AM) -currently unassigned to any subspecies- are, based on current knowledge, impossible to distinguish morphologically from M. olivieri.
4 DISCUSSION
4.1 Phylogenetic relationships and cytonuclear discordance
Our revision of the M. olivieri species complex relies on several phylogenetic approaches to address the previously reported paraphyly of M. olivieri and M. pasteuri (Kapli et al., 2015; Simó-Riudalbas et al., 2019). We used our concatenated mtDNA and nucDNA tree (Figure 3 and Figure S4) as the best hypothesis to delineate the species-level units in the complex; this results in (at least) four species being part of the olivieri species complex, with M. adrarensis sp. nov. and M. simoni now fully monophyletic relative to all the other members of the complex. The relationships between these four species and M. martini are not fully resolved yet and differ between our datasets (mtDNA or nucDNA). This lack of resolution can stem from the (non-exclusive) action of rapid species divergence resulting in short internodes in the species-tree and incongruence among gene trees due to incomplete lineage sorting, interspecific gene flow during or after the speciation processes and/or lack of power due to short sequence data.
Our results are also affected by an instance of cytonuclear discordance. The position of the Algerian clade ALG1 is poorly supported in nucDNA but is recovered as basal to and highly divergent from all M. olivieri and M. pasteuri samples. This same lineage is closely related to and poorly divergent from some of the M. pasteuri samples in mtDNA, resulting in its embedded position in the M. pasteuri mitochondrial diversity (see Figure 2a and Figure S2). Such incongruences in phylogenetic position and amount of divergence in mtDNA and nucDNA may have be due to past introgression of M. pasteuri mtDNA into the nuclear background of the lineage ALG1, resulted in cytonuclear discordance. Incongruences on the genetic divergence between mtDNA and nucDNA have been reported in other taxa, like Podarcis hispanicus species complex (Renoult et al., 2009), Tarentola mauritanica (Rato et al., 2010), in the genus Plethodon (Fisher-Reid & Wiens, 2011) and Salamandra (Burgon et al., 2021) mostly pointing to incomplete lineage sorting or past gene flow between the lineages (Fisher-Reid & Wiens, 2011; Rato et al., 2010).
4.2 Hidden diversity within M. olivieri and M. pasteuri: Linnean and Darwinian shortfalls
None of our datasets (mtDNA, nucDNA, or combined mt + nucDNA trees) recover M. olivieri and M. pasteuri as reciprocally monophyletic. The Algerian clade of M. olivieri ALG1 is recovered as basal to a pasteuri–oliveri group in our concatenated tree (Figure 3), and it has a different position when mtDNA and nucDNA are analyzed separately (Figure 2a,b and Figures S2 and S3). Indeed, lack of monophyly relative to M. pasteuri as well as deep divergences in both mtDNA and nucDNA datasets in M. olivieri are strong evidence that M. olivieri, as understood here, is made of several biological species. For example, the Algerian populations of M. olivieri harbor two highly divergent lineages: ALG1 and ALG2/ALG3, the latter closely related to the Moroccan lineages MOR2 and MOR3. Most of the other deep lineages in M. olivieri have allopatric distributions, from Israel and Egypt to eastern Morocco, based on our still limited sampling. Interestingly, Arnold (1986) already pointed out variation in hemipenal morphology between populations of what we still treat as M. olivieri, suggestive of several species. Beddek et al. (2018) conducted an exhaustive phylogeographic study comparing North African species with an East–West phylogeographic divide, and highlighted several sutures lines and refugia (e.g., the Rifan corridor and the Kabylia mountain region) that generated vicariance events in the Maghreb. The clade subdivision of M. olivieri recovered in our study mirrors some of the suture lines and refugia uncovered in Beddek et al. (2018), reinforcing the idea that, for this species (a Mediterranean or mesic species), longitudinal fragmentation of ancestral areas was the main force shaping its diversification. We are thus convinced that M. olivieri, even after we restricted it here, still contains several valid biological species distributed along an East–West longitudinal diversification pattern. Regarding M. pasteuri, two well-supported (PP = 100%) lineages have been unveiled in North West Africa (Figure 3 and Figure S4): (a) one mostly distributed on the Atlantic Sahara region (13-MAU1 and 14-MAU2 + MOR); (b) one including specimens from inland Mauritania, Algeria, and Niger (15-MAU3 + ALG and 16-MAU4 + NIG). This noteworthy amount of diversity within M. olivieri and M. pasteuri has already been reported in previous studies (Kapli et al., 2015; Simó-Riudalbas et al., 2019). The first step in identifying these candidate species would be to increase both the number of specimens genotyped and the number of nuclear loci employed. This would allow to generate more robust delimitations of candidate species (i.e., using species delimitation methods such as in Rato et al., 2016), and to better understand their distribution before explicit tests of reproductive isolation in contact zones can be realized (as in Dufresnes et al., 2020). The second step to a correct identification of these candidate species would be a redaction of a new dichotomous key. The keys currently available (Hosseinian Yousefkhani et al., 2015; Trape et al., 2012) were found to be incomplete or, sometimes, inaccurate, probably due to the difficulty of the analysis of old and poorly preserved samples and/or because the M. olivieri species complex is composed of several cryptic species (Bickford et al., 2007). Unfortunately, sampling gaps from the central and west regions of North Africa still hampers the identification and analysis of contact zones between these lineages and preclude assessing the species status of many putative species still “hidden” in the M. olivieri complex. Incomplete species inventory (Linnean shortfall; Brown & Lomolino, 1998) is the first obstacle in biodiversity studies (Hortal et al., 2015). This shortfall can be the reason of incomplete phylogenetic knowledge on the evolution of lineages, species, and traits (Darwinian shortfall, Diniz-Filho et al., 2013). Species affected by these shortfalls generally inhabit large and unsurveyed regions of the world (Bush & Lovejoy, 2007; Hopkins, 2007), such as the cases of M. pasteuri or M. olivieri. The distribution of these species encompasses some of the most remote and politically instable regions in the Sahara Desert, where sample collection is very difficult (Brito et al., 2014, 2018).
4.3 Ecological differences and distribution: Wallacean shortfalls
Following the description of M. adrarensis sp. nov. and the revision of the species limits of M. simoni, we have narrowed the distribution of M. olivieri, which now reaches its western limit in eastern Morocco (Figure 4a). We also extended the distribution of M. simoni (previously endemic to Morocco west and north of the Atlas; see Joger et al., 2006 and Martínez del Mármol et al., 2019), which now occupies most of the Saharan climate regions of Morocco from extreme SE Morocco to the north-western border of Mauritania (Figure 4b). The previous southern limit of M. pasteuri in Mauritania was in the Banc d’Arguin National Park (Sow et al., 2014). Our study provides new records for M. pasteuri (Figure 4c), in south-western Mauritania (Diawling National Park), and south of the previously known distribution in south-eastern Niger. We also identified a wide area of sympatry between M. pasteuri and M. adrarensis sp. nov. (Figure S10), extending about 200 km on either side of the north-western Mauritanian border. While no actual syntopy was detected, certainly because the two species have very different habitat requirement (rocky substratum for M. adrarensis sp. nov., sandy substratum for M. pasteuri, see below), populations of both species were found less than 20 km apart in the north-eastern part of the Adrar Atar plateau (Figure S10). We also identified a potential contact zone between M. pasteuri, M. adrarensis sp. nov., and M. simoni saharae ssp. nov. in extreme southern Morocco (Atlantic Sahara; Figure S10). The known distribution limits of these three species are, to date, around 100 km apart, but with no unsuitable habitats in between. This area is still severely under-sampled, and clearly, it is likely that the three species meet in this area (Figure S10). Further sampling efforts are needed to establish accurate range limits and extent of sympatry, but there are obviously no extrinsic barriers to gene flow between M. adrarensis sp. nov., M. pasteuri, and M. simoni in northern Mauritania and southern Atlantic Sahara. This lack of knowledge in species distribution has been named Wallacean shortfall (Lomolino, 2004). This shortfall is particularly accentuated in remote and inaccessible regions (Hortal et al., 2015), such as Mauritania and southern Atlantic Sahara, and North Africa in general (Brito et al., 2018).
The results of the habitat comparisons revealed clear ecological differences between M. adrarensis sp. nov. and the other species of the complex. Mesalina adrarensis sp. nov. exhibits a narrow niche breadth, inhabiting almost exclusively slopes, wadis and plateaus on rocky substratum (Table S9 and Figures 6 and 7j). This distinction is obvious when M. adrarensis sp. nov. is compared to M. olivieri and M. pasteuri, the two species to which the ADR and TAG lineages were associated until now. Mesalina olivieri and M. simoni are soft substratum specialists inhabiting mostly loess or other soil substratum, normally not sand or rocks, although they can locally inhabit stony areas (i.e., soil with scattered stones), mostly on flat grounds. The presence of M. pasteuri in rocky habitat types (Figure 6 and Figure S10; samples 63, 64 and 65), including rocky plateaus, is an artifact of the land-cover upscaling process (from 30 m to 1 km); M. pasteuri is a well-known sand specialist (Bons, 1960; Trape et al., 2012; pers. obs.), which can persist in sandy micro-habitats scattered inside rocky plateaus (Figure S10b,c), but such micro-habitats are mostly overlooked at the geographic scale we used in the habitat analyses.
4.4 Biogeography of Mesalina adrarensis sp. nov.
The diversification of the olivieri complex started in the Late Miocene (ca. 12.08–6.38 Mya; Figure 3) in concomitance with the beginning of the arid period responsible of the formation of the current Sahara Desert dated approximately 7–6 Mya in Chad (Holmes, 2008; Swezey, 2009) or even more recently further west (Schuster et al., 2006), when a gradual decrease in precipitation and increase of the dust flow led to vegetation collapse (Claussen, 2009; Wang et al., 2008). During the Pliocene (5.3–2.6 Mya), multiple dry-wet cycles affected the region and induced a succession of dry-humid periods (reviewed in Brito et al., 2014). Previous studies identified mountains, highlands and coastal areas as refugia during the arid cycles of the Sahara-Sahel (Brito et al., 2014; Gonçalves, Pereira, et al., 2018). We hypothesize that periods of desertification in the area forced the ancestor of the olivieri complex (probably a mesic species) to contract its distribution in several refugia, possibly centered around the Adrar Atar, Tagant (and other Mauritanian massifs) and the Atlantic coastal areas. Eventually, this isolation resulted in a series of vicariance events that led to speciation giving rise to M. adrarensis sp. nov. and M. simoni. In addition, the low elevation of several regions of the Sahara and the presence of sand before the Sahara's desertification started (Swezey, 2009) suggest that parts of the west and center of the Sahara flooded due to the formation of extended hydrological networks (e.g., Tamanrasset paleo river; Skonieczny et al., 2015). In this scenario, today's plateaus (i.e., Adrar Atar) may have been isolated either by dry areas or flooded areas and acted as “islands” were the ancestors of the current species were trapped (see also Gonçalves, Martínez-Freiría, et al., 2018; Velo-Antón et al., 2018). The latitudinal genetic structure recorded for M. adrarensis sp. nov. and M. simoni (i.e., ADR versus TAG or AS, AM, and SOUS, Figures 2 and 3, and Figures S2–S4) could represent more recent range fragmentation due to varying climatic conditions (Brito et al., 2014) and/or dynamic geographical features (such as river basins; e.g., Gonçalves, Martínez-Freiría, et al., 2018; Velo-Antón et al., 2018) or be the result of isolation by distance in continuous populations. Indeed, vicariance and isolation by distance can be difficult to separate when geographical and/or genomic sampling is sparse (Bradburd et al., 2018).
ACKNOWLEDGEMENTS
This work was funded by National Geographic Society (CRE 7629-04/8412-08), Mohamed bin Zayed Species Conservation Fund (11052709, 11052707, 11052499), Fundacão para a Ciência e a Tecnologia (PTDC/BIA-BEC/099934/2008 and PTDC/ BIA-BIC/2903/2012), FEDER funds through the Operational Programme for Competitiveness Factors - COMPETE (FCOMP-01- 0124-FEDER-008917/028276), and by AGRIGEN–NORTE-01-0145-FEDER-000007, supported by Norte Portugal Regional Operational Programme (NORTE2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF). F.M.F., G.V.A. and J.C.B. were supported by FCT (DL57/2016/CP1440/CT0010, IF/01425/2014, and CEECINST/00014/2018/CP1512/CT0001, respectively), C.P. was supported by the Municipality of Martinengo (Determinazione del 16—11-2020 Settore IV – Servizi Socio Culturali; no. generale:872 – No. settoriale 212). Capture permits were issued by the Haut Commissairat aux Eaux et Forêts (278/2012 and 20/2013/HCEFLCD/DLCDPN/DPRN/CFF) and Ministère de l’Environnement et du Développement Durable of Mauritania (460/MDE/PNBA). Logistic support for fieldwork was given by Pedro Santos Lda (Trimble GPS), Off Road Power Shop, P.N. Banc d’Arguin (Mauritania), Association Nature Initiative (Morocco), and Université des Sciences, de Technologie et de Médecine de Nouakchott. We thank BIODESERTS group members for their assistance during fieldwork and A. Pagan and I. Freitas for the support during the analysis. We are also grateful to P. Kapli and P. Lymberakis from the Natural History Museum of Crete for providing additional tissue samples. We also thank O. Peyre, B. Allegrini, J. Marques, J.M. Pleguezuelos, M. Feriche, A.S. Sow, Y. Hingrat, A. Miralles, M. Beddek, M. Siol, G. Léotard, E. Didner, J. Renoult for help with collecting samples.




